Cyclodextrin-Based Nanosponges and Proteins
Definition
:1. Introduction
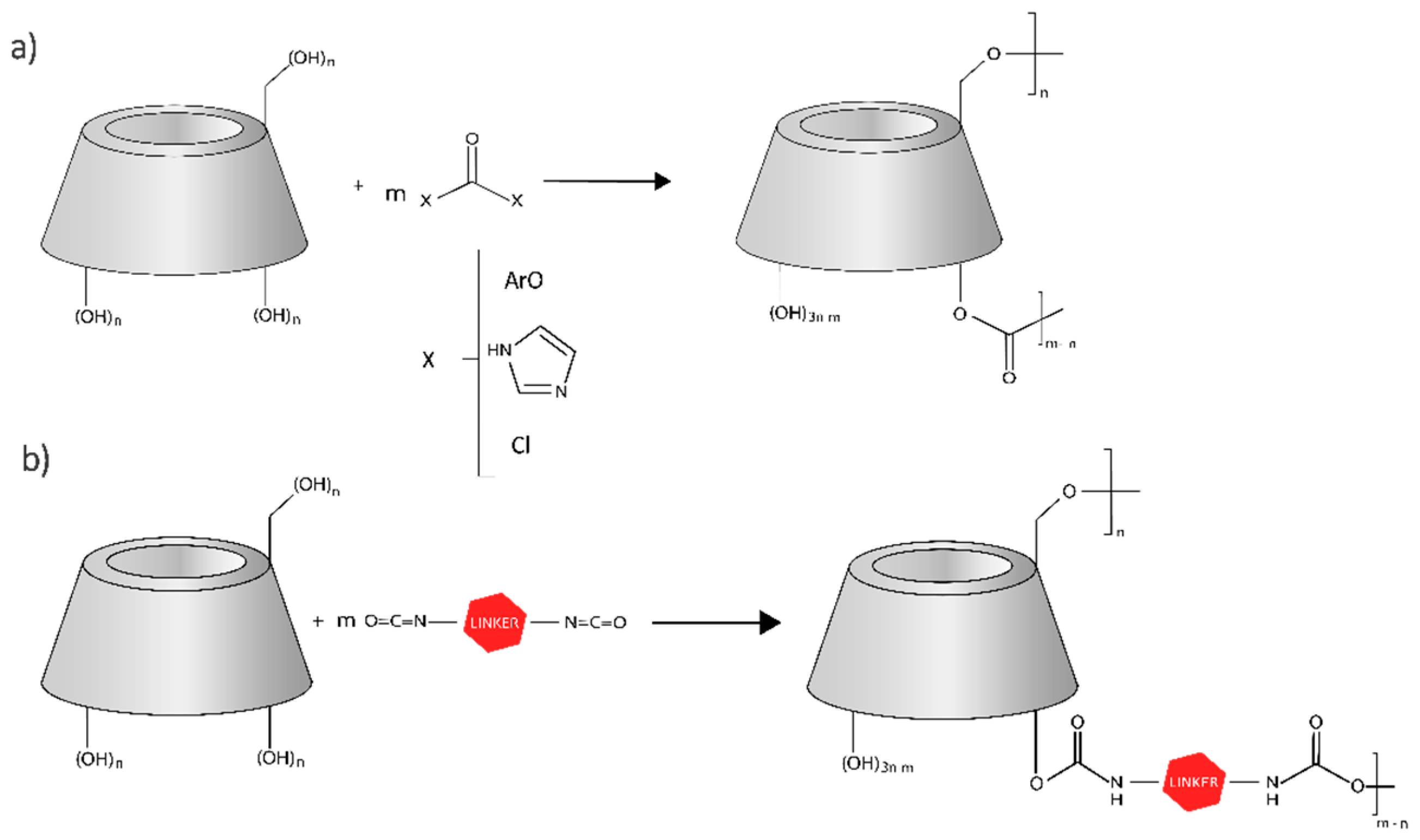
2. Cyclodextrin-Based Nanosponges’ Synthesis and Classification
2.1. General Synthesis Protocol
2.2. General Classification
3. Applications of Cyclodextrin-Based Nanosponges with Proteins and Peptides
3.1. Dioxygenase
3.2. Lipase
3.3. Peroxidase
3.4. Bovine Serum Albumin
3.5. Lysozyme
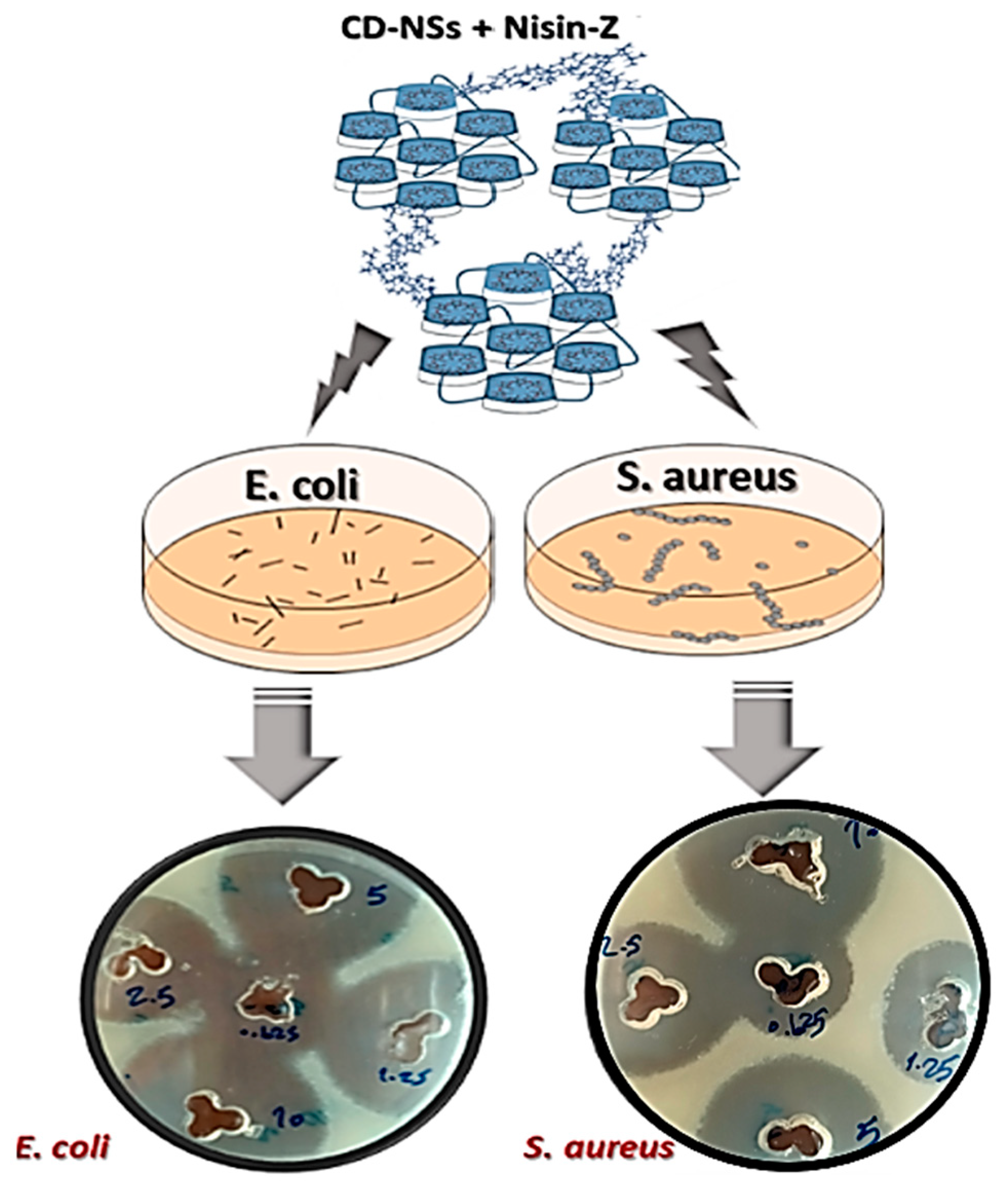
3.6. Nisin
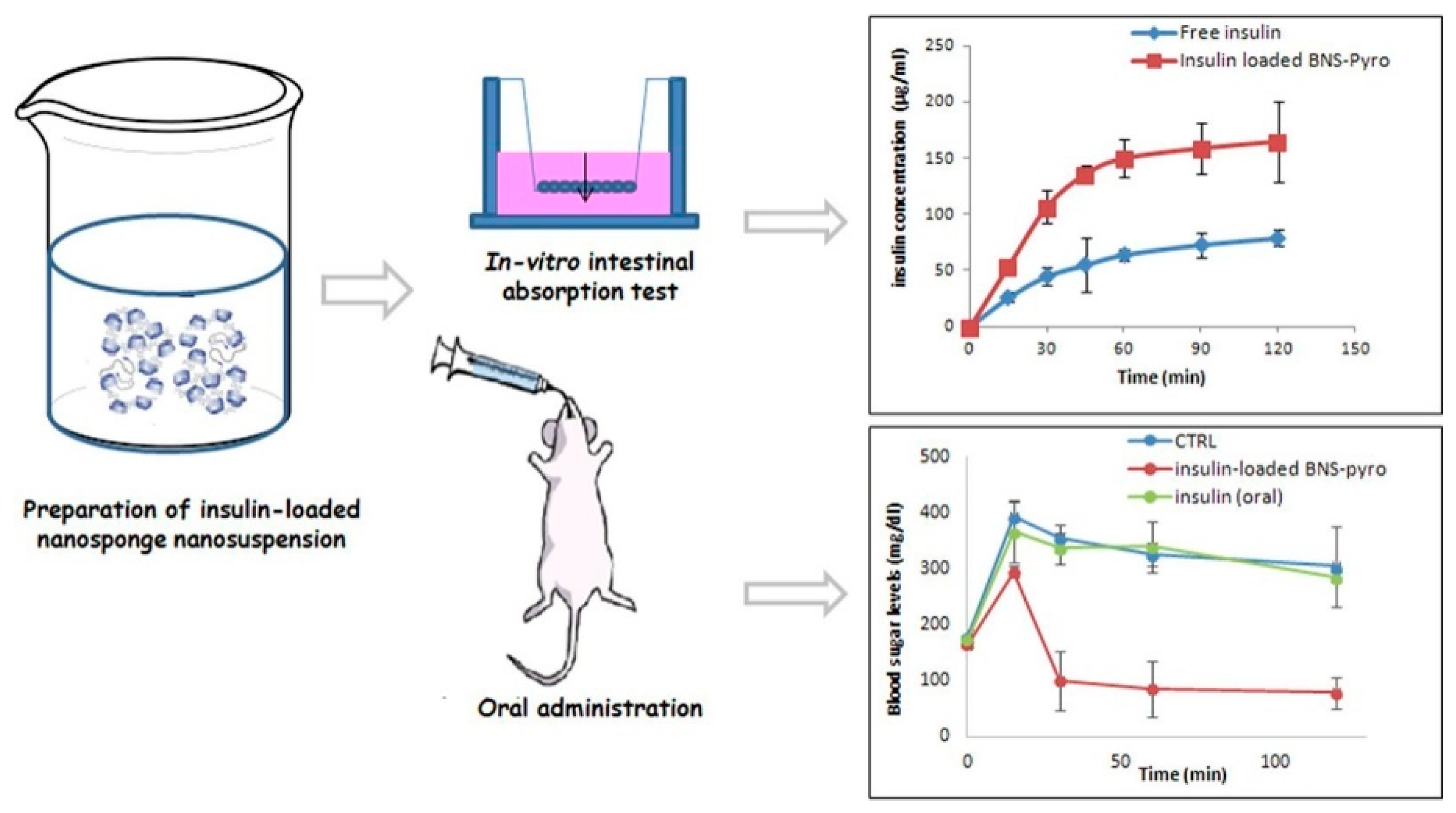
3.7. Insulin
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, Physicochemical Properties and Pharmaceutical Applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as Pharmaceutical Solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.I.A.V.; Ribeiro, A.C.F.; Esteso, M.A. Drug Delivery Systems: Study of Inclusion Complex Formation between Methylxanthines and Cyclodextrins and Their Thermodynamic and Transport Properties. Biomolecules 2019, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; Bermejo-Gimeno, M.J.; García-Carmona, F.; López-Nicolás, J.M. Separating and Identifying the Four Stereoisomers of Methyl Jasmonate by RP-HPLC and Using Cyclodextrins in a Novel Way. Phytochem. Anal. 2017, 28, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; Caldera, F.; Pedrazzo, A.R.; Khazaei Monfared, Y.; Dhakar, N.K.; Trotta, F. A Physicochemical, Thermodynamical, Structural and Computational Evaluation of Kynurenic Acid/Cyclodextrin Complexes. Food Chem. 2021, 356, 129639. [Google Scholar] [CrossRef]
- López-Nicolás, J.M.; García-Carmona, F. Effect of Hydroxypropyl-β-Cyclodextrin on the Aggregation of (E)-Resveratrol in Different Protonation States of the Guest Molecule. Food Chem. 2010, 118, 648–655. [Google Scholar] [CrossRef]
- Cavalli, R.; Trotta, F.; Tumiatti, W. Cyclodextrin-Based Nanosponges for Drug Delivery. J. Incl. Phenom. Macrocycl. Chem. 2006, 56, 209–213. [Google Scholar] [CrossRef]
- Sherje, A.P.; Dravyakar, B.R.; Kadam, D.; Jadhav, M. Cyclodextrin-Based Nanosponges: A Critical Review. Carbohydr. Polym. 2017, 173, 37–49. [Google Scholar] [CrossRef]
- Swaminathan, S.; Cavalli, R.; Trotta, F. Cyclodextrin-Based Nanosponges: A Versatile Platform for Cancer Nanotherapeutics Development. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 579–601. [Google Scholar] [CrossRef]
- Krabicová, I.; Appleton, S.L.; Tannous, M.; Hoti, G.; Caldera, F.; Rubin Pedrazzo, A.; Cecone, C.; Cavalli, R.; Trotta, F. History of Cyclodextrin Nanosponges. Polymers 2020, 12, 1122. [Google Scholar] [CrossRef] [PubMed]
- Mane, P.T.; Wakure, B.S.; Wakte, P.S. Cyclodextrin Based Nanosponges: A Multidimensional Drug Delivery System and Its Biomedical Applications. Curr. Drug Deliv. 2021, 18, 1467–1493. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Kulkarni, Y.A.; Gaud, R.S.; Deshmukh, K.; Cavalli, R.; Trotta, F.; Caldera, F. Acute and Repeated Dose Toxicity Studies of Different β-Cyclodextrin-Based Nanosponge Formulations. J. Pharm. Sci. 2015, 104, 1856–1863. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; Guerrero-Rubio, M.A.; Caldera, F.; Cecone, C.; Trotta, F.; García-Carmona, F.; López-Nicolás, J.M. Lifespan Extension in Caenorhabditis Elegans by Oxyresveratrol Supplementation in Hyper-Branched Cyclodextrin-Based Nanosponges. Int. J. Pharm. 2020, 589, 119862. [Google Scholar] [CrossRef] [PubMed]
- Serno, T.; Geidobler, R.; Winter, G. Protein Stabilization by Cyclodextrins in the Liquid and Dried State. Adv. Drug Deliv. Rev. 2011, 63, 1086–1106. [Google Scholar] [CrossRef]
- Nardo, G.D.; Roggero, C.; Campolongo, S.; Valetti, F.; Trotta, F.; Gilardi, G. Catalytic Properties of Catechol 1,2-Dioxygenase from Acinetobacter Radioresistens S13 Immobilized on Nanosponges. Dalton Trans. 2009, 33, 6507–6512. [Google Scholar] [CrossRef]
- Swaminathan, S.; Cavalli, R.; Trotta, F.; Ferruti, P.; Ranucci, E.; Gerges, I.; Manfredi, A.; Marinotto, D.; Vavia, P.R. In Vitro Release Modulation and Conformational Stabilization of a Model Protein Using Swellable Polyamidoamine Nanosponges of β-Cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2010, 68, 183–191. [Google Scholar] [CrossRef]
- Boscolo, B.; Trotta, F.; Ghibaudi, E. High Catalytic Performances of Pseudomonas Fluorescens Lipase Adsorbed on a New Type of Cyclodextrin-Based Nanosponges. J. Mol. Catal. B Enzym. 2010, 62, 155–161. [Google Scholar] [CrossRef]
- Wajs, E.; Caldera, F.; Trotta, F.; Fragoso, A. Peroxidase-Encapsulated Cyclodextrin Nanosponge Immunoconjugates as a Signal Enhancement Tool in Optical and Electrochemical Assays. Analyst 2013, 139, 375–380. [Google Scholar] [CrossRef]
- Deshmukh, K.; Tanwar, Y.S.; Sharma, S.; Shende, P.; Cavalli, R. Functionalized Nanosponges for Controlled Antibacterial and Antihypocalcemic Actions. Biomed. Pharmacother. 2016, 84, 485–494. [Google Scholar] [CrossRef]
- Appleton, S.L.; Tannous, M.; Argenziano, M.; Muntoni, E.; Rosa, A.C.; Rossi, D.; Caldera, F.; Scomparin, A.; Trotta, F.; Cavalli, R. Nanosponges as Protein Delivery Systems: Insulin, a Case Study. Int. J. Pharm. 2020, 590, 119888. [Google Scholar] [CrossRef] [PubMed]
- Khazaei Monfared, Y.; Mahmoudian, M.; Cecone, C.; Caldera, F.; Zakeri-Milani, P.; Matencio, A.; Trotta, F. Stabilization and Anticancer Enhancing Activity of the Peptide Nisin by Cyclodextrin-Based Nanosponges against Colon and Breast Cancer Cells. Polymers 2022, 14, 594. [Google Scholar] [CrossRef] [PubMed]
- Khazaei Monfared, Y.; Mahmoudian, M.; Hoti, G.; Caldera, F.; López Nicolás, J.M.; Zakeri-Milani, P.; Matencio, A.; Trotta, F. Cyclodextrin-Based Nanosponges as Perse Antimicrobial Agents Increase the Activity of Natural Antimicrobial Peptide Nisin. Pharmaceutics 2022, 14, 685. [Google Scholar] [CrossRef] [PubMed]
- Hoti, G.; Appleton, S.L.; Pedrazzo, A.R.; Cecone, C.; Matencio, A.; Trotta, F.; Caldera, F. Strategies to Develop Cyclodextrin-Based Nanosponges for Smart Drug Delivery; IntechOpen: London, UK, 2021; ISBN 978-1-83969-539-1. [Google Scholar]
- Chilajwar, S.V.; Pednekar, P.P.; Jadhav, K.R.; Gupta, G.J.C.; Kadam, V.J. Cyclodextrin-Based Nanosponges: A Propitious Platform for Enhancing Drug Delivery. Expert Opin. Drug Deliv. 2014, 11, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Tejashri, G.; Amrita, B.; Darshana, J. Cyclodextrin Based Nanosponges for Pharmaceutical Use: A Review. Acta Pharm. 2013, 63, 335–358. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dalal, P.; Rao, R. Cyclodextrin nanosponges: A promising approach for modulating drug delivery. In Colloid Science Pharmaceutical Nanotechnology; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Hoti, G.; Caldera, F.; Cecone, C.; Pedrazzo, A.R.; Anceschi, A.; Appleton, S.L.; Monfared, Y.K.; Trotta, F. Effect of the Cross-Linking Density on the Swelling and Rheological Behavior of Ester-Bridged β-Cyclodextrin Nanosponges. Materials 2021, 14, 478. [Google Scholar] [CrossRef] [PubMed]
- Trotta, F.; Cavalli, R. Characterization and Applications of New Hyper-Cross-Linked Cyclodextrins. Compos. Interfaces 2009, 16, 39–48. [Google Scholar] [CrossRef]
- Cecone, C.; Hoti, G.; Krabicova, I.; Appleton, S.L.; Caldera, F.; Bracco, P.; Zanetti, M.; Trotta, F. Sustainable Synthesis of Cyclodextrin-Based Polymers Exploiting Natural Deep Eutectic Solvents. Green Chem. 2020, 22, 5806–5814. [Google Scholar] [CrossRef]
- Trotta, F. Cyclodextrin nanosponges and their applications. In Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine: Current and Future Industrial Applications; Wiley: Hoboken, NJ, USA, 2011; pp. 323–342. ISBN 9780470474228. [Google Scholar]
- Trotta, F.; Zanetti, M.; Cavalli, R. Cyclodextrin-Based Nanosponges as Drug Carriers. Beilstein J. Org. Chem. 2012, 8, 2091–2099. [Google Scholar] [CrossRef]
- Gamayurova, V.S.; Zinov’eva, M.E.; Shnaider, K.L.; Davletshina, G.A. Lipases in Esterification Reactions: A Review. Catal. Ind. 2021, 13, 58–72. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, P.; Xu, W.; Wang, L.; Tang, K. Lipase-Catalyzed Hydrolysis of (+,−)-2-(4-Methylphenyl) Propionic Methyl Ester Enhanced by Hydroxypropyl-β-Cyclodextrin. J. Chem. Technol. Biotechnol. 2019, 94, 147–158. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, G.; Zhang, P.; Xu, W.; Tang, K. Lipase-Catalyzed Production of (S)-Carprofen Enhanced by Hydroxyethyl-β-Cyclodextrins: Experiment and Optimization. Org. Process Res. Dev. 2019, 23, 891–899. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, M.; Yan, X.; Wan, D.; Zeng, Z.; Yu, P.; Gong, D. Immobilization of Lipase on β-Cyclodextrin Grafted and Aminopropyl-Functionalized Chitosan/Fe3O4 Magnetic Nanocomposites: An Innovative Approach to Fruity Flavor Esters Esterification. Food Chem. 2022, 366, 130616. [Google Scholar] [CrossRef] [PubMed]
- Ozmen, E.Y.; Yilmaz, M. Pretreatment of Candida Rugosa Lipase with Soybean Oil before Immobilization on β-Cyclodextrin-Based Polymer. Colloids Surf. B Biointerfaces 2009, 69, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, A.; Bg, J.; Mr, D. Role of Peroxidase in Clinical Assays: A Short Review. J. Clin. Nutr. Diet. 2017, 3, 14. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Schmidt, K.; Blaivas, L.; Mccoy, J.P.; Wilson, B.S. A Rapid Immunostaining Method Utilizing Preformed Antibody–Avidin–Biotin–Peroxidase Complexes. Am. J. Clin. Pathol. 1985, 83, 636–639. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucia Appleton, S.; Khazaei Monfared, Y.; Vidal-Sánchez, F.J.; Caldera, F.; Cavalli, R.; Trotta, F.; Matencio, A. Cyclodextrin-Based Nanosponges and Proteins. Encyclopedia 2022, 2, 752-760. https://doi.org/10.3390/encyclopedia2020052
Lucia Appleton S, Khazaei Monfared Y, Vidal-Sánchez FJ, Caldera F, Cavalli R, Trotta F, Matencio A. Cyclodextrin-Based Nanosponges and Proteins. Encyclopedia. 2022; 2(2):752-760. https://doi.org/10.3390/encyclopedia2020052
Chicago/Turabian StyleLucia Appleton, Silvia, Yousef Khazaei Monfared, Francisco José Vidal-Sánchez, Fabrizio Caldera, Roberta Cavalli, Francesco Trotta, and Adrián Matencio. 2022. "Cyclodextrin-Based Nanosponges and Proteins" Encyclopedia 2, no. 2: 752-760. https://doi.org/10.3390/encyclopedia2020052
APA StyleLucia Appleton, S., Khazaei Monfared, Y., Vidal-Sánchez, F. J., Caldera, F., Cavalli, R., Trotta, F., & Matencio, A. (2022). Cyclodextrin-Based Nanosponges and Proteins. Encyclopedia, 2(2), 752-760. https://doi.org/10.3390/encyclopedia2020052









