Definition
Eruca sativa Miller (Brassicaceae) is an insect-pollinated diploid annual species which grows spontaneously in the entire Mediterranean basin from semi-arid to arid-hot conditions and is cultivated in Northern America, Europe, and Asia as either salad or oilseed crop. Here, some essential background was provided on this versatile crop, summarizing the present status of Eruca sativa research focusing on the wealth of bioactive ingredients in its seeds, which may find exploitation in agriculture, in the food industries and as nutraceuticals for their antioxidant and anti-inflammatory properties. Fatty acids of Eruca sativa seed oil, gums, glucosinolates and soluble and insoluble phenol and flavonoid fractions in the defatted press cake are the main bioactive compounds considered to date by the scientific literature and that deserve attention for their physical and biological activities.
1. Introduction
Eruca sativa Mill. (Brassicaceae), synonym of E. vesicaria (L.) Cav. subsp. sativa (Mill.) Thell, is the only taxon of Eruca that has been cultivated since Roman times (Figure 1). At present, it is mainly distributed in Southern Europe, North Africa, the Middle East and Asia, where it is typical in Pakistan, Afghanistan and India. It spontaneously grows in the Mediterranean basin, and it is cultivated in Europe and America mostly as a baby-leaf crop [1,2], whilst in Iran and in the Indian subcontinent it is considered an oilseed crop [3]. It is a fast-growing crop (it usually takes 20–30 days after germination for harvesting as a leafy vegetable, and 120–250 days for a complete growing cycle) and can be sowed both in autumn-winter and early spring [4,5]. In recent years, it has been cultivated as a salad via hydroponics and greenhouses to provide higher quality and yields [4,6].

Figure 1.
Eruca sativa cultivated field in the CREA experimental farm located in Bologna (Italy)—flowering time.
The Eruca sativa Mill. genome (2n = 22) and transcriptome have recently been published [7], but rigorous phylogenetic studies are absent from the literature due to the great genetic diversity in the species [1], with the exception of a recent analysis of phylogenetic relationships in the Brassicaceae family based on the complete chloroplast genome determination of E. sativa [8]. Despite ancient reports of its use, very limited breeding activities have been carried out prior to the mid-1990s, when the first meeting of the Rocket genetic resources network was held in Lisbon [9], with the aim of improving germplasm collection, conservation, and characterization. To date, less than 100 varieties are registered in the European Community Plant Variety Office (CPVO) database [10], with the oldest registered one dating to 2004. Reflecting the geographical difference in uses, the CPVO technical protocol for this species primarily focuses, however, on the characteristics of leaves [11], neglecting both seed-related and phytochemical traits. These and other genetic and agro-morphological traits are the subject of scientific studies that, interestingly for breeding purposes, found within the species a wide diversity [12,13,14,15]. Some characteristics of this plant pose challenges to conservation and breeding programs: the relevant degree of self-incompatibility, the allogamy that makes it difficult to keep varieties stable, and the impossibility to transfer genes of interest through intergeneric crosses limit the potential of traditional breeding [16]. Despite these difficulties, E. sativa deserves research attention, as it is a very interesting plant for its high adaptation to arid and semi-arid soils, which are rapidly growing in its cultivation area due to climate changes [17]. Among other uses, E. sativa seeds can be considered as a promising feedstock for biorefinery and, according to a recent life cycle assessment, it may save greenhouse gas emissions by about 150% in comparison to neat diesel [18]. In addition to that, several parts of the plant, and in particular its seeds, possess bioactive compounds which may find several industrial applications and are studied also for their health-promoting activities, which include the antimicrobial, antioxidant, antiproliferative, antiemetic, and antiulcer [19,20,21,22,23].
The steady growth in publications on E. sativa over the last two decades is a proof of the potential of this crop. Here the present status of E. sativa research was provided, highlighting the wealth of bioactive ingredients in its seeds.
2. Components and Bioactive Molecules in Eruca sativa Seeds
The seeds of E. sativa are characterized by oil (30–40%), a significant amount of total carbohydrates (20–25%), crude fibres (20%), and crude protein (20–30%) [5,24,25]. To date the protein fraction has been studied less than the others, however it can have important applications in agriculture as an ingredient for organic fertilizers or as animal feed [26,27]. Moreover, one recent publication brings evidence in E. sativa seeds of a napin, a protein of about 16 kDa that inhibits Fusarium graminearum growth and also shows promising antitumor properties [28]. Part of the carbohydrates and proteins forms a gum or mucilage content (2–4%) [29], and the rest of the E. sativa seeds consist of a wide range of bioactive phytochemical compounds, such as polyphenols and glucosinolates (Figure 2).
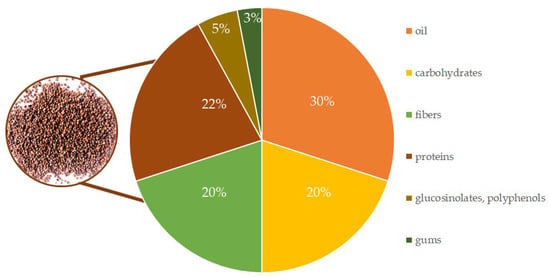
Figure 2.
Main chemical composition of dried raw Eruca sativa seeds.
2.1. Oil
E. sativa is an interesting oilseed crop due to its high content of erucic acid, which is an anti-nutritional compound and limits the oil applications for food and feed purposes [30,31], even if at very low concentrations it could be used as an antidiabetic complement [32] or as a potent antioxidant and antimicrobial oil [33,34,35]. The presence of high erucic acid concentration makes the E. sativa oil suitable for many other different uses: from biofuels to cosmetics and detergents, to polymer production and also as an ingredient for pest management in agriculture [36,37,38,39,40,41].
The oil yield from E. sativa mild oil extraction with cold press methods is 20–30%. The residual oil in the defatted seed meals ranges from 17 to 21%, and its fatty acid profile is similar to that of the extracted oil [42,43,44]. In a study carried out on a cultivar selected for oil production, the oil yield from autumn sowed plants was higher than from spring sowed ones [5], while both in Italy and in Turkey the level of erucic acid proved to be stable in the different field conditions [5,45].
Both the oil content and fatty acid profile of E. sativa seeds were recently evaluated in 66 genotypes originating from several localities. Golkar et al. [13] showed that the oil content in the seeds of selected genotypes varied between a minimum of 16.2% in a sample that came from China and a maximum of 38% in a sample that came from Pakistan. E. sativa seeds’ fatty acid profiles are mainly characterized by oleic, linoleic, linolenic, and erucic acid, with few exceptions for fatty acid profiles of E. sativa oils extracted from genotypes from Syria and Pakistan, which showed a higher concentration of stearic compared to linolenic acid. Erucic acid was the major fatty acid in all of the analysed genotypes, ranging from 25.9% to 53.6% of the total fatty acid composition, except for an accession from Iran that has a fatty acid profile characterized by a high content of linoleic acid, and one genotype from Pakistan whose oil contains more linolenic than erucic acid [13].
E. sativa crude oil is considered as an alternative to mineral oil in many industries, and it has good potential for biodiesel production due to his high productivity and good stability at room temperature [46,47]. Moreover, it finds applications in the production of lubricants, soap, and for cosmetic, diuretic, stimulant, stomachic and depurative uses [48]. Recently, the antifeedant activity of E. sativa cold pressed oil against the plant pest Xanthogaleruca luteola under laboratory conditions was also explored [38].
2.2. Gums
The E. sativa seed’s epidermal cells contain a valuable portion of gum or mucilage, which could have great potential as a hydrocolloid for providing viscosity and stability in the food industry, or as a delivery system for bioactive compounds during food thermal processing and digestion times. They can also be used also in combination with polyvinyl alcohol for producing nanofibers, which may find applications in the food and pharmaceutical industries as bioactive compound encapsulators [49].
Rocket seed gums (RSG) are a cream-colored powder which can be extracted in deionized water from whole E. sativa seeds, and which have a good emulsion stabilizing effect [50,51]. They are anionic polysaccharides mixed to mucilaginous material and their extraction procedures have been studied since 2012, while their chemical characterization has been reported in very recent studies [52]. According to Koocheki et al. [51] the optimum theorical conditions for mucilage extraction to achieve the best yield and viscosity are 60:1 (v/w) water:seed ratio (16.7 g L−1), pH 4 and 65.5 °C. When extracted at 45 °C, pH 4 and with a 20:1 (v/w) water:seed ratio (50 g L−1), RSG contained 67.97% carbohydrates, 9.75% protein, 12.28% moisture, 10% ash, and no fat. These proportions may change depending on the extraction conditions. Kutlu et al. [52] found that RSG extracted in the same water:seed ratio conditions, but at 80 °C had a carbohydrate, protein, moisture, and ash content of 80.38%, 5.81%, 10.26%, and 3.55%, respectively. In another study of the same group, Akcicek et al. [50] obtained an RSG characterized by 57.49% carbohydrates, 0.69% fat, 8.26% ash, 10.5% moisture and a very high content of protein, i.e., 23.01%, starting from the same temperature and water:seed ratio. This water:seed ratio, slightly different from the one predicted in [51], has been recently adopted to obtain RSG for food industry applications, that is for obtaining mucilages which can be used as natural fat replacers in low-fat salad dressings such as new low-fat vegan mayonnaise [29,50,53]. The RSG extraction procedure may also be carried out starting from E. sativa defatted seed meals (DSM), which are by-products of oil extraction. Hijazi et al. [53] starting from E. sativa DSM produced RSG with the same operation procedure used by Kutlu et al. [52], and they obtained a powder characterized by 70.48% carbohydrate, 11.00% protein, 1.94% fat, 9.95% moisture, and 6.63% ash. The monosaccharide composition of RSG, analyzed after acid hydrolysis in H2SO4 by high performance anion exchange chromatography with a pulsed amperometric detector, revealed a high content of mannose (39.12%), and glucose and galactose accounting for 10.26% and 22.08%, respectively [53].
2.3. Glucosinolates
Glucosinolates (GSLs), also known as (Z)-N-hydroximinosulfate esters, are secondary metabolites of Brassicaceae and plants of the Brassicales order consisting of a common glycone group and a variable aglycone side chain (R) derived from amino acids. GSLs have a sulfonate moiety with a pKa value of ca. 2 that makes them hydrophilic, negatively charged compounds at neutral pH [54]. They may be hydrolyzed by a class of endogenous thioglucosidases, the myrosinases, into a wide spectrum of products: isothiocyanates (ITC), nitriles, epithionitriles, hydroxynitriles, oxazolidine-2-thiones, thiocyanates, and indoles, depending on pH, associated proteins, cofactors and other reaction conditions [55]. Myrosinase enzymes are usually present in the same vegetal tissues where GSLs accumulate, but they are compartmentalized in different types of cells. Upon tissue damage in the presence of water, they hydrolyze the GSLs in their active products. Among these, the ITCs, produced mainly at neutral pH, are the most known and studied for their antioxidant, anti-inflammatory, cytostatic and apoptotic characteristics in cancer cells, in addition to their antifungal and bacteriostatic activities [55,56].
E. sativa seeds are characterized by the presence of two main GSLs: 4-methylthiobutyl GSL or glucoerucin, and 4-methylsulfinylbutyl GSL or glucoraphanin, when analyzed as desulfo-GSLs with standard high-performance liquid chromatography (HPLC-UV) procedures [42]. The total GSL content in E. sativa seeds was found to be in the range 108–125 µmol g−1, with glucoerucin accounting for more than 94–95% of total GSLs, and a slightly higher total GSL content in spring sowing in comparison to autumn sowing [5,57,58]. These data are consistent with the first secondary metabolites profiling of E. sativa seeds provided by Bennett et al. [59], who further demonstrated that profiles and amounts of GSLs in the seeds of E. sativa from different suppliers varied very little, suggesting a common genetic origin for most commercial seeds. In 2007, using liquid chromatography coupled to electrospray ionization and a quadrupole ion-trap analyzer (LC/ESI-QIT-MS) for analysis of intact GSLs, Cataldi et al. [54] identified in E. sativa seeds, beside glucoerucin and glucoraphanin amounting to >98% of the total GSLs, a third GSL, the N-heterocycle 4-methoxyglucobrassicin, probably one of the indolic compounds hypothesized by Bennett et al. [59] (Figure 3).

Figure 3.
Glucosinolates in Eruca sativa seeds.
E. sativa DSMs are naturally enriched with GSLs and, depending on the oil extraction procedures and further formulations, they represent interesting and cheap ingredients for several applications, from pest management in agriculture to human and animal health [60,61,62,63,64,65,66,67]. Herein, DSMs according to the method of oil extraction (solvent or mechanical) and the presence or absence of myrosinase activity (active and deactivated DSM, respectively) can be classified. Following these criteria, here the main studies on E. sativa DSMs and on the GSL-enriched extracts that can be obtained from these DSMs were reported.
2.3.1. Active Defatted Seed Meals for Agricultural Uses
Oil extraction with hexane permits to obtaining of defatted meals with a GSL concentration up to 150 µmol g−1 [60,61,62], which usually also retains a myrosinase activity of about 20 U, with one enzyme unit (U) corresponding to 1 µmol g−1 DSM of sinigrin transformed in 1 min [42]. The low level of humidity of the DSM, however, blocks the myrosinase activity, which can be restored only with the addition of water. These DSMs extracted with hexane were successfully used in several experimental formulations for the containment of plant pests, diseases, and weeds [60,61,63], highlighting their potential role in implementing modern cropping systems and agricultural management plans able to achieve good crop yields and at the same time a safer food production chain for the environment and the consumers. In fact, studies on the development of formulations based on E. sativa DSM are ongoing, with the aim of addressing the growing need of farmers to find sustainable solutions. This need is growing due to the continuous phase out of synthetic pesticides in the integrated pest management sector, and even more in organic farming, where, at the same time, the availability of biobased herbicides, for example, is definitely scarce and weed control is mainly carried out with increasingly expensive and time-consuming agronomic techniques. With these assumptions, Matteo et al. [60] proposed a new formulation based on E. sativa DSM and crude glycerin, showing an interesting inhibition of lettuce seed germination (about 90% inhibition compared to the untreated control), also further preventing the development of seedling biomass. However, when the formulation was applied to spontaneous and less sensitive plants, such as Alopecurus myosuroides, the phytotoxic effect greatly declined (a reduction of germination of around 20% compared to the untreated control).
In Giannini et al. [61] the application of E. sativa DSM was tested for its potential weed control activity against both cultivated plants—Cynara cardunculus L. (cardoon) and Eruca sativa cv. Nemat (rocket)—and weeds—Silybum marianum (L.) Gaertn. (milk thistle) and Malva sylvestris L. (mallow)—representative of the Mediterranean flora. The experiments showed that mallow was mainly injured by direct contact through soaking, whilst milk thistle was mainly affected by the volatile compounds released from the DSM. It is reasonable to expect that the most represented compounds in the volatilome of E. sativa DSM include 4-methylthiobutyl ITC (erucin), since the most represented GSL in E. sativa seed is glucoerucin, as discussed. Other studies, for example, observed that hydrodistilled extract from E. sativa green siliques showed values of erucin ranging from 17 to 32 ppm depending on the hydrodistillation method. 4-methylthiobutyl ITC and 5-methylthiopentanenitrile can reach 81.7% and 17.7% of total VOCs, respectively [68]. Among the other results, in Giannini et al. [61] an auto-toxic effect of E. sativa DSM on its seeds was documented for the first time. From the same study it emerges that, although there is an effect due to volatile components, in most cases the phytotoxic effect of E. sativa DSM formulation is determined by contact, affecting development parameters of the seedlings, such as plant development percentage, germination synchronization, average germination time and others [61].
Other studies have shown that E. sativa DSM is one of the possible solutions in the containment of soil-borne parasites of great impact such as nematodes. In 2016, Curto et al. [63] found that amongst 13 hexane DSMs from different plants belonging to the Brassicaceae family, the best results in the containment of Meloidogyne incognita were achieved by the E. sativa DSM containing 121 μmol g−1 total GSL, of which 91% was glucoerucin [63]. The containment of the nematode was even higher than that achieved with other products already on the market and considered the state of the art of biofumigant products, such as sinigrin-containing B. carinata DSM. Hexane extracted E. sativa DSM was also tested, among other Brassicaceae DSMs, for its antimicrobial activity towards pathogenic bacteria in pig manure, with the aim to reduce its bacterial load and to limit the problem of bacterial antibiotic resistance in animal farming and agriculture due to the use of pig manure as fertilizer [62]. In this work, the authors monitored the release from DSM of the active compound erucin in buffer and pig manure solutions. The maximum concentration of erucin was promptly reached within 5 min and was maintained in the range of 80–95% for one hour of incubation in both buffer and pig manure. Erucin, produced in situ from the hydrolysis of pure glucoerucin and E. sativa DSM, also showed good activity against Escherichia coli ATCC 8739, Staphylococcus aureus ATCC 6538, and Enterococcus faecalis ATCC 8043 in in vitro assays, and erucin MIC (minimum inhibitory concentration) was determined as 6.25 mM for the three bacterial pathogens [62].
2.3.2. Active Food-Grade Defatted Seed Meals for a Nutraceutical Purpose: E. sativa Defatted Seed Meals from Cold-Press Oil Production
The use of solvents in oil extraction can impair DSM safety for animal and human uses, but E. sativa DSMs can be also produced by mechanical processes in crusher machines for small seeds, a solvent-free food-grade procedure. The concentration of total GSLs in E. sativa DSM produced in crusher machines was found in the range 90–138 µmol g−1 [42,43,64,65,66]. This ingredient can be interesting when used for formulations in which the ITC release is needed at the time of its administration or application. This occurs when the ingredient comes hydrated. Indeed, without any additional treatment for residual myrosinase deactivation, DSM from seed crushing preserves a mild myrosinase activity [42,43], which can be useful when a sustained release of ITC is necessary to achieve a stronger and prompt biochemical effect [43]. This kind of ingredient was recently tested in several applications: for its therapeutic efficacy against neuropathic pain, both in the case of diabetic neuropathy and in the case of visceral pain due to colitis induced by 2,4-dinitrobenzenesulfonic acid in in vivo models [43,64], and as a starting material to produce GSL enriched lyophilized extracts, which were proved to be effective in the prevention of cardiovascular disorders and metabolic diseases, such as obesity [65,66].
2.3.3. Deactivated Food-Grade Defatted Seed Meals for Nutraceutical Purpose: AutoClaved E. sativa Defatted Seed Meals from Cold-Press Oil Production
E. sativa DSMs produced by mechanical processes in crusher machines for small seeds, if needed, can be treated for myrosinase deactivation, which is autoclaved, to stabilize the concentration of GSLs. In such deactivated DSMs, GSL hydrolysis could be achieved and/or enhanced through the addition of active exogenous myrosinase [43,67] or, depending on the application, thanks to the myrosinase activity that has been observed in microbiomes in the environment, in soil and in the intestine of many animals and of humans [69,70,71,72]. E. sativa DSM autoclaved for 20 min at 120 °C, with a total GSL content in the range of 75–100 µmol g−1, were recently studied as ingredients for human [42,67] and bee health-promoting products [44,70]. For nutraceutical purposes, the naturally GSL enriched deactivated E. sativa DSM was tested as an ingredient for crackers produced in an industrial plant. The addition of only 1% (w/w) of E. sativa DSM to standard industrial recipes of crackers ensured an intake of GLS up to 75 μmol 100 g−1 of product [42]. The bakery products were included in a small pilot study on glucose and lipid metabolism and on systemic markers of inflammation, by asking 19 adult patients to replace the total carbohydrate portions (bread and pasta, or other bakery products) with 150 g day−1 of DSM-enriched crackers for a 4-week period. This preliminary trial showed a significant improvement in inflammation markers such as C-reactive protein and TNF-α, a reduction in cholesterol ratio, but also in Gamma-GT, which is activated in the fatty liver, and a reduction of hepatomegaly after ultrasound examination [67]. Autoclaved E. sativa DSM were also inserted in patties for Apis mellifera feeding. In controlled conditions, formulates enriched with glucoerucin and glucoraphanin from two DSM concentrations, 2 and 4% (w/w), showed good palatability and did not exert toxic effects on bees, while significantly reducing the development of the parasite Nosema ceranae in artificially infected bees [44]. In the field, the treatment with the highest E. sativa DSM concentration of 4% (w/w) in patty formulations was applied to fully developed colonies naturally infected with N. ceranae [70]. In these field trials, even if the treatment with GSL enriched patties did not influence the N. ceranae spread in infected bees, a significant decrease in the number of N. ceranae copies in both foragers and house bees was observed. Interestingly, ITCs and/or ITC-adducts, detected by the cyclocondensation assay, were found in bee guts, indicating the possible presence of a myrosinase-like activity able to hydrolyze ingested GSL from DSM. This enzymatic activity was further demonstrated in in vitro assays performed at pH 6.5 and 25 °C by incubating gut extracts with pure GSLs and detecting formed ITCs by GC-MS. Furthermore, the GSL glucoraphanin and the erucin nitrile, as hydrolysis products derived from glucoerucin, were found in the honey [70].
2.3.4. Extracts from Defatted Seed Meals Enriched in Glucosinolates
Finally, all kinds of DSMs from E. sativa can be used as starting materials for the realization of GSL- enriched extracts, with GSL concentration that can reach 400–520 μmol g−1, and with a glucoerucin/glucoraphanin ratio of about 20, comparable to the ratio in the whole seeds [65,66]. These extracts are oil-free, and the GSL content is stable during storage for a period of about 12 months at −20 °C.
The efficacy in counteracting neuroinflammation in NSC34 motor neurons of an E. sativa seed extract has been reported, but this extract was characterized by a glucoerucin concentration of about a quarter of the one previously mentioned [73].
Numerous studies investigated the hypothesis that the beneficial effects of E. sativa ITC, but also of other Brassicaceae derived ITC, are, at least in part, due to their capability to release H2S [74,75]. The mechanisms underlying the release of the gasotransmitter H2S from ITC have not yet been fully clarified, even if an L-cysteine-mediated reaction was proposed [76]. Experimental evidence proved that in the intracellular environment an increase in H2S release can be sustained also in the presence of unhydrolyzed GSLs: glucoraphanin was able to release H2S in human mesenchymal stromal cells [77], and glucoerucin and glucoraphanin from E. sativa lyophilized extracts were found to slowly release H2S in a phosphate buffer, both in the presence and in the absence of L-cysteine [65]. At the same time a greater efficacy of DSM or extracts in comparison to pure GSLs and GSL-derived ITC was reported [43,65]. Martelli et al. [78] evidenced in in vivo studies that erucin, the ITC produced by the hydrolysis of glucoerucin purified from E. sativa seeds, can reduce systolic blood pressure in spontaneously hypertensive rats by about 25%, at a dose of 10 mg kg−1 or 60 µmol kg−1. A comparable result was obtained in a similar experiment conducted by treating the same animal models with 100 mg kg−1 of E. sativa lyophilized extracts, at 38 µmol kg−1 of glucoerucin, without any bioactivation by exogenous supplementation of myrosinase [65].
These observations support the hypothesis that other substances characterizing the E. sativa seed extracts, such as flavonoids and phenolic acids, may act synergically with GSLs through antioxidant mechanisms and signal transduction, but also favoring the H2S release from sulfur compounds (including intact and/or hydrolyzed GSLs) and in this way they may exert the beneficial effects reported on cardiovascular homeostasis, metabolic diseases, neuropathic pain and gastrointestinal inflammation [43,64,65,66].
2.4. Polyphenols
Polyphenols are plant secondary metabolites known for their antioxidant activity and potential beneficial effects on human health, and their use for prevention and/or treatment of oxidative stress-induced diseases has been extensively investigated [79].
Similarly to what was stated by Bennett et al. back in 2006 [59], there are still limited data on the phenolic and flavonoid content of rocket species and in particular of E. sativa seeds. In the same work, using LC/MS, the authors detected in methanolic extracts of two different seed sources quercetin flavonoids mainly represented by quercetin 2-O-glycoside and quercetin monosinapoyl tri-O-glucoside, and isorhamnetin as isorhamnetin feruloyl tri-O-glucoside. More recently, Sharma et al. [48] found and identified the phenols caffeoyl glucose, 3-caffeoylquinic acid, and sinapic glucoside, and the flavonoids apigenin-7-O-glucoside, isorhamonetin-3-O-rutinoside, kaempferol-3-O-glucuronide and isorhamonetin-3-O-(3″-acetylglucoside), by using UPLC-DAD and UPLC-ESI-QTOF analysis of aqueous methanolic extracts [48]. In 2021 Abd-Elsalam et al. [80] tentatively identified 39 compounds by means of LC-ESI-MS in an E. sativa ethanolic seed extract obtained after a very long extraction (72 h) of powdered seeds without previous defatting. This extract contained fatty acids, GSLs (glucoerucin and glucoalyssin), desulfated GSLs, flavonoid glycosides derived from isorhamnetin, quercetin, kaempferol, myricetin, naringenin, proanthocyanin, and procyanidin, and caffeoyl-O-hexoside, chlorogenic and sinapic acid, but no quantitative analysis information was provided. A protective role of the E. sativa seed extract against toxic effects triggered by acrylamide was reported in the form of antioxidant and anti-apoptotic effects in testicular cells [80].
In a later study, the HPLC-UV analysis of an aqueous E. sativa seed extract revealed a concentration of about 5 mg g−1 of rutin (quercetin-3-rutinoside) and the presence of sinapic, p-hydroxybenzoic, chlorogenic, and p-coumaric acids [81]. Abdelkader et al. [81] characterized and studied the nephroprotective effect of the aqueous extract of E. sativa seeds in comparison to pure rutin in an in vivo experiment after gentamicin treatment. Gentamicin is an aminoglycoside antibiotic that is commonly used for gram-negative bacterial infections, which accumulates in the proximal tubules of the kidney and may induce nephrotoxicity associated with an increase of creatinine and urea in the serum and an unbalance of Na+ and K+ electrolytes. The authors demonstrated that both E. sativa seed extracts and rutin may protect kidneys from gentamicin- induced nephrotoxicity and low doses of E. sativa extracts (150 mg kg−1, that is 750 µg rutin kg−1) and decrease the oxidative damage induced by the antibiotic. On the other hand, a double dose of E. sativa seed extract induced an increase in nitric oxide at the kidney level in gentamicin-nephrotoxic animals, which was not reported after treatment with 50 mg kg−1 and 100 mg kg−1 rutin [81]. Furthermore the E. sativa extract, both at low and high doses, significantly reduced the inflammatory cascade activated by gentamicin, after nephrotoxicity induction, triggering a significant reduction of TNF-α and IL-1β [81]. As discussed above, the main beneficial effects of E. sativa seeds may be related to both the high antioxidant and anti-inflammatory activities of GSL/GSL hydrolysis products and to the presence of flavonoids. They may act directly with a free radical scavenging activity, through the modulation of phase-2 enzyme expression and the consequent detoxification from electrophiles, but also through the regulation of the expression of several inflammatory markers and the release of H2S [73,74,82].
If ITCs from GSLs have already been under observation as natural H2S donors for some time, recent evidence proved that rutin, which is reported to be among the main flavonoids in two E. sativa seed extracts [65,81], showed antidiabetic, antioxidant and anti-inflammatory properties in vivo, and significantly increased H2S levels [83].
Recently, Testai et al. [65] demonstrated in vivo the beneficial effects on the cardiovascular system of a lyophilized ethanolic extract from E. sativa DSM, characterized by the presence of about 170 mg g−1 total GSLs and 20 mg g−1 phenols, with gallic acid, sinapic acid, vanillic acid and vanillin as the main components (1–8 mg g−1), and flavonoids such as luteolin, vitexin, naringenin and rutin (3 mg g−1). Notably, the extract, which was able to release H2S in an L-cysteine-independent manner, had a content of rutin higher than the starting DSM [65]. Other polyphenols isolated from E. sativa showed similar and possibly synergic effects to glucoerucin. For example, kaempferol decreases pain sensitivity in streptozotocin-induced diabetic neuropathy in the same model that is also used to study the effects of glucoerucin and E. sativa DSM [43,84]. A comprehensive overview of the antinociceptive, anti-obesity, anti-inflammatory, anti-hypertensive activities and of protective effects against chemical induced nephrotoxicity and testicular dysfunction in in vivo models was provided (Table 1).

Table 1.
In vivo effects of Eruca sativa defatted seed meals and Eruca sativa seed extracts.
3. Conclusions and Prospects
Eruca sativa seeds have been studied mainly for their fatty acid profiles and for their high glucosinolate content. Recently, their gums and the soluble and insoluble phenols and flavonoids have also attracted scientists’ attention for their interesting applications in the food industry, in agriculture for plant protection and as nutraceuticals for their antioxidant and anti-inflammatory properties. Among all the Eruca sativa seed components, fibers and protein are still lacking in dedicated studies in the literature. Eruca sativa seed co-products represent a sustainable source of biomolecules with applications in agriculture and the food industry, and the perspective of deepening the knowledge of fractionating procedures, analytical separations and high added-value molecule identification is fundamental for a better understanding of their mechanisms of action and for the design and realization of even more innovative bio-based materials and formulations.
Author Contributions
E.P. writing—original draft preparation; L.U., R.M., L.R. writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by CARIPLO FOUNDATION, in the frame of SUSINCER Project (Bioactive Compounds from Brassicaceae and Solanaceae waste for cereals crop protection; Project code. 2019-2538, and carried out within the Agritech National Research Center and received funding from the European Union Next-GenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)—MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4—D.D. 1032 17/06/2022, CN00000022). This manuscript reflects only the authors’ views and opinions, neither the European Union nor the European Commission can be considered responsible for them.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this entry. No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Bell, L.; Wagstaff, C. Rocket science: A review of phytochemical & health-related research in Eruca & Diplotaxis species. Food Chem. X 2019, 1, 100002. [Google Scholar] [CrossRef] [PubMed]
- Bantis, F.; Kaponas, C.; Charalambous, C.; Koukounaras, A. Strategic successive harvesting of rocket and spinach baby leaves enhanced their quality and production efficiency. Agriculture 2021, 11, 465. [Google Scholar] [CrossRef]
- Mirzabe, A.H.; Hajiahmad, A.; Asadollahzadeh, A.H. Moisture-dependent engineering properties of arugula seed relevant in mechanical processing and bulk handling. J. Food Process Eng. 2021, 44, e13704. [Google Scholar] [CrossRef]
- Yang, T.; Samarakoon, U.; Altland, J.; Ling, P. Photosynthesis, biomass production, nutritional quality, and flavor-related phytochemical properties of hydroponic-grown arugula (Eruca sativa Mill.) ‘standard’ under different electrical conductivities of nutrient solution. Agronomy 2021, 11, 1340. [Google Scholar] [CrossRef]
- Lazzeri, L.; Errani, M.; Leoni, O.; Venturi, G. Eruca sativa spp. oleifera: A new non-food crop. Ind. Crops Prod. 2004, 20, 67–73. [Google Scholar] [CrossRef]
- Guffanti, D.; Cocetta, G.; Franchetti, B.M.; Ferrante, A. The effect of flushing on the nitrate content and postharvest quality of lettuce (Lactuca sativa L. var. acephala) and rocket (Eruca sativa mill.) grown in a vertical farm. Horticulturae 2022, 8, 604. [Google Scholar] [CrossRef]
- Bell, L.; Chadwick, M.; Puranik, M.; Tudor, R.; Methven, L.; Kennedy, S.; Wagstaff, C. The Eruca sativa genome and transcriptome: A targeted analysis of sulfur metabolism and glucosinolate biosynthesis pre and postharvest. Front. Plant Sci. 2020, 11, 525102. [Google Scholar] [CrossRef]
- Zhu, B.; Qian, F.; Hou, Y.; Yang, W.; Id, M.C. Complete chloroplast genome features and phylogenetic analysis of e Eruca sativa (Brassicaceae). PLoS ONE 2021, 16, e0248556. [Google Scholar] [CrossRef]
- Padulosi, S. Rocket Genetic Resources Network. Report of the First Meeting, 13–15 November 1994, Lisbon, Portugal; IPGRI: Rome, Italy, 1995. [Google Scholar]
- Community Plant Variety Office Database. Available online: https://online.plantvarieties.eu/ (accessed on 10 August 2022).
- Protocol for Tests on Distinctness, Uniformity and Stability—Eruca sativa Mill. Available online: https://cpvo.europa.eu/sites/default/files/documents/eruca_1.1.pdf (accessed on 10 August 2022).
- Bajpai, P.K.; Weiss, H.; Dvir, G.; Hanin, N.; Wasserstrom, H.; Barazani, O. Phenotypic differentiation and diversifying selection in populations of Eruca sativa along an aridity gradient. BMC Ecol. Evol. 2022, 22, 40. [Google Scholar] [CrossRef]
- Golkar, P.; Bakhtiari, M.A. Evaluation of genetic diversity in the world collection of Eruca sativa L. using oil content, fatty acids and molecular markers. Ind. Crops Prod. 2020, 148, 112280. [Google Scholar] [CrossRef]
- Guijarro-Real, C.; Navarro, A.; Esposito, S.; Festa, G.; Macellaro, R.; Di Cesare, C.; Fita, A.; Rodríguez-Burruezo, A.; Cardi, T.; Prohens, J.; et al. Large scale phenotyping and molecular analysis in a germplasm collection of rocket salad (Eruca vesicaria) reveal a differentiation of the gene pool by geographical origin. Euphytica 2020, 216, 53. [Google Scholar] [CrossRef]
- Zafar-Pashanezhad, M.; Shahbazi, E.; Golkar, P.; Shiran, B. Genetic variation of Eruca sativa L. genotypes revealed by agro-morphological traits and issr molecular markers. Ind. Crops Prod. 2020, 145, 111992. [Google Scholar] [CrossRef]
- Tripodi, P.; Coelho, P.S.; Guijarro-Real, C. Breeding advances and prospects in rocket salad (Eruca vesicaria ssp. sativa Mill.) cultivation. In Advances in Plant Breeding Strategies: Vegetable Crops; Springer International Publishing: Cham, Switzerland, 2021; pp. 95–133. [Google Scholar]
- Hanin, N.; Quaye, M.; Westberg, E.; Barazani, O. Soil seed bank and among-years genetic diversity in arid populations of Eruca sativa Miller (Brassicaceae). J. Arid Environ. 2013, 91, 151–154. [Google Scholar] [CrossRef]
- Rahimi, V.; Karimi, K.; Shafiei, M.; Naghavi, R.; Khoshnevisan, B.; Ghanavati, H.; Mohtasebi, S.S.; Rafiee, S.; Tabatabaei, M. Well-to-wheel life cycle assessment of Eruca sativa-based biorefinery. Renew. Energy 2018, 117, 135–149. [Google Scholar] [CrossRef]
- Rizwana, H.; Alwhibi, M.S.; Khan, F.; Soliman, D.A. Chemical composition and antimicrobial activity of Eruca sativa seeds against pathogenic bacteria and fungi. J. Anim. Plant Sci. 2016, 26, 1859–1871. [Google Scholar]
- Khalil, N.; Gad, H.A.; Al Musayeib, N.M.; Bishr, M.; Ashour, M.L. Correlation of glucosinolates and volatile constituents of six Brassicaceae seeds with their antioxidant activities based on partial least squares regression. Plants 2022, 11, 1116. [Google Scholar] [CrossRef]
- Yehuda, H.; Khatib, S.; Sussan, I.; Musa, R.; Vaya, J.; Tamir, S. Potential skin antiinflammatory effects of 4-methylthiobutylisothiocyanate (mtbi) isolated from rocket (Eruca sativa) seeds. BioFactors 2009, 35, 295–305. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, D.; Singh, G.; Attri, S.; Singh, D.; Sharma, M.; Buttar, H.S.; Bedi, N.; Singh, B.; Arora, S. Pharmacokinetics and toxicity profiling of 4-(methylthio)butyl isothiocyanate with special reference to pre-clinical safety assessment studies. Toxicon 2022, 212, 19–33. [Google Scholar] [CrossRef]
- Qaiyyum, I.A.; Nergis, A. The therapeutic uses and pharmacopeal action of jirjeer (Eruca sativa): A review. CELLMED 2022, 12, 1–8. [Google Scholar]
- Nail, T.N.A.; Ali, M.M.; Salim, E.R.A. Phytochemical studies on sudanese rocket (Eruca sativa) seeds and oil constituents. Am. J. Phytomedicine Clin. Ther. 2017, 5, 1–5. [Google Scholar] [CrossRef]
- Das, S.; Tyagi, A.K.; Singhal, K.K. Chemical composition including amino acid, fatty acid and glucosinolate profile of taramira (Eruca sativa) oilseed. Indian J. Agric. Sci. 2001, 71, 613–615. [Google Scholar]
- Khan, A.U.; Ullah, F.; Khan, N.; Mehmood, S.; Fahad, S.; Datta, R.; Irshad, I.; Danish, S.; Saud, S.; Alaraidh, I.A.; et al. Production of organic fertilizers from rocket seed (Eruca sativa L.), chicken peat and Moringa oleifera leaves for growing linseed under water deficit stress. Sustainability 2021, 13, 59. [Google Scholar] [CrossRef]
- Fagbenro, O.A. Soybean meal replacement by roquette (Eruca sativa Miller) seed meal as protein feedstuff in diets for african catfish, Clarias gariepinus (Burchell 1822), fingerlings. Aquac. Res. 2004, 35, 917–923. [Google Scholar] [CrossRef]
- Khaliq, B.; Falke, S.; Saeed, Q.; Bilal, M.; Munawar, A.; Ali, A.; Baermann, G.; Athar, H.-R.U.R.; Mahmood, S.; Betzel, C.; et al. Eruca sativa seed napin structural insights and thorough functional characterization. Sci. Rep. 2021, 11, 24066. [Google Scholar] [CrossRef]
- Akgul, C.; Akcicek, A.; Karadag, A.; Karasu, S. Formulation optimization of low-fat emulsion stabilized by rocket seed (Eruca sativa mill) gum as novel natural fat replacer: Effect on steady, dynamic and thixotropic behavior. Acta Sci. Technol. 2022, 44, e56006. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Erucic acid in feed and food. EFSA J. 2016, 14, 4593. [Google Scholar] [CrossRef]
- Kumar, M.S.S.; Mawlong, I.; Kumar, A.; Singh, K.H.; Gurung, B.; Rani, R.; Rai, P.K. Evaluation of major anti-nutritional factors in oilseed brassica. Vegetos 2022, 1–8. [Google Scholar] [CrossRef]
- El-Missiry, M.A.; El Gindy, A.M. Amelioration of alloxan induced diabetes mellitus and oxidative stress in rats by oil of Eruca sativa seeds. Ann. Nutr. Metab. 2000, 44, 97–100. [Google Scholar] [CrossRef]
- Bassyouni, R.H.; Kamel, Z.; Algameel, A.A.; Ismail, G.; Gaber, S.N. In-vitro determination of antimicrobial activities of Eruca sativa seed oil against antibiotic-resistant gram-negative clinical isolates from neonates: A future prospect. BMC Complement. Med. Ther. 2022, 22, 229. [Google Scholar] [CrossRef]
- Sanad, R.A.E.B.; Mabrouk, M.I. Development and assessment of stable formulations containing two herbal antimicrobials: Allium sativum L. and Eruca sativa Miller seed oils. Drug Dev. Ind. Pharm. 2016, 42, 958–968. [Google Scholar] [CrossRef]
- Eid, M.A.; Jaradat, A.N.; Al-Masri, M.; Issa, L.; Zubidat, F.; Asrawi, H.; Ahmad, S. Development and antimicrobial evaluation of Eruca sativa oil nanoemulgel with determination of the oil antioxidant, sun protection factor and elastase inhibition. Curr. Pharm. Biotechnol. 2020, 21, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Aghababaie, M.; Beheshti, M.; Razmjou, A.; Bordbar, A.-K. Enzymatic biodiesel production from crude Eruca sativa oil using Candida rugosa lipase in a solvent-free system using response surface methodology. Biofuels 2020, 11, 93–99. [Google Scholar] [CrossRef]
- Elansary, H.O.; Mahmoud, E.A.; Shokralla, S.; Yessoufou, K. Diversity of plants, traditional knowledge, and practices in local cosmetics: A case study from Alexandria, Egypt. Econ. Bot. 2015, 69, 114–126. [Google Scholar] [CrossRef]
- Vahabi Mashhoor, M.; Mikani, A.; Mehrabadi, M.; Moharramipour, S. Antifeedant activity of nanoemulsion formulation of arugula Eruca sativa oil on elm leaf beetle Xanthogaleruca luteola (coleoptera: Chrysomelidae). J. Agric. Sci. Technol. 2021, 23, 125–136. [Google Scholar]
- Aghababaie, M.; Beheshti, M.; Razmjou, A.; Bordbar, A.-K. Two phase enzymatic membrane reactor for the production of biodiesel from crude Eruca sativa oil. Renew. Energy 2019, 140, 104–110. [Google Scholar] [CrossRef]
- Chakrabarti, M.H.; Ali, M.; Baroutian, S.; Saleem, M. Techno-economic comparison between b10 of Eruca sativa L. and other indigenous seed oils in pakistan. Process Saf. Environ. Prot. 2011, 89, 165–171. [Google Scholar] [CrossRef]
- Bateni, H.; Bateni, F.; Karimi, K. Effects of oil extraction on ethanol and biogas production from Eruca sativa seed cake. Waste Biomass Valorization 2017, 8, 1897–1905. [Google Scholar] [CrossRef]
- Franco, P.; Spinozzi, S.; Pagnotta, E.; Lazzeri, L.; Ugolini, L.; Camborata, C.; Roda, A. Development of a liquid chromatography-electrospray ionization-tandem mass spectrometry method for the simultaneous analysis of intact glucosinolates and isothiocyanates in brassicaceae seeds and functional foods. J. Chromatogr. A 2016, 1428, 154–161. [Google Scholar] [CrossRef]
- Lucarini, E.; Pagnotta, E.; Micheli, L.; Parisio, C.; Testai, L.; Martelli, A.; Calderone, V.; Matteo, R.; Lazzeri, L.; Di Cesare Mannelli, L.; et al. Eruca sativa meal against diabetic neuropathic pain: An h2s-mediated effect of glucoerucin. Molecules 2019, 24, 3006. [Google Scholar] [CrossRef]
- Nanetti, A.; Ugolini, L.; Cilia, G.; Pagnotta, E.; Malaguti, L.; Cardaio, I.; Matteo, R.; Lazzeri, L. Seed meals from Brassica nigra and Eruca sativa control artificial Nosema ceranae infections in Apis mellifera. Microorganisms 2021, 9, 949. [Google Scholar] [CrossRef]
- Uğur, A.; Süntar, I.; Aslan, S.; Orhan, I.E.E.; Kartal, M.; Sekeroğlu, N.; Eşiyok, D.; Sener, B.; Uǧur, A.; Süntar, I.; et al. Variations in fatty acid compositions of the seed oil of Eruca sativa Mill. caused by different sowing periods and nitrogen forms. Pharmacogn. Mag. 2010, 6, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Garg, G.; Sharma, V. Eruca sativa (L.): Botanical description, crop improvement, and medicinal properties. J. Herbs. Spices Med. Plants 2014, 20, 171–182. [Google Scholar] [CrossRef]
- Mumtaz, M.W.; Mukhtar, H.; Dilawer, U.A.; Hussain, S.M.; Hussain, M.; Iqbal, M.; Adnan, A.; Nisar, J. Biocatalytic transesterification of Eruca sativa oil for the production of biodiesel. Biocatal. Agric. Biotechnol. 2016, 5, 162–167. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, R.; Arora, R.; Arora, S.; Singh, B.; Sharma, U. Quantitative and qualitative analysis of Eruca sativa and Brassica juncea seeds by UPLC-DAD and UPLC-ESI-QTOF. Nat. Prod. Commun. 2017, 12, 1934578X1701200. [Google Scholar] [CrossRef]
- Tekin, M.D.; Çelikozlu, S.; Aydin, H. Electrospun rocket seed (Eruca sativa Mill) mucilage/polyvinyl alcohol nanofibers: Fabrication and characterization. Iran. Polym. J. 2022, 1–9. [Google Scholar] [CrossRef]
- Akcicek, A.; Bozkurt, F.; Akgül, C.; Karasu, S. Encapsulation of olive pomace extract in rocket seed gum and chia seed gum nanoparticles: Characterization, antioxidant activity and oxidative stability. Foods 2021, 10, 1735. [Google Scholar] [CrossRef]
- Koocheki, A.; Razavi, S.M.A.; Hesarinejad, M.A. Effect of extraction procedures on functional properties of Eruca sativa seed mucilage. Food Biophys. 2012, 7, 84–92. [Google Scholar] [CrossRef]
- Kutlu, G.; Akcicek, A.; Bozkurt, F.; Karasu, S.; Tekin-Cakmak, Z.H. Rocket seed (Eruca sativa Mill) gum: Physicochemical and comprehensive rheological characterization. Food Sci. Technol. 2022, 42. [Google Scholar] [CrossRef]
- Hijazi, T.; Karasu, S.; Tekin-Çakmak, Z.H.; Bozkurt, F. Extraction of natural gum from cold-pressed chia seed, flaxseed, and rocket seed oil by-product and application in low fat vegan mayonnaise. Foods 2022, 11, 363. [Google Scholar] [CrossRef]
- Cataldi, T.R.I.; Rubino, A.; Lelario, F.; Bufo, S.A. Naturally occurring glucosinolates in plant extracts of rocket salad (Eruca sativa L.) identified by liquid chromatography coupled with negative ion electrospray ionization and quadrupole ion-trap mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 2374–2388. [Google Scholar] [CrossRef]
- Parchem, K.; Piekarska, A.; Bartoszek, A. Enzymatic activities behind degradation of glucosinolates. In Glucosinolates: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 79–106. [Google Scholar]
- Wu, J.; Cui, S.; Liu, J.; Tang, X.; Zhao, J.; Zhang, H.; Mao, B.; Chen, W. The recent advances of glucosinolates and their metabolites: Metabolism, physiological functions and potential application strategies. Crit. Rev. Food Sci. Nutr. 2022, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Barillari, J.; Canistro, D.; Paolini, M.; Ferroni, F.; Pedulli, G.F.; Iori, R.; Valgimigli, L. Direct antioxidant activity of purified glucoerucin, the dietary secondary metabolite contained in rocket (Eruca sativa mill.) seeds and sprouts. J. Agric. Food Chem. 2005, 53, 2475–2482. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Ishii, G. Glucosinolate profiles in the seeds, leaves and roots of rocket salad (Eruca sativa mill.) and anti-oxidative activities of intact plant powder and purified 4-methoxyglucobrassicin. Soil Sci. Plant Nutr. 2006, 52, 394–400. [Google Scholar] [CrossRef]
- Bennett, R.N.; Rosa, E.A.S.; Mellon, F.A.; Kroon, P.A. Ontogenic profiling of glucosinolates, flavonoids, and other secondary metabolites in Eruca sativa (salad rocket), Diplotaxis erucoides (wall rocket), Diplotaxis tenuifolia (wild rocket), and Bunias orientalis (turkish rocket). J. Agric. Food Chem. 2006, 54, 4005–4015. [Google Scholar] [CrossRef]
- Matteo, R.; Back, M.A.; Reade, J.P.H.; Ugolini, L.; Pagnotta, E.; Lazzeri, L. Effectiveness of defatted seed meals from brassicaceae with or without crude glycerin against black grass (Alopecurus myosuroides huds.). Ind. Crops Prod. 2018, 111, 506–512. [Google Scholar] [CrossRef]
- Giannini, V.; Melito, S.; Matteo, R.; Lazzeri, L.; Pagnotta, E.; Chahine, S.; Roggero, P.P. Testing Eruca sativa defatted seed meal as a potential bioherbicide on selected weeds and crops. Ind. Crops Prod. 2021, 171, 113834. [Google Scholar] [CrossRef]
- Ugolini, L.; Scarafile, D.; Matteo, R.; Pagnotta, E.; Malaguti, L.; Lazzeri, L.; Modesto, M.; Checcucci, A.; Mattarelli, P.; Braschi, I. Effect of bioactive compounds released from brassicaceae defatted seed meals on bacterial load in pig manure. Environ. Sci. Pollut. Res. 2021, 28, 62353–62367. [Google Scholar] [CrossRef]
- Curto, G.; Dallavalle, E.; Matteo, R.; Lazzeri, L. Biofumigant effect of new defatted seed meals against the southern root-knot nematode, Meloidogyne incognita. Ann. Appl. Biol. 2016, 169, 17–26. [Google Scholar] [CrossRef]
- Lucarini, E.; Micheli, L.; Pagnotta, E.; Matteo, R.; Parisio, C.; Toti, A.; Ferrara, V.; Ciampi, C.; Martelli, A.; Testai, L.; et al. Beneficial effects of Eruca sativa defatted seed meal on visceral pain and intestinal damage resulting from colitis in rats. Foods 2022, 11, 580. [Google Scholar] [CrossRef]
- Testai, L.; Pagnotta, E.; Piragine, E.; Flori, L.; Citi, V.; Martelli, A.; Mannelli, L.D.C.; Ghelardini, C.; Matteo, R.; Suriano, S.; et al. Cardiovascular benefits of Eruca sativa Mill. defatted seed meal extract: Potential role of hydrogen sulfide. Phyther. Res. 2022, 36, 2616–2627. [Google Scholar] [CrossRef]
- Piragine, E.; Flori, L.; Di Cesare Mannelli, L.; Ghelardini, C.; Pagnotta, E.; Matteo, R.; Lazzeri, L.; Martelli, A.; Miragliotta, V.; Pirone, A.; et al. Eruca sativa Mill seed extract promotes anti-obesity and hypoglycemic effects in mice fed with a high-fat diet. Phyther. Res. 2021, 35, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Pagnotta, E.; Lisotti, A.; Ugolini, L.; Matteo, R.; Franco, P.; Roda, G.; Roda, A.; Roda, E. Bakery products enriched with Eruca sativa Mill. defatted seed meal ameliorates systemic markers of inflammation and glucose and lipid metabolism in adults- a pilot study. Acta Sci. Gastrointest. Disord. 2022, 5, 8–14. [Google Scholar] [CrossRef]
- Blaževic, I.; Mastelic, J. Free and bound volatiles of rocket (Eruca sativa Mill.). Flavour Fragr. J. 2008, 23, 278–285. [Google Scholar] [CrossRef]
- Al-Turki, A.I.; Dick, W.A. Myrosinase activity in soil. Soil Sci. Soc. Am. J. 2003, 67, 139–145. [Google Scholar] [CrossRef]
- Ugolini, L.; Cilia, G.; Pagnotta, E.; Malaguti, L.; Capano, V.; Guerra, I.; Zavatta, L.; Albertazzi, S.; Matteo, R.; Lazzeri, L.; et al. Glucosinolate bioactivation by Apis mellifera workers and its impact on Nosema ceranae infection at the colony level. Biomolecules 2021, 11, 1657. [Google Scholar] [CrossRef] [PubMed]
- Youseif, S.H.; Abdel, H.M.K.; Mary, F. A new source of bacterial myrosinase isolated from endophytic Bacillus sp. ngb-b10, and its relevance in biological control activity. World J. Microbiol. Biotechnol. 2022, 38, 215. [Google Scholar] [CrossRef] [PubMed]
- Narbad, A.; Rossiter, J.T. Gut glucosinolate metabolism and isothiocyanate production. Mol. Nutr. Food Res. 2018, 62, 1700991. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Giacoppo, S.; Ficicchia, M.; Aliquo, A.; Bramanti, P.; Mazzon, E. Eruca sativa seed extract: A novel natural product able to counteract neuroinflammation. Mol. Med. Rep. 2018, 17, 6235–6244. [Google Scholar] [CrossRef]
- Piragine, E.; Citi, V.; Lawson, K.; Calderone, V.; Martelli, A. Potential effects of natural H2S-donors in hypertension management. Biomolecules 2022, 12, 581. [Google Scholar] [CrossRef]
- Citi, V.; Martelli, A.; Testai, L.; Marino, A.; Breschi, M.; Calderone, V. Hydrogen sulfide releasing capacity of natural isothiocyanates: Is it a reliable explanation for the multiple biological effects of brassicaceae? Planta Med. 2014, 80, 610–613. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, X.; Lu, Y.; Liang, D.; Huang, D. Isothiocyanates as H2S donors triggered by cysteine: Reaction mechanism and structure and activity relationship. Org. Lett. 2019, 21, 5977–5980. [Google Scholar] [CrossRef] [PubMed]
- Gambari, L.; Barone, M.; Amore, E.; Grigolo, B.; Filardo, G.; Iori, R.; Citi, V.; Calderone, V.; Grassi, F. Glucoraphanin increases intracellular hydrogen sulfide (H2S) levels and stimulates osteogenic differentiation in human mesenchymal stromal cell. Nutrients 2022, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.; Piragine, E.; Citi, V.; Testai, L.; Pagnotta, E.; Ugolini, L.; Lazzeri, L.; Di Cesare Mannelli, L.; Manzo, O.L.; Bucci, M.; et al. Erucin exhibits vasorelaxing effects and antihypertensive activity by H2S-releasing properties. Br. J. Pharmacol. 2020, 177, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Stagos, D. Antioxidant activity of polyphenolic plant extracts. Antioxidants 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elsalam, R.M.; El Badawy, S.A.; Ogaly, H.A.; Ibrahim, F.M.; Farag, O.M.; Ahmed, K.A. Eruca sativa seed extract modulates oxidative stress and apoptosis and up-regulates the expression of bcl-2 and bax genes in acrylamide-induced testicular dysfunction in rats. Environ. Sci. Pollut. Res. 2021, 28, 53249–53266. [Google Scholar] [CrossRef]
- Abdelkader, R.S.-E.; El-Beih, N.M.; Zaahkouk, S.A.; El-Hussieny, E.A. Ameliorative effect of Eruca sativa seeds and its rutin on gentamicin-induced nephrotoxicity in male rats via targeting inflammatory status, oxidative stress and kidney injury molecule-1 (kim-1)/cystatin c expression. Indones. Biomed. J. 2022, 14, 74–83. [Google Scholar] [CrossRef]
- Sarwar Alam, M.; Kaur, G.; Jabbar, Z.; Javed, K.; Athar, M. Eruca sativa seeds possess antioxidant activity and exert a protective effect on mercuric chloride induced renal toxicity. Food Chem. Toxicol. 2007, 45, 910–920. [Google Scholar] [CrossRef]
- Tian, R.; Yang, W.; Xue, Q.; Gao, L.; Huo, J.; Ren, D.; Chen, X. Rutin ameliorates diabetic neuropathy by lowering plasma glucose and decreasing oxidative stress via NRF2 signaling pathway in rats. Eur. J. Pharmacol. 2016, 771, 84–92. [Google Scholar] [CrossRef]
- Kishore, L.; Kaur, N.; Singh, R. Effect of kaempferol isolated from seeds of Eruca sativa on changes of pain sensitivity in streptozotocin-induced diabetic neuropathy. Inflammopharmacology 2018, 26, 993–1003. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).