Oxidation of Antipsychotics
Definition
:1. Introduction
- -
- Nutritional status;
- -
- Hormonal status;
- -
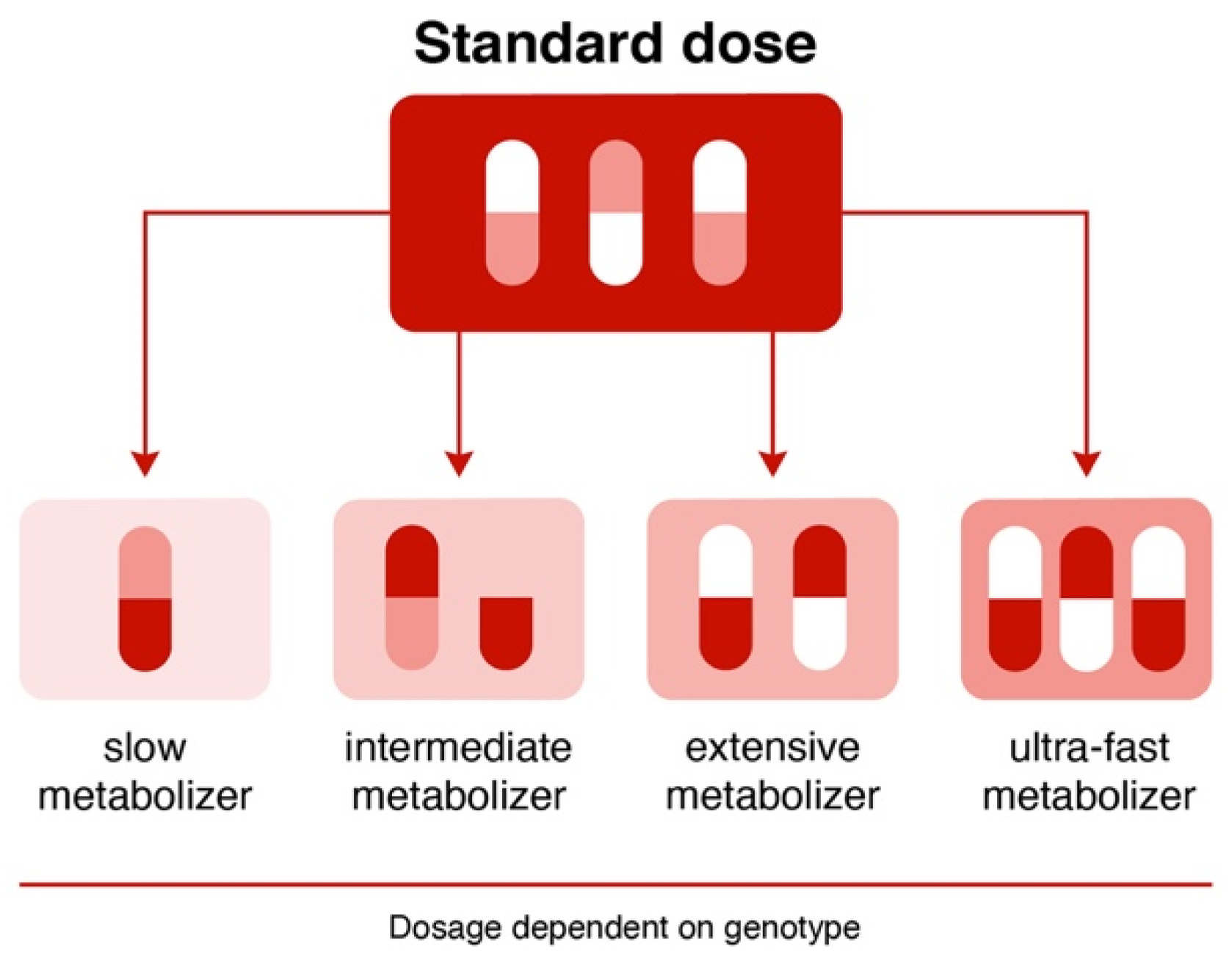
- Genetic factors;
- -
- Previous therapy with Aps or other classes of drugs;
- -
- Concomitant somatic, neurological, or mental status (for example, diseases of the cardiovascular and respiratory systems may decrease biotransformation, etc.);
- -
- I age of the patient (for example, very old patients or children often have a greater sensitivity to APs, due in part to the involutional or immature state of the enzyme systems by which APs are metabolized);
- -
- Functional state of the liver [11].
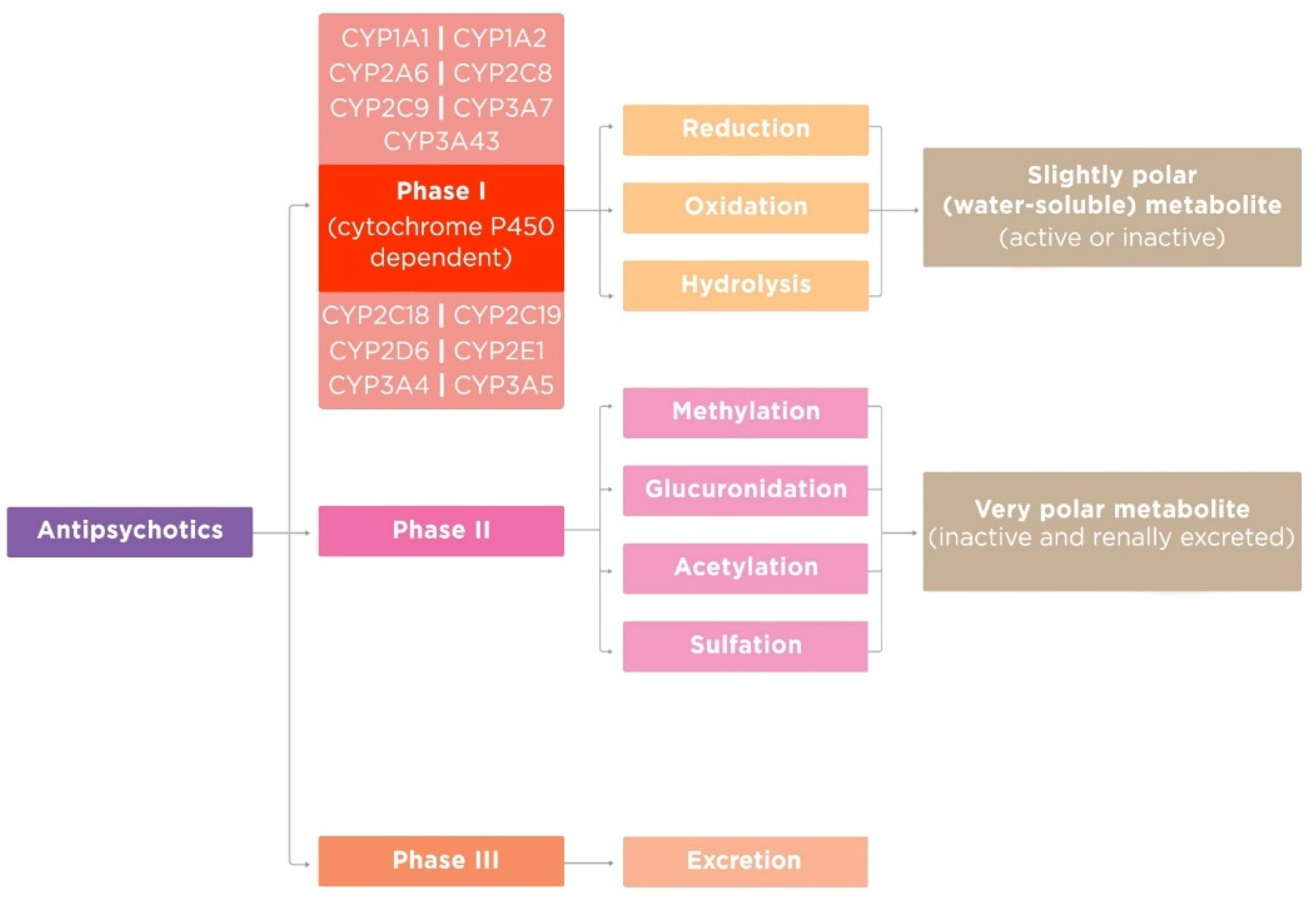
2. Phases of Antipsychotic Metabolism
2.1. Phase I of Antipsychotic Metabolism
- -
- Oxidation;
- -
- Hydrolysis;
- -
- Reduction.
- -
- Other oxidations;
- -
- Dealkylation;
- -
- Deamination;
- -
- Sulfoxidation;
- -
- Oxidation [18].
2.2. Phase II of Antipsychotic Metabolism
- -
- Acetylases;
- -
- Glucuronyl transferase;
- -
- Transacylases;
- -
- Sulfotransferase;
- -
- Ethylase;
- -
- Glutathione transferase;
- -
2.3. Phase III of Antipsychotic Metabolism
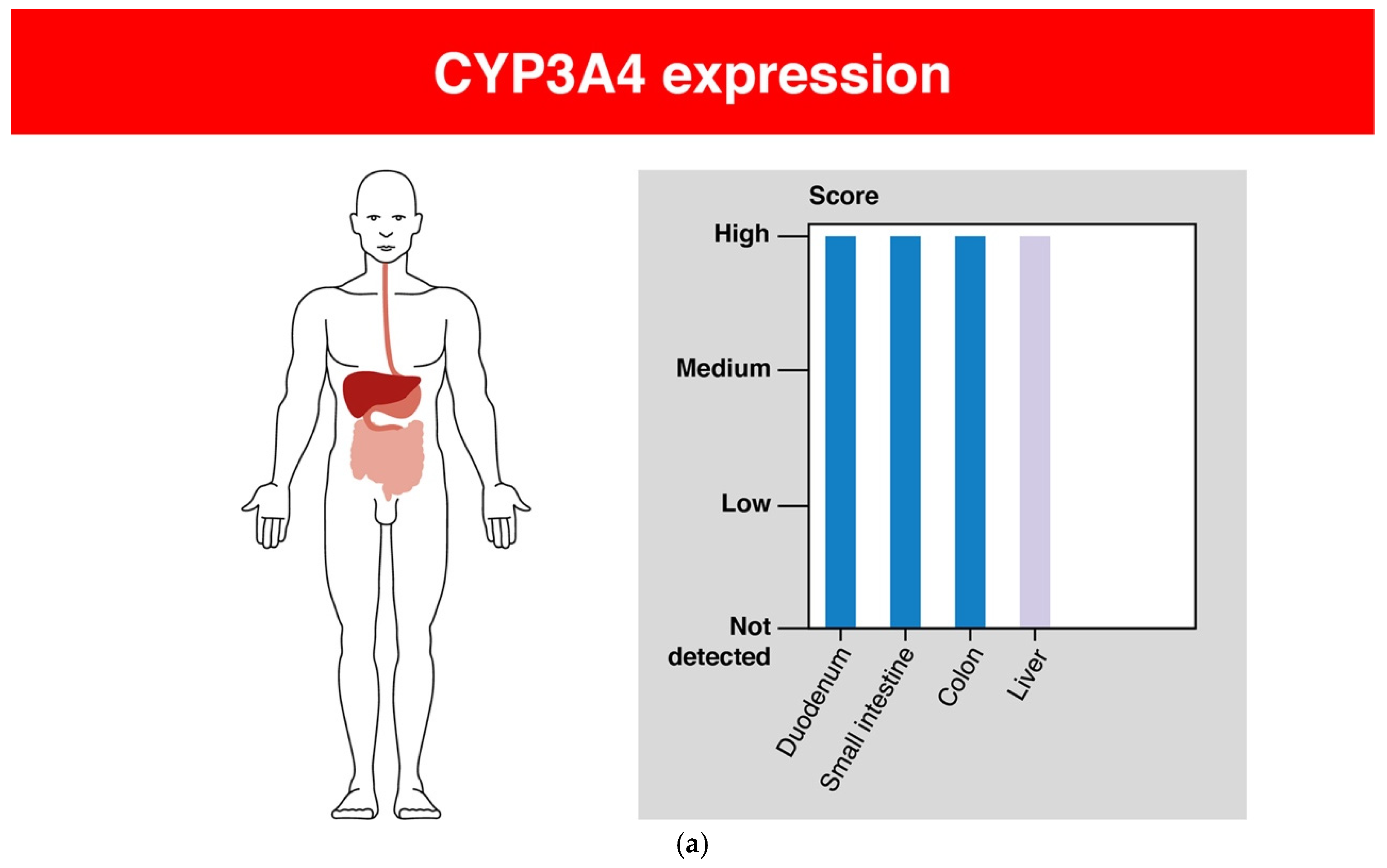
3. Oxidation of Antipsychotics in the Liver
4. Function of Enzymes of Cytochrome P450 and Antipsychotic Metabolism
| Antipsychotics | Other Drugs | ||||
|---|---|---|---|---|---|
| Substrates | Inhibitors | Inducers | Substrates | Inhibitors | Inducers |
| CYP1A1 | |||||
| Partly: Haloperidol Olanzapine Perospirone | Clozapine | Acetaminophen Amiodarone R-Warfarin | Propofol | ||
| CYP1A2 | |||||
| Primary: | Promazine | Acetaminophen | Amiodarone | Carbamazepine | |
| Asenapine | Remoxipride | Alosetron | Cimetidine | Insulin | |
| Clozapine | Caffeine | Ciprofloxacin | Modafinil | ||
| Loxapine | Clomipramine | Citalopram | Nafcillin | ||
| Olanzapine | Duloxetine | Efavirenz | Omeprazole | ||
| Pimozide | Imipramine | Fluvoxamine | Rifampin | ||
| Thiothixene | Melatonin | Ribociclib | Rucaparib | ||
| Trifluoperazine | Mexiletine | Teriflunomide | |||
| Naproxen | Tobacco | ||||
| Partly: | Pirfenidone | ||||
| Chlorpromazine | Theophylline | ||||
| Haloperidol | Tizanidine | ||||
| Lumateperone | |||||
| Perphenazine | |||||
| Promazine | |||||
| Quetiapine | |||||
| Thioridazine Zotepine | |||||
| CYP2A6 | |||||
| Partly: | Cotinine | Methoxsalen | |||
| Clozapine | Nicotine | Pilocarpine | |||
| Promazine | Pilocarpine | Tryptamine | |||
| CYP2C8 | |||||
| Partly: | Amodiaquine | Abiraterone | |||
| Clozapine | Cerivastatin | Clopidogrel | |||
| Lumateperone | Dabrafenib | Deferasirox | |||
| Perospirone | Enzalutamide | Glitazones | |||
| Perphenazine | Olodaterol | Letermovir | |||
| Paclitaxel | Montelukast | ||||
| CYP2C9 | |||||
| Partly: | Amitriptyline | Amiodarone | Bosentan | ||
| Clozapine | Azilsartan | Capecitabine | Carbamazepine | ||
| Haloperidol | Capecitabine | Ceritinib | Enzalutamide | ||
| Olanzapine | Celecoxib | Efavirenz | Nevirapine | ||
| Perphenazine | Clopidogrel | Fenofibrate | Peginterferon alfa-2b | ||
| Promazine | Diclofenac | Fluconazole | Phenobarbital | ||
| Doxepin | Fluvastatin | Rifampin | |||
| Fluoxetine | Fluvoxamine | St. John’s wort | |||
| Fluvastatin | Isoniazid | Tocilizumab | |||
| CYP2C18 | |||||
| Partly: | Mephenytoin | Rifampicin | |||
| Perphenazine | Warfarin | ||||
| CYP2C19 | |||||
| Partly: | Clozapine | Amitriptyline | Armodafinil | Carbamazepine | |
| Clozapine | Olanzapine | Atomoxetine | Cetocanazole | Efavirenz | |
| Haloperidol | Brivaracetam | Cimetidine | Letermovir | ||
| Perphenazine | Carisoprodol | Citalopram | Prednisone | ||
| Promazine | Citalopram | Esomeprazole | Rifampicin | ||
| Pipotiazine | Clobazam | Felbamate | Ritonavir | ||
| Quetiapine | Clomipramine | Fluoxetine | St. John’s wort | ||
| Risperidone | Clopidogrel | Fluvoxamine | |||
| Thioridazine | Diazepam | Isoniazid | |||
| Doxepin | Modafinil | ||||
| Escitalopram | |||||
| CYP2D6 | |||||
| Primary: | Amoxapine | Atomoxetine | Amiodarone | Dexamethasone | |
| Aripiprazole Brexpiprazole Chlorpromazine Fluphenazine Haloperidol Iloperidone Loxapine Perphenazine Pimozide Risperidone Thioridazine Partly: Alimemazine Amoxapine Aripiprazole lauroxil Azenapine Cariprazine Clozapine Clozapine Flupentixol Levomepromazine Mesoridazine Methotrimeprazin Olanzapine Paliperidone Perospirone Pipotiazine Prochlorperazine Promazine Quetiapine Remoxipride Sertindol Trifluperazine Zuclopenthixol | Chlorpromazine Clozapine Fluphenazine Haloperidol Melperone Methotrimeprazine Olanzapine Perphenazine Pimozide Pipotiazine Risperidone Thioridazine Thiothixene | Carvedilol Citalopram Clomipramine Debrisoquine Desipramine Dexfenfluramine Dextromethorphan Donepezil Doxepin Duloxetine Letermovir Lidocaine Metoprolol Nebivolol Perphenazine Propranolol | Bupropion Celecoxib Cimetidine Clobazam Clomipramine Doxepin Duloxetine Fluoxetine Hydroxyzine Methadone Metoclopramide Paroxetine Quinidine Ritonavir Sertraline | Oritavancin Rifampin | |
| CYP2E1 | |||||
| Partly: | Fluphenazine | Acetaminopher | Disulfiram | Ethanol | |
| Clozapine Iloperidone | Methotrimeprazine Thioridazine | Aniline Chlorzoxazone Enflurane Ethanol | Quercetin Ribociclib | Isoniazid | |
| CYP3A4 | |||||
| Primary: Aripiprazole Brexpiprazole Cariprazine Haloperidol Loxapine Lumateperone Lurasidone Perphenazine Pimavanserin Pimozide Quetiapine Ziprasidone Partly: Alimemazine Asenapine Clozapine Fluspirilene Iloperidone Paliperidone Penfluridol Perospirone Pipotiazine Promazine Risperidone Sertindol Zotepine Zuclopenthixol | Clozapine Haloperidol Olanzapine Remoxipride | Chlorpromazine Clozapine | Alprazolam Bupivacaine Buspirone Disopyramide Eszopiclone Etonogestrel Flunisolide Grepafloxacin Indinavir Pantoprazole Ranolazine Terfenadine Voriconazole Zolpidem | Betamethasone Fluconazole Loratadine Quinine Voriconazole | Betamethasone Quinine Rifabutin Rofecoxib |
| CYP3A5 | |||||
| Primary: Aripiprazole Aripiprazole lauroxil Clozapine Haloperidol Iloperidone Olanzapine Paliperidone Partly: Pimavanserin Pimozide Quetiapine Risperidone | Remoxipride Reserpine | Clopidogrel Cyclosporine Indinavir Phenytoin Saquinavir Verapamil | Indinavir Saquinavir Verapamil | Carbamazepine Dexamethasone Phenytoin | |
| CYP3A7 | |||||
| Partly: Aripiprazole Aripiprazole lauroxil Haloperidol Iloperidone Pimozide Quetiapine | Remoxipride | Alprazolam Astemizole Diazepam Triazolam | Erythromycin Nelfinavir Saquinavir | Dexamethasone Phenytoin Triamcinolone | |
| CYP3A43 | |||||
| Olanzapine | Daclatasvir | Cobicistat | Dexamethasone | ||
| Remoxipride | Testosterone | Idelalisib | Rifampicin | ||
5. Discussion of Prospects for Translation of Basic Research into Real Clinical Practice
6. Limitation
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Finkel, R.; Clark, M.A.; Cubeddu, L.X. Pharmacology, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; p. 151. [Google Scholar]
- Lally, J.; MacCabe, J.H. Antipsychotic medication in schizophrenia: A review. Br. Med. Bull. 2015, 114, 1–179. [Google Scholar] [CrossRef] [PubMed]
- Grande, I.; Berk, M.; Birmaher, B.; Vieta, E. Bipolar disorder. Lancet 2016, 387, 1561–1572. [Google Scholar] [CrossRef]
- Caroff, S.N.; Hurford, I.; Lybrand, J.; Campbell, E.C. Movement Disorders Induced by Antipsychotic Drugs: Implications of the CATIE Schizophrenia Trial. Neurol. Clin. 2011, 29, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Sadock, B.J.; Sadock, V.A.; Ruiz, P. Kaplan and Sadock’s Comprehensive Textbook of Psychiatry, 9th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2009; pp. 4113–4119. [Google Scholar]
- Meltzer, H.Y. Update on typical and atypical antipsychotic drugs. An. Rev. Med. 2013, 64, 393–406. [Google Scholar] [CrossRef]
- Sheehan, J.J.; Sliwa, J.K.; Amatniek, J.C.; Grinspan, A.; Canuso, C.M. Atypical antipsychotic metabolism and excretion. Curr. Drug Metab. 2010, 11, 516–525. [Google Scholar] [CrossRef]
- Rourke, J.L.; Sinal, C.J. Biotransformation/Metabolism. Encycl. Toxicol. 2014, 1, 490–502. [Google Scholar]
- Shanu-Wilson, J.; Evans, L.; Wrigley, S.; Steele, J.; Atherton, J.; Boer, J. Biotransformation: Impact and Application of Metabolism in Drug Discovery. ACS Med. Chem. Lett. 2020, 11, 2087–2107. [Google Scholar] [CrossRef]
- Shen, W.W. The Metabolism of Atypical Antipsychotic Drugs: An Update. Ann. Clin. Psychiatry 1999, 11, 145–158. [Google Scholar] [CrossRef]
- Correia, M.A. Drug biotransformation. In Basic & Clinical Pharmacology, 14th ed.; Katzung, B.G., Ed.; McGraw Hill Education: New York, NY, USA, 2017; Volume 1, pp. 56–74. [Google Scholar]
- Josephy, D.P.; Guengerich, P.F.; Miners, J.O. “Phase I and Phase II” drug metabolism: Terminology that we should phase out? Drug Metab. Rev. 2005, 37, 575–580. [Google Scholar] [CrossRef]
- De Bruyn Kops, C.; Šícho, M.; Mazzolari, A.; Kirchmair, J. GLORYx: Prediction of the Metabolites Resulting from Phase 1 and Phase 2 Biotransformations of Xenobiotics. Chem. Res. Toxicol. 2021, 34, 286–299. [Google Scholar] [CrossRef]
- Guengerich, F.P. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem. Res. Toxicol. 2001, 14, 611–650. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, K. Drug Metabolism. Pharmacology 2009, 8, 131–173. [Google Scholar]
- Beedham, C. The role of non-P450 enzymes in drug oxidation. Pharm. World Sci. 1997, 19, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, P.; Nagini, S. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Curr. Drug Targets 2018, 19, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Danielson, P.B. The cytochrome P450 superfamily: Biochemistry, evolution and drug metabolism in humans. Curr. Drug Metab. 2002, 3, 561–597. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.V.; Flück, C.E. NADPH P450 oxidoreductase: Structure, function, and pathology of diseases. Pharmacol. Ther. 2013, 138, 229–254. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.; Møller, B.L. Plant NADPH-cytochrome P450 oxidoreductases. Phytochemistry 2010, 71, 132–141. [Google Scholar] [CrossRef]
- Klein, M.T.; Torry, L.A.; Wu, B.C.; Townsend, S.H.; Paspek, S.C. Hydrolysis in supercritical water: Solvent effects as a probe of the reaction mechanism. J. Supercrit. Fluids 1990, 3, 222–227. [Google Scholar] [CrossRef]
- Jancova, P.; Anzenbacher, P.; Anzenbacherova, E. Phase II drug metabolizing enzymes. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2010, 154, 103–116. [Google Scholar] [CrossRef]
- Jancova, P.; Siller, M. Phase II Drug Metabolism. Top. Drug Metab. 2012, 35–60. [Google Scholar]
- McCarver, D.G. The Ontogeny of Human Drug-Metabolizing Enzymes: Phase II Conjugation Enzymes and Regulatory Mechanisms. J. Pharmacol. Exp. Ther. 2002, 300, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Al-Zoughool, M.; Talaska, G. 4-Aminobiphenyl N-glucuronidation by liver microsomes: Optimization of the reaction conditions and characterization of the UDP-glucoronosyltransferase isoforms. J. Appl. Toxicol. 2006, 26, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, G.C.; Loose, D.S. Pharmacology, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 12–15. [Google Scholar]
- Omiecinski, C.J.; Vanden Heuvel, J.P.; Perdew, G.H.; Peters, J.M. Xenobiotic Metabolism, Disposition, and Regulation by Receptors: From Biochemical Phenomenon to Predictors of Major Toxicities. Toxicol. Sci. 2010, 120, S49–S75. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.R. Cytochrome P450 diversity in the tree of life. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 141–154. [Google Scholar] [CrossRef]
- Nasyrova, R.F.; Neznanov, N.G. Pharmacogenetics of antipsychotics. In Clinical Psychopharmacogenetics; Nasyrova, R.F., Kravtsov, V.V., Dobrodeeva, V.S., Schneider, N.A., Neznanov, N.G., Eds.; DEAN: Saint Petersburg, Russia, 2019; pp. 93–174. (In Russian) [Google Scholar]
- Uno, Y.; Iwasaki, K.; Yamazaki, H.; Nelson, D.R. Macaque cytochromes P450: Nomenclature, transcript, gene, genomic structure, and function. Drug Metab. Rev. 2011, 43, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.P.; Laszlo, K. Rapid Review Pharmacology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 7–9. [Google Scholar]
- Nelson, D.R. Cytochrome P450 Homepage. Hum. Genom. 2009, 4, 59–65. [Google Scholar] [CrossRef]
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ (accessed on 15 January 2022).
- Guengerich, F.P. Mechanisms of Cytochrome P450-Catalyzed Oxidations. ACS Catal. 2018, 7, 10964–10976. [Google Scholar] [CrossRef]
- Rendic, S.; Di Carlo, F.J. Human cytochrome P450 enzymes: A status report summarizing their reactions, substrates, inducers, and inhibitors. Drug Metab. Rev. 1997, 29, 413–580. [Google Scholar] [CrossRef]
- Le, T.; Bhushan, V.; Sochat, M.; Vaidyanathan, V.; Schimansky, S.; Abrams, J.; Kallianos, K. First Aid for the USMLE Step 1, 30th ed.; McGraw Hill Education: New York, NY, USA, 2020; pp. 230, 252. [Google Scholar]
- Drugbak Online. Available online: https://go.drugbank.com/ (accessed on 18 January 2022).
- Hukkanen, J. Induction of cytochrome P450 enzymes: A view on human in vivo findings. Expert Rev. Clin. Pharmacol. 2012, 5, 569–585. [Google Scholar] [CrossRef]
- Drug Interactions Flockhart Table. Available online: https://drug-interactions.medicine.iu.edu/MainTable.aspx (accessed on 18 January 2022).
- Eshmuminov, D.; Leoni, F.; Schneider, M.A.; Becker, D.; Muller, X.; Onder, C.; Hefti, M.; Schuler, M.J.; Dutkowski, P.; Graf, R.; et al. Perfusion settings and additives in liver normothermic machine perfusion with red blood cells as oxygen carrier. A systematic review of human and porcine perfusion protocols. Transpl. Int. 2018, 31, 956–969. [Google Scholar] [CrossRef]
- Pond, S.M.; Tozer, T.N. First-pass elimination basic concepts and clinical consequences. Clin. Pharmacokinet. 1984, 9, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Rowland, M.; Benet, L.Z.; Graham, G.G. Clearance concepts in pharmacokinetics. J. Pharmacokinet. Biopharm. 1973, 1, 123–136. [Google Scholar] [CrossRef] [PubMed]
- First Pass Effect. StatPearls. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551679/ (accessed on 21 February 2022).
- Kaplan Medical. Pharmacokinetics. In USMLE Step 1 Lecture Notes 2019: Pharmacology; Davis, C., Harris, S.R., Eds.; Kaplan Publishing: Berkshire, UK, 2019; Volume 1, p. 7. [Google Scholar]
- Inger, J.; Mangus, I.S. Genetic polymorphism and toxicology--With emphasis on cytochrome p450. Toxicol. Sci. 2011, 120, 1–13. [Google Scholar]
- Medsafe: New Zealand Medicines and Medical Devices Safety Authority. Drug Metabolism–The Importance of Cytochrome P450 3A4. Available online: https://www.medsafe.govt.nz/profs/puarticles/march2014drugmetabolismcytochromep4503a4.htm (accessed on 22 April 2022).
- Werk, A.N.; Cascorbi, I. Functional gene variants of CYP3A4. Clin. Pharmacol. Ther. 2014, 96, 340–348. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Drug Development and Drug Interactions: Table of Substrates, Inhibitors and Inducers. Available online: https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers (accessed on 20 January 2022).
- US Food and Drug Administration. Clinical Drug Interaction Studies—Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions Guidance for Industry, January 2020. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-drug-interaction-studies-cytochrome-p450-enzyme-and-transporter-mediated-drug-interactions (accessed on 20 January 2022).
- Fluphenazine: Drug Information. Available online: https://www.uptodate.com/contents/fluphenazine-drug-information?topicRef=14773&source=see_link (accessed on 20 January 2022).
- Pimozide: Drug Information. Available online: https://www.uptodate.com/contents/pimozide-drug-information?topi69cRef=14773&source=see_link (accessed on 20 January 2022).
- Stimmel, G.L.; Falloon, I.R. Chlorpromazine plasma levels, adverse effects, and tobacco smoking: Case report. Clin. Psychiatry 1998, 44, 420. [Google Scholar]
- Pantuck, E.J.; Pantuck, C.B.; Anderson, K.E.; Conney, A.H.; Kappas, A. Cigarette smoking and chlorpromazine disposition and actions. Clin. Pharmacol. Ther. 1982, 31, 533–538. [Google Scholar] [CrossRef]
- Ereshefsky, L.; Saklad, S.R.; Watanabe, M.D.; Davis, C.M.; Jann, M.W. Thiothixene pharmacokinetic interactions: A study of hepatic enzyme inducers, clearance inhibitors, and demographic variables. J. Clin. Psychopharmacol. 1991, 11, 296. [Google Scholar] [CrossRef]
- Aripiprazole (Oral and Long-Acting Injectable [Abilify Maintena]): Drug Information. Available online: https://www.uptodate.com/contents/aripiprazole-oral-and-long-acting-injectable-abilify-maintena-drug-information?topicRef=14776&source=see_link (accessed on 20 January 2022).
- Asenapine: Drug Information. Available online: https://www.uptodate.com/contents/asenapine-drug-information?topicRef=14776&source=see_link (accessed on 20 January 2022).
- Rexulti (Brexpiprazole): Otsuka America Pharmaceutical, Inc. 2015. Available online: http://www.otsuka-us.com/Products/Documents/Rexulti.PI.pdf (accessed on 20 January 2022).
- Citrome, L.; Stensbøl, T.B.; Maeda, K. The preclinical profile of brexpiprazole: What is its clinical relevance for the treatment of psychiatric disorders? Expert Rev. Neurother. 2015, 15, 1219–1229. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration Safety Communication: Vraylar Package Insert. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/204370lbl.pdf (accessed on 20 January 2022).
- Clozaril (Clozapine): Novartis, Inc. 2014. Available online: https://www.pharma.us.novartis.com/product/pi/pdf/Clozaril.pdf (accessed on 20 January 2022).
- Clozapine-Clozapine Tablet. Remedyrepack Inc. Highlights of Prescribing Information. Available online: https://dailymed.nlm.nih.gov/dailymed/fda/fdadrugxsl.cfm?setid=4bb4e035-7dbc-4582-8e46-3f128f6c2790&type=display (accessed on 22 April 2022).
- Anderson, G.D.; Chan, L.N. Pharmacokinetic Drug Interactions with Tobacco, Cannabinoids and Smoking Cessation Products. Clin. Pharmacokinet. 2016, 55, 1353–1368. [Google Scholar] [CrossRef]
- Fanapt (Iloperidone): Vanda Pharmaceutical, Inc. 2014. Available online: https://www.fanapt.com/product/pi/pdf/fanapt.pdf (accessed on 20 January 2022).
- Lumateperone: Drug Information. Available online: https://www.uptodate.com/contents/lumateperone-drug-information?topicRef=14776&source=see_link (accessed on 20 January 2022).
- Latuda (Lurasidone): Sunovion Pharmaceuticals, Inc. 2013. Available online: http://www.latuda.com/LatudaPrescribingInformation.pdf (accessed on 20 January 2022).
- Zyprexa (Olanzapine). Eli Lilly and Company, Inc. 2015. Available online: http://pi.lilly.com/us/zyprexa-pi.pdf (accessed on 20 January 2022).
- Invega (Paliperidone): Janssen Pharmaceuticals, Inc. 2014. Available online: http://www.invega.com/prescribing-information (accessed on 20 January 2022).
- Nuplazid (Pimavanserin): Full Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/207318lbl.pdf (accessed on 20 January 2022).
- Seroquel (Quetiapine): AstraZeneca 2013. Available online: http://www1.astrazeneca-us.com/pi/seroquel.pdf (accessed on 20 January 2022).
- Seroquel XR (Quetiapine Extended Release): AstraZeneca 2013. Available online: http://www.azpicentral.com/seroquel-xr/seroquelxr.pdf#page=1 (accessed on 20 January 2022).
- Risperdal (Risperidone): Janssen Pharmaceuticals, Inc. 2014. Available online: http://www.janssenpharmaceuticalsinc.com/assets/risperdal.pdf (accessed on 20 January 2022).
- Geodon (Ziprasidone): Pfizer, Inc. 2014. Available online: http://labeling.pfizer.com/ShowLabeling.aspx?id=584 (accessed on 20 January 2022).
- Javaid, J.I. Clinical pharmacokinetics of antipsychotics. J. Clin. Pharmacol. 1994, 34, 286–295. [Google Scholar] [CrossRef]
- Belle, D.J.; Singh, H. Genetic factors in drug metabolism. Am. Fam. Physician 2008, 77, 1553–1560. [Google Scholar] [PubMed]
- Pouget, J.G.; Shams, T.A.; Tiwari, A.K.; Muller, D.J. Pharmacogenetics and outcome with antipsychotic drugs. Dialogues Clin. Neurosci. 2014, 16, 555–566. [Google Scholar] [CrossRef] [PubMed]
- GeneSight Test. Available online: https://genesight.com/product/ (accessed on 22 April 2022).
- Genesight Test. Available online: https://genesight.com/ (accessed on 20 January 2022).
- Genesept Assay. Available online: https://www.dynacare.ca/corporate-clients/wellness-featured-services/mental-health-solution.aspx (accessed on 20 January 2022).
- The Human Protein Atlas: CYP1A1. Available online: https://www.proteinatlas.org/ENSG00000140465-CYP1A1/tissue (accessed on 20 January 2022).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shnayder, N.A.; Abdyrakhmanova, A.K.; Nasyrova, R.F. Oxidation of Antipsychotics. Encyclopedia 2022, 2, 974-989. https://doi.org/10.3390/encyclopedia2020064
Shnayder NA, Abdyrakhmanova AK, Nasyrova RF. Oxidation of Antipsychotics. Encyclopedia. 2022; 2(2):974-989. https://doi.org/10.3390/encyclopedia2020064
Chicago/Turabian StyleShnayder, Natalia A., Aiperi K. Abdyrakhmanova, and Regina F. Nasyrova. 2022. "Oxidation of Antipsychotics" Encyclopedia 2, no. 2: 974-989. https://doi.org/10.3390/encyclopedia2020064








