Abstract
The scare of the ongoing coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), does not seem to fade away, while there is a constant emergence of novel deadly variants including Alpha, Beta, Gamma, Delta and Omicron. Until now, it has claimed approximately 276,436,619 infections, and the number of deaths surpluses to 5,374,744 all over the world. While saving the life has been a priority during the ongoing SARS-CoV-2 pandemic, the post-infection healing and getting back to normalcy has been undermined. Improving general health conditions and immunity with nutritional adequacy is currently of precedence for the government as well as frontline health workers to prevent and assuage infections. Exploring the role of probiotics and prebiotics in managing the after-effects of a viral outbreak could be of great significance, considering the emergence of new variants every now and then. To enhance human immunity, the recent evidence on the connection between gut microbiota and the broad spectrum of the clinical COVID-19 disease is the reason to look at the benefits of probiotics in improving health conditions. This review aims to sketch out the prospective role of probiotics and prebiotics in improving the standard of health in common people.
1. Introduction
Various acute respiratory tract infections caused by viruses, including respiratory syncytial virus, enterovirus, pneumonia-causing viruses, adenovirus and influenza virus, are the main causes of debility and death worldwide []. The main causative agent for these respiratory tract infections (RTIs) are DNA/RNA viruses. However, the RTIs associated with RNA viruses are more virulent in comparison to those that are caused by DNA viruses []. Specifically, coronaviruses belong to a highly significant re-emerging RNA virus family, causing serious life-threatening respiratory infections []. Ever since the onset of the infectious coronavirus disease (popularly known as COVID-19) in Wuhan city of China, the pandemic has increased rapidly in 57 countries, with over 276 million COVID-19 cases and over 5.37 million deaths reported as of 27 December 2021. Additionally, imposing several socio-economic, proper feedback strategies and rigid public health measures globally, involving social distancing, mask wearing, personal hygiene, quarantines, and lockdowns, the number of infections and death due to SARS-CoV-2 virus are constantly rising [].
Given the continuous evolution of the virus that leads towards SARS-CoV-2, WHO, in collaboration with researchers, national authorities, institutions and expert networks, monitored the emergence of variants that posed an increased risk to global public health and prompted the characterization as Variants of Interest (VOIs) and Variants of Concern (VOCs). Currently, using comparative assessment strategies, WHO labeled five variants as VOCs Alpha (United Kingdom, September-2020), Beta (South Africa, May-2020), Gamma (Brazil, November-2020), Delta (India, October-2020)and Omicron (Multiple countries, November-2021), while Lambda (Peru, December-2020) and Mu (Colombia, January-2021) were labeled as VOIs.
With the continuous boost from WHO, current ongoing research trends and developmental attempts are completely focused on developing effective therapy to counter the novel virus []. In this direction, anticoagulants, convalescent plasma, Hydroxychloroquine, Remdesivir, Vasodilators, non-steroidal anti-inflammatory drugs, monoclonal antibodies and Lopinavir/Ritonavir are in distinct phases of trials, research, or approvals. However, none of the above treatments are completely effective against the virus [,,]. In the absence of potent and efficacious vaccines and medicines, the virus is severely transforming and exhibits symptomatic, pre-symptomatic and asymptomatic forms in the affected population. Both asymptomatic and pre-symptomatic exemplifications are certainly one of the principal reasons for the pandemic []. Moreover, WHO released an assessment that this novel disease might persist in staying with the global population for a prolonged period. Therefore, proper investment and constant preparedness in public health and other resources are required for supervising the spread and morbidity caused by SARS-CoV-2.
The novel COVID-19 exhibits wide diversity in disease severity, spanning from minor and ill-defined common cold-like symptoms to pneumonia, and then can lead to life-threatening complications such as acute respiratory distress syndrome (ARDS) and multiple organ failure []. The proliferation and transmission of SARS-CoV-2 are caused through respiratory droplets; however, Ng et al. reported that the gut could also play a major role in the pathogenesis of COVID-19 []. Moreover, it was also reported that some coronaviruses, including the present SARS-CoV-2, could infect enterocytes, thus plating as a potential reservoir for virus proliferation []. Altogether, few clinical reports have revealed that gastrointestinal symptoms are conventional in COVID-19-infected patients, and in few cases, it leads to disease severity [,]. In addition, current pandemic control measures and practices to manage pandemics implement long-term effects on the human microbiome across the world, given the imposition of fleeing endemic areas, physical distancing, scapegoating of certain groups, mask-wearing, personal hygiene, quarantines, and lockdowns that influence overall microbial loss and inability for reinoculation. Rapid depletion and reduction of microbes over generations may lead to the extinction of microbial species ancestrally associated with humans; species may be permanently lost from the microbial pool unless reinoculation from other sources occurs [,]. In this context, various reports have suggested probiotic strains as promising therapeutics to enhance human immunity, thus inhibiting pathogens colonization and further minimizing the incidence and intensity of the infections. Moreover, little clinical evidence also illustrated the significance of probiotics in preventing viral and bacterial infections, including RTIs, sepsis, and gastroenteritis [].
Keeping in view the enormous health and economic burden, repurposing the usage of natural compounds such as probiotics and prebiotics can be an effectual therapeutic approach in blocking and/or reducing SARS-CoV-2 severity. In this review, we describe the existing curative and preventive trial studies focused on the usage of probiotics and prebiotics to combat viral infections. Moreover, the possible application of probiotics bacteria as a prophylactic approach against COVID-19 is also outlined in the present study.
2. SARS-CoV-2 and COVID-19
Morphologically, the enveloped SARS-CoV-2 viruses harbor a single-stranded non-segmented positive-sense ribonucleic acid (RNA) genome. The novel SARS-CoV-2 harbors completely diverse virus from formerly identified coronaviruses, i.e., Middle East respiratory syndrome coronavirus (MERS-CoV) and SARS-CoV []. For that reason, the previously available flu or antiviral therapeutics is ineffectual against it.
The majority of studies revealed that glycoproteins are engaged in binding to the host and consequent virus–host membrane fusion to produce the pathogenesis of the SARS-CoV-2 []. Structurally, the four major glycoproteins involving the membrane protein (M), small envelope protein (E), spike protein (S), and nucleocapsid (N) proteinform the general structure of coronavirus [] (Figure 1). The surface of virus harbors N-linked glycosylated trimeric S of 150 kDa that directs N-terminal signals towards the host endoplasmic reticulum. The M (25–30 kDa) with a higher C-terminal endodomain and lesser N-terminal glycosylated ectodomain is responsible for the shape of the virion []. Several vertebrate reservoirs, including humans, camels, dogs, masked palm civets, bats, cats and mice, are the potent hosts for coronaviruses []. Previous studies have revealed that COVID-19 was initially harbored by bats and then subsequently transmitted to humans through the infection of wild animals; however, the consequent spread of the virus into the human population occurred through human-to-human transmission [,]. The approximate time period between the introduction of the SARS-CoV-2 and its symptom onset in the host species, the incubation period, is 1–14 days []. Moreover, the standardization and estimation of the incubation period for COVID-19 may vary depending on the host’s age, the genetics of the individuals, the environmental conditions [], the pathogenicity of the virus or the long-term use of specific treatment such as glucocorticoids [,]. The usage of glucocorticoids might cause atypical infections and can also increase the incubation period. In addition, variability was also observed in the clinical manifestations of COVID-19 infections, varying from no or minimal symptoms to severe viral pneumonia with failure of respiratory organs and even death []. Earlier reports have revealed that asymptomatic or pre-symptomatic COVID-19 patients can play as promising resources for disease transmission [,]. Some familiar symptoms of COVID-19 include cough, fever, sore throat, fatigue, shortness of breath, aches, myalgia and headache []. Other associated symptoms reported include discoloration of fingers or toes, a rash on the skin or conjunctivitis, and some gastrointestinal symptoms such as vomiting, nausea, diarrhea and abdominal pain []. Person-to-person dissemination of COVID-19 infections was possible via cough- or sneeze-respiratory droplets released from the mouths/noses of SARS-CoV-2-infected patients []. Moreover, direct contact with the contaminated surfaces is an alternative means of COVID-19 transmission in humans []. With the onset of the disease, the infected person faces trouble in breathing, and simultaneously, the disease leads to severe respiratory tract infection and chronic inflammation [].
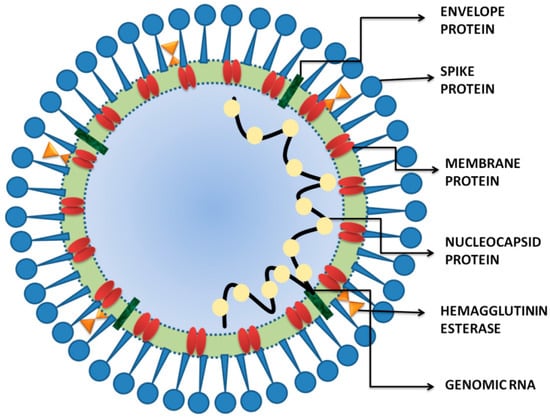
Figure 1.
The schematic structure of SARS-CoV-2. The viral surface proteins, spike, envelope and membrane proteins. The single-stranded positive-sense viral RNA is associated with the nucleocapsid protein.
Depending on the Cryo-EM structural investigation of S, it was revealed that the main reason for the rapid spread of SARS-CoV-2 is the S protein, having a 10–20 times higher affinity to human angiotensin-converting enzyme 2 (ACE2) receptor, in comparison to the previously emerged SARS-CoV []. Moreover, Zou et al. recently revealed that human organs, including epithelial cells from alveolar (lung) and enterocytes belonging to small intestines, are possible targets of the deadlySARS-CoV-2 virus []. Very recently, Guan et al. detected the SARS-CoV-2 viruses in contaminated human stools, recommending the chances of fecal–to-oral transmission []. Supporting this report, Holshue et al. further corroborated in a few patients from US and China that SARS-CoV-2 viruses are able to grow and proliferate in both digestive and respiratory tracts []. Moreover, Wu et al. revealed that the fecal samples of some of the COVID-19 recovered individuals were identified to be positive for the RNA of SARS-CoV-2, even though their respiratory samples were tested negative []. In addition, various current research has confirmed that COVID-19 disease has adversely affected the physiology and anatomy of the gastrointestinal tract for an extended period thus is damaging the gut microbiota [,]. Conticini et al. performed a post-mortem investigation on a patient who had died because of COVID-19 complications involving liver, lung and heart tissue, which revealed that serious damage occurred to the lungs with edema and desquamation []. Additionally, some of the patients infected with SARS-CoV-2 exhibited intestinal and microbial dysbiosis with reduced probiotic species instance, i.e., Bifidobacterium and Lactobacillus, signifying the necessity to look into the gastrointestinal and nutritional function of all patients [,,]. These studies clearly indicate that the development of opportunistic pathogens and simultaneous lessening of beneficial bacteria in GIT can be directly correlated with the severity of SARS-CoV-2 infections.
3. Discussion
The FAO/WHO definition of a probiotic is “live microorganisms which when administered in adequate amounts confer a health benefit on the host”. [,]. Probiotics serveas an enormous metabolic advantage since they play a major role in host immunity by enhancing both specific and non-specific immune system []. Probiotics belonging to the genera Lactobacillaceae, Leuconostocaceae and Bifidobacterium [] have been commonly utilized for their wide variety of benefits to the health by significantly reducing the loss of body weight, pathological symptoms, and viral loading [,,,]. The most commonly used probiotic microorganisms against pathogens include Lactococcuslactis, Streptococcus thermophilus, Lactobacillus helveticus, Lactobacillus acidophilus, Lactobacillus delbrueckii spp. bulgaricus, Lactobacillus gallinarum, Lactobacillus amylovorus, Levilacto bacillusbrevis, Lactobacillus crispatus, Lactobacillus plantarum, Lactobacillus crispatus, Latilactobacillus curvatus, Limosilactobacillus fermentum, Lactobacillus johnsonii, Lacticaseibacillus paracasei subsp. paracasei, Lactobacillus delbrueckii subsp. lactis, Limosilacto bacillusreuteri, Lactobacillu scellobiosus, Lacticaseibacillus rhamnosus, Bifidobacterium laterosporum, Leuconostocmesenteroides, Pediococcus acidilactici, Pediococcus pentosaceus, Bifidobacterium adolescentis, Bifidobacterium animalis, Bifidobacterium breve, Bifidobacterium bifidum, Bifidobacterium infantis, Bifidobacteriumessensis, Bifidobacterium thermophilum, Bifidobacterium cereus, Propionibacterium acidipropionici, B. longumlongum, Alkalihalobacillus alcalophilus, Propionibacterium thoenii, Propionibacteriumjensenii, Propionibacterium freudenreichii, Enterococcus faecalis, Enterococcus faecium, Bacillus Clausii, Bacillus subtilis, Bacillus coagulans, Sporolactobacillus inulinus and Escherichia coli [,,]. Traditionally, probiotics were administered as fermented food and determined to enhance health and nutrition by repairing the microbial balance in the host GIT []. In recent years, various research has illustrated the function of probiotics in controlling immune responses and a wide variety of conditions, exclusively targeting the infections caused by viruses, in various clinical trials and animal models (Figure 2) []. Reports also suggested that dietary meals containing probiotics and fiber supplementation are required to prevent adverse effects of viral infections and maintain a stable immune response system within the host [,,]. Most importantly, probiotic bacterial species, viz., B. bifidum [], Bacillus subtilis [], L. plantarum [,] and L. casei [] were previously reported to play a major role in providing a protective immune response against respiratory tract viral infection in experimental animal models. Moreover, Lehtoranta et al. reviewed that the interventions of probiotics lead in the reduction of viral load in the lungs by easing clinical symptoms, improving health conditions, and increasing survival rates [,,,,,].
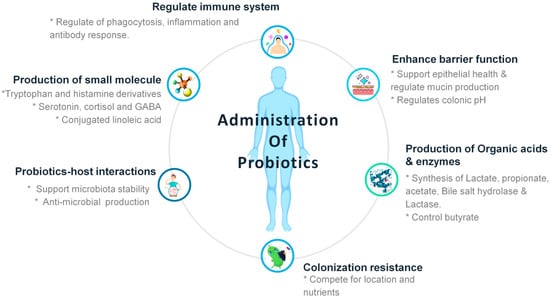
Figure 2.
The schematic diagram representing the benefits of probiotics after administration into human health.
In addition to the GIT, probiotics also colonize at distant mucosal sites, including the lungs, and also enhance the systemic immune responses []. The secreted proteins of probiotics accelerate the production of Antigen-Presenting Cell (APC) that leads to secretion of various interleukins, such asIL10, IL12, IL17 and TNF-α, interferon-α to eradicate foreign and allergic particles that trigger adaptive immunity. Probiotics provide two different immunomodulatory reactions: one is the immunostimulatory effect that activates IL-12 production, induces NK, Th, and Th2 cells, and acts against infection and allergy; and another type is the immunoregulatory effect, which induces IL-10 and Treg cell activation by Th2, DCs, B cells and monocytes for adaptive immunity of the host [] (Figure 3).
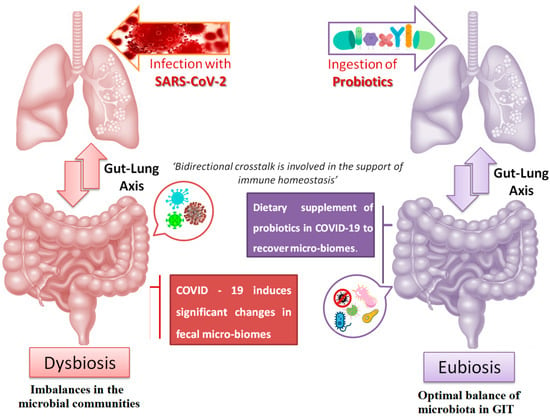
Figure 3.
Schematic representation of the bidirectional crosstalk between the gut–lung axis.
4. COVID-19 Affecting the Gut–Lung Axis Crosstalk
Human microbiota plays a major role in the development and regulation of host metabolism, immune system, brain function and maintenance of a robust and resilient healthy homeostasis []. The gut and lung are among the sections in the human body that harbors microbiota; besides, the lung hosts a smaller amount of microbiota in comparison to gut. This bidirectional crosstalk between gut and lung is involved in supporting the immune homeostasis []. Moreover, previous reports have revealed that dysbiosis of microbiota from the gut is directly affected by various respiratory pathological conditions (Figure 4) [,]. Most importantly, metabolites and microbial components belonging to the gut viz. short-chain fatty acids and lipopolysaccharides are also engaged in the bidirectional communication of the gut–lung axis. In addition to the commonly reported respiratory symptoms such as cough, fever and severe respiratory syndrome caused by the infection COVID-19, research has reported that few COVID-19-infected patients also depicted GIT symptoms such as GI bleeding, loss of appetite, abdominal pain, diarrhea, nausea, and vomiting []. In a two-hospital cohort study, Yeoh et al. revealed that patients infected with COVID-19 were depleted in gut bacteria with known immunomodulatory potential even after disease resolution. Further, these complications lead to the increased concentrations of inflammatory cytokines and blood markers such as C reactive protein, lactate dehydrogenase, aspartate aminotransferase and gamma-glutamyl transferase []. In addition, various COVID-19 risk-reductions measures such as vaccination, masking, physical distancing, intensive hygiene and antibiotics negatively affect microbial diversity and accelerate microbiota loss [].
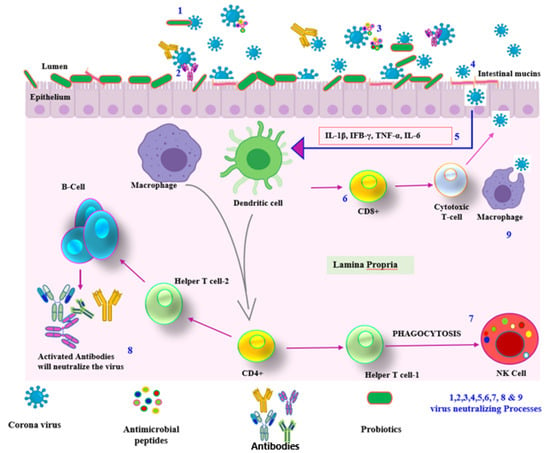
Figure 4.
Schematic diagram representing the immune-modulatory agents regulated by probiotics.
Notably, Chiba et al. reported that SARS-CoV-2-infected patients with GIT symptoms including diarrhea suffered severe respiratory disorders when compared to patients without GIT symptoms []. However, very little information is offered regarding the consequence of lung microbiota on the microbiome of GIT. Moreover, few studies have revealed that acute lung injury regulates the dysbiosis of the lung that directly affects the blood-mediated modulation of the gut microbiota [,]. It was observed that the microbiota population was disrupted in cases of pulmonary allergy []. Keeping this view, we can hypothesize that COVID-19 infection can stimulate disruption of lung microbiota, which further regulates the microbiota from GIT, resulting in various GIT symptoms. Later, a few reports unveiled that the GIT symptoms developed in COVID infected patients might be attributed to the damaged tissues and organs caused by the immune responses [,,,]. In addition to the lung, it is reported that ACE2 is also identified in GIT, and direct colonization of the gut ACE2 receptors through the ingestion of the virus is probably liable for the GIT symptoms in connection with COVID-19. Furthermore, malfunction of apoptosis pathways in the intestine due to infections in the respiratory tract [] is another projected elucidation for COVID-19-related GIT symptoms. Moreover, it can be believed that COVID-19-related GIT symptoms may be the consequence because gut and respiratory tracts have a common embryonic origin, and hence they share a similar structure and interact in a similar way in both pathological and physiological conditions []. Collectively, all the above mechanisms can assist researchers in understanding the GIT disturbances associated with COVID-19.
5. Supporting Evidence of Usage of Probiotics to Combat COVID-19
Despite several probable medications to treat the newly emerging SARS-CoV-2, there is always a constant increment in the number of death cases. Moreover, it has been observed that with intake of an optimized amount of probiotic supplements, most people are withstanding COVID-19 on account of booted immunity. The implication of probiotic strain, specifically Bifidobacteria and Lactobacilli, uplifted the health benefits and a significant stimulation towards recovery [].
Significantly, various studies have depicted that the changes in the lung microbial community also influence the composition of gut microbiota due to a bidirectional relationship. Therefore, any type of infection in the lung can directly affect the intestinal bacterial environment. Hence, in order to maintain the healthy intestinal microbiota and cure the infections in the lungs, consumption of a significant amount of probiotic supplements will not only help the intestine but also stimulate the secretion of metabolites that can cure the contaminationin the lungs []. Previous clinical studies depicted the profit of probiotics towards nullifying the influenza virus present in the respiratory tract and reinforcing the lungs’ immune system []. The administration of probiotics into the body enhances immunity- and anti-inflammatory cytokines, helping to clear the viral infection by minimizing the cell damage in the lungs []. Moreover, a clinical study has proven the exclusive impact through meta-analysis, where they have demonstrated external supplementation with probiotics tremendously improved the respiratory infections in more than 8000 preterm infants []. Several studies have already demonstrated that probiotic supplements can prevent antibiotic-associated diarrhea and infections in the gastrointestinal tract, but also infections at other sites, including sepsis and RTIs [,,,,,,,,]. Table 1 illustrates the relevant pre-clinical and clinical data supporting the use of probiotics against viral diseases.

Table 1.
Pre-clinical and clinical data supporting the use of probiotics against viral diseases.
This supporting evidence strongly supports probiotics’ role in modulating the host immune system, suggesting a potential role for probiotics against viral infections. Supplementation involving probiotics could significantly curtail the extremity of SARS-CoV-2 viruses that causes high morbidity and mortality. In addition, probiotics can be an attractive adjunct, as they can impede cytokine storm by invigorating the innate immunity and evading the exaggeration of adaptive immunity; inventing effective therapy will transform the impact of the pandemic on lives as well as economies across the globe. Therefore, supplementation of probiotics in high-risk and severely ill patients, and frontline health workers, might limit the infection and flatten the COVID-19 curve.
6. Probiotics and COVID-19: Current Perspectives
The affirmative effects of probiotics species on the ACE receptor are well-stated by Robles-Vera et al., focusing on the anti-hypertensive effects of probiotics []. During the period of fermentation of food, probiotics induce the production of significant bioactive peptides with the ability to reduce the activity of ACE enzymes by impeding the active sites [,]. Most importantly, the left-over of the dead probiotics also played as promising ACE inhibitors []. From the above findings, it can be derived that probiotics could be a promising inhibiter to the ACE receptor that plays a major role as an entry for SARS-CoV-2 to infect the GIT. The notion of utilizing medicines for obstruction of the ACE receptors as a treatment approach to combat COVID-19 was proposed by Fernández-Fernández [], regardless of the different opinion expressed by Esler and Esler []. Additionally, Imai and co-authors [] have explained the affirmative impact of utilizing an ACE2-blocker to diminish respiratory-distress-syndrome. Notably, prebiotics might also have a significant impact onCOVID-19 by improving the survivability and growth of probiotics. In 2018, Yeh et al. [] meticulously reviewed twelve studies that scrutinized the effect of probiotic and prebiotic supplements on the infections caused by influenza. Further, the authors concluded that the probiotics and probiotic supplements can enhance the hemagglutination inhibition antibody titers following the vaccination against influenza.
SARS-CoV-2 is a novel emerging virus without any effective therapeutics. Moreover, no research has claimed the promising role of probiotics and prebiotics in preventing/treating COVID-19. Additionally, various registered clinical trials that endeavor to explore the effectiveness of probiotics in treating COVID-19 patients are still ongoing []. Most importantly, a number of patients infected with COVID-19 showed dysbiosis in intestinal microbiota underpinning lower amounts of probiotic species, including Bifidobacterium and Lactobacillus [], indicating weak immunity of COVID-19-infected patients; thus, patients necessitate nutritional maintenance as well as probiotic/prebiotic supplements to maintain the intestinal flora equilibrium and reduce the chance of infection []. As humans have not acquired immunity against the novel COVID-19disease, and the dietary balance at GI microbiota levels is highly essential, a balanced diet involving probiotics-containing foods and immunity-enhancing micronutrients viz., polyphenols; vitamins A, C, and D; and minerals (mainly selenium and zinc) can be highly effective to ease the risk of COVID-19 infection []. Early research suggested that the utilization of fermented milk, including probiotics strains, considerably reduced the occurrence of upper respiratory tract infections among elderly adults, children and healthy infants [,,,].
The existence of probiotics can help to enhance the anti-microbial peptide production, enhance the attachment of mucins, decrease the pathogenic agent from the mucosal layer, stimulate immunomodulatory agent, ACE inhibitor peptide, anti cholesterolemic, enhance the production of lactoferrin, synthesize ca+ binding protein, maintain the pH, help in neutralizing most of the neurotoxins, etc. (Figure 1). Hence, there is a need to have the probiotics to boost the natural immunity [,].
Evidently, based on the aforementioned studies of the impending purpose of probiotics, supplementation involving probiotic bacterial species can be a suitable strategy for treating and inhibiting various viral infections. These interpretations assist the management of probiotics for patients infected with COVID-19. In spite of the absence of any strong evidence supporting these treatments, enhancing the natural immunity of the population using probiotics before, during or after COVID-19 infection is the foremost priority (Figure 5).
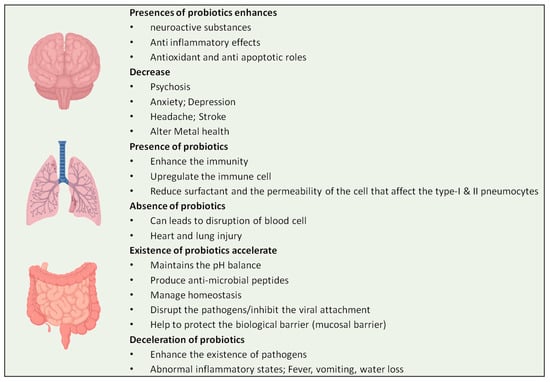
Figure 5.
Effects of probiotics on different organs of human body.
7. Conclusions
Researchers are still in the early stage of understanding the mechanism of SARS-CoV-2 infection in the human body. Evidence is gathering suggesting the benefits of probiotics in regulating the immune system, inhibiting the cytokine storm and boosting adaptive immunity. However, evidence shows that the appropriate usage of current probiotics is safe, even for critically ill and immune-compromised patients [,,,]. Therefore, a clear understanding of the mechanism of probiotics and their mode of use should be determined on an individual basis. In addition, clinical trials, along with biochemical profiling of SARS protein E, are essential before assigning a probiotic in the prophylaxis of COVID-19. When used with caution, probiotic supplementation could reduce the severity of COVID-19 morbidity and mortality. The current situation demands creating awareness among the people about the health benefits of probiotics through social networks at the district, national and international levels to control the spread of COVID-19 infection.
Author Contributions
S.S. and M.S., literature search and data analysis: S.p.D. and S.S.; writing—original draft preparation: S.S. and S.M.; writing—review and editing: N.M. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that no funds, grants or other support was received during the preparation of this manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; Gupta, V.; Abrams, E.M.; Adedoyin, R.A.; Adhikari, T.B.; Advani, S.M.; Agrawal, A.; Ahmadian, E.; et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef]
- Zolnikova, O.; Komkova, I.; Potskherashvili, N.; Trukhmanov, A.; Ivashkin, V. Application of probiotics for acute respiratory tract infections. Ital. J. Med. 2018, 12, 32–38. [Google Scholar] [CrossRef]
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.K.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Masud, M.A.; Ahmed, M.; Rahman, M.H. Optimal control for COVID-19 pandemic with quarantine and antiviral therapy. Sens. Int. 2021, 2, 100131. [Google Scholar] [CrossRef] [PubMed]
- Cevik, M.; Bamford, C.G.; Ho, A. COVID-19pandemic—Afocusedreviewforclinicians. Clin. Microbiol. Infect. 2020, 26, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraj, B.; Siriwattananon, K.; Wangkanont, K.; Phoolcharoen, W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19). Asian Pac. J. Allergy Immunol. 2020, 38, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, R.; Mohanty, S.; Vishwakarma, K.; Nayak, S.K.; Samantaray, D.; Mohapatra, S. Update vision on COVID-19: Structure, immunepathogenesis, treatment and safety assessment. Sens. Int. 2021, 2, 100073. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Singh, K.; Rao, A. Probiotics: A potential immunomodulator in COVID-19 infection management. Nutr. Res. 2021, 87, 1–12. [Google Scholar] [CrossRef]
- Zaim, S.; Chong, J.H.; Sankaranarayanan, V.; Harky, A. COVID-19 and multiorgan response. Curr. Probl. Cardiol. 2020, 45, 100618. [Google Scholar] [CrossRef]
- Ng, S.C.; Tilg, H. COVID-19 and the gastrointestinal tract: More than meets the eye. Gut 2020, 69, 973–974. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Jiang, X.; Zhang, Z.; Huang, S.; Zhang, Z.; Fang, Z.; Gu, Z.; Gao, L.; Shi, H.; Mai, L.; et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 2020, 69, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Lian, J.S.; Hu, J.H.; Gao, J.; Zheng, L.; Zhang, Y.M.; Hao, S.R.; Jia, H.Y.; Cai, H.; Zhang, X.L.; et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut 2020, 69, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Allawadhi, P.; Khurana, A.; Banothu, A.K.; Bharani, K.K. Critical neurological features of COVID-19: Role of imaging methods and biosensors for effective diagnosis. Sens. Int. 2021, 2, 100098. [Google Scholar] [CrossRef]
- Flandroy, L.; Poutahidis, T.; Berg, G.; Clarke, G.; Dao, M.-C.; Decaestecker, E.; Furman, E.; Haahtela, T.; Massart, S.; Plovier, H.; et al. The impact of human activities and lifestyles on the interlinked microbiota and health of humans and of ecosystems. Sci. Total Environ. 2018, 627, 1018–1038. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Baud, D.; Dimopoulou Agri, V.; Gibson, G.R.; Reid, G.; Giannoni, E. Using Probiotics to Flatten the Curve of Coronavirus Disease COVID-2019 Pandemic. Front. Public Health 2020, 8, 186. [Google Scholar] [CrossRef]
- Sahoo, S.; Mahapatra, S.R.; Parida, B.K.; Rath, S.; Dehury, B.; Raina, V.; Mohakud, N.K.; Misra, N.; Suar, M. DBCOVP: A database of coronavirus virulent glycoproteins. Comput. Biol. Med. 2021, 129, 104131. [Google Scholar] [CrossRef]
- Banerjee, N.; Mukhopadhyay, S. Viral glycoproteins: Biological role and application in diagnosis. VirusDisease 2016, 27, 1–11. [Google Scholar] [CrossRef]
- Mahapatra, S.R.; Sahoo, S.; Dehury, B.; Raina, V.; Patro, S.; Misra, N.; Suar, M. Designing an efficient multi-epitope vaccine displaying interactions with diverse HLA molecules for an efficient humoral and cellular immune response to prevent COVID-19 infection. Expert Rev. Vaccines 2020, 19, 871–885. [Google Scholar] [CrossRef]
- Damas, J.; Hughes, G.M.; Keough, K.C.; Painter, C.A.; Persky, N.S.; Corbo, M.; Hiller, M.; Koepfli, K.P.; Pfenning, A.R.; Zhao, H.; et al. Broad host range of SARS -CoV-2 predicted by comparative and structural analysis of ACE2 in vertebrates. Proc. Natl. Acad. Sci. USA 2020, 117, 22311–22322. [Google Scholar] [CrossRef]
- Li, C.; Ji, F.; Wang, L.; Wang, L.; Hao, J.; Dai, M.; Liu, Y.; Pan, X.; Fu, J.; Li, L.; et al. Asymptomatic and human-to-human transmission of SARS-CoV-2 in a 2-family cluster, Xuzhou, China. Emerg. Infect. Dis. 2020, 26, 1626. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, J.S.; Smith, D.W. COVID-19: A novel zoonotic disease caused by a coronavirus from China: What we know and what we don’t. Microbiol. Aust. 2020, 41, 45. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Corona Virus Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 13 April 2022).
- Şahin, M. Impact of weatheron COVID-19 pandemic in Turkey. Sci. Total Environ. 2020, 728, 138810. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Jiang, M.; Xia, D.; He, L.; Lv, X.; Liao, X.; Meng, J. COVID-19 in a patient with long-term use of glucocorticoids: A study of a familial cluster. Clin. Immunol. 2020, 214, 108413. [Google Scholar] [CrossRef]
- Dhouib, W.; Maatoug, J.; Ayouni, I.; Zammit, N.; Ghammem, R.; Ben Fredj, S.; Ghannem, H. The incubation period during the pandemic of COVID-19: A systematic review and meta-analysis. Syst. Rev. 2021, 10, 101. [Google Scholar] [CrossRef]
- Sorci, G.; Faivre, B.; Morand, S. Explaining among-country variation in COVID-19 case fatality rate. Sci. Rep. 2020, 10, 18909. [Google Scholar] [CrossRef]
- Xie, M.; Chen, Q. Insight into 2019 novel coronavirus—An updated intrim review and lessons from SARS-CoV and MERS-CoV. Int. J. Infect. Dis. 2020, 94, 119–124. [Google Scholar] [CrossRef]
- Jiang, F.; Deng, L.; Zhang, L.; Cai, Y.; Cheung, C.W.; Xia, Z. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19). J. Gen. Intern. Med. 2020, 35, 1545–1549. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2020, 19, 141–154. [Google Scholar] [CrossRef]
- Pathak, S.K.; Pandey, S.; Pandey, A.; Salunke, A.A.; Thivari, P.; Ratna, H.V.; Chawla, J. Focus on uncommon symptoms of COVID-19: Potential reason for spread of infection. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1873–1874. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Didelot, X.; Yang, J.; Wong, G.; Shi, Y.; Liu, W.; Gao, G.F.; Bi, Y. Inference of person-to-person transmission of COVID-19 reveals hidden super-spreading events during the early outbreak phase. Nat. Commun. 2020, 11, 5006. [Google Scholar] [CrossRef] [PubMed]
- Karia, R.; Gupta, I.; Khandait, H.; Yadav, A.; Yadav, A. COVID-19 and its Modes of Transmission. SN Compr. Clin. Med. 2020, 2, 1798–1801. [Google Scholar] [CrossRef] [PubMed]
- Alimolaie, A. A review of coronavirus disease-2019 (COVID-19). Biol. Sci. Promot. 2020, 3, 152–157. [Google Scholar]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.; et al. Clinical characteristics of corona-virus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Holshue, M.L.; DeBolt, C.; Lindquist, S.; Lofy, K.H.; Wiesman, J.; Bruce, H.; Spitters, C.; Ericson, K.; Wilkerson, S.; Tural, A.; et al. First Case of 2019 Novel Coronavirus in the United States. N. Engl. J. Med. 2020, 382, 929–936. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, C.; Tang, L.; Hong, Z.; Zhou, J.; Dong, X.; Yin, H.; Xiao, Q.; Tang, Y.; Qu, X.; et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020, 5, 434–435. [Google Scholar] [CrossRef]
- Xiao, F.; Tang, M.; Zheng, X.; Liu, Y.; Li, X.; Shan, H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 2020, 158, 1831–1833. [Google Scholar] [CrossRef]
- Kopel, J.; Perisetti, A.; Gajendran, M.; Boregowda, U.; Goyal, H. Clinical Insights into the Gastrointestinal Manifestations of COVID-19. Am. J. Dig. Dis. 2020, 65, 1932–1939. [Google Scholar] [CrossRef] [PubMed]
- Conticini, E.; Frediani, B.; Caro, D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethal-ity in Northern Italy? Environ. Pollut. 2020, 261, 114465. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Bottari, B.; Castellone, V.; Neviani, E. Probiotics and COVID-19. Int. J. Food Sci. Nutr. 2020, 72, 293–299. [Google Scholar] [CrossRef]
- Sahoo, S.; Mahapatra, S.R.; Misra, N.; Suar, M. Application of genomics, transcriptomics, and proteomics in probiotic research. In Probiotic Beverages; Academic Press: Cambridge, MA, USA, 2021; pp. 235–256. [Google Scholar]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Abdelhamid, A.G.; El-Masry, S.S.; El-Dougdoug, N.K. Probiotic Lactobacillus and Bifidobacterium strains possess safety character-istics, antiviral activities and host adherence factors revealed by genome mining. EPMA J. 2019, 10, 337–350. [Google Scholar] [CrossRef]
- Lu, W.; Fang, Z.; Liu, X.; Li, L.; Zhang, P.; Zhao, J.; Zhang, H.; Chen, W. The Potential Role of Probiotics in Protection against Influenza a Virus Infection in Mice. Foods 2021, 10, 902. [Google Scholar] [CrossRef]
- Izumo, T.; Maekawa, T.; Ida, M.; Noguchi, A.; Kitagawa, Y.; Shibata, H.; Yasui, H.; Kiso, Y. Effect of intranasal administration of Lactobacillus pentosus S-PT84 on influenza virus infection in mice. Int. Immunopharmacol. 2010, 10, 1101–1106. [Google Scholar] [CrossRef]
- Liu, Y.; Tran, D.Q.; Rhoads, J.M. Probiotics in Disease Prevention and Treatment. J. Clin. Pharmacol. 2018, 58, S164–S179. [Google Scholar] [CrossRef]
- Amara, A.A.; Shibl, A. Role of Probiotics in health improvement, infection control and disease treatment and management. Saudi Pharm. J. 2015, 23, 107–114. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.-Y.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Bron, P.A.; Van Baarlen, P.; Kleerebezem, M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat. Rev. Microbiol. 2012, 10, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Saad, N.; Delattre, C.; Urdaci, M.; Schmitter, J.M.; Bressollier, P. An overview of the last advances in probiotic and prebiotic field. LWT Food Sci. Technol. 2013, 50, 1–16. [Google Scholar] [CrossRef]
- Santosa, S.; Farnworth, E.; Jones, P.J.H. Probiotics and their potential health claims. Nutr. Rev. 2006, 64, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented Foods as a Dietary Source of Live Organisms. Front. Microbiol. 2018, 9, 1785. [Google Scholar] [CrossRef]
- Yan, F.; Polk, D.B. Probiotics and immune health. Curr. Opin. Gastroenterol. 2011, 27, 496–501. [Google Scholar] [CrossRef]
- Hardy, H.; Harris, J.; Lyon, E.; Beal, J.; Foey, A.D. Probiotics, prebiotics and immunomodulation of gut mucosal defences: Homeo-stasis and immunopathology. Nutrients 2013, 5, 1869–1912. [Google Scholar] [CrossRef]
- Al Kassaa, I. New Insights on Antiviral Probiotics; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Mahooti, M.; Abdolalipour, E.; Salehzadeh, A.; Mohebbi, S.R.; Gorji, A.; Ghaemi, A. Immunomodulatory and prophylactic effects of Bifidobacteriumbifidum probiotic strain on influenza infection in mice. World J. Microbiol. Biotechnol. 2019, 35, 91. [Google Scholar] [CrossRef]
- Starosila, D.; Rybalko, S.; Varbanetz, L.; Ivanskaya, N.; Sorokulova, I. Anti-influenza Activity of a Bacillus subtilis Probiotic Strain. Antimicrob. Agents Chemother. 2017, 61, e00539-17. [Google Scholar] [CrossRef]
- Kawashima, T.; Hayashi, K.; Kosaka, A.; Kawashima, M.; Igarashi, T.; Tsutsui, H.; Tsuji, N.M.; Nishimura, I.; Hayashi, T.; Obata, A. Lactobacillus plantarum strain YU from fermented foods activates Th1 and protective immune responses. Int. Immunopharmacol. 2011, 11, 2017–2024. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.I.; Bae, J.Y.; Yoo, K.; Kim, H.; Kim, I.H.; Park, M.S.; Lee, I. Effects of heat-killed Lactobacillus plantarum against influ-enza viruses in mice. J. Microbiol. 2018, 56, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Lee, Y.T.; Le Ngo, V.; Cho, Y.H.; Ko, E.J.; Hong, S.M.; Kim, K.H.; Jang, J.H.; Oh, J.S.; Park, M.K.; et al. Heat-killed Lactobacil-lus casei confers broad protection against influenza A virus primary infection and develops heterosubtypic immunity against future secondary infection. Sci. Rep. 2017, 7, 17360. [Google Scholar] [CrossRef] [PubMed]
- Lehtoranta, L.; Pitkäranta, A.; Korpela, R. Probiotics in respiratory virus infections. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1289–1302. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.W.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. The international scientific association and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Guarino, M.P.L.; Altomare, A.; Emerenziani, S.; Di Rosa, C.; Ribolsi, M.; Balestrieri, P.; Iovino, P.; Rocchi, G.; Cicala, M. Mechanisms of Action of Prebiotics and Their Effects on Gastro-Intestinal Disorders in Adults. Nutrients 2020, 12, 1037. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Aolymat, I.; Al-Holy, M.; Ayyash, M.; Abu Ghoush, M.; Al-Nabulsi, A.A.; Osaili, T.; Apostolopoulos, V.; Liu, S.-Q.; Shah, N.P. The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19. npj Sci. Food 2020, 4, 17. [Google Scholar] [CrossRef]
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut–lung axis. Mucosal Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef]
- Hufnagl, K.; Pali-Schöll, I.; Roth-Walter, F.; Jensen-Jarolim, E. Dysbiosis of the gut and lung microbiome has a role in asthma. Semin. Immunopathol. 2020, 42, 75–93. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.S.; Peng, S.H.; Deng, X.Y.; Zhu, D.M.; Javidiparsijani, S.; Wang, G.R.; Li, D.Q.; Li, L.X.; Wang, Y.C.; et al. Gut-lung cross-talk in pulmonary involvement with inflammatory bowel diseases. World J. Gastroenterol. 2013, 19, 6794. [Google Scholar] [CrossRef] [PubMed]
- Smyk, W.; Janik, M.K.; Portincasa, P.; Milkiewicz, P.; Lammert, F.; Krawczyk, M. COVID-19: Focus on the lungs but do not forget the gastrointestinal tract. Eur. J. Clin. Investig. 2020, 50, e13276. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Li, J.; Shen, L.; Zou, Y.; Hou, L.; Zhu, L.; Faden, H.S.; Tang, Z.; Shi, M.; Jiao, N.; et al. Enteric involvement in hospitalised patients with COVID-19 outside Wuhan. Lancet Gastroenterol. Hepatol. 2020, 5, 534–535. [Google Scholar] [CrossRef]
- Szajewska, H.; Kołodziej, M.; Gieruszczak-Białek, D.; Skórka, A.; Ruszczyński, M.; Shamir, R. Systematic review with meta-analysis: Lactobacillus rhamnosus GG for treating acute gastroenteritis in children—A 2019 update. Aliment. Pharmacol. Ther. 2019, 49, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Sze, M.A.; Tsuruta, M.; Yang, S.-W.J.; Oh, Y.; Man, S.F.P.; Hogg, J.C.; Sin, D.D. Changes in the bacterial microbiota in gut, blood, and lungs following acute LPS instillation into mice lungs. PLoS ONE 2014, 9, e111228. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wen, Q.; Yao, F.; Xu, D.; Huang, Y.; Wang, J. Gut–lung axis: The microbial contributions and clinical implications. Crit. Rev. Microbiol. 2017, 43, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Vital, M.; Harkema, J.R.; Rizzo, M.; Tiedje, J.; Brandenberger, C. Alterations of the murine gut microbiome with age and allergic airway disease. J. Immunol. Res. 2015, 2015, 892568. [Google Scholar] [CrossRef]
- Kumar, V.C.; Mukherjee, S.; Harne, P.S.; Subedi, A.; Ganapathy, M.K.; Patthipati, V.S.; Sapkota, B. Novelty in the gut: A systematic re-view and meta-analysis of the gastrointestinal manifestations of COVID-19. BMJ Open Gastroenterol. 2020, 7, e000417. [Google Scholar] [CrossRef]
- Ciaglia, E.; Vecchione, C.; Puca, A.A. COVID-19 Infection and Circulating ACE2 Levels: Protective Role in Women and Children. Front. Pediatr. 2020, 8, 206. [Google Scholar] [CrossRef]
- TeRiet, L.; van Esch, J.H.; Roks, A.J.; van den Meiracker, A.H.; Danser, A.J. Hypertension: Renin–angiotensin–aldosterone system alterations. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef]
- Perrone, E.E.; Jung, E.; Breed, E.; Dominguez, J.A.; Liang, Z.; Clark, A.T.; Dunne, W.M.; Burd, E.M.; Coopersmith, C.M. Mechanisms of Methicillin-Resistant Staphylococcus aureus Pneumonia–Induced Intestinal Epithelial Apoptosis. Shock 2012, 38, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Budden, K.F.; Gellatly, S.L.; Wood, D.L.; Cooper, M.A.; Morrison, M.; Hugenholtz, P.; Hansbro, P.M. Emerging pathogenic links between microbiota and the gut–lung axis. Nat. Rev. Microbiol. 2017, 15, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Zelaya, H.; Alvarez, S.; Kitazawa, H.; Villena, J. Respiratory antiviral immunity and immunobiotics: Beneficial effects on inflamma-tion-coagulation interaction during influenza virus infection. Front. Immunol. 2016, 7, 633. [Google Scholar] [CrossRef] [PubMed]
- Agamennone, V.; Krul, C.A.M.; Rijkers, G.; Kort, R. A practical guide for probiotics applied to the case of antibiotic-associated diarrhea in The Netherlands. BMC Gastroenterol. 2018, 18, 103. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, J.Z.; Yap, C.; Lytvyn, L.; Lo, C.K.-F.; Beardsley, J.; Mertz, D.; Johnston, B.C. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst. Rev. 2017, 12, CD006095. [Google Scholar] [CrossRef]
- Johnston, B.C.; Goldenberg, J.Z.; Vandvik, P.O.; Sun, X.; Guyatt, G.H. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst. Rev. 2011, 11, CD004827. [Google Scholar] [CrossRef]
- Lenoir-Wijnkoop, I.; Gerlier, L.; Roy, D.; Reid, G. The clinical and economic impact of probiotics consumption on respiratory tract infections: Projections for Canada. PLoS ONE 2016, 11, e0166232. [Google Scholar] [CrossRef]
- Pregliasco, F.; Anselmi, G.; Fonte, L.; Giussani, F.; Schieppati, S.; Soletti, L. A New Chance of Preventing Winter Diseases by the Administration of Synbiotic Formulations. J. Clin. Gastroenterol. 2008, 42, S224–S233. [Google Scholar] [CrossRef]
- Guillemard, E.; Tondu, F.; Lacoin, F.; Schrezenmeir, J. Consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114 001 reduces the duration of respiratory infections in the elderly in a randomised controlled trial. Br. J. Nutr. 2009, 103, 58–68. [Google Scholar] [CrossRef]
- Guillemard, E.; Tanguy, J.; Flavigny, A.L.; de la Motte, S.; Schrezenmeir, J. Effects of consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114 001 on common respiratory and gastrointestinal infections in shift workers in a randomized controlled trial. J. Am. Coll. Nutr. 2010, 29, 455–468. [Google Scholar] [CrossRef]
- Issa, I.; Moucari, R. Probiotics for antibiotic-associated diarrhea: Do we have a verdict? World J. Gastroenterol. 2014, 20, 17788–17795. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; He, F.; Kubota, A.; Harata, G.; Hiramatsu, M. Oral administration of Lactobacilli from human intestinal tract protects mice against influenza virus infection. Lett. Appl. Microbiol. 2010, 51, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Saito, T.; Uematsu, T.; Kishi, K.; Toba, M.; Kohda, N.; Suzuki, T. Oral administration of heat-killed Lactobacillus pentosus strain b240 augments protection against influenza virus infection in mice. Int. Immunopharmacol. 2010, 11, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.N.; Lee, D.H.; Lee, Y.N.; Park, J.K.; Yuk, S.S.; Yang, S.Y.; Lee, H.J.; Woo, S.H.; Kim, H.M.; Lee, J.B.; et al. Intranasal administration of live Lactobacillus species facilitates pro-tection against influenza virus infection in mice. Antiviral. Res. 2012, 93, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Oliva, S.; Di Nardo, G.; Ferrari, F.; Mallardo, S.; Rossi, P.; Patrizi, G.; Cucchiara, S.; Stronati, L. Randomised clinical trial: The effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis. Aliment. Pharmacol. Ther. 2011, 35, 327–334. [Google Scholar] [CrossRef]
- Kwak, M.-K.; Liu, R.; Kwon, J.-O.; Kim, M.-K.; Kim, A.H.; Kang, S.-O. Cyclic dipeptides from lactic acid bacteria inhibit proliferation of the influenza a virus. J. Microbiol. 2013, 51, 836–843. [Google Scholar] [CrossRef]
- Goto, H.; Sagitani, A.; Ashida, N.; Kato, S.; Hirota, T.; Shinoda, T.; Yamamoto, N. Anti-influenza virus effects of both live and non-liveLactobacillus acidophilus l-92 accompanied by the activation of innate immunity. Br. J. Nutr. 2013, 110, 1810–1818. [Google Scholar] [CrossRef]
- Smith, T.J.; Rigassio-Radler, D.; Denmark, R.; Haley, T.; Touger-Decker, R. Effect of Lactobacillus rhamnosus LGG(r) and Bifidobacteriumanimalis ssp. lactis bb-12(r) on health-related quality of life in college students affected by upper respiratory infec-tions. Br. J. Nutr. 2013, 109, 1999–2007. [Google Scholar] [CrossRef]
- Zelaya, H.; Tsukida, K.; Chiba, E.; Marranzino, G.; Alvarez, S.; Kitazawa, H.; Agüero, G.; Villena, J. Immunobiotic lactobacilli reduce viral-associated pulmonary damage through the modulation of inflammation–coagulation interactions. Int. Immunopharmacol. 2014, 19, 161–173. [Google Scholar] [CrossRef]
- Nakayama, Y.; Moriya, T.; Sakai, F.; Ikeda, N.; Shiozaki, T.; Hosoya, T.; Nakagawa, H.; Miyazaki, T. Oral administration of Lactobacillus gasseri SBT2055 is effective for preventing influenza in mice. Sci. Rep. 2014, 4, 4638. [Google Scholar] [CrossRef]
- Dietrich, C.G.; Kottmann, T.; Alavi, M. Commercially available probiotic drinks containing Lactobacillus casei DN-114001 reduce antibiotic-associated diarrhea. World J. Gastroenterol. 2014, 20, 15837–15844. [Google Scholar] [CrossRef] [PubMed]
- Bo, L.; Li, J.; Tao, T.; Bai, Y.; Ye, X.; Hotchkiss, R.S.; Kollef, M.H.; Crooks, N.H.; Deng, X. Probiotics for preventing ventilator-associated pneumonia. Cochrane Database Syst. Rev. 2014, 10, CD009066. [Google Scholar] [CrossRef] [PubMed]
- Ang, L.Y.; Too, H.K.; Tan, E.L.; Chow, T.K.; Shek, P.C.; Tham, E.; Alonso, S. Antiviral activity of Lactobacillus reuteriProtectis against Coxsackievirus A and Enterovirus 71 infection in human skeletal muscle and colon cell lines. Virol. J. 2016, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Song, J.A.; Kim, H.J.; Hong, S.K.; Lee, D.H.; Lee, S.W.; Song, C.S.; Kim, K.T.; Choi, I.S.; Lee, J.B.; Park, S.Y. Oral intake of Lactobacillus rhamnosus M21 enhances the survival rate of mice lethally infected with influenza virus. J. Microbiol. Immunol. Infect. 2014, 49, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Ge, T.; Xiao, Y.; Liao, Y.; Cui, Y.; Zhang, Y.; Ho, W.; Yu, G.; Zhang, T. Probiotics for prevention and treatment of respiratory tract infections in children: A systematic review and meta-analysis of randomized controlled trials. Medicine 2016, 95, e4509. [Google Scholar] [CrossRef]
- Chen, M.-F.; Weng, K.-F.; Huang, S.-Y.; Liu, Y.-C.; Tseng, S.-N.; Ojcius, D.M.; Shih, S.-R. Pretreatment with a heat-killed probiotic modulates monocyte chemoattractant protein-1 and reduces the pathogenicity of influenza and enterovirus 71 infections. Mucosal Immunol. 2016, 10, 215–227. [Google Scholar] [CrossRef]
- Shojadoost, B.; Kulkarni, R.; Brisbin, J.T.; Quinteiro-Filho, W.; Alkie, T.N.; Sharif, S. Interactions between lactobacilli and chicken macrophages induce antiviral responses against avian influenza virus. Res. Vet. Sci. 2019, 125, 441–450. [Google Scholar] [CrossRef]
- Pu, F.; Guo, Y.; Li, M.; Zhu, H.; Wang, S.; Shen, X.; He, M.; Huang, C.; He, F. Yogurt supplemented with probiotics can protect the healthy elderly from respiratory infections: A randomized controlled open-label trial. Clin. Interv. Aging 2017, 12, 1223–1231. [Google Scholar] [CrossRef]
- Shida, K.; Sato, T.; Iizuka, R.; Hoshi, R.; Watanabe, O.; Igarashi, T.; Miyazaki, K.; Nanno, M.; Ishikawa, F. Daily intake of fermented milk with Lactobacillus casei strain Shirota reduces the incidence and duration of upper respiratory tract infections in healthy middle-aged office workers. Eur. J. Nutr. 2017, 56, 45–53. [Google Scholar] [CrossRef]
- Zhang, H.; Yeh, C.; Jin, Z.; Ding, L.; Liu, B.Y.; Zhang, L.; Dannelly, H.K. Prospective study of probiotic supplementation results in immune stimulation and improvement of upper respiratory infection rate. Synth. Syst. Biotechnol. 2018, 3, 113–120. [Google Scholar] [CrossRef]
- Eguchi, K.; Fujitani, N.; Nakagawa, H.; Miyazaki, T. Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus gasseri SBT2055. Sci. Rep. 2019, 9, 4812. [Google Scholar] [CrossRef] [PubMed]
- King, S.; Tancredi, D.; Lenoir-Wijnkoop, I.; Gould, K.; Vann, H.; Connors, G.; Sanders, M.E.; A Linder, J.; Shane, A.L.; Merenstein, D. Does probiotic consumption reduce antibiotic utilization for common acute infections? A systematic review and meta-analysis. Eur. J. Public Health 2018, 29, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Robles-Vera, I.; Toral, M.; Romero, M.; Jiménez, R.; Sánchez, M.; Pérez-Vizcaíno, F.; Duarte, J. Antihypertensive effects of probiotics. Curr. Hypertens. Rep. 2017, 19, 26. [Google Scholar] [CrossRef]
- Ayyash, M.M.; Sherkat, F.; Shah, N.P. The effect of NaCl substitution with KCl on Akawi cheese: Chemical composition, prote-olysis, angiotensin-converting enzyme-inhibitory activity, probiotic survival, texture profile, and sensory properties. J. Dairy Sci. 2012, 95, 4747–4759. [Google Scholar] [CrossRef] [PubMed]
- Ayyash, M.; Olaimat, A.; Al-Nabulsi, A.; Liu, S.Q. Bioactive properties of novel probioticLactococcuslactisfermented camel sausages: Cytotoxicity, angiotensin converting enzyme inhibition, antioxidant capacity, and antidiabeticactivity. Food Sci. Anim. Resour. 2020, 40, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Miremadi, F.; Ayyash, M.; Sherkat, F.; Stojanovska, L. Cholesterol reduction mechanisms and fatty acid composition of cellular membranes of probiotic Lactobacilli and Bifidobacteria. J. Funct. Foods 2014, 9, 295–305. [Google Scholar] [CrossRef]
- Fernández-Fernández, F.J. COVID-19, hypertension and angiotensin receptor-blocking drugs. J. Hypertens. 2020, 38, 1191. [Google Scholar] [CrossRef]
- Esler, M.; Esler, D. Can angiotensin receptor-blocking drugs perhaps be harmful in the COVID-19 pandemic? J. Hypertens. 2020, 38, 781–782. [Google Scholar] [CrossRef]
- Imai, Y.; Kuba, K.; Rao, S.; Huan, Y.; Guo, F.; Guan, B.; Yang, P.; Sarao, R.; Wada, T.; Leong-Poi, H.; et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005, 436, 112–116. [Google Scholar] [CrossRef]
- Yeh, T.L.; Shih, P.C.; Liu, S.J.; Lin, C.H.; Liu, J.M.; Lei, W.T.; Lin, C.Y. The influence of prebiotic or probiotic supplementation on antibody titers after influenza vaccination: A systematic review and meta-analysis of randomized controlled trials. Drug Des. Devel. Ther. 2018, 12, 217–230. [Google Scholar] [CrossRef]
- Infusino, F.; Marazzato, M.; Mancone, M.; Fedele, F.; Mastroianni, C.M.; Severino, P.; Ceccarelli, G.; Santinelli, L.; Cavarretta, E.; Marullo, A.G.M.; et al. Diet Supplementation, Probiotics, and Nutraceuticals in SARS-CoV-2 Infection: A Scoping Review. Nutrients 2020, 12, 1718. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Cai, H.; Shen, Y.; Ni, Q.; Chen, Y.; Hu, S.; Li, J.; Wang, H.; Yu, L.; Huang, H.; et al. Management of corona virus disease-19 (COVID-19): The Zhejiang experience. Zhejiang Da Xue Xue Bao. Yi Xue Ban 2020, 49, 147–157. [Google Scholar] [PubMed]
- Gasmi, A.; Noor, S.; Tippairote, T.; Dadar, M.; Menzel, A.; Bjørklund, G. Individual risk management strategy and potential therapeutic options for the COVID-19 pandemic. Clin. Immunol. 2020, 215, 108409. [Google Scholar] [CrossRef]
- Makino, S.; Ikegami, S.; Kume, A.; Horiuchi, H.; Sasaki, H.; Orii, N. Reducing the risk of infection in the elderly by dietary intake of yoghurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. Br. J. Nutr. 2010, 104, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Merenstein, D.; Murphy, M.; Fokar, A.; Hernandez, R.K.; Park, H.; Nsouli, H.; Sanders, M.E.; Davis, B.A.; Niborski, V.; Tondu, F.; et al. Use of a fermented dairy probiotic drink containing Lactobacillus casei (DN-114 001) to decrease the rate of illness in kids: The DRINK study A patient-oriented, double-blind, cluster-randomized, placebo-controlled, clinical trial. Eur. J. Clin. Nutr. 2010, 64, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, D.; Mohapatra, S.; Misra, S.; Sahu, P. Milk derived bioactive peptides and their impact on human health—A review. Saudi J. Biol. Sci. 2015, 23, 577–583. [Google Scholar] [CrossRef]
- Mohanty, D.; Jena, R.; Choudhury, P.K.; Pattnaik, R.; Mohapatra, S.; Saini, M.R. Milk Derived Antimicrobial Bioactive Peptides: A Review. Int. J. Food Prop. 2015, 19, 837–846. [Google Scholar] [CrossRef]
- Van den Nieuwboer, M.; Brummer, R.J.; Guarner, F.; Morelli, L.; Cabana, M.; Claassen, E. The administration of probiot-ics and synbiotics in immune compromised adults: Is it safe? Benef. Microbes 2015, 6, 3–17. [Google Scholar] [CrossRef]
- Nieuwboer, M.V.D.; Claassen, E.; Morelli, L.; Guarner, F.; Brummer, R. Probiotic and synbiotic safety in infants under two years of age. Benef. Microbes 2014, 5, 45–60. [Google Scholar] [CrossRef]
- Larsen, O.F.A.; Van den Nieuwboer, M.; Koks, M.; Flach, J.; Claassen, H.J.H.M. Probiotics for healthy ageing: Innovation barriers and opportunities for bowel habit improvement in nursing homes. Agro Food Ind. Hi Tech 2017, 28, 12–15. [Google Scholar]
- Finlay, B.B.; Amato, K.R.; Azad, M.; Blaser, M.J.; Bosch, T.C.; Chu, H.; Dominguez-Bello, M.G.; Ehrlich, S.D.; Elinav, E.; GevaZatorsky, N.; et al. The hygiene hypothesis, the COVID pandemic, and consequences for the human microbiome. Proc. Natl. Acad. Sci. USA 2021, 118, e2010217118. [Google Scholar] [CrossRef] [PubMed]
- Larsen, O.F.; van de Burgwal, L.H. On the Verge of a Catastrophic Collapse? The Need for a Multi-Ecosystem Approach to Microbiome Studies. Front. Microbiol. 2021, 12, 784797. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).