The Potential for Cellulose Deconstruction in Fungal Genomes
Definition
:1. Introduction
2. Enzymatic Deconstruction of Cellulose
- Endocellulases (aka, endo-1,4-β-D-glucanase, E.C.3.2.1.4) cut randomly in the linear chain of β-1,4-linked glucose units. The complete digestion of cellulose by cellulases produces cello-oligosaccharides and cellobiose (i.e., two glucose units, G2). Per the CAZy database, described protein domains endowed with endocellulase activity belong to the GH families 5, 6, 7, 8, 9, 12, 44, 45, and 48. In addition, few endocellulases have been identified in GH families 10, 51, 74, and 124. However, these last few families are poorly characterized or more frequently associated with other activities such as GH10-xylanases, GH51-arabinofuranosidase, GH74-xyloglucanase.
- Exocellulases (aka, exo-β-1,4-glucanase/cellodextrinase, E.C.3.2.1.74) digest the cellulose and cello-oligosaccharides by the extremities and release glucose (G1). In addition, some exocellulases can also digest the cellobiose (G2). Most described protein domains endowed with exocellulase activity belong to the GH families 5 and 9. In addition, few proteins with domains from the GH families 1 and 3 also display exocellulolytic activities. However, most GH1 and GH3 domains are known as “-osidases” degrading oligosaccharides and producing monosaccharides.
- Cellobiohydrolases (aka, E.C.3.2.1.91) hydrolyze the glucosidic linkages in cellulose and cello-oligosaccharides from their non-reducing end and release cellobiose (G2). Known protein domains with cellobiohydrolases activity are from the GH families 5, 6, and 9.
- β-glucosidases (aka, E.C.3.2.1.21) hydrolyze cellobiose (G2) and release glucose (G1) that can be further processed thru the glycolytic pathways (e.g., Embden–Meyerhof–Parnas or Entner–Doudoroff pathways). Most known domains endowed with β-glucosidase activity belong to the GH families 1 and 3, although few have been identified in the GH families 5, 16, 30, and 39. Although β-glucosidases are not directly targeting the cellulose, these enzymes are essential for overall cellulose deconstruction. Indeed, while hydrolyzing the cellobiose (G2) into glucose, β-glucosidases alleviate the cellulase inhibition by the product (i.e., G2) [20,21,22].
- Auxiliary activities associated with cellulose deconstruction include lytic polysaccharide monooxigenases (LPMOs) from AA families 9 (formerly known as GH61) and 10 (formerly known as CBM33), per the CAZy database. Proteins in AA families 9, 10, and the more recently described AA16 (only one characterized enzyme) are copper-containing enzymes, that cleave the cellulose using an oxidative process (E.C.1.14.99.54, E.C.1.14.99.56). At the end of the reaction, the produced cellulose fragments contain a D-glucono-1,5-lactone residue at the reducing end, which spontaneously hydrolyses to an aldonic acid. In this redox process, the electrons are provided in vivo by the cytochrome-b domain of the associated “cellobiose dehydrogenase” (EC 1.1.99.18) from the AA3 family [23,24,25].
3. MycoCosm and Data Availability
4. Cellulases Distribution across Fungal Phyla
5. Cellulases Distribution across Basidiomycota
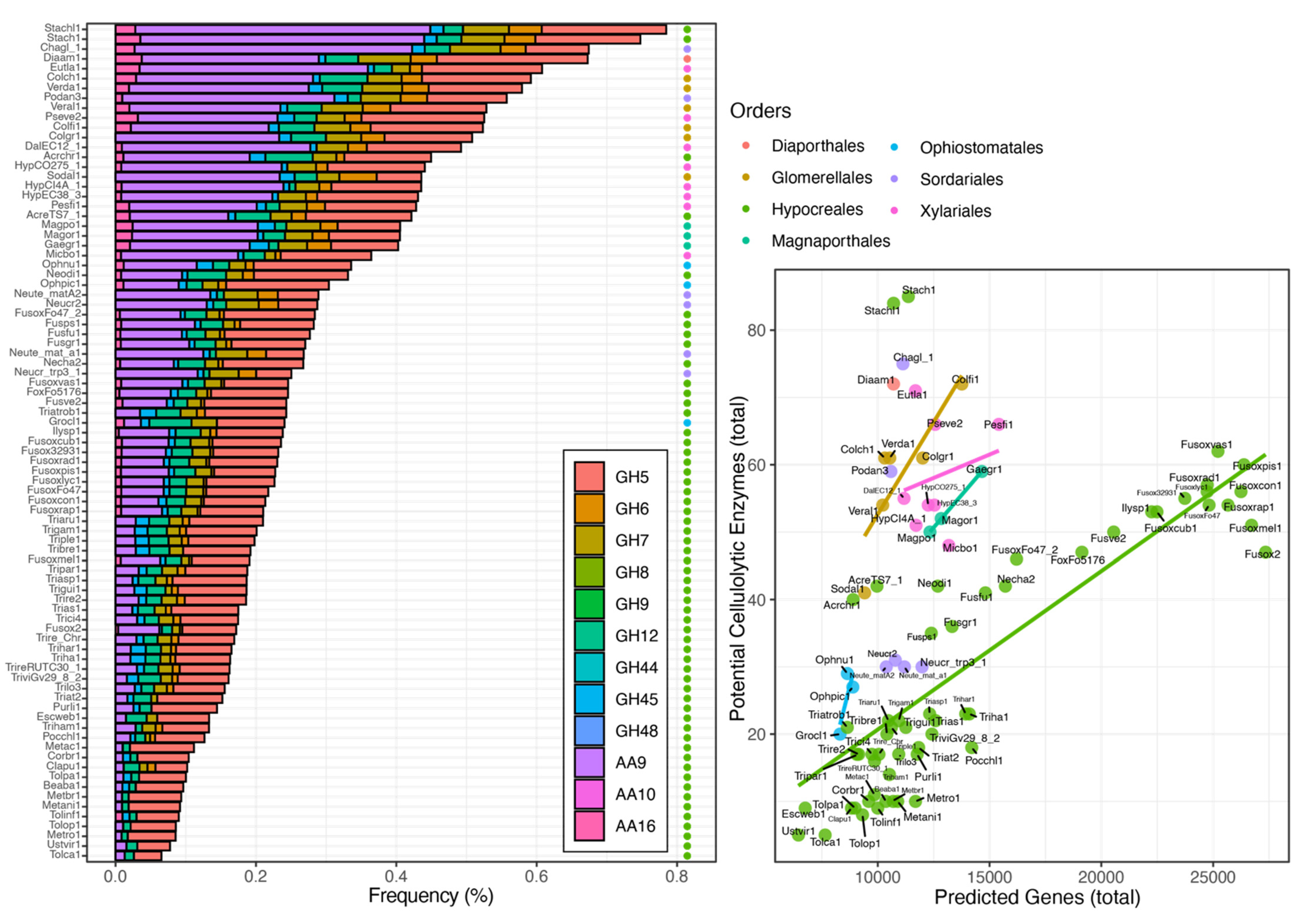
6. Cellulases Distribution across Ascomycota
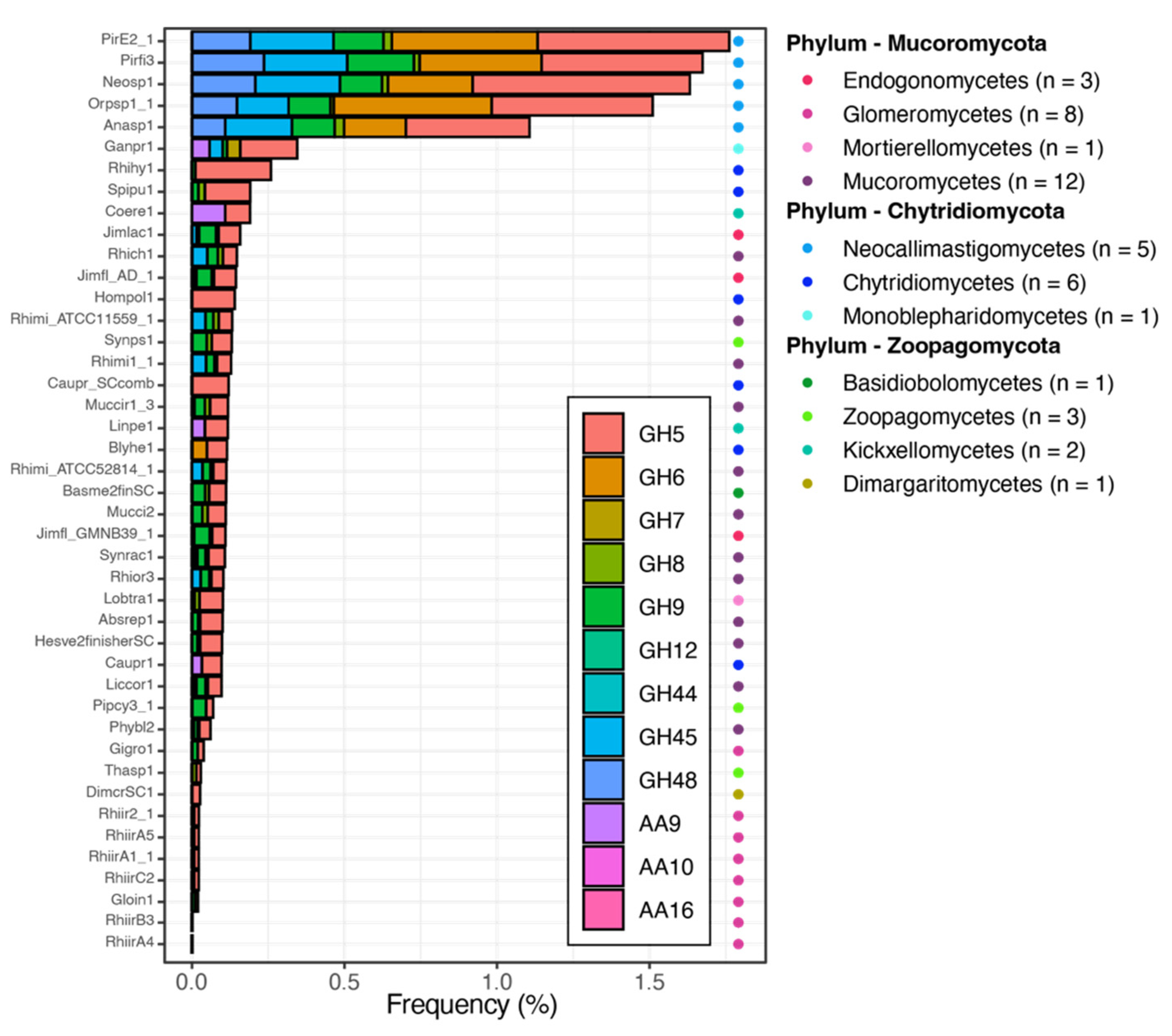
7. Cellulases Distribution across the Other Phyla
8. Conclusions and Prospects
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Treseder, K.K.; Lennon, J.T. Fungal traits that drive ecosystem dynamics on Land. Microbiol. Mol. Biol. Rev. 2015, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Berlemont, R.; Martiny, A.C. Phylogenetic distribution of potential cellulases in bacteria. Appl. Environ. Microbiol. 2013, 79, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Berlemont, R. Distribution and diversity of enzymes for polysaccharide degradation in fungi. Sci. Rep. 2017, 7, 222. [Google Scholar] [CrossRef]
- Talamantes, D.; Biabini, N.; Dang, H.; Abdoun, K.; Berlemont, R. Natural diversity of cellulases, xylanases, and chitinases in bacteria. Biotechnol. Biofuels 2016, 9, 133. [Google Scholar] [CrossRef]
- Brunecky, R.; Donohoe, B.S.; Yarbrough, J.M.; Mittal, A.; Scott, B.R.; Ding, H.; Taylor II, L.E.; Russell, J.F.; Chung, D.; Westpheling, J.; et al. The Multi Domain Caldicellulosiruptor bescii CelA Cellulase Excels at the Hydrolysis of Crystalline Cellulose. Sci. Rep. 2017, 7, 9622. [Google Scholar] [CrossRef]
- Ravachol, J.; Borne, R.; Tardif, C.; de Philip, P.; Fierobe, H.-P. Characterization of all family-9 glycoside hydrolases synthesized by the cellulosome-producing bacterium Clostridium cellulolyticum. J. Biol. Chem. 2014, 289, 7335–7348. [Google Scholar] [CrossRef] [PubMed]
- Payne, C.M.; Knott, B.C.; Mayes, H.B.; Hansson, H.; Himmel, M.E.; Sandgren, M.; Ståhlberg, J.; Beckham, G.T. Fungal cellulases. Chem. Rev. 2015, 115, 1308–1448. [Google Scholar] [CrossRef]
- Terrapon, N.; Lombard, V.; Drula, É.; Lapébie, P.; Al-Masaudi, S.; Gilbert, H.J.; Henrissat, B. PULDB: The expanded database of Polysaccharide Utilization Loci. Nucleic Acids Res. 2018, 46, D677–D683. [Google Scholar] [CrossRef]
- Nguyen, S.; Flores, A.; Talamantes, D.; Dar, F.; Valdez, A.; Schwans, J.; Berlemont, R. GeneHunt for rapid domain-specific annotation of glycoside hydrolases. Sci. Rep. 2019, 9, 10137. [Google Scholar] [CrossRef]
- Yin, Y.; Mao, X.; Yang, J.; Chen, X.; Mao, F.; Xu, Y. dbCAN: A web resource for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2012, 40, W445–W451. [Google Scholar] [CrossRef]
- Grigoriev, I.V.; Nikitin, R.; Haridas, S.; Kuo, A.; Ohm, R.; Otillar, R.; Riley, R.; Salamov, A.; Zhao, X.; Korzeniewski, F.; et al. MycoCosm portal: Gearing up for 1000 fungal genomes. Nucleic Acids Res. 2014, 42, D699–D704. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2016, 44, D67. [Google Scholar] [CrossRef] [PubMed]
- Yates, A.D.; Allen, J.; Amode, R.M.; Azov, A.G.; Barba, M.; Becerra, A.; Bhai, J.; Campbell, L.I.; Carbajo Martinez, M.; Chakiachvili, M.; et al. Ensembl Genomes 2022: An expanding genome resource for non-vertebrates. Nucleic Acids Res. 2022, 50, D996–D1003. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Freund, H.L.; Kasanjian, J.; Berlemont, R. Function, distribution, and annotation of characterized cellulases, xylanases, and chitinases from CAZy. Appl. Microbiol. Biotechnol. 2018, 102, 1629–1637. [Google Scholar] [CrossRef]
- Bradley, E.L.; Ökmen, B.; Doehlemann, G.; Henrissat, B.; Bradshaw, R.E.; Mesarich, C.H. Secreted Glycoside Hydrolase Proteins as Effectors and Invasion Patterns of Plant-Associated Fungi and Oomycetes. Front. Plant Sci. 2022, 13, 562. [Google Scholar] [CrossRef]
- Römling, U.; Galperin, M.Y. Bacterial cellulose biosynthesis: Diversity of operons, subunits, products, and functions. Trends Microbiol. 2015, 23, 545–557. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef]
- Berlemont, R.; Martiny, A.C. Genomic potential for polysaccharide deconstruction in bacteria. Appl. Environ. Microbiol. 2015, 81, 1513–1519. [Google Scholar] [CrossRef]
- Spatafora, J.W.; Aime, M.C.; Grigoriev, I.V.; Martin, F.; Stajich, J.E.; Blackwell, M. The Fungal Tree of Life: From Molecular Systematics to Genome-Scale Phylogenies. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Gefen, G.; Anbar, M.; Morag, E.; Lamed, R.; Bayer, E.A. Enhanced cellulose degradation by targeted integration of a cohesin-fused β-glucosidase into the Clostridium thermocellum cellulosome. Proc. Natl. Acad. Sci. USA 2012, 109, 10298–10303. [Google Scholar] [CrossRef]
- Prawitwong, P.; Waeonukul, R.; Tachaapaikoon, C.; Pason, P.; Ratanakhanokchai, K.; Deng, L.; Sermsathanaswadi, J.; Septiningrum, K.; Mori, Y.; Kosugi, A. Direct glucose production from lignocellulose using Clostridium thermocellum cultures supplemented with a thermostable β-glucosidase. Biotechnol. Biofuels 2013, 6, 184. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.B. Microbial diversity of cellulose hydrolysis. Curr. Opin. Microbiol. 2011, 14, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Hemsworth, G.R.; Henrissat, B.; Davies, G.J.; Walton, P.H. Discovery and characterization of a new family of lytic polysaccharide monooxygenases. Nat. Chem. Biol. 2014, 10, 122–126. [Google Scholar] [CrossRef]
- Labourel, A.; Frandsen, K.E.H.; Zhang, F.; Brouilly, N.; Grisel, S.; Haon, M.; Ciano, L.; Ropartz, D.; Fanuel, M.; Martin, F.; et al. A fungal family of lytic polysaccharide monooxygenase-like copper proteins. Nat. Chem. Biol. 2020, 16, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Filiatrault-Chastel, C.; Heiss-Blanquet, S.; Margeot, A.; Berrin, J.G. From fungal secretomes to enzymes cocktails: The path forward to bioeconomy. Biotechnol. Adv. 2021, 52, 107833. [Google Scholar] [CrossRef]
- Brunecky, R.; Alahuhta, M.; Xu, Q.; Donohoe, B.S.; Crowley, M.F.; Kataeva, I.A.; Yang, S.-J.; Resch, M.G.; Adams, M.W.W.; Lunin, V.V.; et al. Revealing nature’s cellulase diversity: The digestion mechanism of Caldicellulosiruptor bescii CelA. Science 2013, 342, 1513–1516. [Google Scholar] [CrossRef]
- Hervé, C.; Rogowski, A.; Blake, A.W.; Marcus, S.E.; Gilbert, H.J.; Knox, J.P.; Herve, C.; Rogowski, A.; Blake, A.W.; Marcus, S.E.; et al. Carbohydrate-binding modules promote the enzymatic deconstruction of intact plant cell walls by targeting and proximity effects. Proc. Natl. Acad. Sci. USA 2010, 107, 15293–15298. [Google Scholar] [CrossRef]
- Várnai, A.; Mäkelä, M.R.; Djajadi, D.T.; Rahikainen, J.; Hatakka, A.; Viikari, L. Carbohydrate-binding modules of fungal cellulases: Occurrence in nature, function, and relevance in industrial biomass conversion. Adv. Appl. Microbiol. 2014, 88, 103–165. [Google Scholar] [CrossRef]
- Grigoriev, I.V.; Cullen, D.; Goodwin, S.B.; Hibbett, D.; Jeffries, T.W.; Kubicek, C.P.; Kuske, C.; Magnuson, J.K.; Martin, F.; Spatafora, J.W.; et al. Fueling the future with fungal genomics. Mycology 2011, 2, 192–209. [Google Scholar] [CrossRef]
- Segato, F.; Damásio, A.R.L.; de Lucas, R.C.; Squina, F.M.; Prade, R.A. Genomics Review of Holocellulose Deconstruction by Aspergilli. Microbiol. Mol. Biol. Rev. 2014, 78, 588. [Google Scholar] [CrossRef]
- Mondo, S.J.; Jiménez, D.J.; Hector, R.E.; Lipzen, A.; Yan, M.; Labutti, K.; Barry, K.; Van Elsas, J.D.; Grigoriev, I.V.; Nichols, N.N. Genome expansion by allopolyploidization in the fungal strain Coniochaeta 2T2.1 and its exceptional lignocellulolytic machinery. Biotechnol. Biofuels 2019, 12, 229. [Google Scholar] [CrossRef] [PubMed]
- Heitman, J.; Priest, S.J.; Yadav, V. Advances in understanding the evolution of fungal genome architecture. F1000Research 2020, 9, 776. [Google Scholar]
- Nakjang, S.; Williams, T.A.; Heinz, E.; Watson, A.K.; Foster, P.G.; Sendra, K.M.; Heaps, S.E.; Hirt, R.P.; Embley, T.M. Reduction and Expansion in Microsporidian Genome Evolution: New Insights from Comparative Genomics. Genome Biol. Evol. 2013, 5, 2285–2303. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Rochon, D.A.; Sekimoto, S.; Wang, Y.; Chovatia, M.; Sandor, L.; Salamov, A.; Grigoriev, I.V.; Stajich, J.E.; Spatafora, J.W. Genome-scale phylogenetic analyses confirm Olpidium as the closest living zoosporic fungus to the non-flagellated, terrestrial fungi. Sci. Rep. 2021, 11, 3217. [Google Scholar] [CrossRef] [PubMed]
- Midorikawa, G.E.O.; Correa, C.L.; Noronha, E.F.; Filho, E.X.F.; Togawa, R.C.; Costa, M.M.D.C.; Silva-Junior, O.B.; Grynberg, P.; Miller, R.N.G. Analysis of the transcriptome in Aspergillus tamarii during enzymatic degradation of sugarcane bagasse. Front. Bioeng. Biotechnol. 2018, 6, 123. [Google Scholar] [CrossRef]
- Corrêa, C.L.; Midorikawa, G.E.O.; Filho, E.X.F.; Noronha, E.F.; Alves, G.S.C.; Togawa, R.C.; Silva-Junior, O.B.; Costa, M.M.D.C.; Grynberg, P.; Miller, R.N.G. Transcriptome Profiling-Based Analysis of Carbohydrate-Active Enzymes in Aspergillus terreus Involved in Plant Biomass Degradation. Front. Bioeng. Biotechnol. 2020, 8, 1179. [Google Scholar] [CrossRef]
- Tõlgo, M.; Hüttner, S.; Rugbjerg, P.; Thuy, N.T.; Thanh, V.N.; Larsbrink, J.; Olsson, L. Genomic and transcriptomic analysis of the thermophilic lignocellulose-degrading fungus Thielavia terrestris LPH172. Biotechnol. Biofuels 2021, 14, 131. [Google Scholar] [CrossRef]
- Amore, A.; Giacobbe, S.; Faraco, V. Regulation of cellulase and hemicellulase gene expression in fungi. Curr. Genom. 2013, 14, 230–249. [Google Scholar] [CrossRef]
- Binder, M.; Justo, A.; Riley, R.; Salamov, A.; Lopez-Giraldez, F.; Sjökvist, E.; Copeland, A.; Foster, B.; Sun, H.; Larsson, E.; et al. Phylogenetic and phylogenomic overview of the Polyporales. Mycologia 2013, 105, 1350–1373. [Google Scholar] [CrossRef]
- Martin, F.; Aerts, A.; Ahrén, D.; Brun, A.; Danchin, E.G.J.; Duchaussoy, F.; Gibon, J.; Kohler, A.; Lindquist, E.; Pereda, V.; et al. The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis. Nature 2008, 452, 88–92. [Google Scholar] [CrossRef]
- Ohm, R.A.; De Jong, J.F.; Lugones, L.G.; Aerts, A.; Kothe, E.; Stajich, J.E.; De Vries, R.P.; Record, E.; Levasseur, A.; Baker, S.E.; et al. Genome sequence of the model mushroom Schizophyllum commune. Nat. Biotechnol. 2010, 28, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Padamsee, M.; Kumar, A.T.K.; Riley, R.; Binder, M.; Boyd, A.; Calvo, A.M.; Furukawa, K.; Hesse, C.; Hohmann, S.; James, T.Y.; et al. The genome of the xerotolerant mold Wallemia sebi reveals adaptations to osmotic stress and suggests cryptic sexual reproduction. Fungal Genet. Biol. 2012, 49, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Zajc, J.; Liu, Y.; Dai, W.; Yang, Z.; Hu, J.; Gostinčar, C.; Gunde-Cimerman, N. Genome and transcriptome sequencing of the halophilic fungus Wallemia ichthyophaga: Haloadaptations present and absent. BMC Genom. 2013, 14, 617. [Google Scholar] [CrossRef] [PubMed]
- Toome, M.; Ohm, R.A.; Riley, R.W.; James, T.Y.; Lazarus, K.L.; Henrissat, B.; Albu, S.; Boyd, A.; Chow, J.; Clum, A.; et al. Genome sequencing provides insight into the reproductive biology, nutritional mode and ploidy of the fern pathogen Mixia osmundae. New Phytol. 2014, 202, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.; Haridas, S.; Wolfe, K.H.; Lopes, M.R.; Hittinger, C.T.; Göker, M.; Salamov, A.A.; Wisecaver, J.H.; Long, T.M.; Calvey, C.H.; et al. Comparative genomics of biotechnologically important yeasts. Proc. Natl. Acad. Sci. USA 2016, 113, 9882–9887. [Google Scholar] [CrossRef]
- Semeiks, J.; Borek, D.; Otwinowski, Z.; Grishin, N.V. Comparative genome sequencing reveals chemotype-specific gene clusters in the toxigenic black mold Stachybotrys. BMC Genom. 2014, 15, 590. [Google Scholar] [CrossRef]
- Morales-Cruz, A.; Amrine, K.C.; Blanco-Ulate, B.; Lawrence, D.P.; Travadon, R.; Rolshausen, P.E.; Baumgartner, K.; Cantu, D. Distinctive expansion of gene families associated with plant cell wall degradation, secondary metabolism, and nutrient uptake in the genomes of grapevine trunk pathogens. BMC Genom. 2015, 16, 469. [Google Scholar] [CrossRef]
- De Vries, R.P.; Riley, R.; Wiebenga, A.; Aguilar-Osorio, G.; Amillis, S.; Uchima, C.A.; Anderluh, G.; Asadollahi, M.; Askin, M.; Barry, K.; et al. Comparative genomics reveals high biological diversity and specific adaptations in the industrially and medically important fungal genus Aspergillus. Genome Biol. 2017, 18, 28. [Google Scholar] [CrossRef]
- Haridas, S.; Albert, R.; Binder, M.; Bloem, J.; LaButti, K.; Salamov, A.; Andreopoulos, B.; Baker, S.E.; Barry, K.; Bills, G.; et al. 101 Dothideomycetes genomes: A test case for predicting lifestyles and emergence of pathogens. Stud. Mycol. 2020, 96, 141–153. [Google Scholar] [CrossRef]
- Lim, J.J.J.; Koh, J.; Moo, J.R.; Villanueva, E.M.F.; Putri, D.A.; Lim, Y.S.; Seetoh, W.S.; Mulupuri, S.; Ng, J.W.Z.; Nguyen, N.L.U.; et al. Fungi.guru: Comparative genomic and transcriptomic resource for the fungi kingdom. Comput. Struct. Biotechnol. J. 2020, 18, 3788–3795. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, N.; Pearce, R.; Yi, S.; Zhao, X. Comparative Secretomics Analysis Reveals the Major Components of Penicillium oxalicum 16 and Trichoderma reesei RUT-C30. Microorganisms 2021, 9, 2042. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Zhang, H.; Liu, S.; Zhang, L.; Gao, P.; Chen, G.; Wang, L. Comparative Secretome Analysis of Aspergillus niger, Trichoderma reesei, and Penicillium oxalicum During Solid-State Fermentation. Appl. Biochem. Biotechnol. 2015, 177, 1252–1271. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-X.; Liu, L.; Zhang, T.; Luo, X.-M.; Feng, J.-X.; Zhao, S. Three-Dimensional Genome Map of the Filamentous Fungus Penicillium oxalicum. Microbiol. Spectr. 2022, e02121-21. [Google Scholar] [CrossRef] [PubMed]
- Svetlitchnyi, V.A.; Svetlichnaya, T.P.; Falkenhan, D.A.; Swinnen, S.; Knopp, D.; Läufer, A. Direct conversion of cellulose to L-lactic acid by a novel thermophilic Caldicellulosiruptor strain. Biotechnol. Biofuels Bioprod. 2022, 15, 44. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berlemont, R. The Potential for Cellulose Deconstruction in Fungal Genomes. Encyclopedia 2022, 2, 990-1003. https://doi.org/10.3390/encyclopedia2020065
Berlemont R. The Potential for Cellulose Deconstruction in Fungal Genomes. Encyclopedia. 2022; 2(2):990-1003. https://doi.org/10.3390/encyclopedia2020065
Chicago/Turabian StyleBerlemont, Renaud. 2022. "The Potential for Cellulose Deconstruction in Fungal Genomes" Encyclopedia 2, no. 2: 990-1003. https://doi.org/10.3390/encyclopedia2020065
APA StyleBerlemont, R. (2022). The Potential for Cellulose Deconstruction in Fungal Genomes. Encyclopedia, 2(2), 990-1003. https://doi.org/10.3390/encyclopedia2020065





