Recent Advances in Research on Molecular Mechanisms of Fungal Signaling
Abstract
:1. Introduction or History
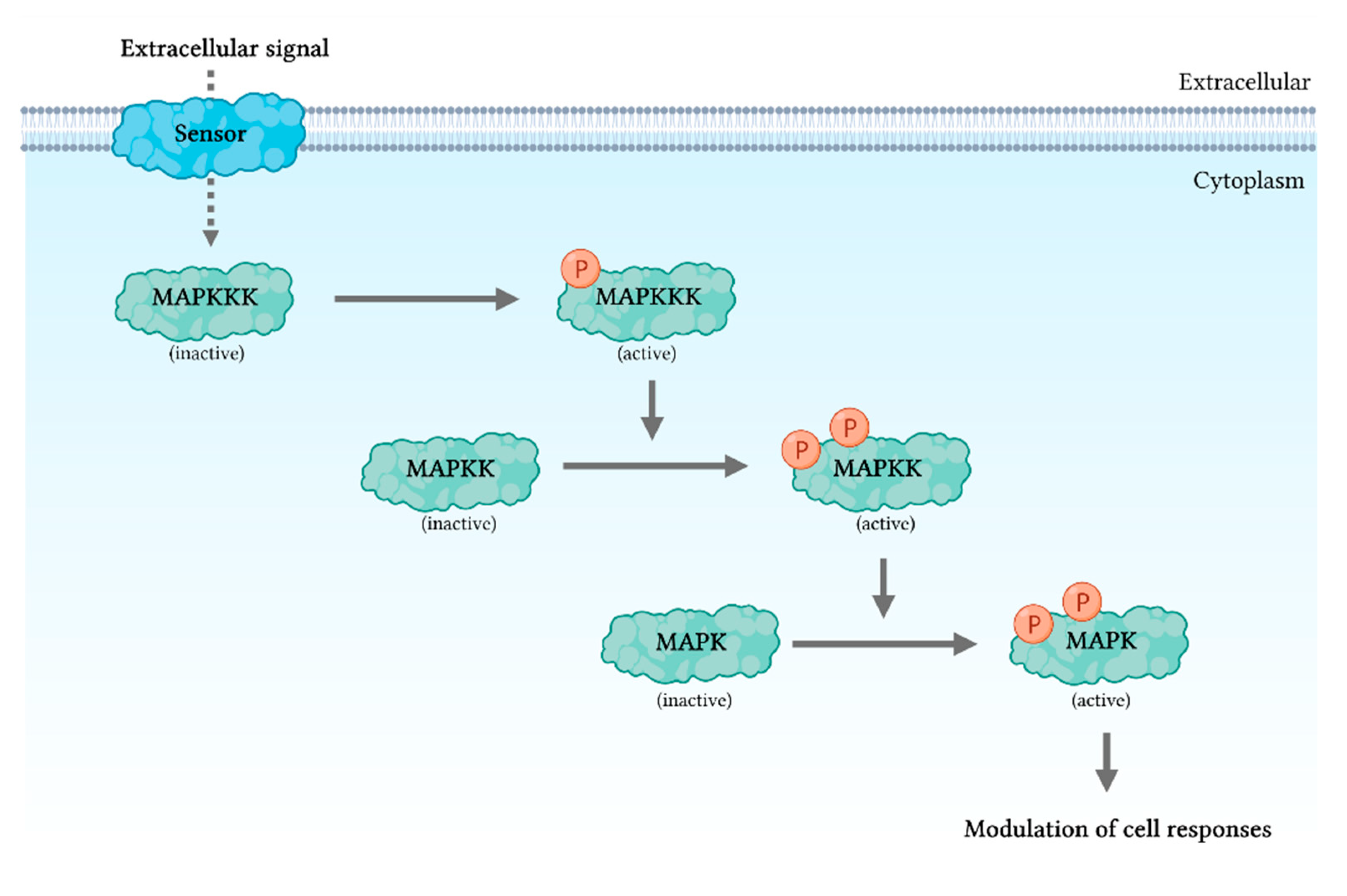
2. Mitogen-Activated Protein Kinase Signaling
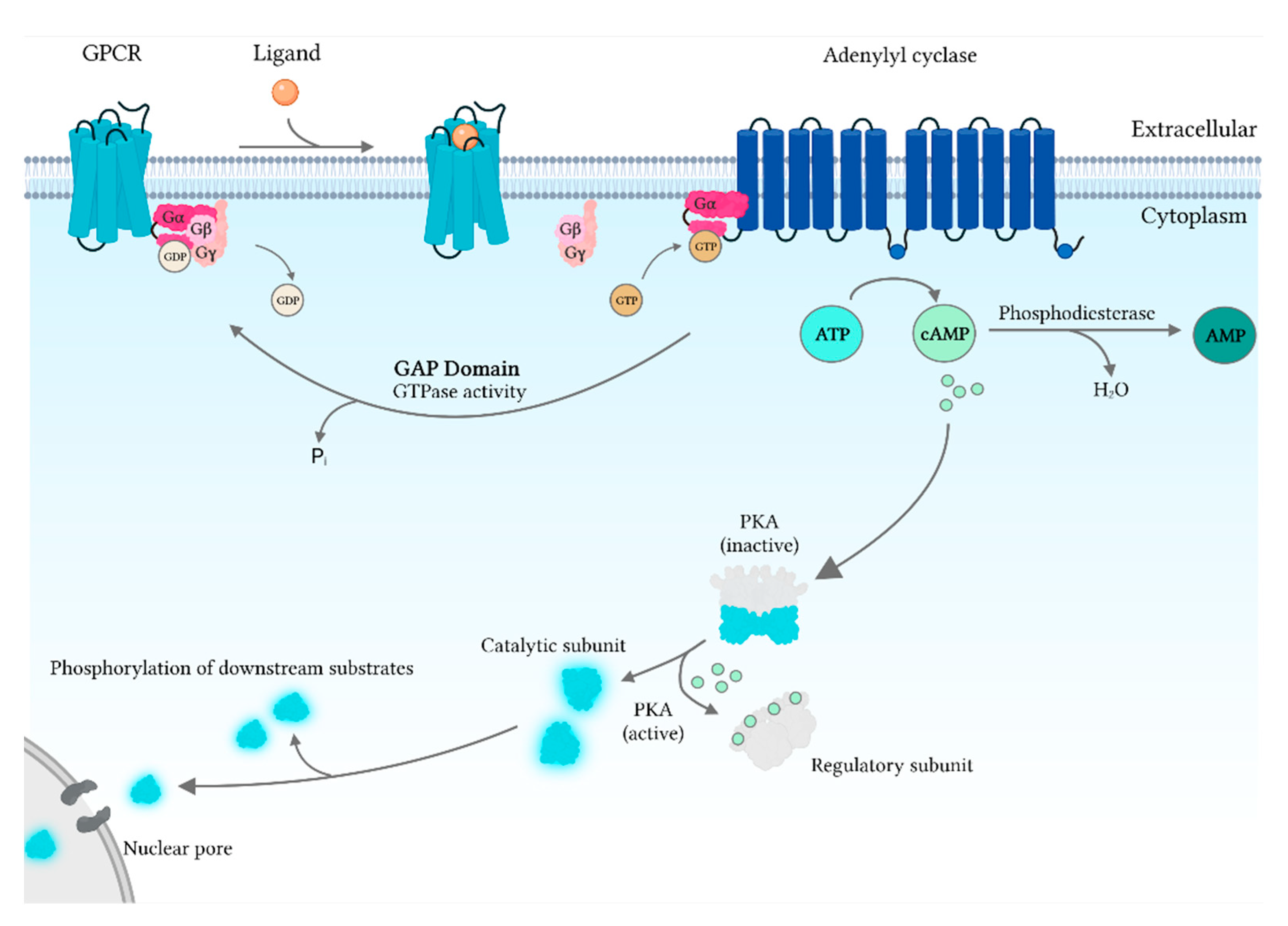
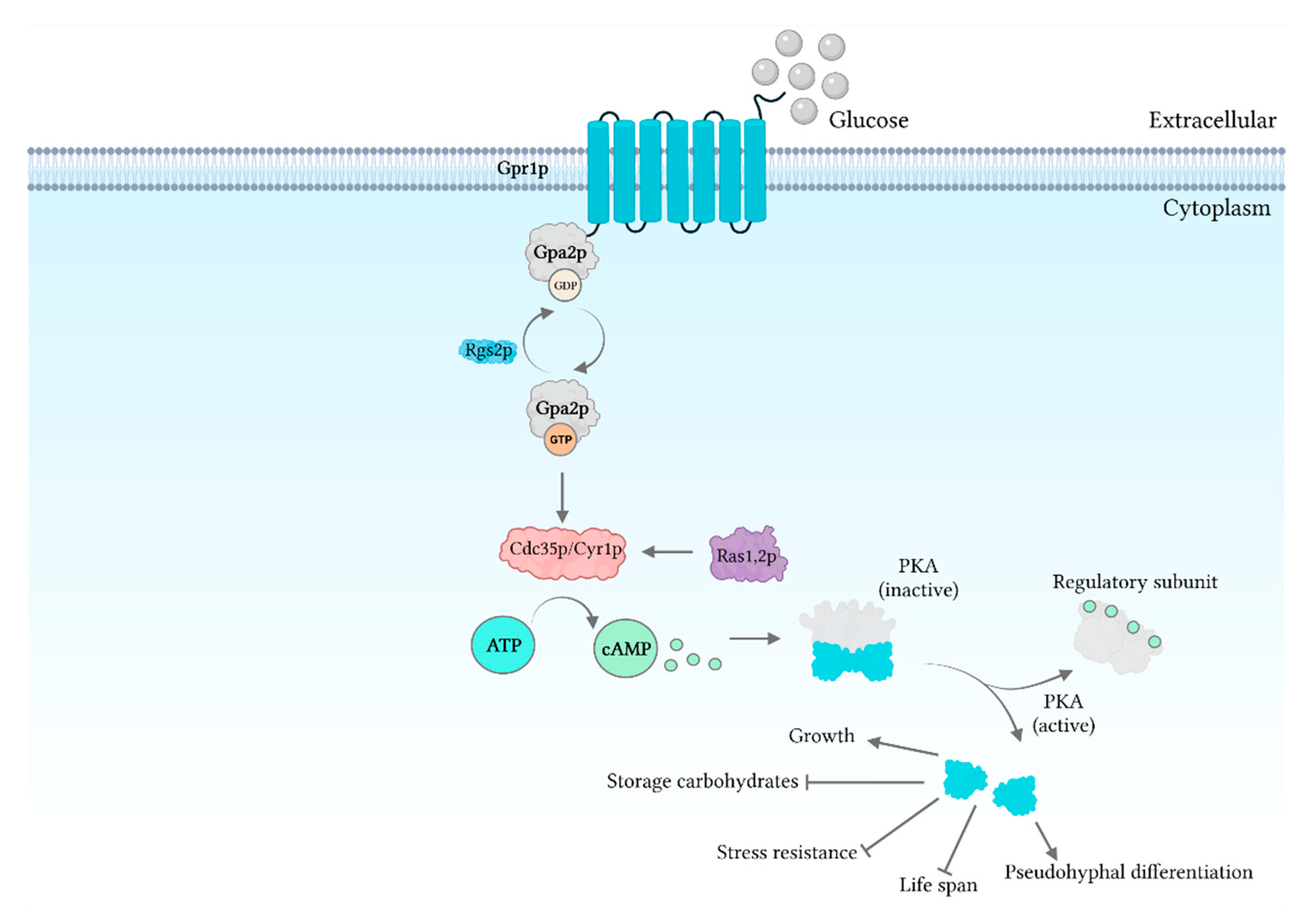
3. cAMP Signaling
4. Quorum Sensing
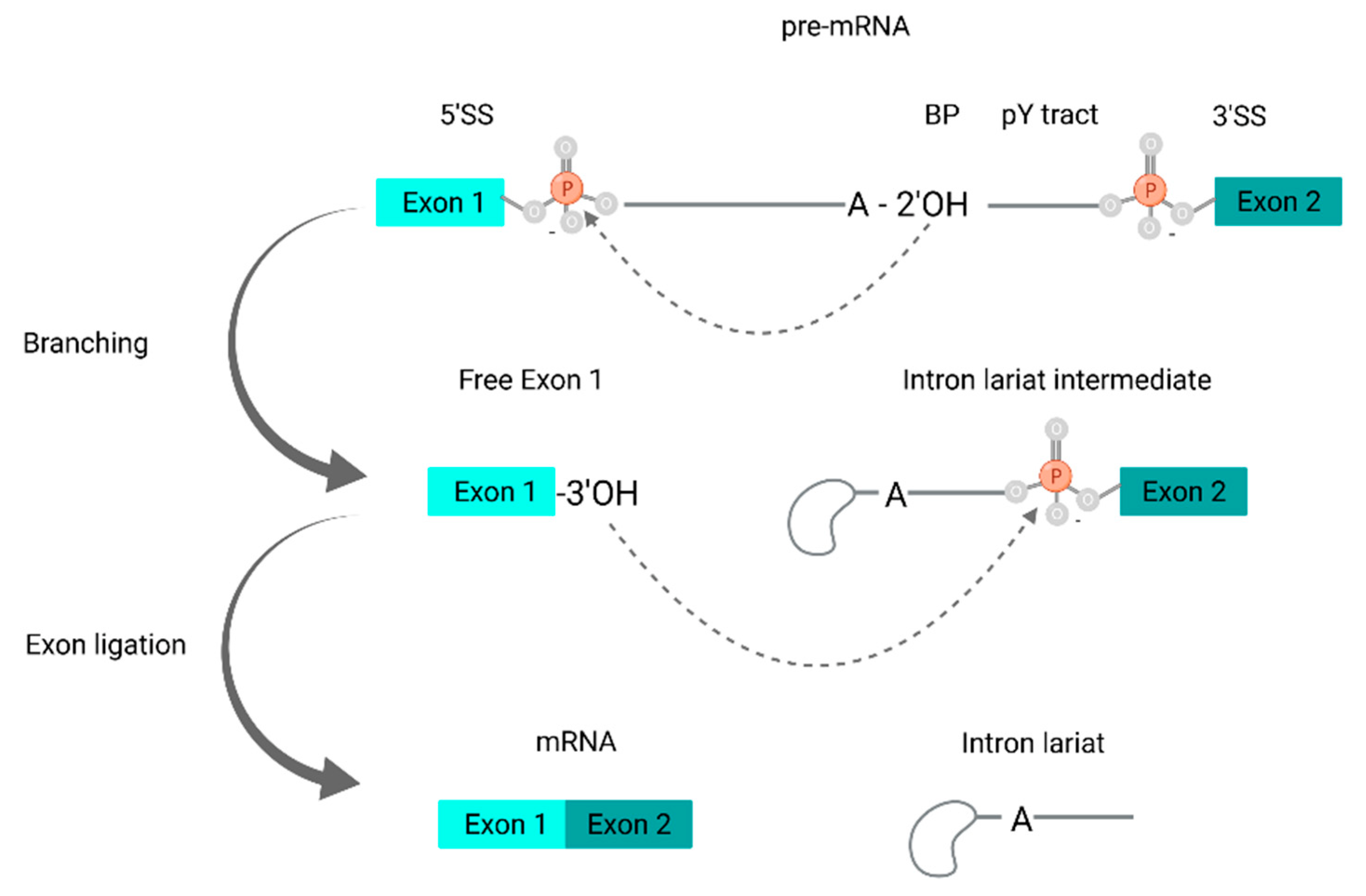
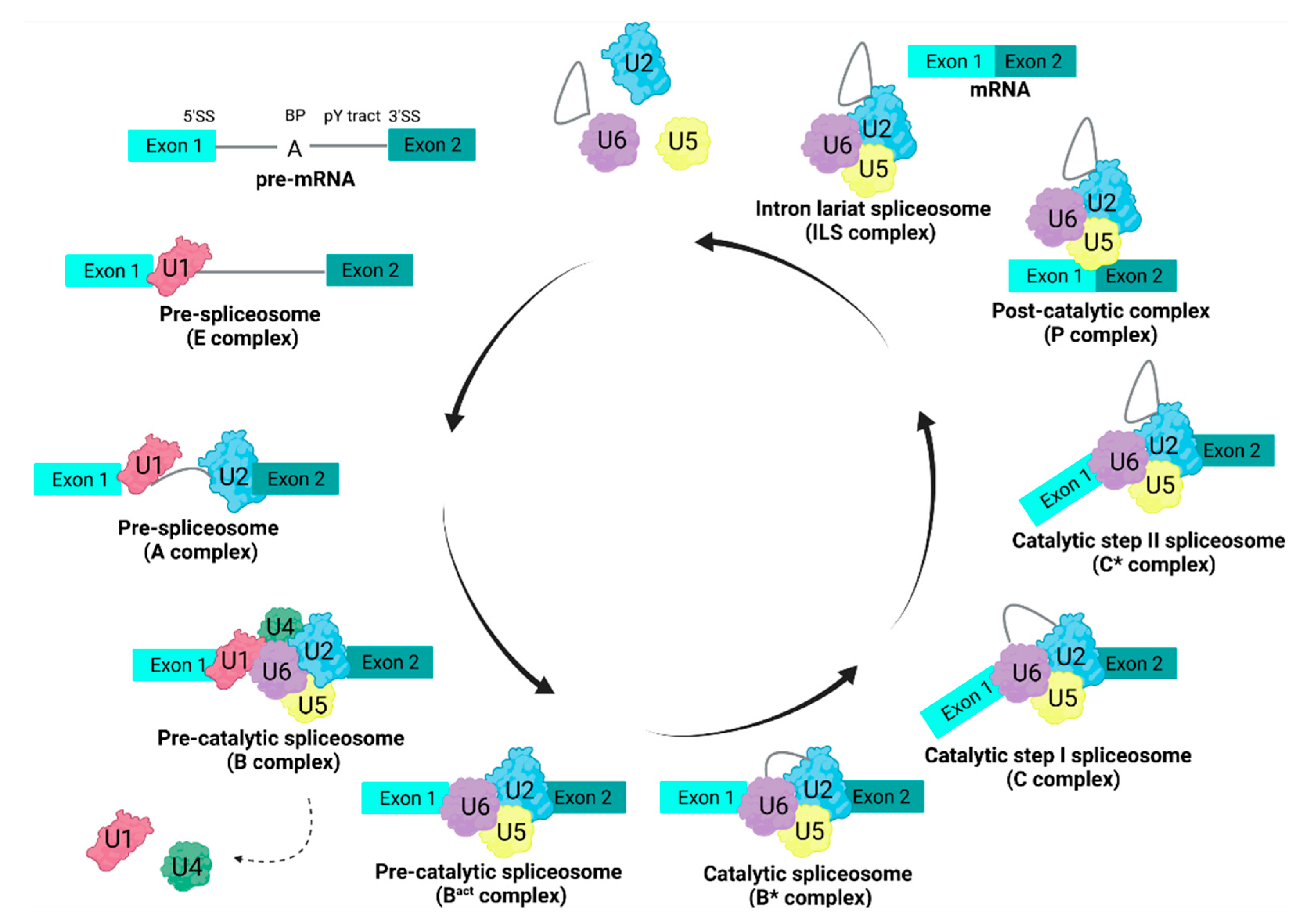
5. Alternative Splicing
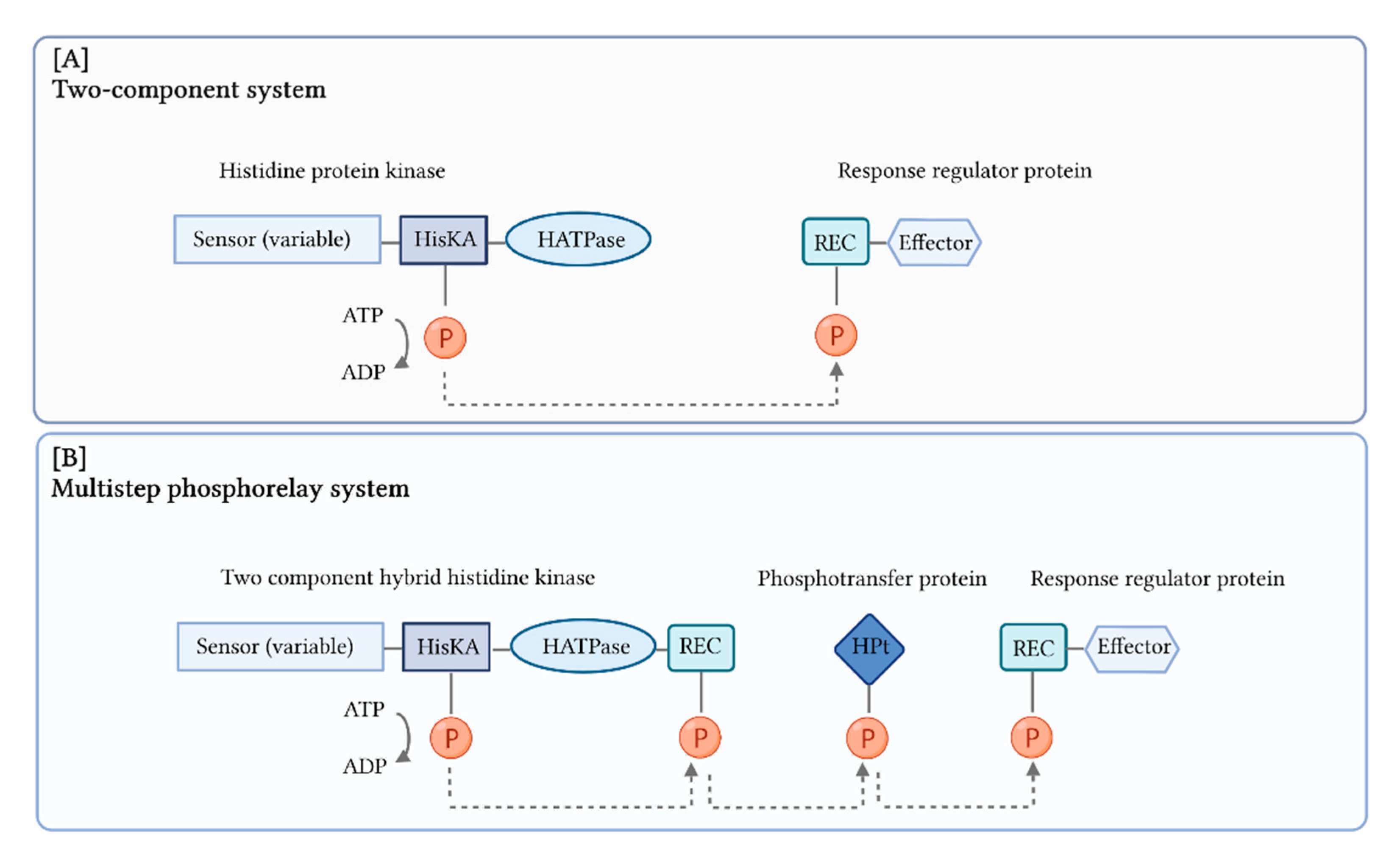
6. Multistep Phosphorelay Systems
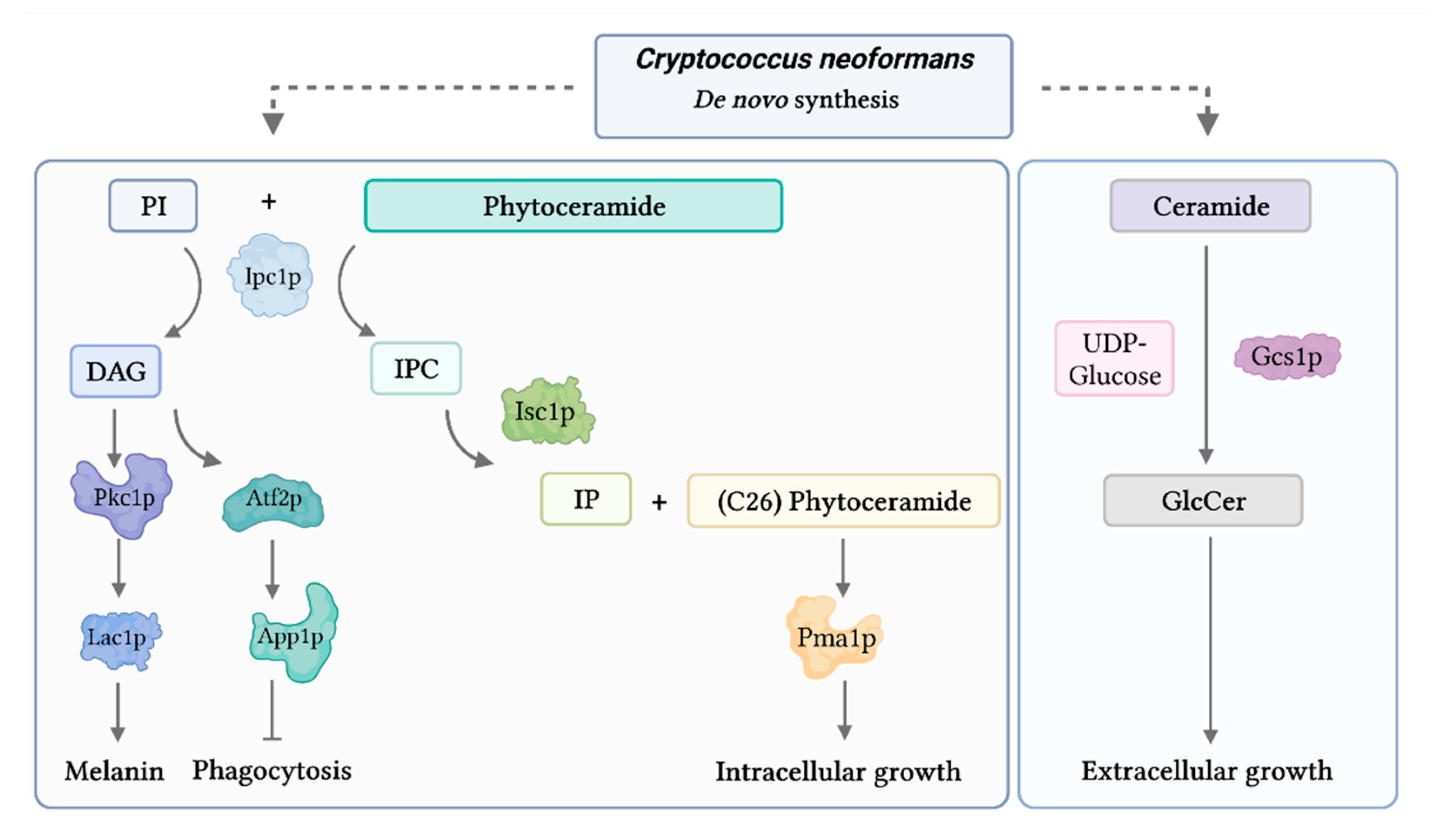
7. Lipid Signaling
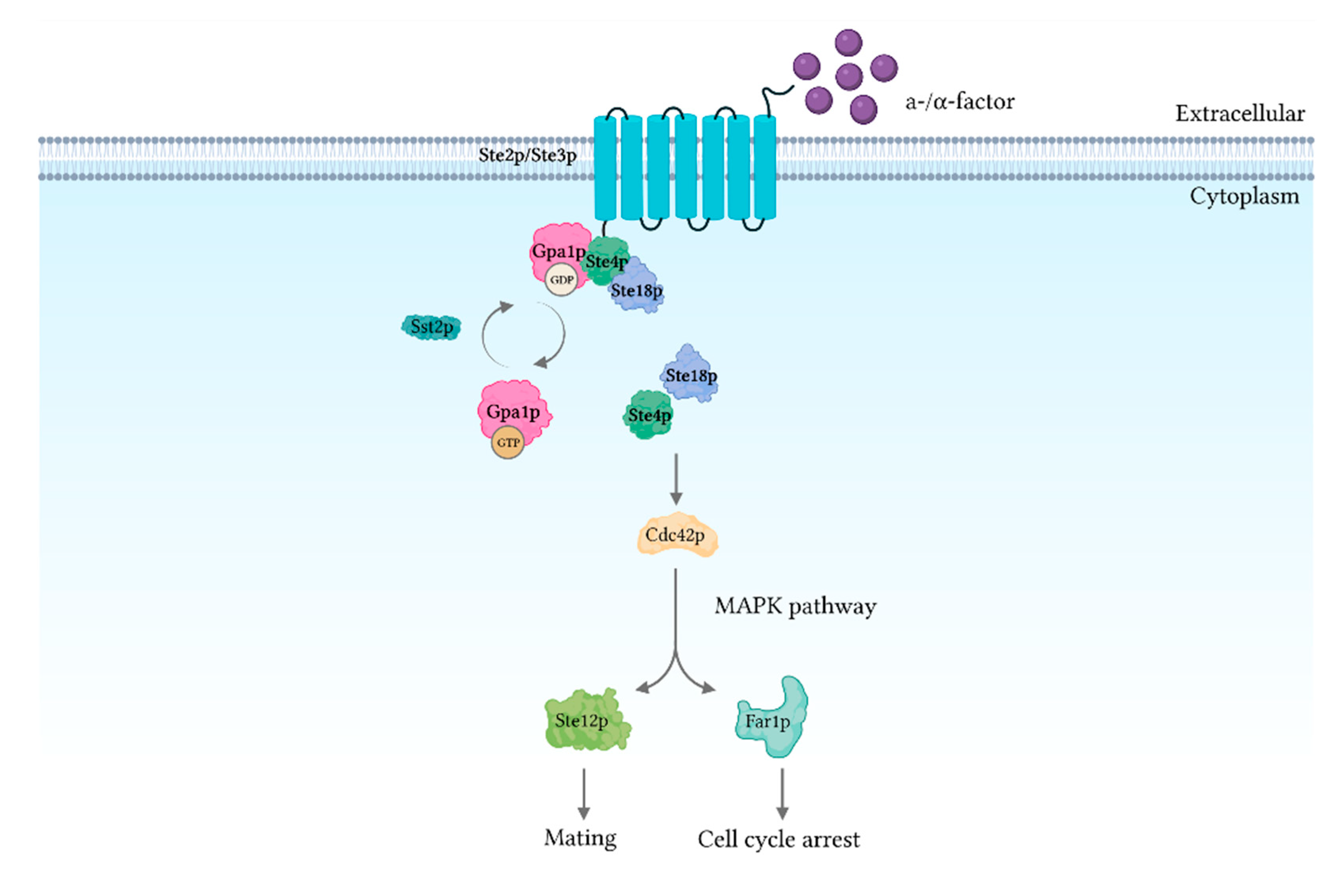
8. Pheromone and Glucose Signaling
9. Light Sensing
10. Concluding Remarks and Future Perspective
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pertseva, M.N.; Shpakov, A.O. The Prokaryotic Origin and Evolution of Eukaryotic Chemosignaling Systems. Neurosci. Behav. Physiol. 2009, 39, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Aravind, L.; Anantharaman, V.; Iyer, L.M. Evolutionary connections between bacterial and eukaryotic signaling systems: A genomic perspective. Curr. Opin. Microbiol. 2003, 6, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Cashin, P.; Goldsack, L.; Hall, D.; O’Toole, R. Contrasting signal transduction mechanisms in bacterial and eukaryotic gene transcription. FEMS Microbiol. Lett. 2006, 261, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Soto, D.; Ruiz-Herrera, J. Functional analysis of the MAPK pathways in fungi. Rev. Iberoam. Micol. 2017, 34, 192–202. [Google Scholar] [CrossRef]
- Cowan, K.J.; Storey, K.B. Mitogen-activated protein kinases: New signaling pathways functioning in cellular responses to environmental stress. J. Exp. Biol. 2003, 206, 1107–1115. [Google Scholar] [CrossRef]
- Papa, S.; Choy, P.M.; Bubici, C. The ERK and JNK pathways in the regulation of metabolic reprogramming. Oncogene 2019, 38, 2223–2240. [Google Scholar] [CrossRef]
- Taylor, S.S.; Meharena, H.S.; Kornev, A.P. Evolution of a dynamic molecular switch. IUBMB Life 2019, 71, 672–684. [Google Scholar] [CrossRef]
- Hoeflich, K.P.; Woodgett, J.R. Chapter 13 Mitogen-activated protein kinases and stress. Cell Mol. Response Stress 2001, 2, 175–193. [Google Scholar] [CrossRef]
- Zhao, X.; Mehrabi, R.; Xu, J.-R. Mitogen-Activated Protein Kinase Pathways and Fungal Pathogenesis. Eukaryot. Cell 2007, 6, 1701–1714. [Google Scholar] [CrossRef]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef]
- Saijo, Y.; Loo, E.P.; Yasuda, S. Pattern recognition receptors and signaling in plant-microbe interactions. Plant J. 2018, 93, 592–613. [Google Scholar] [CrossRef]
- Lopez, E.D.; Burastero, O.; Arcon, J.P.; Defelipe, L.A.; Ahn, N.G.; Marti, M.A.; Turjanski, A.G. Kinase Activation by Small Conformational Changes. J. Chem. Inf. Model. 2020, 60, 821–832. [Google Scholar] [CrossRef]
- Pegram, L.M.; Anderson, J.W.; Ahn, N.G. Dynamic equilibria in protein kinases. Curr. Opin. Struct. Biol. 2021, 71, 215–222. [Google Scholar] [CrossRef]
- Román, E.; Correia, I.; Prieto, D.; Alonso, R.; Pla, J. The HOG MAPK pathway in Candida albicans: More than an osmosensing pathway. Int. Microbiol. 2020, 23, 23–29. [Google Scholar] [CrossRef]
- Kumar, G.S.; Clarkson, M.W.; Kunze, M.B.A.; Granata, D.; Wand, A.J.; Lindorff-Larsen, K.; Page, R.; Peti, W. Dynamic activation and regulation of the mitogen-activated protein kinase p38. Proc. Natl. Acad. Sci. USA 2018, 115, 4655–4660. [Google Scholar] [CrossRef]
- Martínez-Limón, A.; Joaquin, M.; Caballero, M.; Posas, F.; de Nadal, E. The p38 Pathway: From Biology to Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 1913. [Google Scholar] [CrossRef]
- Brewster, J.L.; Gustin, M.C. Hog1: 20 years of discovery and impact. Sci. Signal. 2014, 7, re7. [Google Scholar] [CrossRef]
- Jacob, S.; Foster, A.J.; Yemelin, A.; Thines, E. High osmolarity glycerol (HOG) signalling in Magnaporthe oryzae: Identification of MoYPD1 and its role in osmoregulation, fungicide action, and pathogenicity. Fungal Biol. 2015, 119, 580–594. [Google Scholar] [CrossRef]
- Jacob, S.; Schüffler, A.; Thines, E. Hog1p activation by marasmic acid through inhibition of the histidine kinase Sln1p. Pest Manag. Sci. 2016, 72, 1268–1274. [Google Scholar] [CrossRef]
- Winkler, A.; Arkind, C.; Mattison, C.P.; Burkholder, A.; Knoche, K.; Ota, I. Heat Stress Activates the Yeast High-Osmolarity Glycerol Mitogen-Activated Protein Kinase Pathway, and Protein Tyrosine Phosphatases Are Essential under Heat Stress. Eukaryot. Cell 2002, 1, 163–173. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, E.; An, J.; Lee, Y.; Choi, E.; Choi, W.; Moon, E.; Kim, W. Dissection of the HOG pathway activated by hydrogen peroxide in S accharomyces cerevisiae. Environ. Microbiol. 2017, 19, 584–597. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Wang, D. Regulation of the Response of Caenorhabditis elegans to Simulated Microgravity by p38 Mitogen-Activated Protein Kinase Signaling. Sci. Rep. 2018, 8, 857. [Google Scholar] [CrossRef]
- Maik-Rachline, G.; Zehorai, E.; Hanoch, T.; Blenis, J.; Seger, R. The nuclear translocation of the kinases p38 and JNK promotes inflammation-induced cancer. Sci. Signal. 2018, 11, eaao3428. [Google Scholar] [CrossRef]
- González-Rubio, G.; Sellers-Moya, Á.; Martín, H.; Molina, M. Differential Role of Threonine and Tyrosine Phosphorylation in the Activation and Activity of the Yeast MAPK Slt2. Int. J. Mol. Sci. 2021, 22, 1110. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, Y.Y.; Lu, C.; Zhang, W.; Deng, H.; Wu, J.W.; Wang, J.; Wang, Z.X. Kinetic and mechanistic studies of p38α MAP kinase phosphorylation by MKK6. FEBS J. 2019, 286, 1030–1052. [Google Scholar] [CrossRef]
- Wolf, A.; Beuerlein, K.; Eckart, C.; Weiser, H.; Dickkopf, B.; Müller, H.; Sakurai, H.; Kracht, M. Identification and Functional Characterization of Novel Phosphorylation Sites in TAK1-Binding Protein (TAB) 1. PLoS ONE 2011, 6, e29256. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Mei, Z.-Q.; Wu, J.-W.; Wang, Z.-X. Enzymatic activity and substrate specificity of mitogen-activated protein kinase p38alpha in different phosphorylation states. J. Biol. Chem. 2008, 283, 26591–26601. [Google Scholar] [CrossRef]
- Bell, M.; Engelberg, D. Phosphorylation of Tyr-176 of the yeast MAPK Hog1/p38 is not vital for Hog1 biological activity. J. Biol. Chem. 2003, 278, 14603–14606. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.B.; Agarwal, S.R.; Harvey, R.D.; Ostrom, R.S. cAMP Signaling Compartmentation: Adenylyl Cyclases as Anchors of Dynamic Signaling Complexes. Mol. Pharmacol. 2018, 93, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Zaccolo, M.; Zerio, A.; Lobo, M.J. Subcellular Organization of the cAMP Signaling Pathway. Pharmacol. Rev. 2021, 73, 278–309. [Google Scholar] [CrossRef] [PubMed]
- Musheshe, N.; Schmidt, M.; Zaccolo, M. cAMP: From Long-Range Second Messenger to Nanodomain Signalling. Trends Pharmacol. Sci. 2018, 39, 209–222. [Google Scholar] [CrossRef]
- Portela, P.; Rossi, S. cAMP-PKA signal transduction specificity in Saccharomyces cerevisiae. Curr. Genet. 2020, 66, 1093–1099. [Google Scholar] [CrossRef]
- Taylor, S.S.; Zhang, P.; Steichen, J.M.; Keshwani, M.M.; Kornev, A.P. PKA: Lessons learned after twenty years. Biochim. Biophys. Acta—Proteins Proteom. 2013, 1834, 1271–1278. [Google Scholar] [CrossRef]
- Lee, N.; D’Souza, C.A.; Kronstad, J.W. OF SMUTS, BLASTS, MILDEWS, AND BLIGHTS: cAMP Signaling in Phytopathogenic Fungi. Annu. Rev. Phytopathol. 2003, 41, 399–427. [Google Scholar] [CrossRef]
- Skålhegg, B.S.; Tasken, K. Specificity in the cAMP/PKA signaling pathway. Differential expression, regulation, and subcellular localization of subunits of PKA. Front. Biosci 2000, 5, 678–693. [Google Scholar]
- Zhang, X.; Pizzoni, A.; Hong, K.; Naim, N.; Qi, C.; Korkhov, V.; Altschuler, D.L. CAP1 binds and activates adenylyl cyclase in mammalian cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2024576118. [Google Scholar] [CrossRef]
- Clapham, D.E.; Neer, E.J. New roles for G-protein (βγ-dimers in transmembrane signalling. Nature 1993, 365, 403–406. [Google Scholar] [CrossRef]
- Ribeiro, L.F.C.; Chelius, C.; Boppidi, K.R.; Naik, N.S.; Hossain, S.; Ramsey, J.J.J.; Kumar, J.; Ribeiro, L.F.; Ostermeier, M.; Tran, B.; et al. Comprehensive analysis of aspergillus nidulans PKA phosphorylome identifies a novel mode of CreA regulation. mBio 2019, 10, e02825-18. [Google Scholar] [CrossRef]
- Cai, E.; Sun, S.; Deng, Y.; Huang, P.; Sun, X.; Wang, Y.; Chang, C.; Jiang, Z. Histidine Kinase Sln1 and cAMP/PKA Signaling Pathways Antagonistically Regulate Sporisorium scitamineum Mating and Virulence via Transcription Factor Prf1. J. Fungi 2021, 7, 610. [Google Scholar] [CrossRef]
- Pan, X.; Heitman, J. Protein Kinase A Operates a Molecular Switch That Governs Yeast Pseudohyphal Differentiation. Mol. Cell. Biol. 2002, 22, 3981–3993. [Google Scholar] [CrossRef]
- Palecek, S.P.; Parikh, A.S.; Kron, S.J. Sensing, signalling and integrating physical processes during Saccharomyces cerevisiae invasive and filamentous growth. Microbiology 2002, 148, 893–907. [Google Scholar] [CrossRef]
- Rolland, F.; Winderickx, J.; Thevelein, J.M. Glucose-sensing mechanisms in eukaryotic cells. Trends Biochem. Sci. 2001, 26, 310–317. [Google Scholar] [CrossRef]
- Wendland, J. Comparison of Morphogenetic Networks of Filamentous Fungi and Yeast. Fungal Genet. Biol. 2001, 34, 63–82. [Google Scholar] [CrossRef]
- D’Souza, C.A.; Heitman, J. Conserved cAMP signaling cascades regulate fungal development and virulence. FEMS Microbiol. Rev. 2001, 25, 349–364. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, K.; Qin, Q.; Lin, G.; Hu, T.; Xu, Z.; Wang, S. G Protein α Subunit GpaB is Required for Asexual Development, Aflatoxin Biosynthesis and Pathogenicity by Regulating cAMP Signaling in Aspergillus flavus. Toxins 2018, 10, 117. [Google Scholar] [CrossRef]
- Fillinger, S.; Chaveroche, M.-K.; Shimizu, K.; Keller, N.; D’Enfert, C. cAMP and ras signalling independently control spore germination in the filamentous fungus Aspergillus nidulans. Mol. Microbiol. 2002, 44, 1001–1016. [Google Scholar] [CrossRef] [PubMed]
- Saudohar, M.; Bencina, M.; van de Vondervoort, P.J.; Panneman, H.; Legisa, M.; Visser, J.; Ruijter, G.J.G. Cyclic AMP-dependent protein kinase is involved in morphogenesis of Aspergillus niger a aThe EMBL accession number for the sequence reported in this paper is AJ296317. Microbiology 2002, 148, 2635–2645. [Google Scholar] [CrossRef]
- Shimizu, K.; Keller, N.P. Genetic involvement of a cAMP-dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics 2001, 157, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Mowat, E.; Jones, B.; Williams, C.; Lopez-Ribot, J. Our Current Understanding of Fungal Biofilms. Crit. Rev. Microbiol. 2009, 35, 340–355. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.; Casadevall, A. Quorum sensing in fungi—A review. Med. Mycol. 2012, 50, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Bassler, B.L.; Losick, R. Bacterially Speaking. Cell 2006, 125, 237–246. [Google Scholar] [CrossRef]
- Tian, X.; Ding, H.; Ke, W.; Wang, L. Quorum Sensing in Fungal Species. Annu. Rev. Microbiol. 2021, 75, 449–469. [Google Scholar] [CrossRef]
- Miller, M.B.; Bassler, B.L. Quorum Sensing in Bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef]
- De Kievit, T.R.; Iglewski, B.H. Bacterial Quorum Sensing in Pathogenic Relationships. Infect. Immun. 2000, 68, 4839–4849. [Google Scholar] [CrossRef]
- Padder, S.A.; Prasad, R.; Shah, A.H. Quorum sensing: A less known mode of communication among fungi. Microbiol. Res. 2018, 210, 51–58. [Google Scholar] [CrossRef]
- Wongsuk, T.; Pumeesat, P.; Luplertlop, N. Fungal quorum sensing molecules: Role in fungal morphogenesis and pathogenicity. J. Basic Microbiol. 2016, 56, 440–447. [Google Scholar] [CrossRef]
- Hornby, J.M.; Jensen, E.C.; Lisec, A.D.; Tasto, J.J.; Jahnke, B.; Shoemaker, R.; Dussault, P.; Nickerson, K.W. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 2001, 67, 2982–2992. [Google Scholar] [CrossRef]
- Kebaara, B.W.; Langford, M.L.; Navarathna, D.H.M.L.P.; Dumitru, R.; Nickerson, K.W.; Atkin, A.L. Candida albicans Tup1 Is Involved in Farnesol-Mediated Inhibition of Filamentous-Growth Induction. Eukaryot. Cell 2008, 7, 980–987. [Google Scholar] [CrossRef]
- Chen, H.; Fujita, M.; Feng, Q.; Clardy, J.; Fink, G.R. Tyrosol is a quorum-sensing molecule in Candida albicans. Proc. Natl. Acad. Sci. USA 2004, 101, 5048–5052. [Google Scholar] [CrossRef]
- Lindsay, A.K.; Deveau, A.; Piispanen, A.E.; Hogan, D.A. Farnesol and Cyclic AMP Signaling Effects on the Hypha-to-Yeast Transition in Candida albicans. Eukaryot. Cell 2012, 11, 1219–1225. [Google Scholar] [CrossRef]
- Swetha, T.K.; Priya, A.; Pandian, S.K. Natural molecules against QS-associated biofilm formation of pathogens. Microb. Nat. Macromol. 2021, 317–348. [Google Scholar] [CrossRef]
- Chen, H.; Fink, G.R. Feedback control of morphogenesis in fungi by aromatic alcohols. Genes Dev. 2006, 20, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Avbelj, M.; Zupan, J.; Kranjc, L.; Raspor, P. Quorum-Sensing Kinetics in Saccharomyces cerevisiae: A Symphony of ARO Genes and Aromatic Alcohols. J. Agric. Food Chem. 2015, 63, 8544–8550. [Google Scholar] [CrossRef]
- Wuster, A.; Babu, M.M. Transcriptional control of the quorum sensing response in yeast. Mol. BioSyst. 2009, 6, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Vinod, P.K.; Sengupta, N.; Bhat, P.J.; Venkatesh, K.V. Integration of Global Signaling Pathways, cAMP-PKA, MAPK and TOR in the Regulation of FLO11. PLoS ONE 2008, 3, e1663. [Google Scholar] [CrossRef] [PubMed]
- Barrales, R.R.; Jimenez, J.; Ibeas, J.I. Identification of Novel Activation Mechanisms for FLO11 Regulation in Saccharomyces cerevisiae. Genetics 2008, 178, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Valerius, O.; Rupprecht, H.; Dumkow, M.; Krappmann, S.; Braus, G.H. Posttranscriptional regulation of FLO11 upon amino acid starvation in Saccharomyces cerevisiae. FEMS Yeast Res. 2008, 8, 225–236. [Google Scholar] [CrossRef]
- Winters, M.; Arneborg, N.; Appels, R.; Howell, K. Can community-based signalling behaviour in Saccharomyces cerevisiae be called quorum sensing? A critical review of the literature. FEMS Yeast Res. 2019, 19, foz046. [Google Scholar] [CrossRef]
- Kelemen, O.; Convertini, P.; Zhang, Z.; Wen, Y.; Shen, M.; Falaleeva, M.; Stamm, S. Function of alternative splicing. Gene 2013, 514, 1–30. [Google Scholar] [CrossRef]
- Nilsen, T.W.; Graveley, B.R. Expansion of the eukaryotic proteome by alternative splicing. Nature 2010, 463, 457–463. [Google Scholar] [CrossRef]
- Fang, S.; Hou, X.; Qiu, K.; He, R.; Feng, X.; Liang, X. The occurrence and function of alternative splicing in fungi. Fungal Biol. Rev. 2020, 34, 178–188. [Google Scholar] [CrossRef]
- Baralle, F.E.; Giudice, J. Alternative splicing as a regulator of development and tissue identity. Nat. Rev. Mol. Cell Biol. 2017, 18, 437. [Google Scholar] [CrossRef]
- Matera, A.G.; Wang, Z. A day in the life of the spliceosome. Nat. Rev. Mol. Cell Biol. 2014, 15, 108. [Google Scholar] [CrossRef]
- Wahl, M.C.; Will, C.L.; Lührmann, R. The Spliceosome: Design Principles of a Dynamic RNP Machine. Cell 2009, 136, 701–718. [Google Scholar] [CrossRef]
- Wilkinson, M.E.; Charenton, C.; Nagai, K. RNA Splicing by the Spliceosome. Annu. Rev. Biochem. 2020, 89, 359–388. [Google Scholar] [CrossRef]
- Fica, S.M.; Nagai, K. Cryo-electron microscopy snapshots of the spliceosome: Structural insights into a dynamic ribonucleoprotein machine. Nat. Struct. Mol. Biol. 2017, 24, 791–799. [Google Scholar] [CrossRef]
- Morais, P.; Adachi, H.; Yu, Y.T. Spliceosomal snRNA Epitranscriptomics. Front. Genet. 2021, 12, 249. [Google Scholar] [CrossRef]
- Liu, X.; Pan, X.; Chen, D.; Yin, C.; Peng, J.; Shi, W.; Qi, L.; Wang, R.; Zhao, W.; Zhang, Z.; et al. Prp19-associated splicing factor Cwf15 regulates fungal virulence and development in the rice blast fungus. Environ. Microbiol. 2021, 23, 5901–5916. [Google Scholar] [CrossRef]
- Wan, R.; Yan, C.; Bai, R.; Wang, L.; Huang, M.; Wong, C.C.L.; Shi, Y. The 3.8 Å structure of the U4/U6.U5 tri-snRNP: Insights into spliceosome assembly and catalysis. Science 2016, 351, 466–475. [Google Scholar] [CrossRef]
- Huang, W.; Huang, Y.; Xu, J.; Liao, J. Lou How Does the Spliceosome Catalyze Intron Lariat Formation? Insights from Quantum Mechanics/Molecular Mechanics Free-Energy Simulations. J. Phys. Chem. B 2019, 123, 6049–6055. [Google Scholar] [CrossRef]
- Shi, Y. The Spliceosome: A Protein-Directed Metalloribozyme. J. Mol. Biol. 2017, 429, 2640–2653. [Google Scholar] [CrossRef]
- Zhang, X.; Zhan, X.; Yan, C.; Zhang, W.; Liu, D.; Lei, J.; Shi, Y. Structures of the human spliceosomes before and after release of the ligated exon. Cell Res. 2019, 29, 274–285. [Google Scholar] [CrossRef]
- Pan, Q.; Shai, O.; Lee, L.J.; Frey, B.J.; Blencowe, B.J. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 2008, 40, 1413–1415. [Google Scholar] [CrossRef]
- Wang, E.T.; Sandberg, R.; Luo, S.; Khrebtukova, I.; Zhang, L.; Mayr, C.; Kingsmore, S.F.; Schroth, G.P.; Burge, C.B. Alternative isoform regulation in human tissue transcriptomes. Nature 2008, 456, 470–476. [Google Scholar] [CrossRef]
- Bonnal, S.C.; López-Oreja, I.; Valcárcel, J. Roles and mechanisms of alternative splicing in cancer—Implications for care. Nat. Rev. Clin. Oncol. 2020, 17, 457–474. [Google Scholar] [CrossRef]
- Reviejo, M.; Soto, M.; Lozano, E.; Asensio, M.; Martínez-Augustin, O.; de Medina, F.S.; Marin, J.J.G. Impact of alternative splicing on mechanisms of resistance to anticancer drugs. Biochem. Pharmacol. 2021, 193, 114810. [Google Scholar] [CrossRef]
- Kempken, F. Alternative splicing in ascomycetes. Appl. Microbiol. Biotechnol. 2013, 97, 4235–4241. [Google Scholar] [CrossRef] [PubMed]
- Ganie, S.A.; Reddy, A.S.N. Stress-induced changes in alternative splicing landscape in rice: Functional significance of splice isoforms in stress tolerance. Biology 2021, 10, 309. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.S.; Weinstein, B.N.; Roy, S.W.; Brown, C.M. Analysis of Fungal Genomes Reveals Commonalities of Intron Gain or Loss and Functions in Intron-Poor Species. Mol. Biol. Evol. 2021, 38, 4166–4186. [Google Scholar] [CrossRef] [PubMed]
- Grützmann, K.; Szafranski, K.; Pohl, M.; Voigt, K.; Petzold, A.; Schuster, S. Fungal Alternative Splicing is Associated with Multicellular Complexity and Virulence: A Genome-Wide Multi-Species Study. DNA Res. 2014, 21, 27. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Bae, H. The diversity of fungal genome. Biol. Proced. Online 2015, 17, 8. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Peng, J.; Cheng, Z.; Liu, X.; Zhang, Z.; Bhadauria, V.; Zhao, W.; Peng, Y.L. Transcriptional Landscapes of Long Non-coding RNAs and Alternative Splicing in Pyricularia oryzae Revealed by RNA-Seq. Front. Plant Sci. 2021, 12, 1974. [Google Scholar] [CrossRef]
- Jin, L.; Li, G.; Yu, D.; Huang, W.; Cheng, C.; Liao, S.; Wu, Q.; Zhang, Y. Transcriptome analysis reveals the complexity of alternative splicing regulation in the fungus Verticillium dahliae. BMC Genom. 2017, 18, 130. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Bai, N.; Yang, X.; Zhang, K.-Q.; Yang, J. Transcriptomic Analysis Reveals That Rho GTPases Regulate Trap Development and Lifestyle Transition of the Nematode-Trapping Fungus Arthrobotrys oligospora. Microbiol. Spectr. 2022, 10, e01759-21. [Google Scholar] [CrossRef]
- Muzafar, S.; Sharma, R.D.; Chauhan, N.; Prasad, R. Intron distribution and emerging role of alternative splicing in fungi. FEMS Microbiol. Lett. 2021, 368, fnab135. [Google Scholar] [CrossRef]
- Kumar, S.; Mutturi, S. Alternative splicing regulates the α-glucosidase synthesis in Aspergillus neoniger NCIM 1400. Fungal Biol. 2021, 125, 658–665. [Google Scholar] [CrossRef]
- Zhao, C.; Waalwijk, C.; de Wit, P.J.G.M.; Tang, D.; van der Lee, T. RNA-Seq analysis reveals new gene models and alternative splicing in the fungal pathogen Fusarium graminearum. BMC Genom. 2013, 14, 21. [Google Scholar] [CrossRef]
- Liu, X.Y.; Fan, L.; Gao, J.; Shen, X.Y.; Hou, C.L. Global identification of alternative splicing in Shiraia bambusicola and analysis of its regulation in hypocrellin biosynthesis. Appl. Microbiol. Biotechnol. 2020, 104, 211–223. [Google Scholar] [CrossRef]
- Freitag, J.; Ast, J.; Bölker, M. Cryptic peroxisomal targeting via alternative splicing and stop codon read-through in fungi. Nature 2012, 485, 522–525. [Google Scholar] [CrossRef]
- Wang, S.; Liang, H.; Wei, Y.; Zhang, P.; Dang, Y.; Li, G.; Zhang, S.H. Alternative Splicing of MoPTEN Is Important for Growth and Pathogenesis in Magnaporthe oryzae. Front. Microbiol. 2021, 12, 715773. [Google Scholar] [CrossRef]
- Rossi, A.; Cruz, A.H.S.; Santos, R.S.; Silva, P.M.; Silva, E.M.; Mendes, N.S.; Martinez-Rossi, N.M. Ambient pH sensing in filamentous fungi: Pitfalls in elucidating regulatory hierarchical signaling networks. IUBMB Life 2013, 65, 930–935. [Google Scholar] [CrossRef]
- Schreiber, K.; Csaba, G.; Haslbeck, M.; Zimmer, R. Alternative Splicing in Next Generation Sequencing Data of Saccharomyces cerevisiae. PLoS ONE 2015, 10, e0140487. [Google Scholar] [CrossRef]
- Muzafar, S.; Sharma, R.D.; Shah, A.H.; Gaur, N.A.; Dasgupta, U.; Chauhan, N.; Prasad, R. Identification of Genomewide Alternative Splicing Events in Sequential, Isogenic Clinical Isolates of Candida albicans Reveals a Novel Mechanism of Drug Resistance and Tolerance to Cellular Stresses. mSphere 2020, 5, e00608-20. [Google Scholar] [CrossRef]
- Gonzalez-Hilarion, S.; Paulet, D.; Lee, K.T.; Hon, C.C.; Lechat, P.; Mogensen, E.; Moyrand, F.; Proux, C.; Barboux, R.; Bussotti, G.; et al. Intron retention-dependent gene regulation in Cryptococcus neoformans. Sci. Rep. 2016, 6, 32252. [Google Scholar] [CrossRef]
- Sieber, P.; Voigt, K.; Kämmer, P.; Brunke, S.; Schuster, S.; Linde, J. Comparative study on alternative splicing in human fungal pathogens suggests its involvement during host invasion. Front. Microbiol. 2018, 9, 2313. [Google Scholar] [CrossRef]
- Gehrmann, T.; Pelkmans, J.F.; Lugones, L.G.; Wösten, H.A.B.; Abeel, T.; Reinders, M.J.T. Schizophyllum commune has an extensive and functional alternative splicing repertoire. Sci. Rep. 2016, 6, 33640. [Google Scholar] [CrossRef]
- Wilson, R.A.; Talbot, N.J. Under pressure: Investigating the biology of plant infection by Magnaporthe oryzae. Nat. Rev. Microbiol. 2009, 7, 185–195. [Google Scholar] [CrossRef]
- Hérivaux, A.; So, Y.S.; Gastebois, A.; Latgé, J.P.; Bouchara, J.P.; Bahn, Y.S.; Papon, N. Major Sensing Proteins in Pathogenic Fungi: The Hybrid Histidine Kinase Family. PLOS Pathog. 2016, 12, e1005683. [Google Scholar] [CrossRef]
- Defosse, T.A.; Sharma, A.; Mondal, A.K.; de Bernonville, T.D.; Latgé, J.P.; Calderone, R.; Giglioli-Guivarc’h, N.; Courdavault, V.; Clastre, M.; Papon, N. Hybrid histidine kinases in pathogenic fungi. Mol. Microbiol. 2015, 95, 914–924. [Google Scholar] [CrossRef]
- Kabbara, S.; Herivaux, A.; De Bernonville, T.D.; Courdavault, V.; Clastre, M.; Gastebois, A.; Osman, M.; Hamze, M.; Mark Cock, J.; Schaap, P.; et al. Diversity and Evolution of Sensor Histidine Kinases in Eukaryotes. Genome Biol. Evol. 2019, 11, 86–108. [Google Scholar] [CrossRef]
- Catlett, N.L.; Yoder, O.C.; Turgeon, B.G. Whole-Genome Analysis of Two-Component Signal Transduction Genes in Fungal Pathogens. Eukaryot. Cell 2003, 2, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Foster, A.J.; Yemelin, A.; Thines, E. Histidine kinases mediate differentiation, stress response, and pathogenicity in Magnaporthe oryzae. MicrobiologyOpen 2014, 3, 668–687. [Google Scholar] [CrossRef] [PubMed]
- Papon, N.; Stock, A.M. Two-component systems. Curr. Biol. 2019, 29, 715–737. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Thines, E. Multistep phosphorelay in fungi: The enigma of multiple signals and a limited number of signaling pathways. Mycol. Prog. 2017, 16, 1007–1013. [Google Scholar] [CrossRef]
- Stock, A.M.; Robinson, V.L.; Goudreau, P.N. Two-Component Signal Transduction. Annu. Rev. Biochem. 2003, 69, 183–215. [Google Scholar] [CrossRef]
- Huo, R.; Liu, Z.; Yu, X.; Li, Z. The Interaction Network and Signaling Specificity of Two-Component System in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 4898. [Google Scholar] [CrossRef]
- Bourret, R.B.; Foster, C.A.; Goldman, W.E. Predicted Functional and Structural Diversity of Receiver Domains in Fungal Two-Component Regulatory Systems. mSphere 2021, 6, e00722-21. [Google Scholar] [CrossRef]
- Nongpiur, R.C.; Gupta, P.; Sharan, A.; Singh, D.; Pareek, A.; Singla-Pareek, S.L.; Nongpiur, R.C.; Gupta, P.; Sharan, A.; Singh, D.; et al. The Two-Component System: Transducing Environmental and Hormonal Signals. In Sensory Biology of Plants; Springer: Berlin/Heidelberg, Germany, 2019; pp. 247–278. [Google Scholar] [CrossRef]
- Alvarez, A.F.; Barba-Ostria, C.; Silva-Jiménez, H.; Georgellis, D. Organization and mode of action of two component system signaling circuits from the various kingdoms of life. Environ. Microbiol. 2016, 18, 3210–3226. [Google Scholar] [CrossRef]
- Liao, B.; Ye, X.; Chen, X.; Zhou, Y.; Cheng, L.; Zhou, X.; Ren, B. The two-component signal transduction system and its regulation in Candida albicans. Virulence 2021, 12, 1884–1899. [Google Scholar] [CrossRef]
- Singh, D.; Gupta, P.; Singla-Pareek, S.L.; Siddique, K.H.M.; Pareek, A. The Journey from Two-Step to Multi-Step Phosphorelay Signaling Systems. Curr. Genom. 2021, 22, 59–74. [Google Scholar] [CrossRef]
- Bersching, K.; Jacob, S. The Molecular Mechanism of Fludioxonil Action Is Different to Osmotic Stress Sensing. J. Fungi 2021, 7, 393. [Google Scholar] [CrossRef]
- Kaur, H.; Sasan, S.P.; Yadav, A.; Martoliya, Y.; Mondal, A.K. Distinct role of HAMP and HAMP-like linker domains in regulating the activity of Hik1p, a hybrid histidine kinase 3 from Magnaporthe oryzae. Mol. Genet. Genom. 2021, 296, 1135–1145. [Google Scholar] [CrossRef]
- Motoyama, T.; Kadokura, K.; Ohira, T.; Ichiishi, A.; Fujimura, M.; Yamaguchi, I.; Kudo, T. A two-component histidine kinase of the rice blast fungus is involved in osmotic stress response and fungicide action. Fungal Genet. Biol. 2005, 42, 200–212. [Google Scholar] [CrossRef]
- Bohnert, S.; Heck, L.; Gruber, C.; Neumann, H.; Distler, U.; Tenzer, S.; Yemelin, A.; Thines, E.; Jacob, S. Fungicide resistance toward fludioxonil conferred by overexpression of the phosphatase gene MoPTP2 in Magnaporthe oryzae. Mol. Microbiol. 2019, 111, 662–677. [Google Scholar] [CrossRef]
- Bohnert, S.; Neumann, H.; Thines, E.; Jacob, S. Visualizing fungicide action: An in vivo tool for rapid validation of fungicides with target location HOG pathway. Pest Manag. Sci. 2019, 75, 772–778. [Google Scholar] [CrossRef]
- El-Mowafy, M.; Bilitewski, U. Identification of possible Ser/Thr/Tyr phosphorylation sites in the fungal histidine kinase CaNik1p by peptide array technique. Bull. Fac. Pharm. Cairo Univ. 2018, 56, 68–72. [Google Scholar] [CrossRef]
- El-Mowafy, M.; Bahgat, M.M.; Bilitewski, U. Deletion of the HAMP domains from the histidine kinase CaNik1p of Candida albicans or treatment with fungicides activates the MAP kinase Hog1p in S. cerevisiae transformants. BMC Microbiol. 2013, 13, 209. [Google Scholar] [CrossRef]
- Mavrianos, J.; Desai, C.; Chauhan, N. Two-component histidine phosphotransfer protein Ypd1 is not essential for viability in Candida albicans. Eukaryot. Cell 2014, 13, 452–460. [Google Scholar] [CrossRef]
- Menon, V.; Li, D.; Chauhan, N.; Rajnarayanan, R.; Dubrovska, A.; West, A.H.; Calderone, R. Functional studies of the Ssk1p response regulator protein of Candida albicans as determined by phenotypic analysis of receiver domain point mutants. Mol. Microbiol. 2006, 62, 997–1013. [Google Scholar] [CrossRef]
- Chauhan, N.; Latge, J.P.; Calderone, R. Signalling and oxidant adaptation in Candida albicans and Aspergillus fumigatus. Nat. Rev. Microbiol. 2006, 4, 435–444. [Google Scholar] [CrossRef]
- Masi, A.; Mach, R.L.; Mach-Aigner, A.R. The pentose phosphate pathway in industrially relevant fungi: Crucial insights for bioprocessing. Appl. Microbiol. Biotechnol. 2021, 105, 4017–4031. [Google Scholar] [CrossRef]
- Corrochano, L.M. Light in the Fungal World: From Photoreception to Gene Transcription and Beyond. Annu. Rev. Genet. 2019, 53, 149–170. [Google Scholar] [CrossRef]
- Day, A.M.; Smith, D.A.; Ikeh, M.A.C.; Haider, M.; Herrero-de-Dios, C.M.; Brown, A.J.P.; Morgan, B.A.; Erwig, L.P.; MacCallum, D.M.; Quinn, J. Blocking two-component signalling enhances Candida albicans virulence and reveals adaptive mechanisms that counteract sustained SAPK activation. PLoS Pathog. 2017, 13, e1006131. [Google Scholar] [CrossRef]
- Bahn, Y.S. Master and commander in fungal pathogens: The two-component system and the HOG signaling pathway. Eukaryot. Cell 2008, 7, 2017–2036. [Google Scholar] [CrossRef]
- Singh, A.; Del Poeta, M. Lipid signalling in pathogenic fungi. Cell. Microbiol. 2011, 13, 177–185. [Google Scholar] [CrossRef]
- Green, C.D.; Maceyka, M.; Cowart, L.A.; Spiegel, S. Sphingolipids in metabolic disease: The good, the bad, and the unknown. Cell Metab. 2021, 33, 1293–1306. [Google Scholar] [CrossRef]
- Luo, X.; Zhao, X.; Cheng, C.; Li, N.; Liu, Y.; Cao, Y. The implications of signaling lipids in cancer metastasis. Exp. Mol. Med. 2018, 50, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bielawski, J.; Pierce, J.S.; Snider, J.; Rembiesa, B.; Szulc, Z.M.; Bielawska, A. Sphingolipid Analysis by High Performance Liquid Chromatography-Tandem Mass Spectrometry (HPLC-MS/MS). In Sphingolipids as Signaling and Regulatory Molecules; Springer: New York, NY, USA, 2010; pp. 46–59. [Google Scholar]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An Overview of Sphingolipid Metabolism: From Synthesis to Breakdown. In Sphingolipids as Signaling and Regulatory Molecules; Springer: New York, NY, USA, 2010; pp. 1–23. [Google Scholar]
- Gómez-Muñoz, A.; Gangoiti, P.; Granado, M.H.; Arana, L.; Ouro, A. Ceramide-1-Phosphate in Cell Survival and Inflammatory Signaling. In Sphingolipids as Signaling and Regulatory Molecules; Springer: New York, NY, USA, 2010; pp. 118–130. [Google Scholar]
- Messner, M.C.; Cabot, M.C. Glucosylceramide in Humans. In Sphingolipids as Signaling and Regulatory Molecules; Springer: New York, NY, USA, 2010; pp. 156–164. [Google Scholar]
- Fox, T.E.; Kester, M. Therapeutic Strategies for Diabetes and Complications: A Role for Sphingolipids? In Sphingolipids as Signaling and Regulatory Molecules; Springer: New York, NY, USA, 2010; pp. 206–216. [Google Scholar]
- Holthuis, J.C.M.; Luberto, C. Tales and Mysteries of the Enigmatic Sphingomyelin Synthase Family. In Sphingolipids as Signaling and Regulatory Molecules; Springer: New York, NY, USA, 2010; pp. 72–85. [Google Scholar]
- Wymann, M.P.; Schneiter, R. Lipid signalling in disease. Nat. Rev. Mol. Cell Biol. 2008, 9, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Ali, U.; Li, H.; Wang, X.; Guo, L. Emerging Roles of Sphingolipid Signaling in Plant Response to Biotic and Abiotic Stresses. Mol. Plant 2018, 11, 1328–1343. [Google Scholar] [CrossRef]
- Lynch, D.V.; Dunn, T.M. An introduction to plant sphingolipids and a review of recent advances in understanding their metabolism and function. New Phytol. 2004, 161, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Alden, K.P.; Dhondt-Cordelier, S.; McDonald, K.L.; Reape, T.J.; Ng, C.K.-Y.; McCabe, P.F.; Leaver, C.J. Sphingolipid long chain base phosphates can regulate apoptotic-like programmed cell death in plants. Biochem. Biophys. Res. Commun. 2011, 410, 574–580. [Google Scholar] [CrossRef]
- Song, P.; Jia, Q.; Chen, L.; Jin, X.; Xiao, X.; Li, L.; Chen, H.; Qu, Y.; Su, Y.; Zhang, W.; et al. Involvement of Arabidopsis phospholipase D δ in regulation of ROS-mediated microtubule organization and stomatal movement upon heat shock. J. Exp. Bot. 2020, 71, 6555–6570. [Google Scholar] [CrossRef]
- Guo, L.; Mishra, G.; Markham, J.E.; Li, M.; Tawfall, A.; Welti, R.; Wang, X. Connections between sphingosine kinase and phospholipase D in the abscisic acid signaling pathway in Arabidopsis. J. Biol. Chem. 2012, 287, 8286–8296. [Google Scholar] [CrossRef]
- Dutilleul, C.; Benhassaine-Kesri, G.; Demandre, C.; Rézé, N.; Launay, A.; Pelletier, S.; Renou, J.; Zachowski, A.; Baudouin, E.; Guillas, I. Phytosphingosine-phosphate is a signal for AtMPK6 activation and Arabidopsis response to chilling. New Phytol. 2012, 194, 181–191. [Google Scholar] [CrossRef]
- Chao, D.Y.; Gable, K.; Chen, M.; Baxter, I.; Dietrich, C.R.; Cahoon, E.B.; Guerinot, M.L.; Lahner, B.; Lü, S.; Markham, J.E.; et al. Sphingolipids in the Root Play an Important Role in Regulating the Leaf Ionome in Arabidopsis thaliana. Plant Cell 2011, 23, 1061–1081. [Google Scholar] [CrossRef]
- Michaelson, L.V.; Napier, J.A.; Molino, D.; Faure, J.D. Plant sphingolipids: Their importance in cellular organization and adaption. Biochim. Biophys. Acta—Mol. Cell Biol. Lipids 2016, 1861, 1329–1335. [Google Scholar] [CrossRef]
- Msanne, J.; Chen, M.; Luttgeharm, K.D.; Bradley, A.M.; Mays, E.S.; Paper, J.M.; Boyle, D.L.; Cahoon, R.E.; Schrick, K.; Cahoon, E.B. Glucosylceramides are critical for cell-type differentiation and organogenesis, but not for cell viability in Arabidopsis. Plant J. 2015, 84, 188–201. [Google Scholar] [CrossRef]
- Lenarčič, T.; Albert, I.; Böhm, H.; Hodnik, V.; Pirc, K.; Zavec, A.B.; Podobnik, M.; Pahovnik, D.; Žagar, E.; Pruitt, R.; et al. Eudicot plant-specific sphingolipids determine host selectivity of microbial NLP cytolysins. Science 2017, 358, 1431–1434. [Google Scholar] [CrossRef]
- Rhome, R.; Del Poeta, M. Sphingolipid Signaling in Fungal Pathogens. Adv. Exp. Med. Biol. 2010, 688, 232–237. [Google Scholar] [CrossRef]
- Rhome, R.; McQuiston, T.; Kechichian, T.; Bielawska, A.; Hennig, M.; Drago, M.; Morace, G.; Luberto, C.; Del Poeta, M. Biosynthesis and immunogenicity of glucosylceramide in Cryptococcus neoformans and other human pathogens. Eukaryot. Cell 2007, 6, 1715–1726. [Google Scholar] [CrossRef]
- Luberto, C.; Toffaletti, D.L.; Wills, E.A.; Tucker, S.C.; Casadevall, A.; Perfect, J.R.; Hannun, Y.A.; Del Poeta, M. Roles for inositol-phosphoryl ceramide synthase 1 (IPC1) in pathogenesis of C. neoformans. Genes Dev. 2001, 15, 201–212. [Google Scholar] [CrossRef]
- Heung, L.J.; Luberto, C.; Plowden, A.; Hannun, Y.A.; Del Poeta, M. The Sphingolipid Pathway Regulates Pkc1 through the Formation of Diacylglycerol in Cryptococcus neoformans. J. Biol. Chem. 2004, 279, 21144–21153. [Google Scholar] [CrossRef]
- Tommasino, N.; Villani, M.; Qureshi, A.; Henry, J.; Luberto, C.; Del Poeta, M. Atf2 transcription factor binds to the APP1 promoter in Cryptococcus neoformans: Stimulatory effect of diacylglycerol. Eukaryot. Cell 2008, 7, 294–301. [Google Scholar] [CrossRef]
- Mare, L.; Iatta, R.; Montagna, M.T.; Luberto, C.; Del Poeta, M. APP1 Transcription Is Regulated by Inositol-phosphorylceramide Synthase 1-Diacylglycerol Pathway and Is Controlled by ATF2 Transcription Factor in Cryptococcus neoformans. J. Biol. Chem. 2005, 280, 36055–36064. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Kraus, P.R.; Boily, M.J.; Heitman, J. Cryptococcus neoformans gene expression during murine macrophage infection. Eukaryot. Cell 2005, 4, 1420–1433. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Shea, J.; Alvarez-Vasquez, F.; Qureshi, A.; Luberto, C.; Voit, E.O.; Del Poeta, M. Mathematical modeling of pathogenicity of Cryptococcus neoformans. Mol. Syst. Biol. 2008, 4, 183. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.J.; Esterhazy, D.; Kim, S.H.; Lemetre, C.; Aguilar, R.R.; Gordon, E.A.; Pickard, A.J.; Cross, J.R.; Emiliano, A.B.; Han, S.M.; et al. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature 2017, 549, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.A.; Schrevens, S.; Van Dijck, P.; Goldman, G.H. Fungal G-protein-coupled receptors: Mediators of pathogenesis and targets for disease control. Nat. Microbiol. 2018, 3, 402–414. [Google Scholar] [CrossRef]
- Bahn, Y.S.; Xue, C.; Idnurm, A.; Rutherford, J.C.; Heitman, J.; Cardenas, M.E. Sensing the environment: Lessons from fungi. Nat. Rev. Microbiol. 2007, 5, 57–69. [Google Scholar] [CrossRef]
- Dos Reis, T.F.; Mellado, L.; Lohmar, J.M.; Silva, L.P.; Zhou, J.J.; Calvo, A.M.; Goldman, G.H.; Brown, N.A. GPCR-mediated glucose sensing system regulates light-dependent fungal development and mycotoxin production. PLoS Genet. 2019, 15, e1008419. [Google Scholar] [CrossRef]
- Bardwell, L. A walk-through of the yeast mating pheromone response pathway. Peptides 2005, 26, 339–350. [Google Scholar] [CrossRef]
- Han, K.-H.; Seo, J.-A.; Yu, J.-H. A putative G protein-coupled receptor negatively controls sexual development in Aspergillus nidulans. Mol. Microbiol. 2004, 51, 1333–1345. [Google Scholar] [CrossRef]
- Lemaire, K.; Van De Velde, S.; Van Dijck, P.; Thevelein, J.M. Glucose and Sucrose Act as Agonist and Mannose as Antagonist Ligands of the G Protein-Coupled Receptor Gpr1 in the Yeast Saccharomyces cerevisiae. Mol. Cell 2004, 16, 293–299. [Google Scholar] [CrossRef]
- Jones, S.K.; Bennett, R.J. Fungal mating pheromones: Choreographing the dating game. Fungal Genet. Biol. 2011, 48, 668–676. [Google Scholar] [CrossRef]
- Xue, C.; Hsueh, Y.P.; Heitman, J. Magnificent seven: Roles of G protein-coupled receptors in extracellular sensing in fungi. FEMS Microbiol. Rev. 2008, 32, 1010–1032. [Google Scholar] [CrossRef]
- Billerbeck, S.; Brisbois, J.; Agmon, N.; Jimenez, M.; Temple, J.; Shen, M.; Boeke, J.D.; Cornish, V.W. A scalable peptide-GPCR language for engineering multicellular communication. Nat. Commun. 2018, 9, 5057. [Google Scholar] [CrossRef]
- Gao, J.; Xu, X.; Huang, K.; Liang, Z. Fungal G-Protein-Coupled Receptors: A Promising Mediator of the Impact of Extracellular Signals on Biosynthesis of Ochratoxin A. Front. Microbiol. 2021, 12, 193. [Google Scholar] [CrossRef]
- Naider, F.; Becker, J.M.; Zerbe, O.; Mcphee, D.J. A Paradigm for Peptide Hormone-GPCR Analyses. Molecules 2020, 25, 4272. [Google Scholar] [CrossRef]
- Velazhahan, V.; Ma, N.; Pándy-Szekeres, G.; Kooistra, A.J.; Lee, Y.; Gloriam, D.E.; Vaidehi, N.; Tate, C.G. Structure of the class D GPCR Ste2 dimer coupled to two G proteins. Nature 2020, 589, 148–153. [Google Scholar] [CrossRef]
- Chen, R.E.; Thorner, J. Function and regulation in MAPK signaling pathways: Lessons learned from the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta—Mol. Cell Res. 2007, 1773, 1311–1340. [Google Scholar] [CrossRef]
- Herskowitz, I. MAP kinase pathways in yeast: For mating and more. Cell 1995, 80, 187–197. [Google Scholar] [CrossRef]
- Krishnan, A.; Almén, M.S.; Fredriksson, R.; Schiöth, H.B. The Origin of GPCRs: Identification of Mammalian like Rhodopsin, Adhesion, Glutamate and Frizzled GPCRs in Fungi. PLoS ONE 2012, 7, e29817. [Google Scholar] [CrossRef]
- Kulkarni, R.D.; Thon, M.R.; Pan, H.; Dean, R.A. Novel G-protein-coupled receptor-like proteins in the plant pathogenic fungus Magnaporthe grisea. Genome Biol. 2005, 6, R24. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Borkovich, K.A. A pheromone receptor gene, pre-1, is essential for mating type-specific directional growth and fusion of trichogynes and female fertility in Neurospora crassa. Mol. Microbiol. 2004, 52, 1781–1798. [Google Scholar] [CrossRef] [PubMed]
- Rolland, F.; Winderickx, J.; Thevelein, J.M. Glucose-sensing and -signalling mechanisms in yeast. FEMS Yeast Res. 2002, 2, 183–201. [Google Scholar] [CrossRef]
- Brown, N.A.; dos Reis, T.F.; Ries, L.N.A.; Caldana, C.; Mah, J.H.; Yu, J.H.; Macdonald, J.M.; Goldman, G.H. G-protein coupled receptor-mediated nutrient sensing and developmental control in Aspergillus nidulans. Mol. Microbiol. 2015, 98, 420–439. [Google Scholar] [CrossRef] [PubMed]
- Maidan, M.M.; De Rop, L.; Serneels, J.; Exler, S.; Rupp, S.; Tournu, H.; Thevelein, J.M.; Van Dijck, P. The G protein-coupled receptor Gpr1 and the Gα protein Gpa2 act through the cAMP-protein kinase A pathway to induce morphogenesis in Candida albicans. Mol. Biol. Cell 2005, 16, 1971–1986. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Borkovich, K.A. GPR-4 is a predicted G-protein-coupled receptor required for carbon source-dependent asexual growth and development in Neurospora crassa. Eukaryot. Cell 2006, 5, 1287–1300. [Google Scholar] [CrossRef] [PubMed]
- Nijland, J.G.; Driessen, A.J.M. Engineering of Pentose Transport in Saccharomyces cerevisiae for Biotechnological Applications. Front. Bioeng. Biotechnol. 2020, 7, 464. [Google Scholar] [CrossRef]
- Scharff-Poulsen, P.; Moriya, H.; Johnston, M. Genetic Analysis of Signal Generation by the Rgt2 Glucose Sensor of Saccharomyces cerevisiae. G3 Genes Genomes Genet. 2018, 8, 2685–2696. [Google Scholar] [CrossRef]
- Bharatula, V.; Broach, J.R. The Nutrient Stress Response in Yeast. In Stress Response Mechanisms in Fungi; Springer: Berlin/Heidelberg, Germany, 2018; pp. 131–159. [Google Scholar] [CrossRef]
- Yu, Z.; Fischer, R. Light sensing and responses in fungi. Nat. Rev. Microbiol. 2018, 17, 25–36. [Google Scholar] [CrossRef]
- Streng, C.; Hartmann, J.; Leister, K.; Krauß, N.; Lamparter, T.; Frankenberg-Dinkel, N.; Weth, F.; Bastmeyer, M.; Yu, Z.; Fischer, R. Fungal phytochrome chromophore biosynthesis at mitochondria. EMBO J. 2021, 40, e108083. [Google Scholar] [CrossRef]
- Igbalajobi, O.; Yu, Z.; Fischer, R. Red-and blue-light sensing in the plant pathogen alternaria alternata depends on phytochrome and the white-collar protein LreA. mBio 2019, 10, e00371-19. [Google Scholar] [CrossRef]
- Krobanan, K.; Liang, S.W.; Chiu, H.C.; Shen, W.C. The blue-light photoreceptor SfWC-1 gene regulates the phototropic response and fruiting-body development in the homothallic ascomycete Sordaria fimicola. Appl. Environ. Microbiol. 2019, 85, e02206-18. [Google Scholar] [CrossRef]
- García-Martínez, J.; Brunk, M.; Avalos, J.; Terpitz, U. The CarO rhodopsin of the fungus Fusarium fujikuroi is a light-driven proton pump that retards spore germination. Sci. Rep. 2015, 5, 7798. [Google Scholar] [CrossRef]
- Bayram, Ö.; Feussner, K.; Dumkow, M.; Herrfurth, C.; Feussner, I.; Braus, G.H. Changes of global gene expression and secondary metabolite accumulation during light-dependent Aspergillus nidulans development. Fungal Genet. Biol. 2016, 87, 30–53. [Google Scholar] [CrossRef]
- Yu, Z.; Armant, O.; Fischer, R. Fungi use the SakA (HogA) pathway for phytochrome-dependent light signalling. Nat. Microbiol. 2016, 1, 16019. [Google Scholar] [CrossRef]
- Fuller, K.K.; Ringelberg, C.S.; Loros, J.J.; Dunlap, J.C. The fungal pathogen Aspergillus fumigatus regulates growth, metabolism, and stress resistance in response to light. mBio 2013, 4, e00142-13. [Google Scholar] [CrossRef]
- Ruger-Herreros, C.; Rodríguez-Romero, J.; Fernández-Barranco, R.; Olmedo, M.; Fischer, R.; Corrochano, L.M.; Canovas, D. Regulation of Conidiation by Light in Aspergillus nidulans. Genetics 2011, 188, 809–822. [Google Scholar] [CrossRef]
- Baek, M.; Virgilio, S.; Lamb, T.M.; Ibarra, O.; Andrade, J.M.; Gonçalves, R.D.; Dovzhenok, A.; Lim, S.; Bell-Pedersen, D.; Bertolini, M.C.; et al. Circadian clock regulation of the glycogen synthase (gsn) gene by WCC is critical for rhythmic glycogen metabolism in Neurospora crassa. Proc. Natl. Acad. Sci. USA 2019, 116, 10435–10440. [Google Scholar] [CrossRef]
- Froehlich, A.C.; Liu, Y.; Loros, J.J.; Dunlap, J.C. White collar-1, a circadian blue light photoreceptor, binding to the frequency promoter. Science 2002, 297, 815–819. [Google Scholar] [CrossRef]
- Wang, B.; Kettenbach, A.N.; Zhou, X.; Loros, J.J.; Dunlap, J.C. The Phospho-Code Determining Circadian Feedback Loop Closure and Output in Neurospora. Mol. Cell 2019, 74, 771–784.e3. [Google Scholar] [CrossRef]
- Liu, Y.; Bell-Pedersen, D. Circadian rhythms in Neurospora crassa and other filamentous fungi. Eukaryot. Cell 2006, 5, 1184–1193. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, X.; Loros, J.J.; Dunlap, J.C. Alternative Use of DNA Binding Domains by the Neurospora White Collar Complex Dictates Circadian Regulation and Light Responses. Mol. Cell. Biol. 2016, 36, 781–793. [Google Scholar] [CrossRef]
- Losi, A.; Gärtner, W. A light life together: Photosensing in the plant microbiota. Photochem. Photobiol. Sci. 2021, 20, 451–473. [Google Scholar] [CrossRef]
- Brenna, A.; Talora, C. WC-1 and the Proximal GATA Sequence Mediate a Cis-/Trans-Acting Repressive Regulation of Light-Dependent Gene Transcription in the Dark. Int. J. Mol. Sci. 2019, 20, 2854. [Google Scholar] [CrossRef]
- Ballario, P.; Talora, C.; Galli, D.; Linden, H.; Macino, G. Roles in dimerization and blue light photoresponse of the PAS and LOV domains of Neurospora crassa white collar proteins. Mol. Microbiol. 1998, 29, 719–729. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, F.; Yang, Y.; Wang, Y.; Dong, C. CmVVD is involved in fruiting body development and carotenoid production and the transcriptional linkage among three blue-light receptors in edible fungus Cordyceps militaris. Environ. Microbiol. 2020, 22, 466–482. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Yuan, Y.; Li, R.; Zhang, X.; Xin, Q. Structure prediction and function characterization of WC-2 proteins in Blakeslea trispora. Int. Microbiol. 2021, 24, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, B.; Coiro, P.; Filetici, P.; Berge, E.; Dobosy, J.R.; Freitag, M.; Selker, E.U.; Ballario, P. The Neurospora crassa white collar-1 dependent blue light response requires acetylation of histone H3 lysine 14 by NGF-1. Mol. Biol. Cell 2006, 17, 4576–4583. [Google Scholar] [CrossRef] [PubMed]
- Brenna, A.; Grimaldi, B.; Filetici, P.; Ballario, P. Physical association of the WC-1 photoreceptor and the histone acetyltransferase NGF-1 is required for blue light signal transduction in Neurospora crassa. Mol. Biol. Cell 2012, 23, 3863–3872. [Google Scholar] [CrossRef]
- Xin, G.; Tian-qi, C.; Xing-wang, L.; Lu-han, X.; Qi, X. Research Progress on Blue-light Photoreceptors in Zygomyceta. Biotechnol. Bull. 2018, 34, 43. [Google Scholar] [CrossRef]
- Liversage, J.; Coetzee, M.P.A.; Bluhm, B.H.; Berger, D.K.; Crampton, B.G. LOVe across kingdoms: Blue light perception vital for growth and development in plant–fungal interactions. Fungal Biol. Rev. 2018, 32, 86–103. [Google Scholar] [CrossRef]
- Qi, Y.; Sun, X.; Ma, L.; Wen, Q.; Qiu, L.; Shen, J. Identification of two Pleurotus ostreatus blue light receptor genes (PoWC-1 and PoWC-2) and in vivo confirmation of complex PoWC-12 formation through yeast two hybrid system. Fungal Biol. 2020, 124, 8–14. [Google Scholar] [CrossRef]
- Salichos, L.; Rokas, A. The diversity and evolution of circadian clock proteins in fungi. Mycologia 2017, 102, 269–278. [Google Scholar] [CrossRef]
- Dasgupta, A.; Fuller, K.K.; Dunlap, J.C.; Loros, J.J. Seeing the world differently: Variability in the photosensory mechanisms of two model fungi. Environ. Microbiol. 2016, 18, 5–20. [Google Scholar] [CrossRef]
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular Mechanisms of Chitosan Interactions with Fungi and Plants. Int. J. Mol. Sci. 2019, 20, 332. [Google Scholar] [CrossRef]
- Zhao, Y.; Lim, J.; Xu, J.; Yu, J.H.; Zheng, W. Nitric oxide as a developmental and metabolic signal in filamentous fungi. Mol. Microbiol. 2020, 113, 872–882. [Google Scholar] [CrossRef]
- Gupta, M.; Outten, C.E. Iron–sulfur cluster signaling: The common thread in fungal iron regulation. Curr. Opin. Chem. Biol. 2020, 55, 189–201. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacob, S.; Bühring, S.; Bersching, K. Recent Advances in Research on Molecular Mechanisms of Fungal Signaling. Encyclopedia 2022, 2, 840-863. https://doi.org/10.3390/encyclopedia2020055
Jacob S, Bühring S, Bersching K. Recent Advances in Research on Molecular Mechanisms of Fungal Signaling. Encyclopedia. 2022; 2(2):840-863. https://doi.org/10.3390/encyclopedia2020055
Chicago/Turabian StyleJacob, Stefan, Sri Bühring, and Katharina Bersching. 2022. "Recent Advances in Research on Molecular Mechanisms of Fungal Signaling" Encyclopedia 2, no. 2: 840-863. https://doi.org/10.3390/encyclopedia2020055
APA StyleJacob, S., Bühring, S., & Bersching, K. (2022). Recent Advances in Research on Molecular Mechanisms of Fungal Signaling. Encyclopedia, 2(2), 840-863. https://doi.org/10.3390/encyclopedia2020055





