Effect of Probiotics on Host-Microbial Crosstalk: A Review on Strategies to Combat Diversified Strain of Coronavirus
Abstract
:1. Introduction
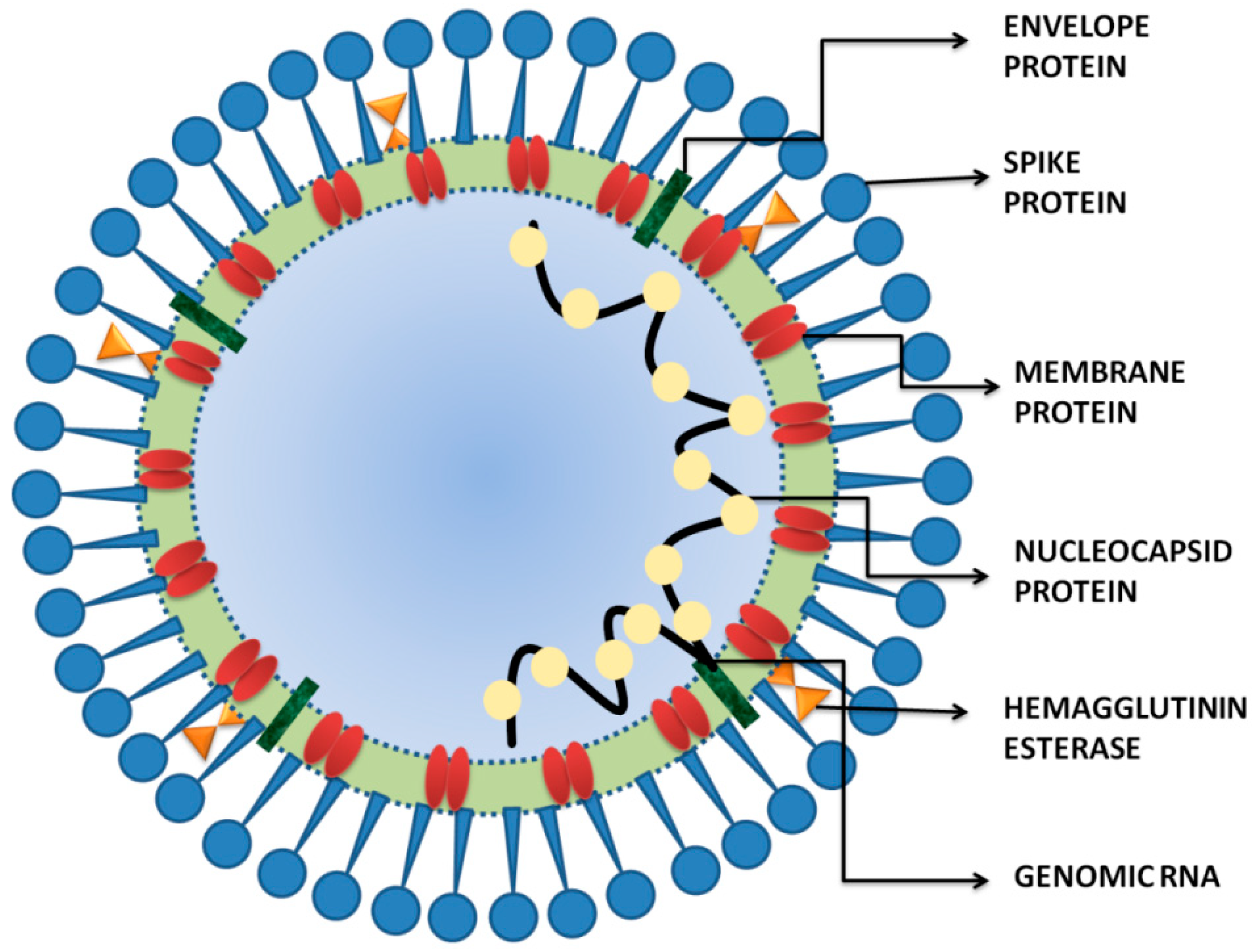
2. SARS-CoV-2 and COVID-19
3. Discussion
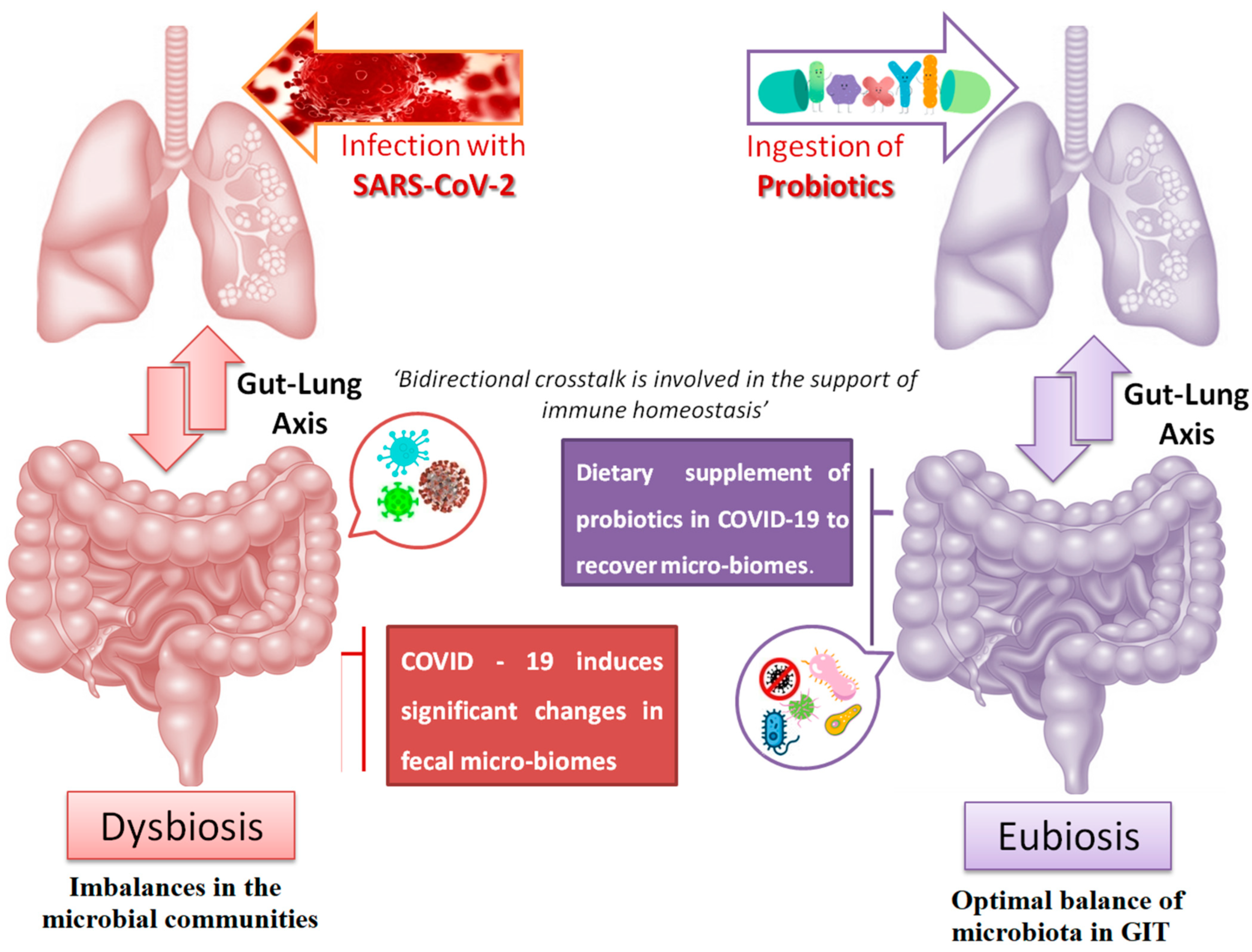
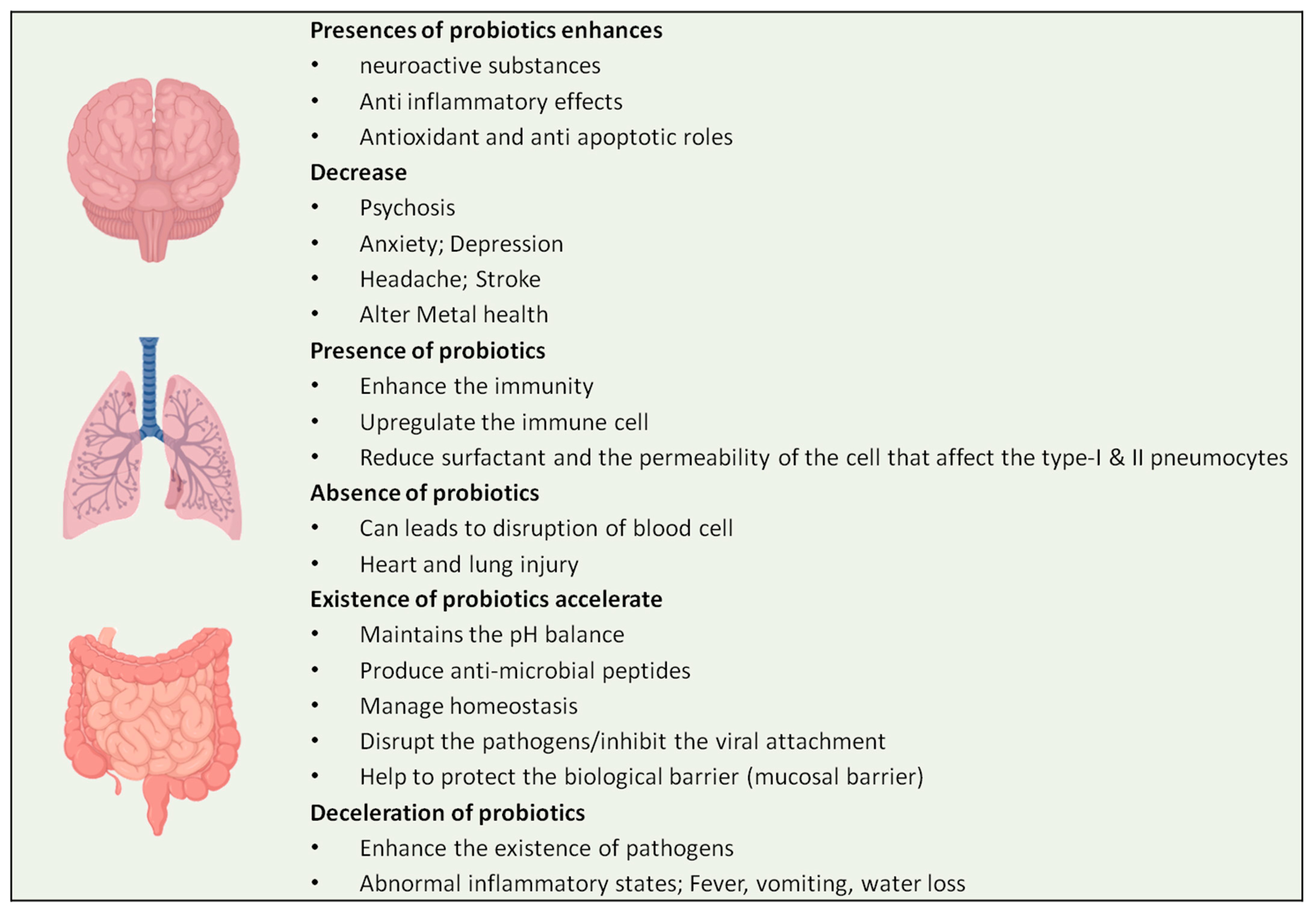
4. COVID-19 Affecting the Gut–Lung Axis Crosstalk
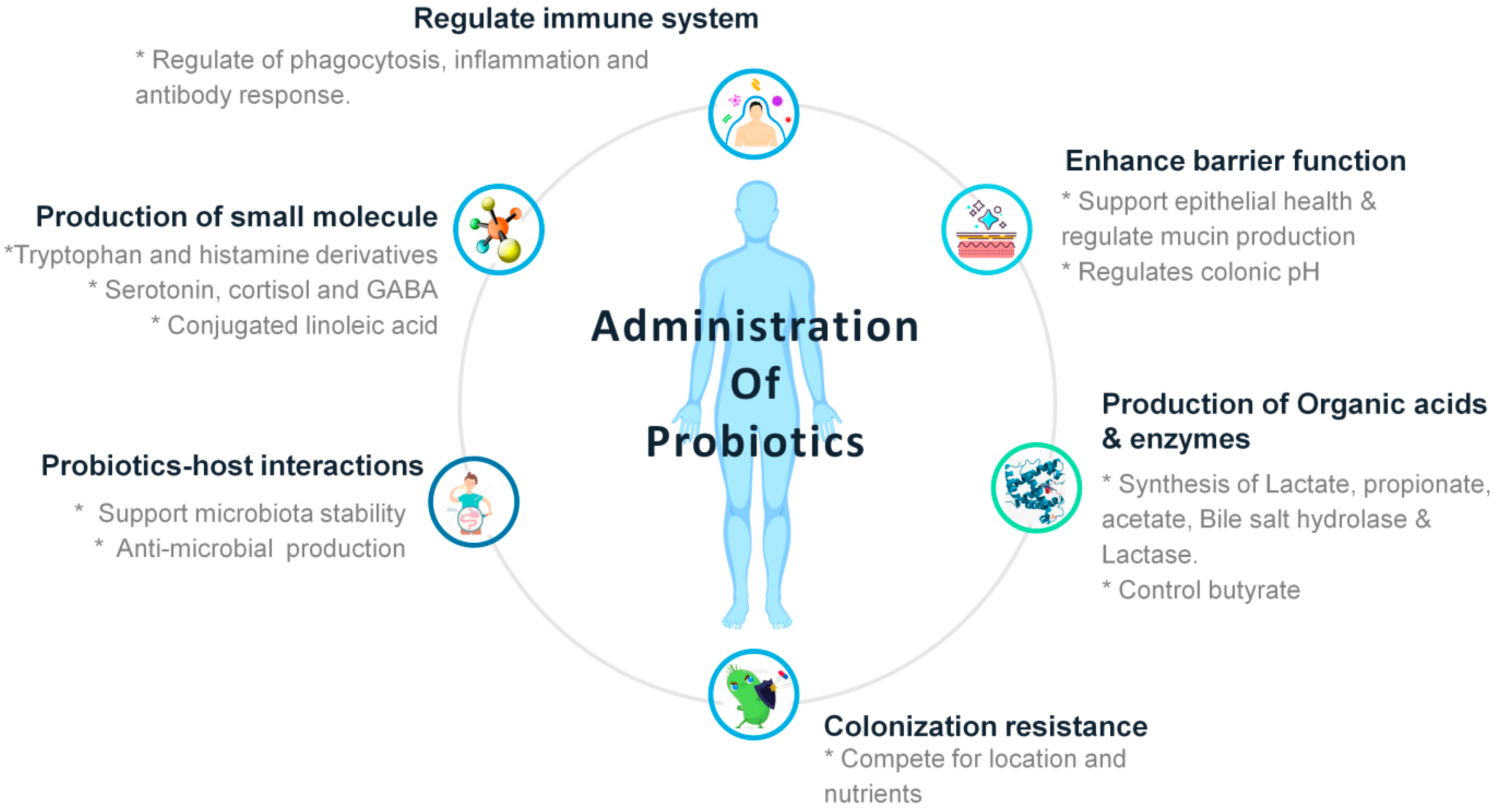
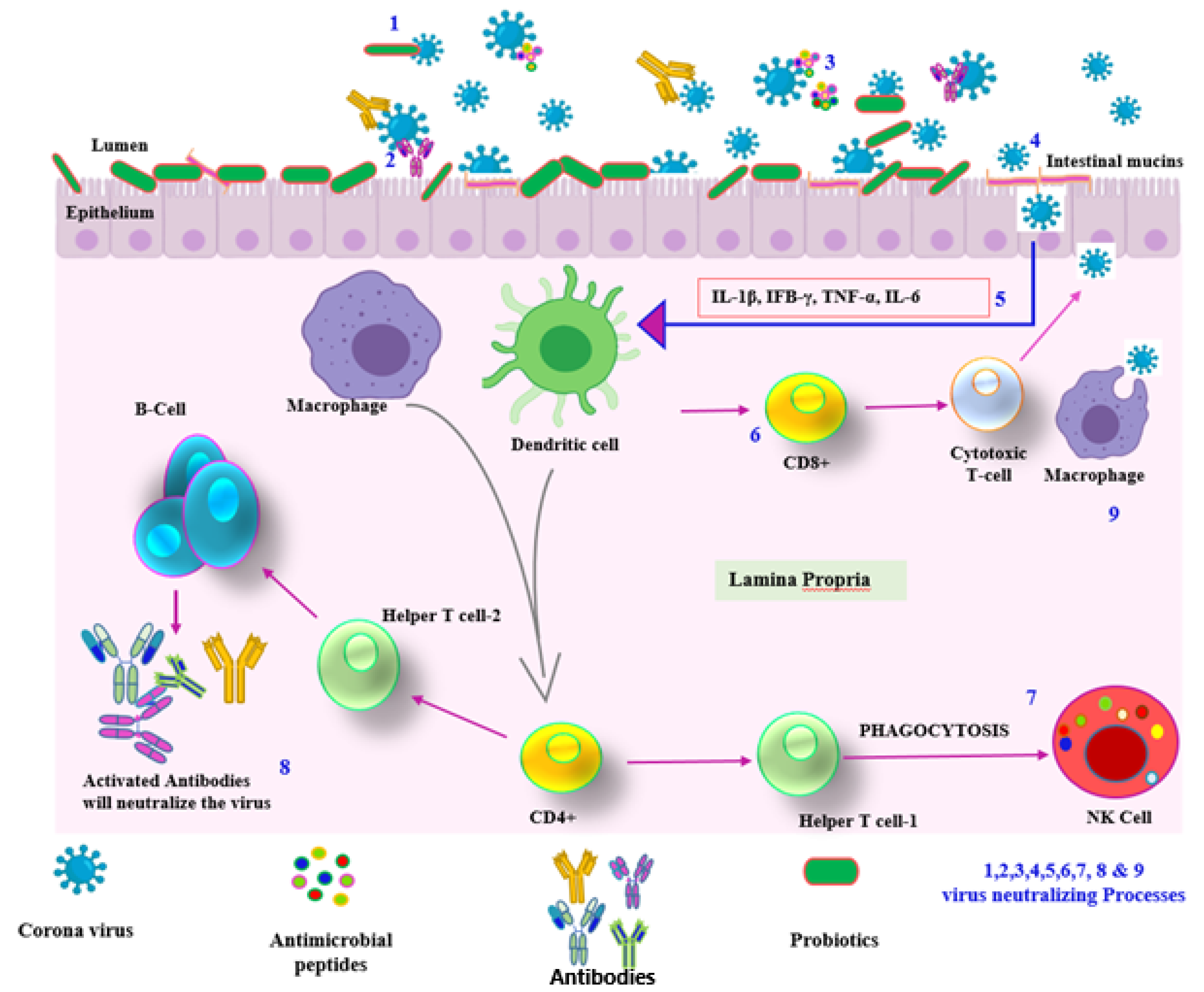
5. Supporting Evidence of Usage of Probiotics to Combat COVID-19
6. Probiotics and COVID-19: Current Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; Gupta, V.; Abrams, E.M.; Adedoyin, R.A.; Adhikari, T.B.; Advani, S.M.; Agrawal, A.; Ahmadian, E.; et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef]
- Zolnikova, O.; Komkova, I.; Potskherashvili, N.; Trukhmanov, A.; Ivashkin, V. Application of probiotics for acute respiratory tract infections. Ital. J. Med. 2018, 12, 32–38. [Google Scholar] [CrossRef]
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.K.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Masud, M.A.; Ahmed, M.; Rahman, M.H. Optimal control for COVID-19 pandemic with quarantine and antiviral therapy. Sens. Int. 2021, 2, 100131. [Google Scholar] [CrossRef] [PubMed]
- Cevik, M.; Bamford, C.G.; Ho, A. COVID-19pandemic—Afocusedreviewforclinicians. Clin. Microbiol. Infect. 2020, 26, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraj, B.; Siriwattananon, K.; Wangkanont, K.; Phoolcharoen, W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19). Asian Pac. J. Allergy Immunol. 2020, 38, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, R.; Mohanty, S.; Vishwakarma, K.; Nayak, S.K.; Samantaray, D.; Mohapatra, S. Update vision on COVID-19: Structure, immunepathogenesis, treatment and safety assessment. Sens. Int. 2021, 2, 100073. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Singh, K.; Rao, A. Probiotics: A potential immunomodulator in COVID-19 infection management. Nutr. Res. 2021, 87, 1–12. [Google Scholar] [CrossRef]
- Zaim, S.; Chong, J.H.; Sankaranarayanan, V.; Harky, A. COVID-19 and multiorgan response. Curr. Probl. Cardiol. 2020, 45, 100618. [Google Scholar] [CrossRef]
- Ng, S.C.; Tilg, H. COVID-19 and the gastrointestinal tract: More than meets the eye. Gut 2020, 69, 973–974. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Jiang, X.; Zhang, Z.; Huang, S.; Zhang, Z.; Fang, Z.; Gu, Z.; Gao, L.; Shi, H.; Mai, L.; et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 2020, 69, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Lian, J.S.; Hu, J.H.; Gao, J.; Zheng, L.; Zhang, Y.M.; Hao, S.R.; Jia, H.Y.; Cai, H.; Zhang, X.L.; et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut 2020, 69, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Allawadhi, P.; Khurana, A.; Banothu, A.K.; Bharani, K.K. Critical neurological features of COVID-19: Role of imaging methods and biosensors for effective diagnosis. Sens. Int. 2021, 2, 100098. [Google Scholar] [CrossRef]
- Flandroy, L.; Poutahidis, T.; Berg, G.; Clarke, G.; Dao, M.-C.; Decaestecker, E.; Furman, E.; Haahtela, T.; Massart, S.; Plovier, H.; et al. The impact of human activities and lifestyles on the interlinked microbiota and health of humans and of ecosystems. Sci. Total Environ. 2018, 627, 1018–1038. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Baud, D.; Dimopoulou Agri, V.; Gibson, G.R.; Reid, G.; Giannoni, E. Using Probiotics to Flatten the Curve of Coronavirus Disease COVID-2019 Pandemic. Front. Public Health 2020, 8, 186. [Google Scholar] [CrossRef]
- Sahoo, S.; Mahapatra, S.R.; Parida, B.K.; Rath, S.; Dehury, B.; Raina, V.; Mohakud, N.K.; Misra, N.; Suar, M. DBCOVP: A database of coronavirus virulent glycoproteins. Comput. Biol. Med. 2021, 129, 104131. [Google Scholar] [CrossRef]
- Banerjee, N.; Mukhopadhyay, S. Viral glycoproteins: Biological role and application in diagnosis. VirusDisease 2016, 27, 1–11. [Google Scholar] [CrossRef]
- Mahapatra, S.R.; Sahoo, S.; Dehury, B.; Raina, V.; Patro, S.; Misra, N.; Suar, M. Designing an efficient multi-epitope vaccine displaying interactions with diverse HLA molecules for an efficient humoral and cellular immune response to prevent COVID-19 infection. Expert Rev. Vaccines 2020, 19, 871–885. [Google Scholar] [CrossRef]
- Damas, J.; Hughes, G.M.; Keough, K.C.; Painter, C.A.; Persky, N.S.; Corbo, M.; Hiller, M.; Koepfli, K.P.; Pfenning, A.R.; Zhao, H.; et al. Broad host range of SARS -CoV-2 predicted by comparative and structural analysis of ACE2 in vertebrates. Proc. Natl. Acad. Sci. USA 2020, 117, 22311–22322. [Google Scholar] [CrossRef]
- Li, C.; Ji, F.; Wang, L.; Wang, L.; Hao, J.; Dai, M.; Liu, Y.; Pan, X.; Fu, J.; Li, L.; et al. Asymptomatic and human-to-human transmission of SARS-CoV-2 in a 2-family cluster, Xuzhou, China. Emerg. Infect. Dis. 2020, 26, 1626. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, J.S.; Smith, D.W. COVID-19: A novel zoonotic disease caused by a coronavirus from China: What we know and what we don’t. Microbiol. Aust. 2020, 41, 45. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Corona Virus Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 13 April 2022).
- Şahin, M. Impact of weatheron COVID-19 pandemic in Turkey. Sci. Total Environ. 2020, 728, 138810. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Jiang, M.; Xia, D.; He, L.; Lv, X.; Liao, X.; Meng, J. COVID-19 in a patient with long-term use of glucocorticoids: A study of a familial cluster. Clin. Immunol. 2020, 214, 108413. [Google Scholar] [CrossRef]
- Dhouib, W.; Maatoug, J.; Ayouni, I.; Zammit, N.; Ghammem, R.; Ben Fredj, S.; Ghannem, H. The incubation period during the pandemic of COVID-19: A systematic review and meta-analysis. Syst. Rev. 2021, 10, 101. [Google Scholar] [CrossRef]
- Sorci, G.; Faivre, B.; Morand, S. Explaining among-country variation in COVID-19 case fatality rate. Sci. Rep. 2020, 10, 18909. [Google Scholar] [CrossRef]
- Xie, M.; Chen, Q. Insight into 2019 novel coronavirus—An updated intrim review and lessons from SARS-CoV and MERS-CoV. Int. J. Infect. Dis. 2020, 94, 119–124. [Google Scholar] [CrossRef]
- Jiang, F.; Deng, L.; Zhang, L.; Cai, Y.; Cheung, C.W.; Xia, Z. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19). J. Gen. Intern. Med. 2020, 35, 1545–1549. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2020, 19, 141–154. [Google Scholar] [CrossRef]
- Pathak, S.K.; Pandey, S.; Pandey, A.; Salunke, A.A.; Thivari, P.; Ratna, H.V.; Chawla, J. Focus on uncommon symptoms of COVID-19: Potential reason for spread of infection. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1873–1874. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Didelot, X.; Yang, J.; Wong, G.; Shi, Y.; Liu, W.; Gao, G.F.; Bi, Y. Inference of person-to-person transmission of COVID-19 reveals hidden super-spreading events during the early outbreak phase. Nat. Commun. 2020, 11, 5006. [Google Scholar] [CrossRef] [PubMed]
- Karia, R.; Gupta, I.; Khandait, H.; Yadav, A.; Yadav, A. COVID-19 and its Modes of Transmission. SN Compr. Clin. Med. 2020, 2, 1798–1801. [Google Scholar] [CrossRef] [PubMed]
- Alimolaie, A. A review of coronavirus disease-2019 (COVID-19). Biol. Sci. Promot. 2020, 3, 152–157. [Google Scholar]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.; et al. Clinical characteristics of corona-virus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Holshue, M.L.; DeBolt, C.; Lindquist, S.; Lofy, K.H.; Wiesman, J.; Bruce, H.; Spitters, C.; Ericson, K.; Wilkerson, S.; Tural, A.; et al. First Case of 2019 Novel Coronavirus in the United States. N. Engl. J. Med. 2020, 382, 929–936. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, C.; Tang, L.; Hong, Z.; Zhou, J.; Dong, X.; Yin, H.; Xiao, Q.; Tang, Y.; Qu, X.; et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020, 5, 434–435. [Google Scholar] [CrossRef]
- Xiao, F.; Tang, M.; Zheng, X.; Liu, Y.; Li, X.; Shan, H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 2020, 158, 1831–1833. [Google Scholar] [CrossRef]
- Kopel, J.; Perisetti, A.; Gajendran, M.; Boregowda, U.; Goyal, H. Clinical Insights into the Gastrointestinal Manifestations of COVID-19. Am. J. Dig. Dis. 2020, 65, 1932–1939. [Google Scholar] [CrossRef] [PubMed]
- Conticini, E.; Frediani, B.; Caro, D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethal-ity in Northern Italy? Environ. Pollut. 2020, 261, 114465. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Bottari, B.; Castellone, V.; Neviani, E. Probiotics and COVID-19. Int. J. Food Sci. Nutr. 2020, 72, 293–299. [Google Scholar] [CrossRef]
- Sahoo, S.; Mahapatra, S.R.; Misra, N.; Suar, M. Application of genomics, transcriptomics, and proteomics in probiotic research. In Probiotic Beverages; Academic Press: Cambridge, MA, USA, 2021; pp. 235–256. [Google Scholar]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Abdelhamid, A.G.; El-Masry, S.S.; El-Dougdoug, N.K. Probiotic Lactobacillus and Bifidobacterium strains possess safety character-istics, antiviral activities and host adherence factors revealed by genome mining. EPMA J. 2019, 10, 337–350. [Google Scholar] [CrossRef]
- Lu, W.; Fang, Z.; Liu, X.; Li, L.; Zhang, P.; Zhao, J.; Zhang, H.; Chen, W. The Potential Role of Probiotics in Protection against Influenza a Virus Infection in Mice. Foods 2021, 10, 902. [Google Scholar] [CrossRef]
- Izumo, T.; Maekawa, T.; Ida, M.; Noguchi, A.; Kitagawa, Y.; Shibata, H.; Yasui, H.; Kiso, Y. Effect of intranasal administration of Lactobacillus pentosus S-PT84 on influenza virus infection in mice. Int. Immunopharmacol. 2010, 10, 1101–1106. [Google Scholar] [CrossRef]
- Liu, Y.; Tran, D.Q.; Rhoads, J.M. Probiotics in Disease Prevention and Treatment. J. Clin. Pharmacol. 2018, 58, S164–S179. [Google Scholar] [CrossRef]
- Amara, A.A.; Shibl, A. Role of Probiotics in health improvement, infection control and disease treatment and management. Saudi Pharm. J. 2015, 23, 107–114. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.-Y.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Bron, P.A.; Van Baarlen, P.; Kleerebezem, M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat. Rev. Microbiol. 2012, 10, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Saad, N.; Delattre, C.; Urdaci, M.; Schmitter, J.M.; Bressollier, P. An overview of the last advances in probiotic and prebiotic field. LWT Food Sci. Technol. 2013, 50, 1–16. [Google Scholar] [CrossRef]
- Santosa, S.; Farnworth, E.; Jones, P.J.H. Probiotics and their potential health claims. Nutr. Rev. 2006, 64, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented Foods as a Dietary Source of Live Organisms. Front. Microbiol. 2018, 9, 1785. [Google Scholar] [CrossRef]
- Yan, F.; Polk, D.B. Probiotics and immune health. Curr. Opin. Gastroenterol. 2011, 27, 496–501. [Google Scholar] [CrossRef]
- Hardy, H.; Harris, J.; Lyon, E.; Beal, J.; Foey, A.D. Probiotics, prebiotics and immunomodulation of gut mucosal defences: Homeo-stasis and immunopathology. Nutrients 2013, 5, 1869–1912. [Google Scholar] [CrossRef]
- Al Kassaa, I. New Insights on Antiviral Probiotics; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Mahooti, M.; Abdolalipour, E.; Salehzadeh, A.; Mohebbi, S.R.; Gorji, A.; Ghaemi, A. Immunomodulatory and prophylactic effects of Bifidobacteriumbifidum probiotic strain on influenza infection in mice. World J. Microbiol. Biotechnol. 2019, 35, 91. [Google Scholar] [CrossRef]
- Starosila, D.; Rybalko, S.; Varbanetz, L.; Ivanskaya, N.; Sorokulova, I. Anti-influenza Activity of a Bacillus subtilis Probiotic Strain. Antimicrob. Agents Chemother. 2017, 61, e00539-17. [Google Scholar] [CrossRef]
- Kawashima, T.; Hayashi, K.; Kosaka, A.; Kawashima, M.; Igarashi, T.; Tsutsui, H.; Tsuji, N.M.; Nishimura, I.; Hayashi, T.; Obata, A. Lactobacillus plantarum strain YU from fermented foods activates Th1 and protective immune responses. Int. Immunopharmacol. 2011, 11, 2017–2024. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.I.; Bae, J.Y.; Yoo, K.; Kim, H.; Kim, I.H.; Park, M.S.; Lee, I. Effects of heat-killed Lactobacillus plantarum against influ-enza viruses in mice. J. Microbiol. 2018, 56, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Lee, Y.T.; Le Ngo, V.; Cho, Y.H.; Ko, E.J.; Hong, S.M.; Kim, K.H.; Jang, J.H.; Oh, J.S.; Park, M.K.; et al. Heat-killed Lactobacil-lus casei confers broad protection against influenza A virus primary infection and develops heterosubtypic immunity against future secondary infection. Sci. Rep. 2017, 7, 17360. [Google Scholar] [CrossRef] [PubMed]
- Lehtoranta, L.; Pitkäranta, A.; Korpela, R. Probiotics in respiratory virus infections. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1289–1302. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.W.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. The international scientific association and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Guarino, M.P.L.; Altomare, A.; Emerenziani, S.; Di Rosa, C.; Ribolsi, M.; Balestrieri, P.; Iovino, P.; Rocchi, G.; Cicala, M. Mechanisms of Action of Prebiotics and Their Effects on Gastro-Intestinal Disorders in Adults. Nutrients 2020, 12, 1037. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Aolymat, I.; Al-Holy, M.; Ayyash, M.; Abu Ghoush, M.; Al-Nabulsi, A.A.; Osaili, T.; Apostolopoulos, V.; Liu, S.-Q.; Shah, N.P. The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19. npj Sci. Food 2020, 4, 17. [Google Scholar] [CrossRef]
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut–lung axis. Mucosal Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef]
- Hufnagl, K.; Pali-Schöll, I.; Roth-Walter, F.; Jensen-Jarolim, E. Dysbiosis of the gut and lung microbiome has a role in asthma. Semin. Immunopathol. 2020, 42, 75–93. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.S.; Peng, S.H.; Deng, X.Y.; Zhu, D.M.; Javidiparsijani, S.; Wang, G.R.; Li, D.Q.; Li, L.X.; Wang, Y.C.; et al. Gut-lung cross-talk in pulmonary involvement with inflammatory bowel diseases. World J. Gastroenterol. 2013, 19, 6794. [Google Scholar] [CrossRef] [PubMed]
- Smyk, W.; Janik, M.K.; Portincasa, P.; Milkiewicz, P.; Lammert, F.; Krawczyk, M. COVID-19: Focus on the lungs but do not forget the gastrointestinal tract. Eur. J. Clin. Investig. 2020, 50, e13276. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Li, J.; Shen, L.; Zou, Y.; Hou, L.; Zhu, L.; Faden, H.S.; Tang, Z.; Shi, M.; Jiao, N.; et al. Enteric involvement in hospitalised patients with COVID-19 outside Wuhan. Lancet Gastroenterol. Hepatol. 2020, 5, 534–535. [Google Scholar] [CrossRef]
- Szajewska, H.; Kołodziej, M.; Gieruszczak-Białek, D.; Skórka, A.; Ruszczyński, M.; Shamir, R. Systematic review with meta-analysis: Lactobacillus rhamnosus GG for treating acute gastroenteritis in children—A 2019 update. Aliment. Pharmacol. Ther. 2019, 49, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Sze, M.A.; Tsuruta, M.; Yang, S.-W.J.; Oh, Y.; Man, S.F.P.; Hogg, J.C.; Sin, D.D. Changes in the bacterial microbiota in gut, blood, and lungs following acute LPS instillation into mice lungs. PLoS ONE 2014, 9, e111228. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wen, Q.; Yao, F.; Xu, D.; Huang, Y.; Wang, J. Gut–lung axis: The microbial contributions and clinical implications. Crit. Rev. Microbiol. 2017, 43, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Vital, M.; Harkema, J.R.; Rizzo, M.; Tiedje, J.; Brandenberger, C. Alterations of the murine gut microbiome with age and allergic airway disease. J. Immunol. Res. 2015, 2015, 892568. [Google Scholar] [CrossRef]
- Kumar, V.C.; Mukherjee, S.; Harne, P.S.; Subedi, A.; Ganapathy, M.K.; Patthipati, V.S.; Sapkota, B. Novelty in the gut: A systematic re-view and meta-analysis of the gastrointestinal manifestations of COVID-19. BMJ Open Gastroenterol. 2020, 7, e000417. [Google Scholar] [CrossRef]
- Ciaglia, E.; Vecchione, C.; Puca, A.A. COVID-19 Infection and Circulating ACE2 Levels: Protective Role in Women and Children. Front. Pediatr. 2020, 8, 206. [Google Scholar] [CrossRef]
- TeRiet, L.; van Esch, J.H.; Roks, A.J.; van den Meiracker, A.H.; Danser, A.J. Hypertension: Renin–angiotensin–aldosterone system alterations. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef]
- Perrone, E.E.; Jung, E.; Breed, E.; Dominguez, J.A.; Liang, Z.; Clark, A.T.; Dunne, W.M.; Burd, E.M.; Coopersmith, C.M. Mechanisms of Methicillin-Resistant Staphylococcus aureus Pneumonia–Induced Intestinal Epithelial Apoptosis. Shock 2012, 38, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Budden, K.F.; Gellatly, S.L.; Wood, D.L.; Cooper, M.A.; Morrison, M.; Hugenholtz, P.; Hansbro, P.M. Emerging pathogenic links between microbiota and the gut–lung axis. Nat. Rev. Microbiol. 2017, 15, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Zelaya, H.; Alvarez, S.; Kitazawa, H.; Villena, J. Respiratory antiviral immunity and immunobiotics: Beneficial effects on inflamma-tion-coagulation interaction during influenza virus infection. Front. Immunol. 2016, 7, 633. [Google Scholar] [CrossRef] [PubMed]
- Agamennone, V.; Krul, C.A.M.; Rijkers, G.; Kort, R. A practical guide for probiotics applied to the case of antibiotic-associated diarrhea in The Netherlands. BMC Gastroenterol. 2018, 18, 103. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, J.Z.; Yap, C.; Lytvyn, L.; Lo, C.K.-F.; Beardsley, J.; Mertz, D.; Johnston, B.C. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst. Rev. 2017, 12, CD006095. [Google Scholar] [CrossRef]
- Johnston, B.C.; Goldenberg, J.Z.; Vandvik, P.O.; Sun, X.; Guyatt, G.H. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst. Rev. 2011, 11, CD004827. [Google Scholar] [CrossRef]
- Lenoir-Wijnkoop, I.; Gerlier, L.; Roy, D.; Reid, G. The clinical and economic impact of probiotics consumption on respiratory tract infections: Projections for Canada. PLoS ONE 2016, 11, e0166232. [Google Scholar] [CrossRef]
- Pregliasco, F.; Anselmi, G.; Fonte, L.; Giussani, F.; Schieppati, S.; Soletti, L. A New Chance of Preventing Winter Diseases by the Administration of Synbiotic Formulations. J. Clin. Gastroenterol. 2008, 42, S224–S233. [Google Scholar] [CrossRef]
- Guillemard, E.; Tondu, F.; Lacoin, F.; Schrezenmeir, J. Consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114 001 reduces the duration of respiratory infections in the elderly in a randomised controlled trial. Br. J. Nutr. 2009, 103, 58–68. [Google Scholar] [CrossRef]
- Guillemard, E.; Tanguy, J.; Flavigny, A.L.; de la Motte, S.; Schrezenmeir, J. Effects of consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114 001 on common respiratory and gastrointestinal infections in shift workers in a randomized controlled trial. J. Am. Coll. Nutr. 2010, 29, 455–468. [Google Scholar] [CrossRef]
- Issa, I.; Moucari, R. Probiotics for antibiotic-associated diarrhea: Do we have a verdict? World J. Gastroenterol. 2014, 20, 17788–17795. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; He, F.; Kubota, A.; Harata, G.; Hiramatsu, M. Oral administration of Lactobacilli from human intestinal tract protects mice against influenza virus infection. Lett. Appl. Microbiol. 2010, 51, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Saito, T.; Uematsu, T.; Kishi, K.; Toba, M.; Kohda, N.; Suzuki, T. Oral administration of heat-killed Lactobacillus pentosus strain b240 augments protection against influenza virus infection in mice. Int. Immunopharmacol. 2010, 11, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.N.; Lee, D.H.; Lee, Y.N.; Park, J.K.; Yuk, S.S.; Yang, S.Y.; Lee, H.J.; Woo, S.H.; Kim, H.M.; Lee, J.B.; et al. Intranasal administration of live Lactobacillus species facilitates pro-tection against influenza virus infection in mice. Antiviral. Res. 2012, 93, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Oliva, S.; Di Nardo, G.; Ferrari, F.; Mallardo, S.; Rossi, P.; Patrizi, G.; Cucchiara, S.; Stronati, L. Randomised clinical trial: The effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis. Aliment. Pharmacol. Ther. 2011, 35, 327–334. [Google Scholar] [CrossRef]
- Kwak, M.-K.; Liu, R.; Kwon, J.-O.; Kim, M.-K.; Kim, A.H.; Kang, S.-O. Cyclic dipeptides from lactic acid bacteria inhibit proliferation of the influenza a virus. J. Microbiol. 2013, 51, 836–843. [Google Scholar] [CrossRef]
- Goto, H.; Sagitani, A.; Ashida, N.; Kato, S.; Hirota, T.; Shinoda, T.; Yamamoto, N. Anti-influenza virus effects of both live and non-liveLactobacillus acidophilus l-92 accompanied by the activation of innate immunity. Br. J. Nutr. 2013, 110, 1810–1818. [Google Scholar] [CrossRef]
- Smith, T.J.; Rigassio-Radler, D.; Denmark, R.; Haley, T.; Touger-Decker, R. Effect of Lactobacillus rhamnosus LGG(r) and Bifidobacteriumanimalis ssp. lactis bb-12(r) on health-related quality of life in college students affected by upper respiratory infec-tions. Br. J. Nutr. 2013, 109, 1999–2007. [Google Scholar] [CrossRef]
- Zelaya, H.; Tsukida, K.; Chiba, E.; Marranzino, G.; Alvarez, S.; Kitazawa, H.; Agüero, G.; Villena, J. Immunobiotic lactobacilli reduce viral-associated pulmonary damage through the modulation of inflammation–coagulation interactions. Int. Immunopharmacol. 2014, 19, 161–173. [Google Scholar] [CrossRef]
- Nakayama, Y.; Moriya, T.; Sakai, F.; Ikeda, N.; Shiozaki, T.; Hosoya, T.; Nakagawa, H.; Miyazaki, T. Oral administration of Lactobacillus gasseri SBT2055 is effective for preventing influenza in mice. Sci. Rep. 2014, 4, 4638. [Google Scholar] [CrossRef]
- Dietrich, C.G.; Kottmann, T.; Alavi, M. Commercially available probiotic drinks containing Lactobacillus casei DN-114001 reduce antibiotic-associated diarrhea. World J. Gastroenterol. 2014, 20, 15837–15844. [Google Scholar] [CrossRef] [PubMed]
- Bo, L.; Li, J.; Tao, T.; Bai, Y.; Ye, X.; Hotchkiss, R.S.; Kollef, M.H.; Crooks, N.H.; Deng, X. Probiotics for preventing ventilator-associated pneumonia. Cochrane Database Syst. Rev. 2014, 10, CD009066. [Google Scholar] [CrossRef] [PubMed]
- Ang, L.Y.; Too, H.K.; Tan, E.L.; Chow, T.K.; Shek, P.C.; Tham, E.; Alonso, S. Antiviral activity of Lactobacillus reuteriProtectis against Coxsackievirus A and Enterovirus 71 infection in human skeletal muscle and colon cell lines. Virol. J. 2016, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Song, J.A.; Kim, H.J.; Hong, S.K.; Lee, D.H.; Lee, S.W.; Song, C.S.; Kim, K.T.; Choi, I.S.; Lee, J.B.; Park, S.Y. Oral intake of Lactobacillus rhamnosus M21 enhances the survival rate of mice lethally infected with influenza virus. J. Microbiol. Immunol. Infect. 2014, 49, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Ge, T.; Xiao, Y.; Liao, Y.; Cui, Y.; Zhang, Y.; Ho, W.; Yu, G.; Zhang, T. Probiotics for prevention and treatment of respiratory tract infections in children: A systematic review and meta-analysis of randomized controlled trials. Medicine 2016, 95, e4509. [Google Scholar] [CrossRef]
- Chen, M.-F.; Weng, K.-F.; Huang, S.-Y.; Liu, Y.-C.; Tseng, S.-N.; Ojcius, D.M.; Shih, S.-R. Pretreatment with a heat-killed probiotic modulates monocyte chemoattractant protein-1 and reduces the pathogenicity of influenza and enterovirus 71 infections. Mucosal Immunol. 2016, 10, 215–227. [Google Scholar] [CrossRef]
- Shojadoost, B.; Kulkarni, R.; Brisbin, J.T.; Quinteiro-Filho, W.; Alkie, T.N.; Sharif, S. Interactions between lactobacilli and chicken macrophages induce antiviral responses against avian influenza virus. Res. Vet. Sci. 2019, 125, 441–450. [Google Scholar] [CrossRef]
- Pu, F.; Guo, Y.; Li, M.; Zhu, H.; Wang, S.; Shen, X.; He, M.; Huang, C.; He, F. Yogurt supplemented with probiotics can protect the healthy elderly from respiratory infections: A randomized controlled open-label trial. Clin. Interv. Aging 2017, 12, 1223–1231. [Google Scholar] [CrossRef]
- Shida, K.; Sato, T.; Iizuka, R.; Hoshi, R.; Watanabe, O.; Igarashi, T.; Miyazaki, K.; Nanno, M.; Ishikawa, F. Daily intake of fermented milk with Lactobacillus casei strain Shirota reduces the incidence and duration of upper respiratory tract infections in healthy middle-aged office workers. Eur. J. Nutr. 2017, 56, 45–53. [Google Scholar] [CrossRef]
- Zhang, H.; Yeh, C.; Jin, Z.; Ding, L.; Liu, B.Y.; Zhang, L.; Dannelly, H.K. Prospective study of probiotic supplementation results in immune stimulation and improvement of upper respiratory infection rate. Synth. Syst. Biotechnol. 2018, 3, 113–120. [Google Scholar] [CrossRef]
- Eguchi, K.; Fujitani, N.; Nakagawa, H.; Miyazaki, T. Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus gasseri SBT2055. Sci. Rep. 2019, 9, 4812. [Google Scholar] [CrossRef] [PubMed]
- King, S.; Tancredi, D.; Lenoir-Wijnkoop, I.; Gould, K.; Vann, H.; Connors, G.; Sanders, M.E.; A Linder, J.; Shane, A.L.; Merenstein, D. Does probiotic consumption reduce antibiotic utilization for common acute infections? A systematic review and meta-analysis. Eur. J. Public Health 2018, 29, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Robles-Vera, I.; Toral, M.; Romero, M.; Jiménez, R.; Sánchez, M.; Pérez-Vizcaíno, F.; Duarte, J. Antihypertensive effects of probiotics. Curr. Hypertens. Rep. 2017, 19, 26. [Google Scholar] [CrossRef]
- Ayyash, M.M.; Sherkat, F.; Shah, N.P. The effect of NaCl substitution with KCl on Akawi cheese: Chemical composition, prote-olysis, angiotensin-converting enzyme-inhibitory activity, probiotic survival, texture profile, and sensory properties. J. Dairy Sci. 2012, 95, 4747–4759. [Google Scholar] [CrossRef] [PubMed]
- Ayyash, M.; Olaimat, A.; Al-Nabulsi, A.; Liu, S.Q. Bioactive properties of novel probioticLactococcuslactisfermented camel sausages: Cytotoxicity, angiotensin converting enzyme inhibition, antioxidant capacity, and antidiabeticactivity. Food Sci. Anim. Resour. 2020, 40, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Miremadi, F.; Ayyash, M.; Sherkat, F.; Stojanovska, L. Cholesterol reduction mechanisms and fatty acid composition of cellular membranes of probiotic Lactobacilli and Bifidobacteria. J. Funct. Foods 2014, 9, 295–305. [Google Scholar] [CrossRef]
- Fernández-Fernández, F.J. COVID-19, hypertension and angiotensin receptor-blocking drugs. J. Hypertens. 2020, 38, 1191. [Google Scholar] [CrossRef]
- Esler, M.; Esler, D. Can angiotensin receptor-blocking drugs perhaps be harmful in the COVID-19 pandemic? J. Hypertens. 2020, 38, 781–782. [Google Scholar] [CrossRef]
- Imai, Y.; Kuba, K.; Rao, S.; Huan, Y.; Guo, F.; Guan, B.; Yang, P.; Sarao, R.; Wada, T.; Leong-Poi, H.; et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005, 436, 112–116. [Google Scholar] [CrossRef]
- Yeh, T.L.; Shih, P.C.; Liu, S.J.; Lin, C.H.; Liu, J.M.; Lei, W.T.; Lin, C.Y. The influence of prebiotic or probiotic supplementation on antibody titers after influenza vaccination: A systematic review and meta-analysis of randomized controlled trials. Drug Des. Devel. Ther. 2018, 12, 217–230. [Google Scholar] [CrossRef]
- Infusino, F.; Marazzato, M.; Mancone, M.; Fedele, F.; Mastroianni, C.M.; Severino, P.; Ceccarelli, G.; Santinelli, L.; Cavarretta, E.; Marullo, A.G.M.; et al. Diet Supplementation, Probiotics, and Nutraceuticals in SARS-CoV-2 Infection: A Scoping Review. Nutrients 2020, 12, 1718. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Cai, H.; Shen, Y.; Ni, Q.; Chen, Y.; Hu, S.; Li, J.; Wang, H.; Yu, L.; Huang, H.; et al. Management of corona virus disease-19 (COVID-19): The Zhejiang experience. Zhejiang Da Xue Xue Bao. Yi Xue Ban 2020, 49, 147–157. [Google Scholar] [PubMed]
- Gasmi, A.; Noor, S.; Tippairote, T.; Dadar, M.; Menzel, A.; Bjørklund, G. Individual risk management strategy and potential therapeutic options for the COVID-19 pandemic. Clin. Immunol. 2020, 215, 108409. [Google Scholar] [CrossRef]
- Makino, S.; Ikegami, S.; Kume, A.; Horiuchi, H.; Sasaki, H.; Orii, N. Reducing the risk of infection in the elderly by dietary intake of yoghurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. Br. J. Nutr. 2010, 104, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Merenstein, D.; Murphy, M.; Fokar, A.; Hernandez, R.K.; Park, H.; Nsouli, H.; Sanders, M.E.; Davis, B.A.; Niborski, V.; Tondu, F.; et al. Use of a fermented dairy probiotic drink containing Lactobacillus casei (DN-114 001) to decrease the rate of illness in kids: The DRINK study A patient-oriented, double-blind, cluster-randomized, placebo-controlled, clinical trial. Eur. J. Clin. Nutr. 2010, 64, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, D.; Mohapatra, S.; Misra, S.; Sahu, P. Milk derived bioactive peptides and their impact on human health—A review. Saudi J. Biol. Sci. 2015, 23, 577–583. [Google Scholar] [CrossRef]
- Mohanty, D.; Jena, R.; Choudhury, P.K.; Pattnaik, R.; Mohapatra, S.; Saini, M.R. Milk Derived Antimicrobial Bioactive Peptides: A Review. Int. J. Food Prop. 2015, 19, 837–846. [Google Scholar] [CrossRef]
- Van den Nieuwboer, M.; Brummer, R.J.; Guarner, F.; Morelli, L.; Cabana, M.; Claassen, E. The administration of probiot-ics and synbiotics in immune compromised adults: Is it safe? Benef. Microbes 2015, 6, 3–17. [Google Scholar] [CrossRef]
- Nieuwboer, M.V.D.; Claassen, E.; Morelli, L.; Guarner, F.; Brummer, R. Probiotic and synbiotic safety in infants under two years of age. Benef. Microbes 2014, 5, 45–60. [Google Scholar] [CrossRef]
- Larsen, O.F.A.; Van den Nieuwboer, M.; Koks, M.; Flach, J.; Claassen, H.J.H.M. Probiotics for healthy ageing: Innovation barriers and opportunities for bowel habit improvement in nursing homes. Agro Food Ind. Hi Tech 2017, 28, 12–15. [Google Scholar]
- Finlay, B.B.; Amato, K.R.; Azad, M.; Blaser, M.J.; Bosch, T.C.; Chu, H.; Dominguez-Bello, M.G.; Ehrlich, S.D.; Elinav, E.; GevaZatorsky, N.; et al. The hygiene hypothesis, the COVID pandemic, and consequences for the human microbiome. Proc. Natl. Acad. Sci. USA 2021, 118, e2010217118. [Google Scholar] [CrossRef] [PubMed]
- Larsen, O.F.; van de Burgwal, L.H. On the Verge of a Catastrophic Collapse? The Need for a Multi-Ecosystem Approach to Microbiome Studies. Front. Microbiol. 2021, 12, 784797. [Google Scholar] [CrossRef] [PubMed]
| Probiotics Strains | Against Diseases and Viral Infections | Clinically Tested on | Results from the Clinical Studies | Ref. |
|---|---|---|---|---|
| B.infantis 35624 | Inflammatory-bowel diseases | Clinical trial on 192 participants | Significant reduction in C reactive protein levels and proinflammatory markers (TNF-α and IL-6) | [9] |
| B. bifidum | Influenza virus—(H1N1) | Female mice | Elevated survival rate along with the induction of both humoral and cellular immune responses | [9] |
| B. lactis | RTI | Clinical trial on 109 participants | Neonates receiving probiotics had a lower (65%) incidence of respiratory infections as compared to 94% of infants in the control group | [9] |
| Bacillus subtilis3 | Influenza virus(H1N1) | Mice | Reduced viralload in lungs and improved survival rate of infected mice | [9] |
| L. pentosus | Influenza virus(H1N1) | Female mice | Higher survivalrate and lower viralload in lungs alongwith increased NK cellactivity along with a high expression of IL-12 and IFN-α in the lung | [95] |
| L. rhamnosus GG and L. gasseri | Influenza virus(H1N1) | Female mice | Improved clinical symptoms and lower virus load in the lungs | [96] |
| L. pentosus | Influenza virus(H1N1) | Female mice | Alleviate survival rate and decreased virus load in the lungs along with increased production of IgA and IgG in bronchoalveolar lavage fluid and plasma | [97] |
| L. rhamnosus | Influenza virus(H1N1) | Female mice | Alleviate survival rate with increased secretory IgA production and reduced the expression levels of TNF-α and IL-6 | [98] |
| L. reuteri | Inflammatory-bowel diseases | Clinical trial on 40 participants | Useful in improving mucosal inflammation along with increased cytokine expression level of IL-10 and decreased levels of TNF-α, IL-1β and IL-8 | [99] |
| L. plantarum | Influenza virus A/PR/8/34 (H1N1) | Female mice | decreased weight loss, increased clinical symptoms and reduced virus load in the lungs of infected mice | [9] |
| L. plantarum | Influenza virusH3N2 | Madin–Darby canine kidney cells | Inhibited viral infectivity and proliferation successfully | [100] |
| L. acidophilus | Influenza virus(H1N1) | Female mice | Increased expression of antiviral cytokines and chemokines with prevented weight lossand reduced viral load in the lungs | [101] |
| L. rhamnosus and B. lactis | Upper-respiratory tract infection | Clinical trial on 231candidates | Lower severity in the probiotics group | [102] |
| L. rhamnosus | Influenza virus (H1N1) andrespiratory syncytial virus | Male mice | Decreased risk of lung injury | [103] |
| L. gasseri | Influenza virus(H1N1) | Male mice | Reduced expression of IL-6 in the lung tissue and decreased virus load | [104] |
| L. casei | Antibiotic-associated diarrhea | Clinical trial on 258 candidates | Effective in the treatment of antibiotic-associated diarrhea in adults and infants | [105] |
| B. longum, L. rhamnosus, and L. plantarum | Ventilator-associated pneumonia | Clinical trials on 1083 candidates | Revealed the beneficial role of probiotic strains in reducing the risk of ventilator-associated pneumoniain patients | [106] |
| L. reuteri Protectis | Coxsackie-viruses and enterovirus | Human rhabdomyosarcoma and Caco-2 cell lines | Revealed antiviral activity Coxsackievirus and Enterovirus | [107] |
| L. rhamnosus | Influenza virus(H1N1) | Female mice | Increased production of IFN-γ, IL-2and IgA; the increased survival rate and lower viral titer in lungs of infected mice | [108] |
| Streptococcus thermophilus, L. acidophilus, L. rhamnosus 1, and B. lactis Bb-12. | Upper-respiratory tract infection | Clinical trials on 6269 participants | Decrease in the prevalence of respiratory tract infections along with the improved quality of life | [109] |
| Enterococcus faecalis | Influenza virus and enterovirus | Male mice | Low viral load and improved survival rate | [110] |
| L. salivarius, L. reuteri, and L. acidophilus | Influenza virus(H4N6) | Madin–Darby canine kidney cells | Improved expression of IL-1β, IFN-γand IFN-α resulted in protective responses against infection | [65] |
| L. casei | Influenza virus(H3N2) | Female mice | Prevented weight loss and higher survival rate | [111] |
| L. paracasei | Upper respiratory tract infection | Clinical trial on 233 candidates | Reduced provenance | [112] |
| L. casei | Upper respiratory tract infection | Clinical trial on 96 female candidates | Lower incidence of respiratory infections | [113] |
| L. plantarum | Influenza H1N1 andH3N2 | Female mice | Significantly lower viral proliferation and increased survival rate | [9] |
| L. fermentum, L. casei, and L. paracase. | Upper respiratory tract infection | Clinical trial on 136 patients | 50–60% reduced prevalence of common cold and increased levels of IFN-γ andIgA | [51] |
| B. infantis, L. reuteri, and L. rhamnosus GG | Multiple diseases | Meta-analysis trials | Probiotics wereeffectivein combating necrotizing enterocolitis, infant colic, antibiotic-associated diarrhea, acute infectious diarrhea and acute respiratory tract infections | [114] |
| L. gasseri | Respiratory syncytial virus-A2 strain | Female mice | Eeduced expression of proinflammatory cytokines, with decreased risk of weight loss and lower viral load in the lungs | [115] |
| B. lactis Bb-12L. rhamnosus GG, L. casei | Acute otitis andacute respiratory tract infections | - | Reduction in the prevalence of common acute infections and antibiotics utilization | [116] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahoo, S.; Mohapatra, S.; Dalai, S.p.; Misra, N.; Suar, M. Effect of Probiotics on Host-Microbial Crosstalk: A Review on Strategies to Combat Diversified Strain of Coronavirus. Encyclopedia 2022, 2, 1138-1153. https://doi.org/10.3390/encyclopedia2020076
Sahoo S, Mohapatra S, Dalai Sp, Misra N, Suar M. Effect of Probiotics on Host-Microbial Crosstalk: A Review on Strategies to Combat Diversified Strain of Coronavirus. Encyclopedia. 2022; 2(2):1138-1153. https://doi.org/10.3390/encyclopedia2020076
Chicago/Turabian StyleSahoo, Susrita, Swati Mohapatra, Swayam prava Dalai, Namrata Misra, and Mrutyunjay Suar. 2022. "Effect of Probiotics on Host-Microbial Crosstalk: A Review on Strategies to Combat Diversified Strain of Coronavirus" Encyclopedia 2, no. 2: 1138-1153. https://doi.org/10.3390/encyclopedia2020076
APA StyleSahoo, S., Mohapatra, S., Dalai, S. p., Misra, N., & Suar, M. (2022). Effect of Probiotics on Host-Microbial Crosstalk: A Review on Strategies to Combat Diversified Strain of Coronavirus. Encyclopedia, 2(2), 1138-1153. https://doi.org/10.3390/encyclopedia2020076






