Fungal Metabolites in Human Health and Diseases—An Overview
Definition
:1. Introduction
2. Mycotoxin Involved in Human Diseases
2.1. Mycotoxin Involved in Liver Diseases
2.2. Mycotoxins Involved in Kidney Diseases
2.3. Mycotoxins Involved in Genotoxicity, Immunotoxicity, and Neurotoxicity
2.4. Mycotoxins Involved in Damage to Organs
2.5. Mycotoxins Involved Gastrointestinal, Skin, Thyroid, and Bone Marrow Disorders
2.6. Mycotoxins Involved in Cancer and Heart Failure
2.7. Mycotoxins Involved in Oesophageal Cancer and Hematologic Disorder
2.8. Mycotoxins Involved in Cause Respiratory Illness
3. Fungal Metabolites against Human Disease
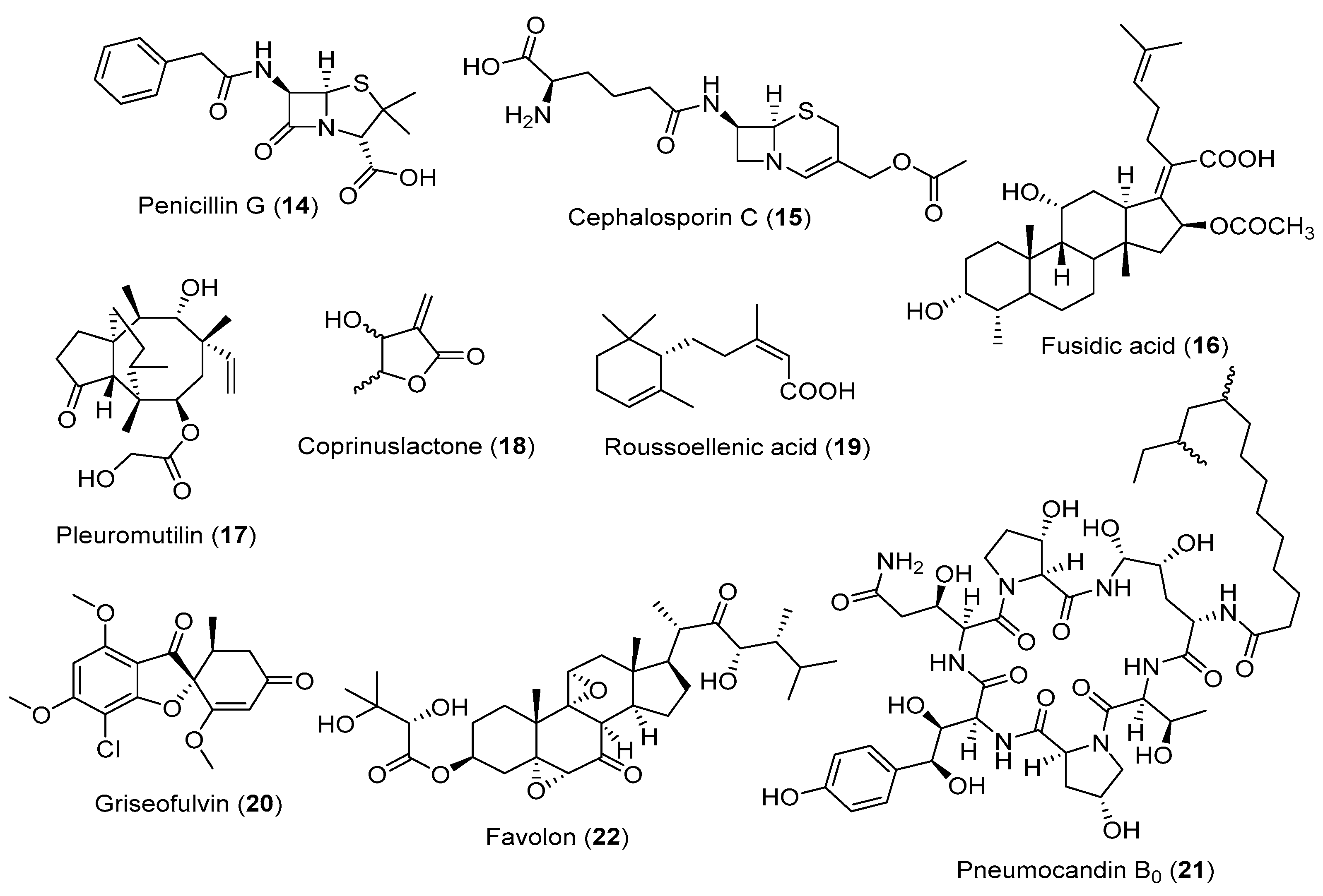
3.1. Fungal Metabolites Used as Antibiotics
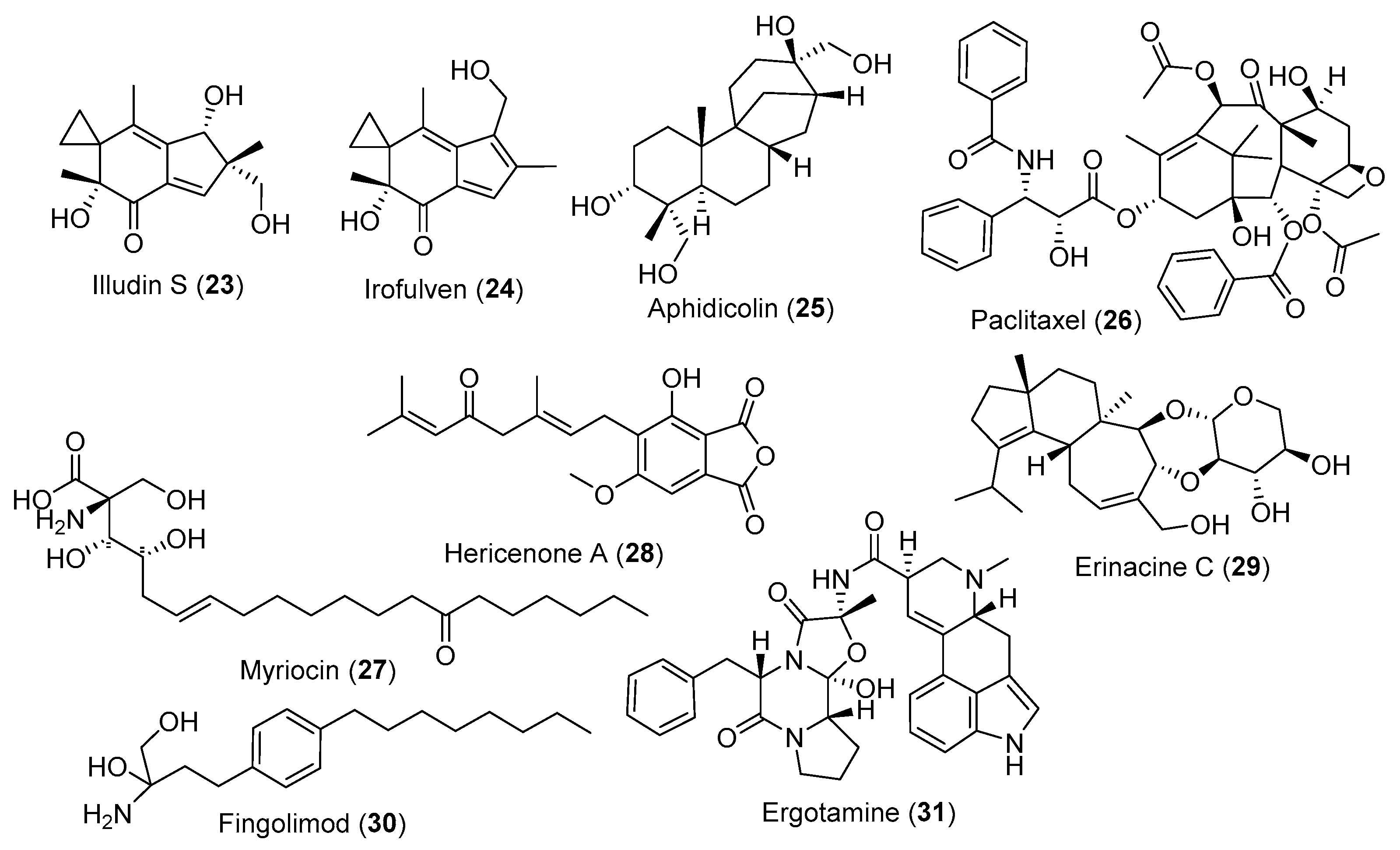
3.2. Fungal Metabolites Used as Anticancer Agents
3.3. Fungal Metabolites Used as CNS-Disease-Related Agents
3.4. Fungal Metabolites Control Cardiovascular Diseases
3.5. Fungal Metabolites Used as Immunomodulatory Agents
3.6. Fungal Metabolites Used as Antiviral Agents
4. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Naranjo-Ortiz, M.; Gabaldón, T. Fungal evolution: Cellular, genomic and metabolic complexity. Biol. Rev. 2020, 95, 1198–1232. [Google Scholar] [CrossRef] [PubMed]
- Boruta, T. Uncovering the repertoire of fungal secondary metabolites: From Fleming’s laboratory to the International Space Station. Bioengineered 2017, 9, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Eshelli, M.; Qader, M.M.; Jambi, E.J.; Hursthouse, A.S.; Rateb, M.E. Current Status and Future Opportunities of Omics Tools in Mycotoxin Research. Toxins 2018, 10, 433. [Google Scholar] [CrossRef]
- Peraica, M.; Radić, B.; Lucić, A.; Pavlović, M. Toxic effects of mycotoxins in humans. Bull. World Health Organ. 1999, 77, 754–766. [Google Scholar]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Liew, W.P.P.; Mohd-Redzwan, S. Mycotoxin: Its impact on gut health and microbiota. Front. Cell. Infect. Microbiol. 2018, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Janik, E.; Niemcewicz, M.; Ceremuga, M.; Stela, M.; Saluk-Bijak, J.; Siadkowski, A.; Bijak, M. Molecular Aspects of Mycotoxins—A Serious Problem for Human Health. Int. J. Mol. Sci. 2020, 21, 8187. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, A.; A Parsi, M. Aflatoxins, hepatocellular carcinoma and public health. World J. Gastroenterol. 2013, 19, 1508–1512. [Google Scholar] [CrossRef] [PubMed]
- Tola, M.; Kebede, B. Occurrence, importance and control of mycotoxins: A review. Cogent Food Agric. 2016, 2, 1191103. [Google Scholar] [CrossRef]
- Karwehl, S.; Stadler, M. Exploitation of fungal biodiversity for discovery of novel antibiotics. Curr. Top. Microbiol. Immunol. 2016, 398, 303–338. [Google Scholar]
- De Carvalho, A.; do Amaral, M.W. Coprinuslactone Protects the Edible Mushroom Coprinus Comatus against Biofilm Infections by Blocking Both Quorum-sensing and MurA. Wiley Online Libr. 2016, 18, 4254–4264. [Google Scholar]
- Phukhamsakda, C.; Macabeo, A.P.G.; Yuyama, K.T.; Hyde, K.D.; Stadler, M. Biofilm Inhibitory Abscisic Acid Derivatives from the Plant-Associated Dothideomycete Fungus, Roussoella sp. Molecules 2018, 23, 2190. [Google Scholar] [CrossRef]
- Grove, J.F.; MacMillan, J.; Mulholland, T.P.C.; Rogers, M.A.T. 762. Griseofulvin. Part IV. Structure. J. Chem. Soc. 1952, 3977–3987. [Google Scholar] [CrossRef]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A Global Concern for Food Safety, Human Health and Their Management. Front. Microbiol. 2017, 7, 2170. [Google Scholar] [CrossRef] [PubMed]
- Hamid, A.S.; Tesfamariam, I.G.; Zhang, Y.; Zhang, Z.G. Aflatoxin B1-induced hepatocellular carcinoma in developing countries: Geographical distribution, mechanism of action and prevention. Oncol. Lett. 2013, 5, 1087–1092. [Google Scholar] [CrossRef]
- Walte, H.-G.; Schwake-Anduschus, C.; Geisen, R.; Fritsche, J. Aflatoxin: Food chain transfer from feed to milk. J. Für Verbrauch. Und Lebensm. 2016, 11, 295–297. [Google Scholar] [CrossRef]
- Schrenk, D.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C.; Nebbia, C.S. Risk assessment of ochratoxin A in food. EFSA J. 2020, 18, e06113. [Google Scholar] [CrossRef]
- EL Khoury, A.; Atoui, A. Ochratoxin A: General Overview and Actual Molecular Status. Toxins 2010, 2, 461–493. [Google Scholar] [CrossRef]
- Bui-Klimke, T.R.; Wu, F. Ochratoxin A and Human Health Risk: A Review of the Evidence. Crit. Rev. Food Sci. Nutr. 2013, 55, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (Contam). Scientific Opinion on the risks for public and animal health related to the presence of citrinin in food and feed. EFSA J. 2012, 10, 2605. [Google Scholar] [CrossRef]
- Silva, L.J.G.; Pereira, A.M.P.T.; Pena, A.; Lino, C.M. Citrinin in Foods and Supplements: A Review of Occurrence and Analytical Methodologies. Foods 2020, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Atapattu, S.N.; Poole, C.F. Recent advances in analytical methods for the determination of citrinin in food matrices. J. Chromatogr. A 2020, 1627, 461399. [Google Scholar] [CrossRef]
- Barreira, M.J.; Alvito, P.; Almeida, C.M.M. Occurrence of patulin in apple-based-foods in Portugal. Food Chem. 2010, 121, 653–658. [Google Scholar] [CrossRef]
- Mahato, D.K.; Kamle, M.; Sharma, B.; Pandhi, S.; Devi, S.; Dhawan, K.; Selvakumar, R.; Mishra, D.; Kumar, A.; Arora, S.; et al. Patulin in food: A mycotoxin concern for human health and its management strategies. Toxicon 2021, 198, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Janik, E.; Niemcewicz, M.; Podogrocki, M.; Ceremuga, M.; Stela, M.; Bijak, M. T-2 Toxin—The Most Toxic Trichothecene Mycotoxin: Metabolism, Toxicity, and Decontamination Strategies. Molecules 2021, 26, 6868. [Google Scholar] [CrossRef]
- Ueno, Y. Toxicological features of T-2 toxin and related trichothecenes. Fundam. Appl. Toxicol. 1984, 4, S124–S132. [Google Scholar] [CrossRef]
- Voss, K.A.; Riley, R.T.; Norred, W.P.; Bacon, C.W.; Meredith, F.I.; Howard, P.C.; Plattner, R.D.; Collins, T.F.; Hansen, D.K.; Porter, J.K. An overview of rodent toxicities: Liver and kidney effects of fumonisins and Fusarium moniliforme. Environ. Health Perspect. 2001, 109, 259–266. [Google Scholar] [CrossRef]
- Chen, J.; Wen, J.; Tang, Y.; Shi, J.; Mu, G.; Yan, R.; Cai, J.; Long, M. Research Progress on Fumonisin B1 Contamination and Toxicity: A Review. Molecules 2021, 26, 5238. [Google Scholar] [CrossRef]
- Wu, Q.-H.; Wang, X.; Yang, W.; Nüssler, A.K.; Xiong, L.-Y.; Kuča, K.; Dohnal, V.; Zhang, X.-J.; Yuan, Z.-H. Oxidative stress-mediated cytotoxicity and metabolism of T-2 toxin and deoxynivalenol in animals and humans: An update. Arch. Toxicol. 2014, 88, 1309–1326. [Google Scholar] [CrossRef]
- Peles, F.; Sipos, P.; Győri, Z.; Pfliegler, W.P.; Giacometti, F.; Serraino, A.; Pagliuca, G.; Gazzotti, T.; Pócsi, I. Adverse Effects, Transformation and Channeling of Aflatoxins Into Food Raw Materials in Livestock. Front. Microbiol. 2019, 10, 2861. [Google Scholar] [CrossRef] [PubMed]
- Rogowska, A.; Pomastowski, P.; Sagandykova, G.; Buszewski, B. Zearalenone and its metabolites: Effect on human health, metabolism and neutralisation methods. Toxicon 2019, 162, 46–56. [Google Scholar] [CrossRef] [PubMed]
- A Meronuck, R.; A Steele, J.; Mirocha, C.J.; Christensen, C.M. Tenuazonic acid, a toxic produced by Alternaria alternata. Appl. Microbiol. 1972, 23, 613–617. [Google Scholar] [CrossRef]
- Hollander, D.D.; Holvoet, C.; Demeyere, K.; De Zutter, N.; Audenaert, K.; Meyer, E.; Croubels, S. Cytotoxic Effects of Alternariol, Alternariol Monomethyl-Ether, and Tenuazonic Acid and Their Relevant Combined Mixtures on Human Enterocytes and Hepatocytes. Front. Microbiol. 2022, 13, 849243. [Google Scholar] [CrossRef]
- Ulrich, S.; Lang, K.; Niessen, L.; Baschien, C.; Kosicki, R.; Twarużek, M.; Straubinger, R.K.; Ebel, F. The Evolution of the Satratoxin and Atranone Gene Clusters of Stachybotrys chartarum. J. Fungi 2022, 8, 340. [Google Scholar] [CrossRef]
- Dyląg, M.; Spychała, K.; Zielinski, J.; Łagowski, D.; Gnat, S. Update on Stachybotrys chartarum—Black Mold Perceived as Toxigenic and Potentially Pathogenic to Humans. Biology 2022, 11, 352. [Google Scholar] [CrossRef]
- Coppock, R.W.; Christian, R.G.; Jacobsen, B.J. Aflatoxins. In Veterinary Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 983–994. [Google Scholar] [CrossRef]
- Baranyi, N.; Kocsubé, S.; Varga, J. Aflatoxins: Climate change and biodegradation. Curr. Opin. Food Sci. 2015, 5, 60–66. [Google Scholar] [CrossRef]
- Eshelli, M.; Harvey, L.; Edrada-Ebel, R.; McNeil, B. Metabolomics of the Bio-Degradation Process of Aflatoxin B1 by Actinomycetes at an Initial pH of 6.0. Toxins 2015, 7, 439–456. [Google Scholar] [CrossRef]
- Milićević, D.R.; Spirić, D.; Radičević, T.; Velebit, B.; Stefanović, S.; Milojević, L.; Janković, S. A review of the current situation of aflatoxin M1 in cow’s milk in Serbia: Risk assessment and regulatory aspects. Food Addit. Contam. Part A 2017, 34, 1617–1631. [Google Scholar] [CrossRef]
- Filazi, A.; Tansel, U. Occurrence of Aflatoxins in Food. In Aflatoxins—Recent Advances and Future Prospects; Intechopen: London, UK, 2013. [Google Scholar] [CrossRef]
- Kimanya, M.E.; Routledge, M.N.; Mpolya, E.; Ezekiel, C.N.; Shirima, C.P.; Gong, Y.Y. Estimating the risk of aflatoxin-induced liver cancer in Tanzania based on biomarker data. PLoS ONE 2021, 16, e0247281. [Google Scholar] [CrossRef]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 Years of Research. Toxins 2016, 8, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vettorazzi, A.; van Delft, J.; de Cerain, A.L. A review on ochratoxin A transcriptomic studies. Food Chem. Toxicol. 2013, 59, 766–783. [Google Scholar] [CrossRef] [PubMed]
- Nan, M.; Xue, H.; Bi, Y. Contamination, Detection and Control of Mycotoxins in Fruits and Vegetables. Toxins 2022, 14, 309. [Google Scholar] [CrossRef] [PubMed]
- Sudakin, D.L. Trichothecenes in the environment: Relevance to human health. Toxicol. Lett. 2003, 143, 97–107. [Google Scholar] [CrossRef]
- Foroud, N.A.; Baines, D.; Gagkaeva, T.Y.; Thakor, N.; Badea, A.; Steiner, B.; Bürstmayr, M.; Bürstmayr, H. Trichothecenes in Cereal Grains—An Update. Toxins 2019, 11, 634. [Google Scholar] [CrossRef]
- Proctor, R.; Brown, D.; Busman, M.; Naumann, T. Genomic and Metabolomic Approaches for Detection and Control of Fusarium, Fumonisins and Other Mycotoxins on Corn. Available online: https://www.ars.usda.gov/research/project/?accnNo=430343 (accessed on 20 June 2022).
- Dall’Asta, C.; Battilani, P. Fumonisins and their modified forms, a matter of concern in future scenario? World Mycotoxin J. 2016, 9, 727–739. [Google Scholar] [CrossRef]
- Alexander, J.; Benford, D.; Boobis, A.; Ceccatelli, S.; Cottrill, B.; Cravedi, J.-P.; Di Domenico, A.; Doerge, D.; Dogliotti, E.; Edler, L.; et al. Scientific Opinion on the risks for animal and public health related to the presence ofAlternariatoxins in feed and food. EFSA J. 2011, 9, 2407. [Google Scholar] [CrossRef]
- Escrivá, L.; Oueslati, S.; Font, G.; Manyes, L. Alternaria Mycotoxins in Food and Feed: An Overview. J. Food Qual. 2017, 2017, 1569748. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Mañes, J.; Berrada, H.; Juan, C. Development and Validation of a LC-ESI-MS/MS Method for the Determination of Alternaria Toxins Alternariol, Alternariol Methyl-Ether and Tentoxin in Tomato and Tomato-Based Products. Toxins 2016, 8, 328. [Google Scholar] [CrossRef]
- De Souza, G.D.; Mithöfer, A.; Daolio, C.; Schneider, B.; Rodrigues-Filho, E. Identification of Alternaria alternata Mycotoxins by LC-SPE-NMR and Their Cytotoxic Effects to Soybean (Glycine max) Cell Suspension Culture. Molecules 2013, 18, 2528–2538. [Google Scholar] [CrossRef]
- Meena, M.; Samal, S. Alternaria host-specific (HSTs) toxins: An overview of chemical characterization, target sites, regulation and their toxic effects. Toxicol. Rep. 2019, 6, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Hövelmann, Y.; Hickert, S.; Cramer, B.; Humpf, H.-U. Determination of Exposure to the Alternaria Mycotoxin Tenuazonic Acid and Its Isomer allo-Tenuazonic Acid in a German Population by Stable Isotope Dilution HPLC-MS3. J. Agric. Food Chem. 2016, 64, 6641–6647. [Google Scholar] [CrossRef] [PubMed]
- Islam, Z.; Harkema, J.R.; Pestka, J.J. Satratoxin G from the Black Mold Stachybotrys chartarum Evokes Olfactory Sensory Neuron Loss and Inflammation in the Murine Noseand Brain. Environ. Health Perspect. 2006, 114, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Carey, S.A.; Plopper, C.G.; Hyde, D.M.; Islam, Z.; Pestka, J.J.; Harkema, J.R. Satratoxin-G from the Black Mold Stachybotrys chartarum Induces Rhinitis and Apoptosis of Olfactory Sensory Neurons in the Nasal Airways of Rhesus Monkeys. Toxicol. Pathol. 2012, 40, 887–898. [Google Scholar] [CrossRef]
- Bills, G.F.; Gloer, J.B. Biologically Active Secondary Metabolites from the Fungi. Microbiol. Spectr. 2016, 4, 1087–1119. [Google Scholar] [CrossRef]
- Godtfredsen, W.O.; Jahnsen, S.; Lorck, H.; Roholt, K.; Tybring, L. Fusidic Acid: A New Antibiotic. Nature 1962, 193, 987. [Google Scholar] [CrossRef]
- Newton, G.G.F.; Abraham, E.P. Cephalosporin C, a New Antibiotic containing Sulphur and D-α-Aminoadipic Acid. Nature 1955, 175, 548. [Google Scholar] [CrossRef]
- Novak, R.; Shlaes, D.M. The Pleuromutilin Antibiotics: A New Class for Human Use. Curr. Opin. Investig. Drugs 2010, 11, 182–191. [Google Scholar]
- Jayawardena, R.S.; Hyde, K.D.; McKenzie, E.H.C.; Jeewon, R.; Phillips, A.J.L.; Perera, R.H.; de Silva, N.I.; Maharachchikumburua, S.S.N.; Samarakoon, M.C.; Ekanayake, A.H.; et al. One stop shop III: Taxonomic update with molecular phylogeny for important phytopathogenic genera: 51–75 (2019). Fungal Divers. 2019, 98, 77–160. [Google Scholar] [CrossRef]
- Bentley, R. Mycophenolic Acid: A One Hundred Year Odyssey from Antibiotic to Immunosuppressant. Chem. Rev. 2000, 100, 3801–3826. [Google Scholar] [CrossRef]
- Hesterkamp, T. Antibiotics Clinical Development and Pipeline. Curr. Top. Microbiol. 2016, 398, 447–474. [Google Scholar] [CrossRef]
- Denning, D.W. Echinocandins: A new class of antifungal. J. Antimicrob. Chemother. 2002, 49, 889–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anke, H.; Stadler, M.; Mayer, A.; Sterner, O. Secondary metabolites with nematicidal and antimicrobial activity from nematophagous fungi and Ascomycetes. Can. J. Bot. 1995, 73, 932–939. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, Y.; Zhan, H.; Bhatt, P.; Chen, S. An Overview of Strobilurin Fungicide Degradation:Current Status and Future Perspective. Front. Microbiol. 2020, 11, 389. [Google Scholar] [CrossRef]
- Nakanishi, K.; Ohashi, M.; Tada, M.; Yamada, Y. Illudin s (lampterol). Tetrahedron 1965, 21, 1231–1246. [Google Scholar] [CrossRef]
- Movassaghi, M.; Piizzi, G.; Siegel, D.S.; Piersanti, G. Enantioselective Total Synthesis of (−)-Acylfulvene and (−)-Irofulven. Angew. Chem. 2006, 118, 5991–5995. [Google Scholar] [CrossRef]
- Bucknall, R.A.; Moores, H.; Simms, R.; Hesp, B. Antiviral Effects of Aphidicolin, a New Antibiotic Produced by Cephalosporium aphidicola. Antimicrob. Agents Chemother. 1973, 4, 294–298. [Google Scholar] [CrossRef]
- Stierle, A.; Strobel, G.; Stierle, D.; Grothaus, P.; Bignami, G. The Search for a Taxol-Producing Microorganism Among the Endophytic Fungi of the Pacific Yew, Taxus brevifolia. J. Nat. Prod. 1995, 58, 1315–1324. [Google Scholar] [CrossRef]
- Stierle, A.A.; Stierle, D.B. Bioactive Secondary Metabolites Produced by the Fungal Endophytes of Conifers. Nat. Prod. Commun. 2015, 10, 1671–1682. [Google Scholar] [CrossRef]
- Thongbai, B.; Rapior, S.; Hyde, K.D.; Wittstein, K.; Stadler, M. Hericium erinaceus, an amazing medicinal mushroom. Mycol. Prog. 2015, 14, 91. [Google Scholar] [CrossRef]
- Strader, C.R.; Pearce, C.J.; Oberlies, N.H. Fingolimod (FTY720): A Recently Approved Multiple Sclerosis Drug Based on a Fungal Secondary Metabolite. J. Nat. Prod. 2011, 74, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L.; Panaccione, D.G.; Tudzynski, P. Chapter 2 Ergot Alkaloids—Biology and Molecular Biology. Alkaloids Chem. Biol. 2006, 63, 45–86. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Faust, J.R.; Goldstein, J.L.; Kaneko, I.; Endo, A. Induction of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Activity in Human Fibroblasts Incubated with Compactin (ML-236B), a competitive inhibitor of the reductase. J. Biol. Chem. 1978, 253, 1121–1128. [Google Scholar] [CrossRef]
- Endo, A.; Kuroda, M.; Tsujita, Y. ML-236A, ML-236B, and ML-236C, New Inhibitors of Cholesterogenesis Produced by Penicillium Citrinium. J. Antibiot. 1976, 29, 1346–1348. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, M.F.; Liang, J.B.; Ho, Y.W.; Mohamad, R.; Goh, Y.M.; Shokryazdan, P. Lovastatin Production byAspergillus terreusUsing Agro-Biomass as Substrate in Solid State Fermentation. J. Biomed. Biotechnol. 2012, 2012, 196264. [Google Scholar] [CrossRef]
- Wiesinger, D.; Borel, J. Studies on the Mechanism of Action of Cyclosporin A. Immunobiology 1980, 156, 454–463. [Google Scholar] [CrossRef]
- Allison, A.C.; Eugui, E.M. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 2000, 47, 85–118. [Google Scholar] [CrossRef]
- Singh, S.B.; Ondeyka, J.G.; Tsipouras, N.; Ruby, C.; Sardana, V.; Schulman, M.; Sanchez, M.; Pelaez, F.; Stahlhut, M.W.; Munshi, S.; et al. Hinnuliquinone, a C2-symmetric dimeric non-peptide fungal metabolite inhibitor of HIV-1 protease. Biochem. Biophys. Res. Commun. 2004, 324, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, L.; Zhu, T.; Ba, M.; Li, G.; Gu, Q.; Guo, Y.; Li, D. Phenylspirodrimanes with Anti-HIV Activity from the Sponge-Derived Fungus Stachybotrys chartarum MXH-X73. J. Nat. Prod. 2013, 76, 2298–2306. [Google Scholar] [CrossRef]
- Minagawa, K.; Kouzuki, S.; Yoshimoto, J.; Kawamura, Y.; Tani, H.; Iwata, T.; Terui, Y.; Nakai, H.; Yagi, S.; Hattori, N.; et al. Stachyflin and Acetylstachyflin, Novel Anti-influenza A Virus Substances, Produced by Stachybotrys sp. RF-7260. I. Isolation, Structure Elucidation and Biological Activities. J. Antibiot. 2002, 55, 155–164. [Google Scholar] [CrossRef]
- Kaneko, M.; Watashi, K.; Kamisuki, S.; Matsunaga, H.; Iwamoto, M.; Kawai, F.; Ohashi, H.; Tsukuda, S.; Shimura, S.; Suzuki, R.; et al. A Novel Tricyclic Polyketide, Vanitaracin A, Specifically Inhibits the Entry of Hepatitis B and D Viruses by Targeting Sodium Taurocholate Cotransporting Polypeptide. J. Virol. 2015, 89, 11945–11953. [Google Scholar] [CrossRef] [PubMed]
- Sandargo, B.; Michehl, M.; Praditya, D.; Steinmann, E.; Stadler, M.; Surup, F. Antiviral Meroterpenoid Rhodatin and Sesquiterpenoids Rhodocoranes A-E from the Wrinkled Peach Mushroom, Rhodotus Palmatus. Org. Lett. 2019, 21, 3286–3289. [Google Scholar] [CrossRef] [PubMed]


| Fungal Species | Toxin * | Toxin Classification | Chronic Effect | Refs. |
|---|---|---|---|---|
| Aspergillus flavus | Aflatoxins B1, B2 | Aspergillus toxins | Hepatotoxic | [8,17] |
| A. parasiticus | Aflatoxins B1, B2, G1, G2 | Penicillium toxins | ||
| A. ochraceus | Ochratoxin A | Aspergillus toxins | Nephrotoxic | [18,19,20] [21,22,23] |
| Penicillium verrucosum | Ochratoxin A, Citrinin | Penicillium toxins | ||
| P. purpurogenum | Ochratoxin A, Citrinin | |||
| P. expansum | Patulin, Citrinin | Cytotoxic effects | [21,22,23,24,25] | |
| Fusarium sporotrichiodes F. graminearum F.verticillioides F.proliferatum | T-2 toxin, Fumonisin | Fusarium toxins | Gastrointestinal, skin, thyroid, and bone marrow, disorders | [26,27,28,29,30,31,32] |
| Alternaria alternata | Alternariol, Aternariol monomethyl-ether, Tenuazonic acid | Alternaria toxins | Oesophageal cancer, hematologic disorder | [33,34] |
| Stachybotrys atra | Satratoxins | Stachybotrys | Sick building syndrome | [35,36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esheli, M.; Thissera, B.; El-Seedi, H.R.; Rateb, M.E. Fungal Metabolites in Human Health and Diseases—An Overview. Encyclopedia 2022, 2, 1590-1601. https://doi.org/10.3390/encyclopedia2030108
Esheli M, Thissera B, El-Seedi HR, Rateb ME. Fungal Metabolites in Human Health and Diseases—An Overview. Encyclopedia. 2022; 2(3):1590-1601. https://doi.org/10.3390/encyclopedia2030108
Chicago/Turabian StyleEsheli, Manal, Bathini Thissera, Hesham R. El-Seedi, and Mostafa E. Rateb. 2022. "Fungal Metabolites in Human Health and Diseases—An Overview" Encyclopedia 2, no. 3: 1590-1601. https://doi.org/10.3390/encyclopedia2030108
APA StyleEsheli, M., Thissera, B., El-Seedi, H. R., & Rateb, M. E. (2022). Fungal Metabolites in Human Health and Diseases—An Overview. Encyclopedia, 2(3), 1590-1601. https://doi.org/10.3390/encyclopedia2030108








