Prevalence and Predictors of Long COVID-19 and the Average Time to Diagnosis in the General Population: A Systematic Review, Meta-Analysis and Meta-Regression
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Terms and Criteria for Inclusion
2.2. Data Extraction
2.3. Outcome Measures
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Prevalence of Long COVID-19 Sequelae in General Population
3.2. Average Time to and Association with Long COVID-19
3.3. Sub-Group and Meta-Regression Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, e21–e33. [Google Scholar] [CrossRef]
- Wijeratne, T.; Crewther, S. Post-COVID 19 Neurological Syndrome (PCNS); a novel syndrome with challenges for the global neurology community. J. Neurol. Sci. 2020, 419, 117179. [Google Scholar] [CrossRef]
- Rando, H.M.; Bennett, T.D.; Byrd, J.B.; Bramante, C.; Callahan, T.J.; Chute, C.G.; Davis, H.E.; Deer, R.; Gagnier, J.; Koraishy, F.M.; et al. Challenges in defining Long COVID: Striking differences across literature, Electronic Health Records, and patient-reported information. medRxiv 2021. [Google Scholar] [CrossRef]
- Blomberg, B.; Mohn, K.G.I.; Brokstad, K.A.; Zhou, F.; Linchausen, D.W.; Hansen, B.A.; Lartey, S.; Onyango, T.B.; Kuwelker, K.; Sævik, M.; et al. Long COVID in a prospective cohort of home-isolated patients. Nat. Med. 2021, 27, 1607–1613. [Google Scholar] [CrossRef]
- Galeotti, C.; Bayry, J. Autoimmune and inflammatory diseases following COVID-19. Nat. Rev. Rheumatol. 2020, 16, 413–414. [Google Scholar] [CrossRef]
- Ramakrishnan, R.K.; Kashour, T.; Hamid, Q.; Halwani, R.; Tleyjeh, I.M. Unraveling the Mystery Surrounding Post-Acute Sequelae of COVID-19. Front. Immunol. 2021, 12, 686029. [Google Scholar] [CrossRef]
- Vu, T.; McGill, S.C. An Overview of Post–COVID-19 Condition (Long COVID). Can. J. Health Technol. 2021, 1, 1–31. [Google Scholar] [CrossRef]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid—Mechanisms, risk factors, and management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef]
- Mumoli, N.; Conte, G.; Evangelista, I.; Cei, M.; Mazzone, A.; Colombo, A. Post-COVID or long-COVID: Two different conditions or the same? J. Infect. Public Health 2021, 14, 1349. [Google Scholar] [CrossRef]
- Kamal, M.; Abo Omirah, M.; Hussein, A.; Saeed, H. Assessment and characterisation of post-COVID-19 manifestations. Int. J. Clin. Pract. 2021, 75, e13746. [Google Scholar] [CrossRef]
- Ryan, F.J.; Hope, C.M.; Masavuli, M.G.; Lynn, M.A.; Mekonnen, Z.A.; Yeow, A.E.L.; Garcia-Valtanen, P.; Al-Delfi, Z.; Gummow, J.; Ferguson, C.; et al. Long-term perturbation of the peripheral immune system months after SARS-CoV-2 infection. BMC Med. 2022, 20, 26. [Google Scholar] [CrossRef]
- Garg, M.; Maralakunte, M.; Garg, S.; Dhooria, S.; Sehgal, I.; Bhalla, A.S.; Vijayvergiya, R.; Grover, S.; Bhatia, V.; Jagia, P.; et al. The conundrum of ‘long-COVID-19′: A narrative review. Int. J. Gen. Med. 2021, 14, 2491–2506. [Google Scholar] [CrossRef]
- Tawfik, G.M.; Dila, K.A.S.; Mohamed, M.Y.F.; Tam, D.N.H.; Kien, N.D.; Ahmed, A.M.; Huy, N.T. A step by step guide for conducting a systematic review and meta-analysis with simulation data. Trop. Med. Health 2019, 47, 46. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Bagias, C.; Sukumar, N.; Weldeselassie, Y.; Oyebode, O.; Saravanan, P. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Int. J. Environ. Res. Public Health 2021, 18, 1–4. [Google Scholar]
- Schreck, N.; Piepho, H.P.; Schlather, M. Best prediction of the additive genomic variance in random-effects models. Genetics 2019, 213, 379–394. [Google Scholar] [CrossRef]
- Cochrane Review Manager (RevMan) [Computer Program]; Version 5.4; The Nordic Cochrane Centre: Copenhagen, Denmark; Cochrane Collaboration: Oxford, UK, 2020.
- Goel, N.; Goyal, N.; Kumar, R. Clinico-radiological evaluation of post COVID-19 at a tertiary pulmonary care centre in Delhi, India. Monaldi Arch. Chest Dis. Arch. Monaldi per le Mal. del Torace 2021, 91, 386–391. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Wanga, V.; Chevinsky, J.R.; Dimitrov, L.V.; Gerdes, M.E.; Whitfield, G.P.; Bonacci, R.A.; Nji, M.A.M.; Hernandez-Romieu, A.C.; Rogers-Brown, J.S.; McLeod, T.; et al. Long-Term Symptoms Among Adults Tested for SARS-CoV-2—United States, January 2020–April 2021. MMWR Recomm. Rep. 2021, 70, 1235–1241. [Google Scholar] [CrossRef]
- Somani, S.S.; Richter, F.; Fuster, V.; De Freitas, J.K.; Naik, N.; Sigel, K.; Bottinger, E.P.; Levin, M.A.; Fayad, Z.; Just, A.C.; et al. Characterization of Patients Who Return to Hospital Following Discharge from Hospitalization for COVID-19. J. Gen. Intern. Med. 2020, 35, 2838–2844. [Google Scholar] [CrossRef]
- Perlis, R.H.; Green, J.; Santillana, M.; Lazer, D.; Ognyanova, K.; Simonson, M.; Baum, M.A.; Quintana, A.; Chwe, H.; Druckman, J.; et al. Persistence of symptoms up to 10 months following acute COVID-19 illness. medRxiv 2021. [Google Scholar] [CrossRef]
- Leijte, W.T.; Wagemaker, N.M.M.; van Kraaij, T.D.A.; de Kruif, M.D.; Mostard, G.J.M.; Leers, M.P.G.; Mostard, R.L.M.; Buijs, J.; van Twist, D.J.L. [Mortality and re-admission after hospitalization with COVID-19]. Ned. Tijdschr. Geneeskd. 2020, 164, D5423. [Google Scholar] [PubMed]
- Pinato, D.J.; Tabernero, J.; Bower, M.; Scotti, L.; Patel, M.; Colomba, E.; Dolly, S.; Loizidou, A.; Chester, J.; Mukherjee, U.; et al. Prevalence and impact of COVID-19 sequelae on treatment and survival of patients with cancer who recovered from SARS-CoV-2 infection: Evidence from the OnCovid retrospective, multicentre registry study. Lancet Oncol. 2021, 22, 1669–1680. [Google Scholar] [CrossRef] [PubMed]
- Sathyamurthy, P.; Madhavan, S.; Pandurangan, V. Prevalence, Pattern and Functional Outcome of Post COVID-19 Syndrome in Older Adults. Cureus 2021, 13, e17189. [Google Scholar] [CrossRef]
- Guarin, G.; Lo, K.B.; Bhargav, R.; Salacup, G.; Wattoo, A.; Coignet, J.G.; DeJoy, R.; Azmaiparashvili, Z.; Patarroyo-Aponte, G.; Eiger, G.; et al. Factors associated with hospital readmissions among patients with COVID-19: A single-center experience. J. Med. Virol. 2021, 93, 5582–5587. [Google Scholar] [CrossRef] [PubMed]
- Logue, J.K.; Franko, N.M.; McCulloch, D.J.; McDonald, D.; Magedson, A.; Wolf, C.R.; Chu, H.Y. Sequelae in Adults at 6 Months After COVID-19 Infection. JAMA Netw. Open 2021, 4, e210830. [Google Scholar] [CrossRef] [PubMed]
- Günster, C.; Busse, R.; Spoden, M.; Rombey, T.; Schillinger, G.; Hoffmann, W.; Weber-Carstens, S.; Schuppert, A.; Karagiannidis, C. 6-month mortality and readmissions of hospitalized COVID-19 patients: A nationwide cohort study of 8,679 patients in Germany. PLoS ONE 2021, 16, e0255427. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Guijarro, C.; Plaza-Canteli, S.; Hernández-Barrera, V.; Torres-Macho, J. Prevalence of Post-COVID-19 Cough One Year After SARS-CoV-2 Infection: A Multicenter Study. Lung 2021, 199, 249–253. [Google Scholar] [CrossRef]
- Zayet, S.; Zahra, H.; Royer, P.Y.; Tipirdamaz, C.; Mercier, J.; Gendrin, V.; Lepiller, Q.; Marty-Quinternet, S.; Osman, M.; Belfeki, N.; et al. Post-COVID-19 syndrome: Nine months after SARS-CoV-2 infection in a cohort of 354 patients: Data from the first wave of COVID-19 in nord franche-comté hospital, France. Microorganisms 2021, 9, 1719. [Google Scholar] [CrossRef]
- Myall, K.J.; Mukherjee, B.; Castanheira, A.M.; Lam, J.L.; Benedetti, G.; Mak, S.M.; Preston, R.; Thillai, M.; Dewar, A.; Molyneaux, P.L.; et al. Persistent post–COVID-19 interstitial lung disease: An observational study of corticosteroid treatment. Ann. Am. Thorac. Soc. 2021, 18, 799–806. [Google Scholar] [CrossRef]
- Naik, S.; Haldar, S.N.; Soneja, M.; Mundadan, N.G.; Garg, P.; Mittal, A.; Desai, D.; Trilangi, P.K.; Chakraborty, S.; Begam, N.N.; et al. Post COVID-19 sequelae: A prospective observational study from Northern India. Drug Discov. Ther. 2021, 15, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Peghin, M.; Palese, A.; Venturini, M.; De Martino, M.; Gerussi, V.; Graziano, E.; Bontempo, G.; Marrella, F.; Tommasini, A.; Fabris, M.; et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin. Microbiol. Infect. 2021, 27, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Menges, D.; Ballouz, T.; Anagnostopoulos, A.; Aschmann, H.E.; Domenghino, A.; Fehr, J.S.; Puhan, M.A. Burden of post-COVID-19 syndrome and implications for healthcare service planning: A population-based cohort study. PLoS ONE 2021, 16, e0254523. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, R.; Rahman, M.M.; Rassel, M.A.; Monayem, F.B.; Sayeed, S.K.J.B.; Islam, M.S.; Islam, M.M. Post-COVID-19 syndrome among symptomatic COVID-19 patients: A prospective cohort study in a tertiary care center of Bangladesh. PLoS ONE 2021, 16, e0249644. [Google Scholar] [CrossRef] [PubMed]
- Khodeir, M.M.; Shabana, H.A.; Rasheed, Z.; Alkhamiss, A.S.; Khodeir, M.; Alkhowailed, M.S.; Alharbi, S.; Alsoghair, M.; Alsagaby, S.A.; Al Abdulmonem, W. COVID-19: Post-recovery long-term symptoms among patients in Saudi Arabia. PLoS ONE 2021, 16, e0260259. [Google Scholar] [CrossRef] [PubMed]
- Kayaaslan, B.; Eser, F.; Kalem, A.K.; Kaya, G.; Kaplan, B.; Kacar, D.; Hasanoglu, I.; Coskun, B.; Guner, R. Post-COVID syndrome: A single-center questionnaire study on 1007 participants recovered from COVID-19. J. Med. Virol. 2021, 93, 6566–6574. [Google Scholar] [CrossRef] [PubMed]
- Maestre-Muñiz, M.M.; Arias, Á.; Mata-Vázquez, E.; Martín-Toledano, M.; López-Larramona, G.; Ruiz-Chicote, A.M.; Nieto-Sandoval, B.; Lucendo, A.J. Long-term outcomes of patients with coronavirus disease 2019 at one year after hospital discharge. J. Clin. Med. 2021, 10, 2945. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Pérez, O.; Merino, E.; Leon-Ramirez, J.M.; Andres, M.; Ramos, J.M.; Arenas-Jiménez, J.; Asensio, S.; Sanchez, R.; Ruiz-Torregrosa, P.; Galan, I.; et al. Post-acute COVID-19 syndrome. Incidence and risk factors: A Mediterranean cohort study. J. Infect. 2021, 82, 378–383. [Google Scholar] [CrossRef]
- Venturelli, S.; Benatti, S.V.; Casati, M.; Binda, F.; Zuglian, G.; Imeri, G.; Conti, C.; Biffi, A.M.; Spada, S.; Bondi, E.; et al. Surviving COVID-19 in Bergamo Province: A post-Acute outpatient re-evaluation. Epidemiol. Infect. 2021, 149, e32. [Google Scholar] [CrossRef]
- Hirschtick, J.L.; Titus, A.R.; Slocum, E.; Power, L.E.; Hirschtick, R.E.; Elliott, M.R.; McKane, P.; Fleischer, N.L. Population-Based Estimates of Post-acute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection (PASC) Prevalence and Characteristics. Clin. Infect. Dis. 2021, 73, 2055–2064. [Google Scholar] [CrossRef]
- Boscolo-Rizzo, P.; Guida, F.; Polesel, J.; Marcuzzo, A.V.; Capriotti, V.; D’Alessandro, A.; Zanelli, E.; Marzolino, R.; Lazzarin, C.; Antonucci, P.; et al. Sequelae in adults at 12 months after mild-to-moderate coronavirus disease 2019 (COVID-19). Int. Forum Allergy Rhinol. 2021, 11, 1685–1688. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.S.; Kristiansen, M.F.; Hanusson, K.D.; Danielsen, M.E.; Á Steig, B.; Gaini, S.; Strøm, M.; Weihe, P. Long COVID in the Faroe Islands: A Longitudinal Study among Nonhospitalized Patients. Clin. Infect. Dis. 2021, 73, e4058–e4063. [Google Scholar] [CrossRef] [PubMed]
- Elkan, M.; Dvir, A.; Zaidenstein, R.; Keller, M.; Kagansky, D.; Hochman, C.; Koren, R. Patient-reported outcome measures after hospitalization during the COVID-19 pandemic: A survey among COVID-19 and non-COVID-19 patients. Int. J. Gen. Med. 2021, 2021, 4829–4836. [Google Scholar] [CrossRef] [PubMed]
- Maamar, M.; Artime, A.; Pariente, E.; Fierro, P.; Ruiz, Y.; Gutiérrez, S.; Tobalina, M.; Díaz-Salazar, S.; Ramos, C.; Olmos, J.M.; et al. Post-COVID-19 syndrome, low-grade inflammation and inflammatory markers: A cross-sectional study. Curr. Med. Res. Opin. 2022, 38, 901–909. [Google Scholar] [CrossRef]
- Tleyjeh, I.M.; Saddik, B.; AlSwaidan, N.; AlAnazi, A.; Ramakrishnan, R.K.; Alhazmi, D.; Aloufi, A.; AlSumait, F.; Berbari, E.; Halwani, R. Prevalence and predictors of Post-Acute COVID-19 Syndrome (PACS) after hospital discharge: A cohort study with 4 months median follow-up. PLoS ONE 2021, 16, e0260568. [Google Scholar] [CrossRef] [PubMed]
- Ogoina, D.; James, H.I.; Ogoinja, S.Z. Post-discharge symptoms among hospitalized COVID-19 patients in Nigeria: A single-center study. Am. J. Trop. Med. Hyg. 2021, 105, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Dryden, M.T.G.; Mudara, C.; Vika, C.; Blumberg, L.; Mayet, N.; Cohen, C.; Tempia, S.; Parker, A.; Nel, J.; Perumal, R.; et al. Post COVID-19 Condition in South Africa: 3-Month Follow-Up after Hospitalisation with SARS-CoV-2. Lancet Glob. Health 2022, 10, E1247–E1256. [Google Scholar] [CrossRef]
- Bell, M.L.; Catalfamo, C.J.; Farland, L.V.; Ernst, K.C.; Jacobs, E.T.; Klimentidis, Y.C.; Jehn, M.; Pogreba-Brown, K. Post-acute sequelae of COVID-19 in a non-hospitalized cohort: Results from the Arizona CoVHORT. PLoS ONE 2021, 16, e0254347. [Google Scholar] [CrossRef]
- Becker, C.; Beck, K.; Zumbrunn, S.; Memma, V.; Herzog, N.; Bissmann, B.; Gross, S.; Loretz, N.; Mueller, J.; Amacher, S.A.; et al. Long COVID 1 year after hospitalisation for COVID-19: A prospective bicentric cohort study. Swiss Med. Wkly. 2021, 151, W30091. [Google Scholar] [CrossRef]
- Wang, G.; Cao, K.; Liu, K.; Xue, Y.; Roberts, A.I.; Li, F.; Han, Y.; Rabson, A.B.; Wang, Y.; Shi, Y. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 2018, 25, 1209–1223. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.T.; Hamilton, F.W.; Milne, A.; Morley, A.J.; Viner, J.; Attwood, M.; Noel, A.; Gunning, S.; Hatrick, J.; Hamilton, S.; et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: Results from a prospective UK cohort. Thorax 2021, 76, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Ayoubkhani, D.; Khunti, K.; Nafilyan, V.; Maddox, T.; Humberstone, B.; Diamond, I.; Banerjee, A. Post-covid syndrome in individuals admitted to hospital with COVID-19: Retrospective cohort study. BMJ 2021, 372, n693. [Google Scholar] [CrossRef] [PubMed]
- Osikomaiya, B.; Erinoso, O.; Wright, K.O.; Odusola, A.O.; Thomas, B.; Adeyemi, O.; Bowale, A.; Adejumo, O.; Falana, A.; Abdus-salam, I.; et al. ‘Long COVID’: Persistent COVID-19 symptoms in survivors managed in Lagos State, Nigeria. BMC Infect. Dis. 2021, 21, 304. [Google Scholar] [CrossRef] [PubMed]
- Sigfrid, L.; Drake, T.M.; Pauley, E.; Jesudason, E.C.; Olliaro, P.; Lim, W.S.; Gillesen, A.; Berry, C.; Lowe, D.J.; McPeake, J.; et al. Long Covid in adults discharged from UK hospitals after COVID-19: A prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg. Health-Eur. 2021, 8, 100186. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Dercon, Q.; Luciano, S.; Geddes, J.R.; Husain, M.; Harrison, P.J. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021, 18, e1003773. [Google Scholar] [CrossRef] [PubMed]
- Wynberg, E.; van Willigen, H.D.G.; Dijkstra, M.; Boyd, A.; Kootstra, N.A.; van den Aardweg, J.G.; van Gils, M.J.; Matser, A.; de Wit, M.R.; Leenstra, T.; et al. Evolution of Coronavirus Disease 2019 (COVID-19) Symptoms During the First 12 Months after Illness Onset. Clin. Infect. Dis. 2022, 75, e482–e490. [Google Scholar] [CrossRef]
- Osmanov, I.M.; Spiridonova, E.; Bobkova, P.; Gamirova, A.; Shikhaleva, A.; Andreeva, M.; Blyuss, O.; El-Taravi, Y.; DunnGalvin, A.; Comberiati, P.; et al. Risk factors for long covid in previously hospitalised children using the ISARIC Global follow-up protocol: A prospective cohort study. Eur. Respir. J. 2022, 59, 2101341. [Google Scholar] [CrossRef]
- Pereira, C.; Harris, B.H.L.; Di Giovannantonio, M.; Rosadas, C.; Short, C.E.; Quinlan, R.; Sureda-Vives, M.; Fernandez, N.; Day-Weber, I.; Khan, M.; et al. The Association Between Antibody Response to Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Post-COVID-19 Syndrome in Healthcare Workers. J. Infect. Dis. 2021, 223, 1671–1676. [Google Scholar] [CrossRef]
- Abdelrahman, M.M.; Abd-Elrahman, N.M.; Bakheet, T.M. Persistence of symptoms after improvement of acute COVID-19 infection, a longitudinal study. J. Med. Virol. 2021, 93, 5942–5946. [Google Scholar] [CrossRef]
- Fernández-De-las-peñas, C.; Palacios-Ceña, D.; Gómez-Mayordomo, V.; Cuadrado, M.L.; Florencio, L.L. Defining post-covid symptoms (Post-acute covid, long covid, persistent post-covid): An integrative classification. Int. J. Environ. Res. Public Health 2021, 18, 2621. [Google Scholar] [CrossRef] [PubMed]
- Vinhaes, C.L.; Araujo-Pereira, M.; Tibúrcio, R.; Cubillos-Angulo, J.M.; Demitto, F.O.; Akrami, K.M.; Andrade, B.B. Systemic inflammation associated with immune reconstitution inflammatory syndrome in persons living with hiv. Life 2021, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, X.; Wu, S.; Chen, S.; Li, Y.; Nong, L.; Lie, P.; Huang, L.; Cheng, L.; Lin, Y.; et al. Definition and Risks of Cytokine Release Syndrome in 11 Critically Ill COVID-19 Patients with Pneumonia: Analysis of Disease Characteristics. J. Infect. Dis. 2020, 222, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- King, C.G.; Guyette, R.W.; Piotrowski, C. Online exams and cheating: An empirical analysis of business students’ views. J. Educ. Online 2009, 6, n1. [Google Scholar] [CrossRef]
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global Prevalence of Post-Acute Sequelae of COVID-19 (PASC) or Long COVID: A Meta-Analysis and Systematic Review. medRxiv 2021. [Google Scholar] [CrossRef]
- Kingery, J.R.; Safford, M.M.; Martin, P.; Lau, J.D.; Rajan, M.; Wehmeyer, G.T.; Li, H.A.; Alshak, M.N.; Jabri, A.; Kofman, A.; et al. Health Status, Persistent Symptoms, and Effort Intolerance One Year After Acute COVID-19 Infection. J. Gen. Intern. Med. 2022, 37, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Fortini, A.; Torrigiani, A.; Sbaragli, S.; Lo Forte, A.; Crociani, A.; Cecchini, P.; Innocenti Bruni, G.; Faraone, A. COVID-19: Persistence of symptoms and lung alterations after 3–6 months from hospital discharge. Infection 2021, 49, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- ONS. Prevalence of Ongoing Symptoms Following Coronavirus (COVID-19) Infection in the UK: 2 December 2021; Office for National Statistics: London, UK, 2021.
- Sugiyama, A.; Miwata, K.; Kitahara, Y.; Okimoto, M.; Abe, K.; Ouoba, S.; Akita, T.; Tanimine, N.; Ohdan, H.; Kubo, T.; et al. Long COVID occurrence in COVID-19 survivors. Sci. Rep. 2022, 12, 6039. [Google Scholar] [CrossRef]
- Förster, C.; Colombo, M.G.; Wetzel, A.J.; Martus, P.; Joos, S. Persisting Symptoms after COVID-19: Prevalence and Risk Factors in a Population-Based Cohort. Dtsch. Arztebl. Int. 2022, 119, 167–174. [Google Scholar] [CrossRef]
- Groff, D.; Sun, A.; Ssentongo, A.E.; Ba, D.M.; Parsons, N.; Poudel, G.R.; Lekoubou, A.; Oh, J.S.; Ericson, J.E.; Ssentongo, P.; et al. Short-term and Long-term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review. JAMA Netw. Open 2021, 4, e2128568. [Google Scholar] [CrossRef]
- Lavery, A.M.; Preston, L.E.; Ko, J.Y.; Chevinsky, J.R.; DeSisto, C.L.; Pennington, A.F.; Kompaniyets, L.; Datta, S.D.; Click, E.S.; Golden, T.; et al. Characteristics of Hospitalized COVID-19 Patients Discharged and Experiencing Same-Hospital Readmission—United States, March–August 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1695–1699. [Google Scholar] [CrossRef]
- Munblit, D.; Bobkova, P.; Spiridonova, E.; Shikhaleva, A.; Gamirova, A.; Blyuss, O.; Nekliudov, N.; Bugaeva, P.; Andreeva, M.; DunnGalvin, A.; et al. Risk factors for long-term consequences of COVID-19 in hospitalised adults in Moscow using the ISARIC Global follow-up protocol: StopCOVID cohort study. medRxiv 2021. [Google Scholar] [CrossRef]
- Vimercati, L.; De Maria, L.; Quarato, M.; Caputi, A.; Gesualdo, L.; Migliore, G.; Cavone, D.; Sponselli, S.; Pipoli, A.; Inchingolo, F.; et al. Association between long COVID and overweight/obesity. J. Clin. Med. 2021, 10, 4143. [Google Scholar] [CrossRef] [PubMed]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of Long-COVID: Analysis of COVID cases and their symptoms collected by the Covid Symptoms Study App. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.P.; Wang, X.Q.; Iwashyna, T.J.; Prescott, H.C. Readmission and Death after Initial Hospital Discharge among Patients with COVID-19 in a Large Multihospital System. JAMA—J. Am. Med. Assoc. 2021, 325, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, E.A.; Santos, C.V.; Sharma, M.; Szpunar, S.M.; Saravolatz, L.; Bhargava, A. 36. Clinical Features of and Risk Factors for 30-day Readmission after an Initial Hospitalization with COVID-19. Open Forum Infect. Dis. 2021, 8, S26–S27. [Google Scholar] [CrossRef]
- Goërtz, Y.M.J.; Van Herck, M.; Delbressine, J.M.; Vaes, A.W.; Meys, R.; Machado, F.V.C.; Houben-Wilke, S.; Burtin, C.; Posthuma, R.; Franssen, F.M.E.; et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: The post-COVID-19 syndrome? ERJ Open Res. 2020, 6, 00542–2020. [Google Scholar] [CrossRef] [PubMed]
- Gaber, T.A.Z.K.; Ashish, A.; Unsworth, A. Persistent post-covid symptoms in healthcare workers. Occup. Med. 2021, 71, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Pazukhina, E.; Andreeva, M.; Spiridonova, E.; Bobkova, P.; Shikhaleva, A.; El-Taravi, Y.; Rumyantsev, M.; Gamirova, A.; Bairashevskaia, A.; Petrova, P.; et al. Prevalence and Risk Factors of Post-COVID-19 Condition in Adults and Children at 6 and 12 Months after Hospital Discharge: A Prospective, Cohort Study in Moscow (Stop COVID). SSRN Electron. J. 2022, 20, 244. [Google Scholar] [CrossRef]
- Durstenfeld, M.S.; Sun, K.; Ma, Y.; Rodriguez, F.; Secemsky, E.A.; Parikh, R.V.; Hsue, P.Y. Association of HIV with COVID-19 Outcomes among Hospitalized Adults. AIDS 2022, 36, 391–398. [Google Scholar] [CrossRef]

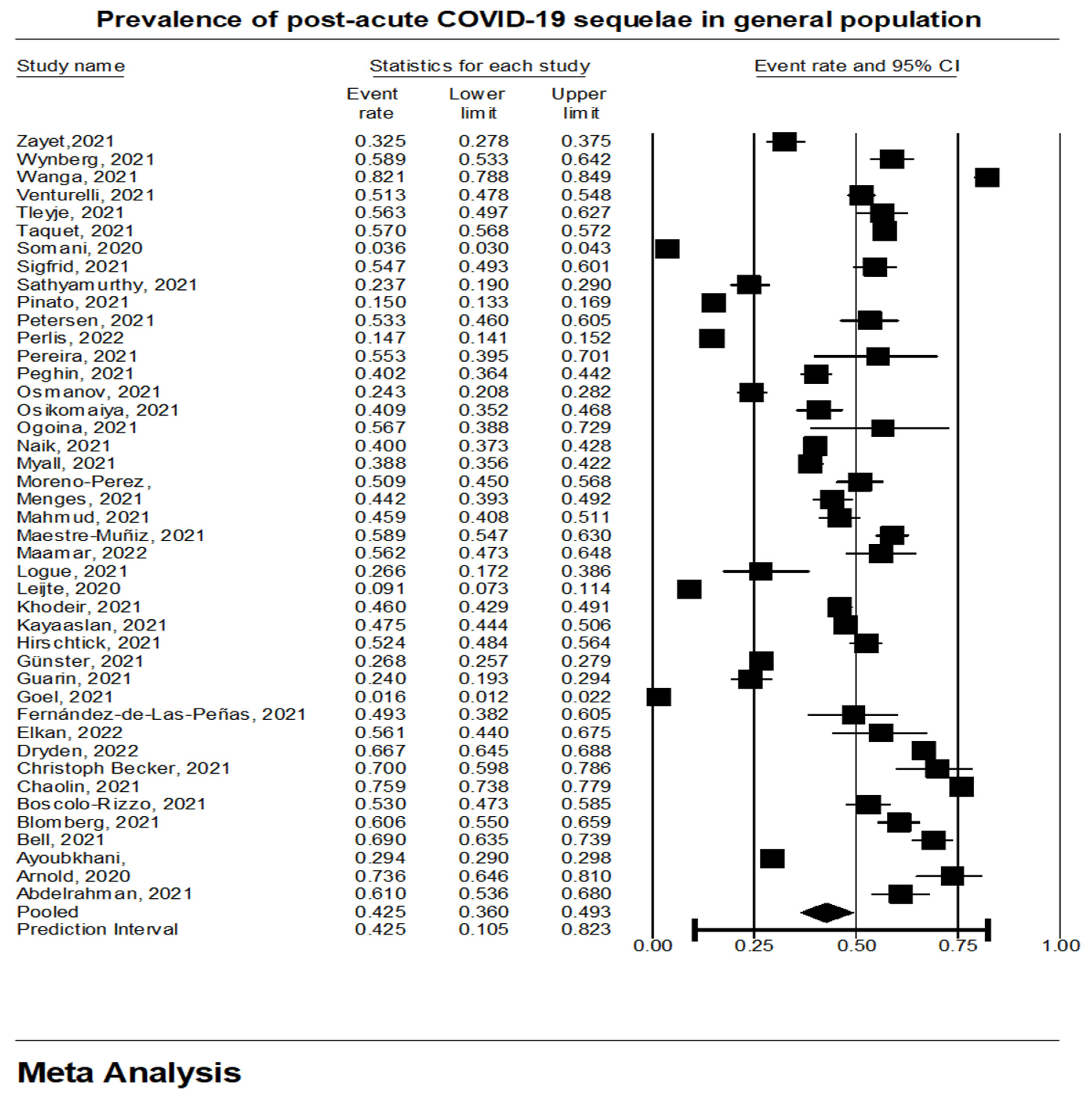
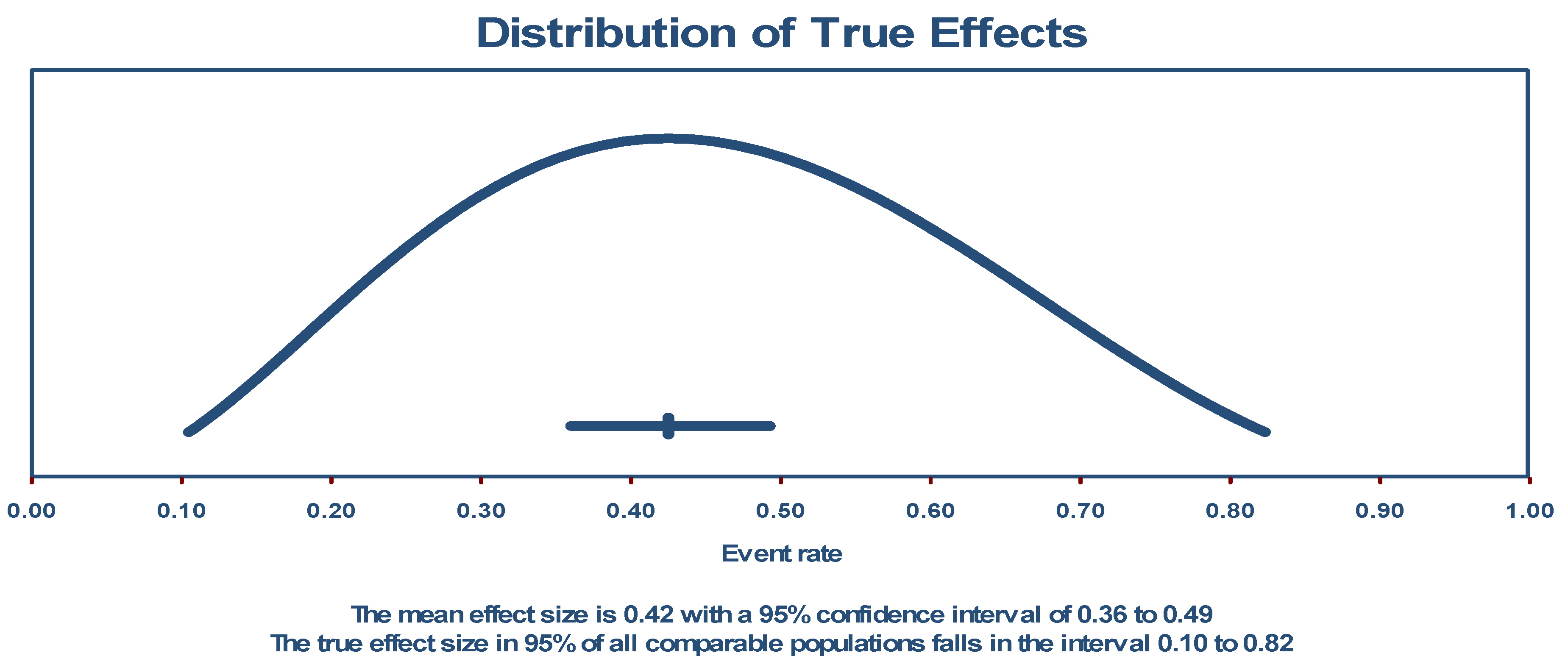

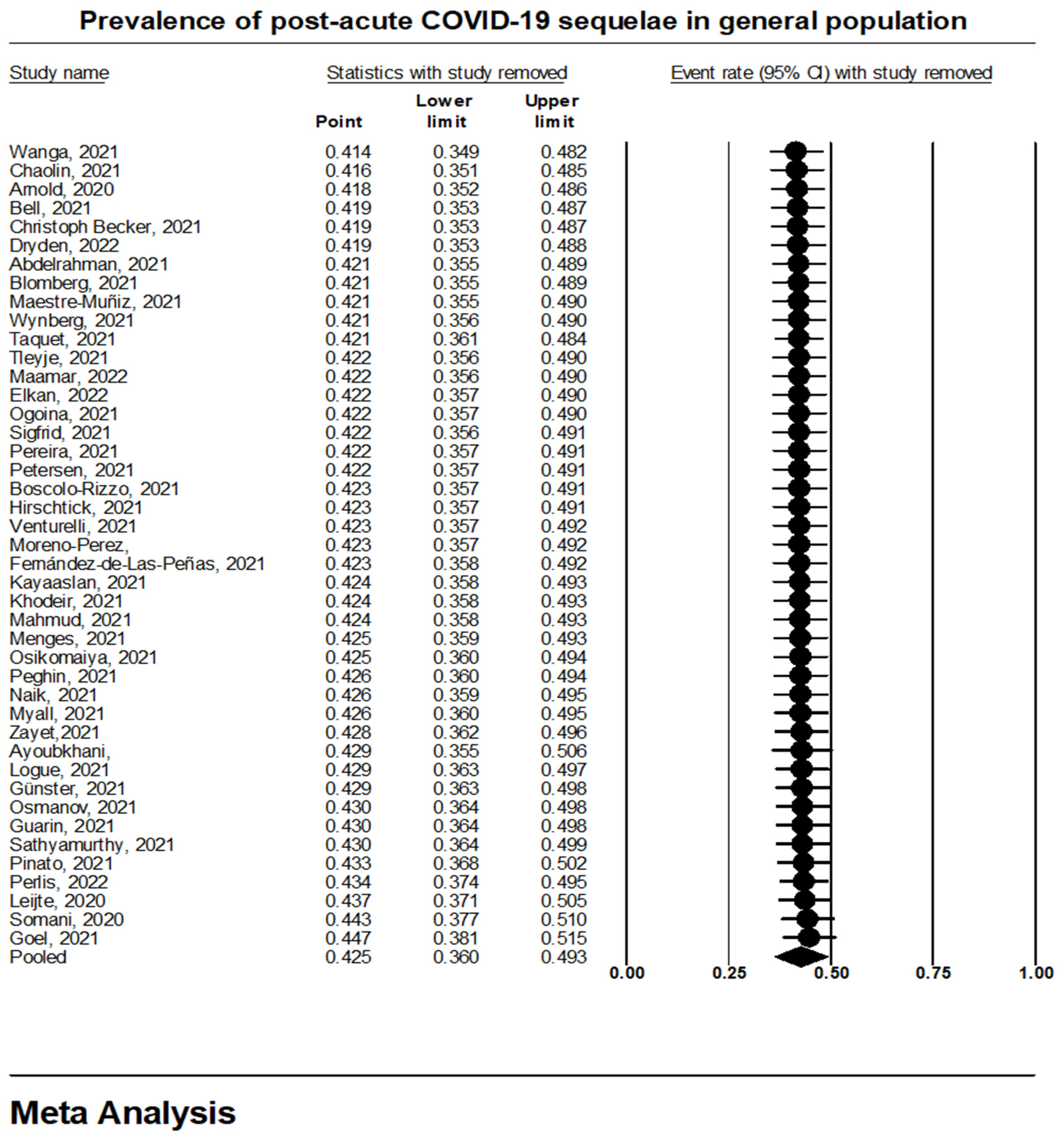
| Study | Region | Study Design | Study Setting | Average Time of Diagnosis (Months) | |
|---|---|---|---|---|---|
| 1. | [61] | Africa | Prospective | Single | 2 |
| 2. | [53] | Europe | Prospective | Single | 3 |
| 3. | [54] | Europe | Retrospective | Multicenter | 5 |
| 4. | [50] | USA | Prospective | Multicenter | 2 |
| 5. | [4] | Europe | Prospective | Single | 6 |
| 6. | [42] | Europe | Prospective | Multicenter | 12 |
| 7. | [1] | Asia | Prospective | Single | 6 |
| 8. | [51] | Europe | Prospective | Multicenter | 12 |
| 9. | [49] | Africa | Prospective | Single | 3 |
| 10. | [44] | Asia | Retrospective | Single | 9 |
| 11. | [29] | Europe | Cross-sectional | Single | 11 |
| 12. | [18] | Asia | Retrospective | Single | - |
| 13. | [26] | USA | Prospective | Single | 4 |
| 14. | [28] | Europe | Retrospective | Multicenter | 6 |
| 15. | [41] | USA | Retrospective | Multicenter | 1 |
| 16. | [37] | Asia | Prospective | Multicenter | 3 |
| 17. | [36] | Asia | Cross-sectional | Multicenter | 3 |
| 18. | [23] | USA | Retrospective | Single | - |
| 19. | [27] | USA | Prospective | Single | 6 |
| 20. | [45] | USA | Cross-sectional | Single | - |
| 21. | [38] | Europe | Retrospective | Single | 12 |
| 22. | [35] | Asia | Prospective | Single | - |
| 23. | [34] | Europe | Prospective | Multicenter | 7 |
| 24. | [39] | Europe | Prospective | Multicenter | 3 |
| 25. | [31] | Europe | Prospective | Single | 1 |
| 26. | [32] | Asia | Prospective | Single | - |
| 27. | [47] | Africa | Retrospective | Single | 5 |
| 28. | [55] | Africa | Retrospective | Single | 5 |
| 29. | [59] | Asia | Prospective | Single | - |
| 30. | [33] | Europe | Bidirectional prospective | Single | 6 |
| 31. | [60] | Europe | Cross-sectional | Single | 7 |
| 32. | [22] | USA | Retrospective | Multicenter | 5 |
| 33. | [43] | Europe | Prospective | Single | 4 |
| 34. | [24] | Europe | Retrospective | Multicenter | 4 |
| 35. | [25] | Asia | Prospective | Single | 3 |
| 36. | [56] | Europe | Prospective | Multicenter | 3 |
| 37. | [21] | USA | Retrospective | Single | - |
| 38. | [57] | USA | Retrospective | Multicenter | 6 |
| 39. | [46] | Asia | Cross-sectional | Single | 4 |
| 40. | [40] | Europe | Prospective | Single | 3 |
| 41. | [20] | USA | Prospective | Multicenter | - |
| 42. | [58] | Europe | Prospective | Single | 3 |
| 43. | [30] | Europe | Retrospective | Single | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muthuka, J.K.; Nzioki, J.M.; Kelly, J.O.; Musangi, E.N.; Chebungei, L.C.; Nabaweesi, R.; Kiptoo, M.K. Prevalence and Predictors of Long COVID-19 and the Average Time to Diagnosis in the General Population: A Systematic Review, Meta-Analysis and Meta-Regression. COVID 2024, 4, 968-981. https://doi.org/10.3390/covid4070067
Muthuka JK, Nzioki JM, Kelly JO, Musangi EN, Chebungei LC, Nabaweesi R, Kiptoo MK. Prevalence and Predictors of Long COVID-19 and the Average Time to Diagnosis in the General Population: A Systematic Review, Meta-Analysis and Meta-Regression. COVID. 2024; 4(7):968-981. https://doi.org/10.3390/covid4070067
Chicago/Turabian StyleMuthuka, John Kyalo, Japeth Mativo Nzioki, Jack Oluoch Kelly, Everlyn Nyamai Musangi, Lucy Chepkemei Chebungei, Rosemary Nabaweesi, and Michael Kibet Kiptoo. 2024. "Prevalence and Predictors of Long COVID-19 and the Average Time to Diagnosis in the General Population: A Systematic Review, Meta-Analysis and Meta-Regression" COVID 4, no. 7: 968-981. https://doi.org/10.3390/covid4070067
APA StyleMuthuka, J. K., Nzioki, J. M., Kelly, J. O., Musangi, E. N., Chebungei, L. C., Nabaweesi, R., & Kiptoo, M. K. (2024). Prevalence and Predictors of Long COVID-19 and the Average Time to Diagnosis in the General Population: A Systematic Review, Meta-Analysis and Meta-Regression. COVID, 4(7), 968-981. https://doi.org/10.3390/covid4070067










