New Onset Generalized Myasthenia Gravis Evolving Following SARS-CoV-2 Infection
Abstract
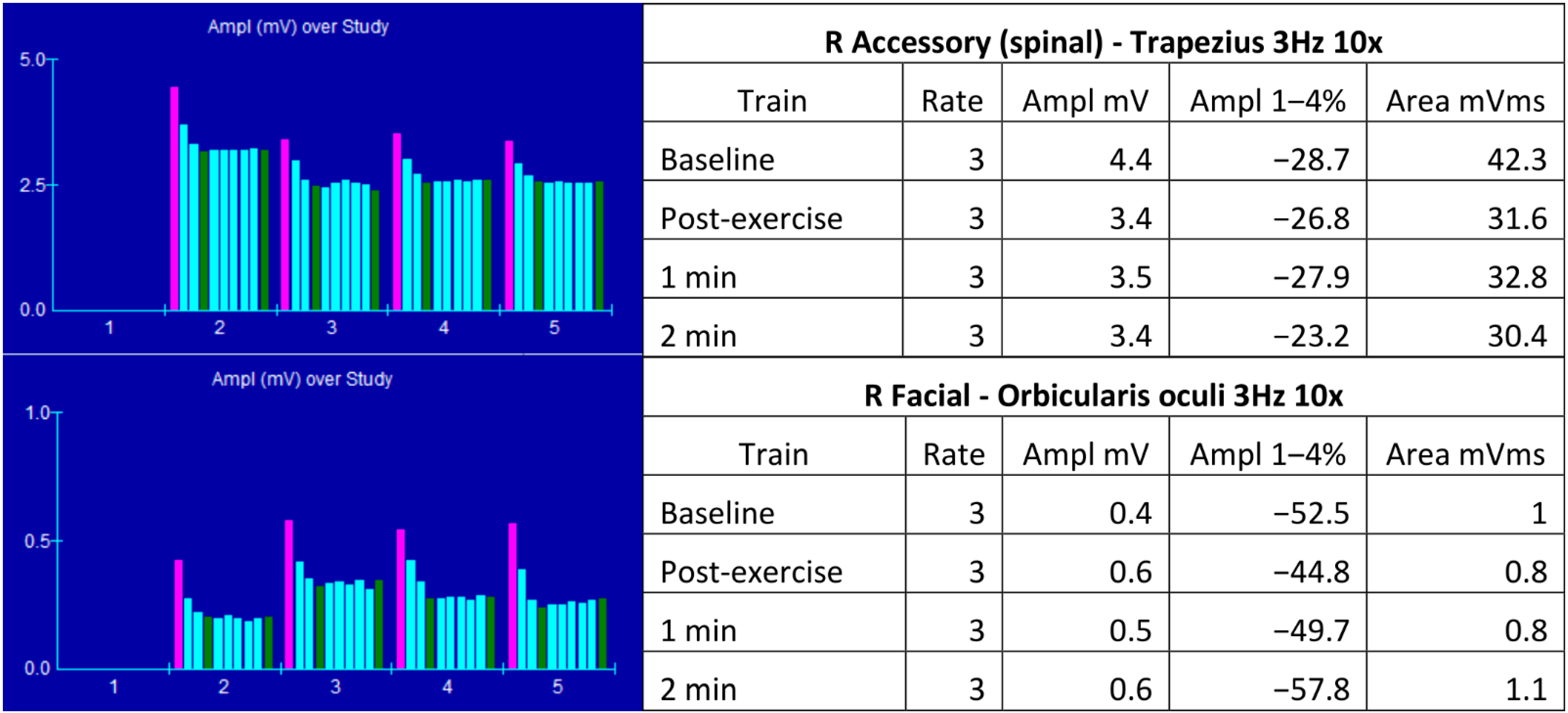
:1. Case Report
2. Overview of Myasthenia Gravis and SARS-CoV-2 Infection
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carr, A.S.; Cardwell, C.; McCarron, P.; McConville, J. A systematic review of population based epidemiological studies in Myasthenia Gravis. BMC Neurol. 2010, 10, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilhus, N.E.; Tzartos, S.; Evoli, A.; Palace, J.; Burns, T.M.; Verschuuren, J. Myasthenia gravis. Nat. Rev. Dis. Primers 2019, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Tzartos, S.J.; Lindstrom, J.M. Monoclonal antibodies used to probe acetylcholine receptor structure: Localization of the main immunogenic region and detection of similarities between subunits. Proc. Natl. Acad. Sci. USA 1980, 77, 755–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindstrom, J. An assay for antibodies to human acetylcholine receptor in serum from patients with myasthenia gravis. Clin. Immunol. Immunopathol. 1977, 7, 36–43. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Yang, H.; Han, J.; Zhao, X.; Han, S.; Zhang, Y.; Lv, J.; Zhang, J.; Li, M.; et al. No correlation between acetylcholine receptor antibody concentration and individual clinical symptoms of myasthenia gravis: A systematic retrospective study involving 67 patients. Brain Behav. 2021, 11, e02203. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.M.; Gajdos, P.; Eymard, B.; Tranchant, C.; Warter, J.M.; Gomez, L.; Bourquin, C.; Bach, J.-F.; Garchon, H.-J. Anti-titin antibodies in myasthenia gravis: Tight association with thymoma and heterogeneity of nonthymoma patients. Arch. Neurol. 2001, 58, 885–890. [Google Scholar] [CrossRef] [Green Version]
- Romi, F.; Skeie, G.O.; Aarli, J.A.; Gilhus, N.E. The severity of myasthenia gravis correlates with the serum concentration of titin and ryanodine receptor antibodies. Arch. Neurol. 2000, 57, 1596–1600. [Google Scholar] [CrossRef] [Green Version]
- Skeie, G.O.; Mygland, Å.; Aarli, J.A.; Gilhus, N.E. Titin Antibodies in Patients with Late Onset Myasthenia Gravis: Clinical Correlations. Autoimmunity 1995, 20, 99–104. [Google Scholar] [CrossRef]
- Gautel, M.; Lakey, A.; Barlow, D.P.; Holmes, Z.; Scales, S.; Leonard, K.; Labeit, S.; Mygland, A.; Gilhus, N.E.; Aarli, J.A. Titin antibodies in myasthenia gravis: Identification of a major immunogenic region of titin. Neurology 1993, 43, 1581. [Google Scholar] [CrossRef]
- Meriggioli, M.N.; Sanders, D.B. Muscle autoantibodies in myasthenia gravis: Beyond diagnosis? Expert Rev. Clin. Immunol. 2012, 8, 427–438. [Google Scholar] [CrossRef] [Green Version]
- Sieb, J.P. Myasthenia gravis: An update for the clinician. Clin. Exp. Immunol. 2014, 175, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Bubuioc, A.-M.; Kudebayeva, A.; Turuspekova, S.; Lisnic, V.; Leone, M.A. The epidemiology of myasthenia gravis. J. Med. Life 2021, 14, 7–16. [Google Scholar] [CrossRef]
- Muppidi, S.; Guptill, J.T.; Jacob, S.; Li, Y.; Farrugia, M.E.; Guidon, A.C.; Tavee, J.O.; Kaminski, H.; Howard, J.F.; Cutter, G.; et al. COVID-19-associated risks and effects in myasthenia gravis (CARE-MG). Lancet Neurol. 2020, 19, 970–971. [Google Scholar] [CrossRef]
- Assini, A.; Gandoglia, I.; Damato, V.; Rikani, K.; Evoli, A.; Del Sette, M. Myasthenia gravis associated with anti-MuSK antibodies developed after SARS-CoV-2 infection. Eur. J. Neurol. 2021, 28, 3537–3539. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Rogozinski, S.; Puppe, W.; Framme, C.; Höglinger, G.; Hufendiek, K.; Wegner, F. Postinfectious Onset of Myasthenia Gravis in a COVID-19 Patient. Front. Neurol. 2020, 11, 576153. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, L.; Baheerathan, A.; Cao, M.; Leite, M.I.; Viegas, S. MuSK Antibody-Associated Myasthenia Gravis With SARS-CoV-2 Infection: A Case Report. Ann. Intern. Med. 2021, 174, 872–873. [Google Scholar] [CrossRef]
- Reddy, M.Y.; Kumar, S.B.; Osman, S.; Murthy, J.M.K. Temporal association between SARS-CoV-2 and new-onset myasthenia gravis: Is it causal or coincidental? BMJ Case Rep. 2021, 14, e244146. [Google Scholar] [CrossRef]
- Restivo, D.A.; Centonze, D.; Alesina, A.; Marchese-Ragona, R. Myasthenia Gravis Associated With SARS-CoV-2 Infection. Ann. Intern. Med. 2020, 173, 1027–1028. [Google Scholar] [CrossRef]
- Sriwastava, S.; Tandon, M.; Kataria, S.; Daimee, M.; Sultan, S. New onset of ocular myasthenia gravis in a patient with COVID-19: A novel case report and literature review. J. Neurol. 2021, 268, 2690–2696. [Google Scholar] [CrossRef]
- Perez-Alvarez, A.I.; Suarez-Cuervo, C.; Fernandez-Menendez, S. SARS-CoV-2 infection associated with diplopia and an-ti-acetylcholine receptor antibodies. Neurologia 2020, 35, 264–265. [Google Scholar]
- Elsner, R.A.; Shlomchik, M.J. Germinal Center and Extrafollicular B Cell Responses in Vaccination, Immunity, and Autoimmunity. Immunity 2020, 53, 1136–1150. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.C.; Ramonell, R.P.; Nguyen, D.C.; Cashman, K.S.; Saini, A.S.; Haddad, N.S.; Ley, A.M.; Kyu, S.; Howell, J.C.; Ozturk, T.; et al. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat. Immunol. 2020, 21, 1506–1516. [Google Scholar] [CrossRef] [PubMed]
- Fichtner, M.L.; Jiang, R.; Bourke, A.; Nowak, R.J.; O’Connor, K.C. Autoimmune Pathology in Myasthenia Gravis Disease Subtypes Is Governed by Divergent Mechanisms of Immunopathology. Front. Immunol. 2020, 11, 776. [Google Scholar] [CrossRef] [PubMed]
- Bannard, O.; Cyster, J.G. Germinal centers: Programmed for affinity maturation and antibody diversification. Curr. Opin. Immunol. 2017, 45, 21–30. [Google Scholar] [CrossRef]
- Laidlaw, B.J.; Ellebedy, A.H. The germinal centre B cell response to SARS-CoV-2. Nat. Rev. Immunol. 2022, 22, 7–18. [Google Scholar] [CrossRef]
- Zagoriti, Z.; Kambouris, M.E.; Patrinos, G.P.; Tzartos, S.J.; Poulas, K. Recent Advances in Genetic Predisposition of Myasthenia Gravis. BioMed Res. Int. 2013, 2013, 404053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousefipour, G.A.; Salami, Z.; Farjadian, S. Association of HLA-DQA1*0101/2 and DQB1*0502 with myasthenia gravis in southern Iranian patients. Iran J. Immunol. 2009, 6, 99–102. [Google Scholar] [PubMed]
- Maniaol, A.H.; Elsais, A.; Lorentzen-Åslaug, R.; Owe, J.F.; Viken, M.K.; Sæther, H.; Flåm, S.T.; Bråthen, G.; Kampman, M.T.; Midgard, R.; et al. Late Onset Myasthenia Gravis Is Associated with HLA DRB1*15:01 in the Norwegian Population. PLoS ONE 2012, 7, e36603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, H.; Zhao, C.; Luo, S. HLA in myasthenia gravis: From superficial correlation to underlying mechanism. Autoimmun. Rev. 2019, 18, 102349. [Google Scholar] [CrossRef] [PubMed]
- Alahgholi-Hajibehzad, M.; Yilmaz, V.; Gulsen-Parman, Y.; Aysal, F.; Oflazer, P.; Deymeer, F.; Saruhan-Direskeneli, G. Association of HLA-DRB1 *14, -DRB1 *16 and -DQB1 *05 with MuSK-myasthenia gravis in patients from Turkey. Hum. Immunol. 2013, 74, 1633–1635. [Google Scholar] [CrossRef]
- Kanai, T.; Uzawa, A.; Kawaguchi, N.; Sakamaki, T.; Yoshiyama, Y.; Himuro, K.; Oda, F.; Kuwabara, S. HLA-DRB1*14 and DQB1*05 are associated with Japanese anti-MuSK antibody-positive myasthenia gravis patients. J. Neurol. Sci. 2016, 363, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Kanduc, D. Anti-SARS-CoV-2 Immune Response and Sudden Death: Titin as a Link. Adv. Stud. Biol. 2021, 13, 37–44. [Google Scholar] [CrossRef]
- Farrugia, M.E.; Goodfellow, J.A. A Practical Approach to Managing Patients With Myasthenia Gravis—Opinions and a Review of the Literature. Front. Neurol. 2020, 11, 604. [Google Scholar] [CrossRef]

| Author | Age/Gender | COVID-19 Infection Severity | Latency of MG Symptom Onset | MG Presentation | MG Serological Findings | Thymus Pathology |
|---|---|---|---|---|---|---|
| Restivo et al., 2020 [18] | 64/M | mild | 5 days | generalized | AChR Abs + | none |
| 68/M | mild | 7 days | generalized | AChR Abs + | none | |
| 71/M | severe | 5 days | generalized | AChR Abs + | none | |
| Sriwastawa et al., 2021 [19] | 65/F | severe | 11 days | ocular | AChR Abs + | none |
| Huber et al., 2020 [15] | 21/F | mild | 10 days | ocular | AChR Abs +, titin Abs - | none |
| Muralidhar Reddy et al., 2021 [17] | 65/M | mild | 42 days | generalized | AChR Abs + | none |
| Perez Alvarez et al., 2020 [20] | 48/M | mild | 15 days | ocular | AChR Abs + | none |
| Assini et al., 2021 [14] | 77/M | NA | 56 days | oculobulbar | MuSK Abs + | none |
| Muhammed et al., 2021 [16] | 24/F | mild | 28 days | generalized | MuSK Abs + | none |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jõgi, K.; Sabre, L.; Rosental, M.; Leheste, A.-R.; Vilisaar, J. New Onset Generalized Myasthenia Gravis Evolving Following SARS-CoV-2 Infection. COVID 2022, 2, 464-471. https://doi.org/10.3390/covid2040033
Jõgi K, Sabre L, Rosental M, Leheste A-R, Vilisaar J. New Onset Generalized Myasthenia Gravis Evolving Following SARS-CoV-2 Infection. COVID. 2022; 2(4):464-471. https://doi.org/10.3390/covid2040033
Chicago/Turabian StyleJõgi, Karl, Liis Sabre, Merit Rosental, Alo-Rainer Leheste, and Janek Vilisaar. 2022. "New Onset Generalized Myasthenia Gravis Evolving Following SARS-CoV-2 Infection" COVID 2, no. 4: 464-471. https://doi.org/10.3390/covid2040033
APA StyleJõgi, K., Sabre, L., Rosental, M., Leheste, A.-R., & Vilisaar, J. (2022). New Onset Generalized Myasthenia Gravis Evolving Following SARS-CoV-2 Infection. COVID, 2(4), 464-471. https://doi.org/10.3390/covid2040033






