Abstract
Since 2019, COVID-19 began spreading globally and has significantly affected peoples’ daily lifestyles. The public was asked to stay at home for constant quarantine and community containment starting on 23 January 2020. To assess the circadian rhythms and sleep changes and their influential factors during the COVID-19 outbreak, a questionnaire was administered to 451 Chinese participants during 20–31 January 2020. The changes in circadian rhythm, sleep–wake cycle, dining, and exercise of the participants and their correlation with negative emotions were analyzed. Furthermore, the effects of three factors (holiday, quarantine, and concerns regarding the pandemic situation) on these changes were assessed. We found that 34.6% of the participants reported circadian rhythm disturbance. Moreover, 67.2% presented negative emotions (worry, fear, downheartedness, anxiety, depression, and stupefaction) regarding the pandemic situation, among which worry was the most prevalent. Gender and age were significant factors for changes in the circadian phases and emotions. There was a correlation between circadian rhythm alterations and negative emotions. In addition, holiday, quarantine, and concerns regarding the pandemic situation had significant effects on circadian rhythms and sleep in a substantial part of the population. Regression analysis demonstrated reciprocal influences between many of these variables. Our findings suggest that circadian rhythms, sleep, and negative emotions in the normal population need to be considered during the pandemic period and that the adjustment of circadian rhythms could help promote sleep, restore emotions and improve public health.
1. Introduction
COVID-19 (novel coronavirus pneumonia) is a newly emerging severe acute respiratory infectious disease that is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. To suppress COVID-19 spread in China, 31 provinces, autonomous regions, and municipalities declared a level 1 health emergency; furthermore, the public was required to stay at home for quarantine and community containment [2,3]. This resulted in extensive lifestyle changes, with the public continuously staying at home for long periods since 23 January 2020, and only leaving discreetly to shop for food and necessities was allowed. As the pandemic continues to spread, lockdown measures are also implemented in many other countries.
The circadian clock controls the rhythmicity of physiology and behavior, which endows the adaptability to cycling environmental cues with a period of 24 h [4,5,6]. Circadian rhythm disturbances cause sleep disorders, metabolic diseases, and decreased immunity [7]. Circadian typology is tightly related to mental health aspects including emotion regulation, and circadian misalignment accounts for increased mood vulnerability [8,9]. Moreover, circadian desynchronization is related to several mood disorders, including depression, seasonal affective disorder, and bipolar syndrome [10,11,12,13,14]. In addition to circadian rhythm, there is a bidirectional relationship between emotion and sleep—aberrant sleep leads to emotional stresses and vice versa [15]. These facts suggest that circadian rhythms, sleep, and emotions are tightly interlocked.
Circadian rhythms are affected by social cues, including working schedules, shift work, and jet lag [16]. Confinement is also a social factor affecting circadian rhythms and sleep. In the Mars500 mission that mimicked the impact of a confined cabin, Earth–Mars communication, and space tasks, the crew members showed prolonged sleep duration (8.4%), disturbed sleep–wake cycles, and decreased sleep quality during the simulated voyage [17].
In history, the survivors of illness experienced isolation during the outbreaks of different pandemics (e.g., SARS in 2003, Ebola in 2014, and Zika in 2016) experienced mental stresses [18]. During the outbreak of COVID-19 in China, multiple factors, including confinement due to quarantine and community containment, irregular lifestyle during the Spring Festival, and concerns regarding the pandemic situation, may have imposed interwoven influences on the circadian rhythm, sleep, and mood of the public. Specially, in China, since the pandemic period covered the Spring Festival holiday, the irregular lifestyle during the Spring Festival may be an additional influential factor. Understanding how the population’s circadian rhythms, sleep, and emotions are affected by such events is critical for developing proper countermeasures to improve the public health during this pandemic era.
To this end, we conducted a nationwide survey of public sleep–wake schedules, exercising, dining, and mood from 20 January 2020 to 31 January 2020, which revealed that alterations in circadian rhythms, sleep, and negative emotion occurred in a substantial proportion of participants. Our findings revealed the alterations in circadian rhythms, sleep, and negative emotion in a substantial proportion of participants, and their correlations were analyzed.
2. Materials and Methods
2.1. Measurements and Procedures
Due to the COVID-19 outbreak, a lockdown was implemented in Wuhan on 23 January; from this time, Chinese people across the country began staying at home for quarantine. We designed a questionnaire to collect information regarding circadian rhythms, sleep, and negative emotions. The surveyed period was between 20 January 2020 and 31 January 2020, which comprised nine days after the emergency was launched. Furthermore, the Spring Festival holiday was between 20 January and 2 February. To discriminate changes between the period after and before (control) the emergency launch, most sections consisted of questions comparing changes in variables between the period 20 January 2020 and 31 January 2020 and the period of one week before 20 January 2020.
The questionnaire obtained information on characteristics, circadian rhythms and sleep, negative emotions, and the subjective evaluation of the effects of three factors (Spring Festival holiday, concerns regarding pandemic situations, and constant quarantine) on circadian rhythms and sleep (Supplemental File S1). We assessed sleepiness with the Karolinska Sleepiness Scale (KSS), and we used the simplified Beck Depression Inventory (BDI) to evaluate changes in emotions [19,20]. The questions assessing circadian typology were designed by referring to the Morningness-Eveningness Questionnaire [21]. The questionnaire was administered on the Wen Juan Xing platform website (www.wjx.cn, accessed on 31 January 2020) with 451 filled forms being collected. The content of the questionnaire was validated by Sun Yat-sen University.
2.2. Participants and Ethics
All of the participants were Chinese. In the 451 participants, 448 Chinese responses were obtained from participants in 31 provinces, autonomous regions, municipalities, and Special Administrative Regions by random. Three participants were Chinese nationals living overseas (Table S1). The minimum sample size was calculated using the G*Power 3.1 software (Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany). According to the G*Power for Wilcoxon signed-rank test (matched pairs), a sample size of 164 was suggested with α = 0.05, power = 0.8, effect size = 0.2. Besides, given the parameters of α = 0.05, effect size = 0.2, and the actual total sample size of 451, the power was high (1 − β = 0.99). These data suggest that the sample size in the present study was appropriate. There were 170 (37.7%) male and 281 (62.3%) female participants, respectively. The participants were in different ages, education levels, and occupations (Table S1). None of the participants reported being patients of COVID-19 novel coronavirus pneumonia. This anonymous survey was approved by the Committee of Ethics, University of Sun Yat-sen University. All the participants were informed of the basic information about the survey, including the objective and content in a written form before they filled the forms. Written consents were obtained from the respondents and from guardians of respondents under eighteen. All participants gave their informed consent for inclusion before they participated in the study.
2.3. Statistics
The Kolmogorov–Smirnov test was conducted to assess the normality of samples [22]. The Wilcoxon signed-rank test was performed to assess between-period differences in circadian rhythms and sleep [23]. Between-group differences were analyzed using the Mann–Whitney U test with statistical significance set at p < 0.05 [24]. Bivariate (Spearman) correlation analysis was used to assess the between-variable correlation for each comparison [25]. In addition, we performed linear regression to further quantify the strength of the relationships among circadian rhythms, sleep, and negative emotions and to evaluate the influence of Spring Festival Holiday, concerns regarding the pandemic situation, and constant quarantine or community containment on the changes in circadian rhythms and sleep. SPSS 26 (IBM, Chigao, USA), GraphPad Prism 8 (Graphpad Software Inc., San Diego, USA), and RStudio 1.2.1335 (RStudio Inc., Boston, USA) were used for statistical analysis and picture drawing. p < 0.05 was considered significant with the significant values being * p < 0.05 and ** p < 0.01. Additional details regarding the method and the results of correlation coefficient analysis can be found online in the Supplementary Materials.
3. Results
3.1. Changes in the Circadian Rhythm, Sleep–Wake Cycle, Dining, and Exercise
To assess the changes in the circadian phases, we classified the participants into three chronotypes according to arbitrary cut-off scores based on their sleep onset time during the survey period as follows: early-type, slept before 22:00; intermediate type, slept between 22:00 and 24:00; late-type, slept after 24:00. In addition, irregular type denotes people who showed no fixed sleep time. Both the time of falling asleep and waking up are circadian phase references. In this study, we used the former parameter to assess the changes in the circadian phase since there was a significant positive correlation between both parameters (Table S2).
Among the 451 participants, during the quarantine period, 34.6% (156) of the participants reported chronotype changes. Among these 156 individuals, 81 individuals (18.0%) showed a delayed phase while 33 (7.3%) showed an advanced phase (Figure 1A). The most prevalent (13.7% participants [62/451]) changed from the intermediate type to the late type (62/451) (Figure 1B). During the quarantine period, the proportion of participants who woke up prior to 7:00 before and during the quarantine period was 35.5% (160) and 10.6% (48), respectively. In contrast, the fraction of participants who woke up after 9:00 before and during the quarantine period was 12.9% (58) and 42.1% (190), respectively (Table S3). The sleep schedule became irregular and regular in 31.0% (140) and 14.6% (66) of the participants (Figure 1C). Compared with the parameters before the quarantine period, 16 (3.6%) and 119 (26.4%) participants showed an increase and decrease in exercise, respectively. A total of 20 (4.3%) and 95 (21.1%) participants reported increased and decreased dining frequencies, respectively (Figure 1D).
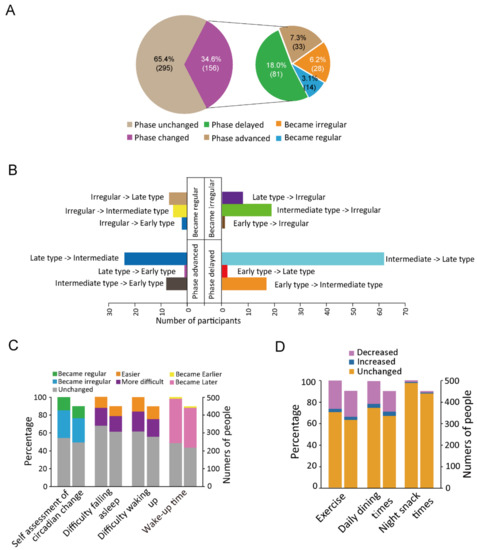
Figure 1.
Changes in the circadian rhythm characteristics. (A): The proportions of participants who reported changes in their circadian rhythms. (B): The bar chart shows the number of people who reported each of the 4 phase-change types (12 subtypes). (C): The percentages and numbers of people who reported each of the four types of circadian rhythm changes. (D): The percentages and numbers of people who reported changes in exercise, daily dining times, and night snack times. For each stacked bar chart (C,D), in two parallel bars, the one on the left represents proportion and the one on the right represents the number of people.
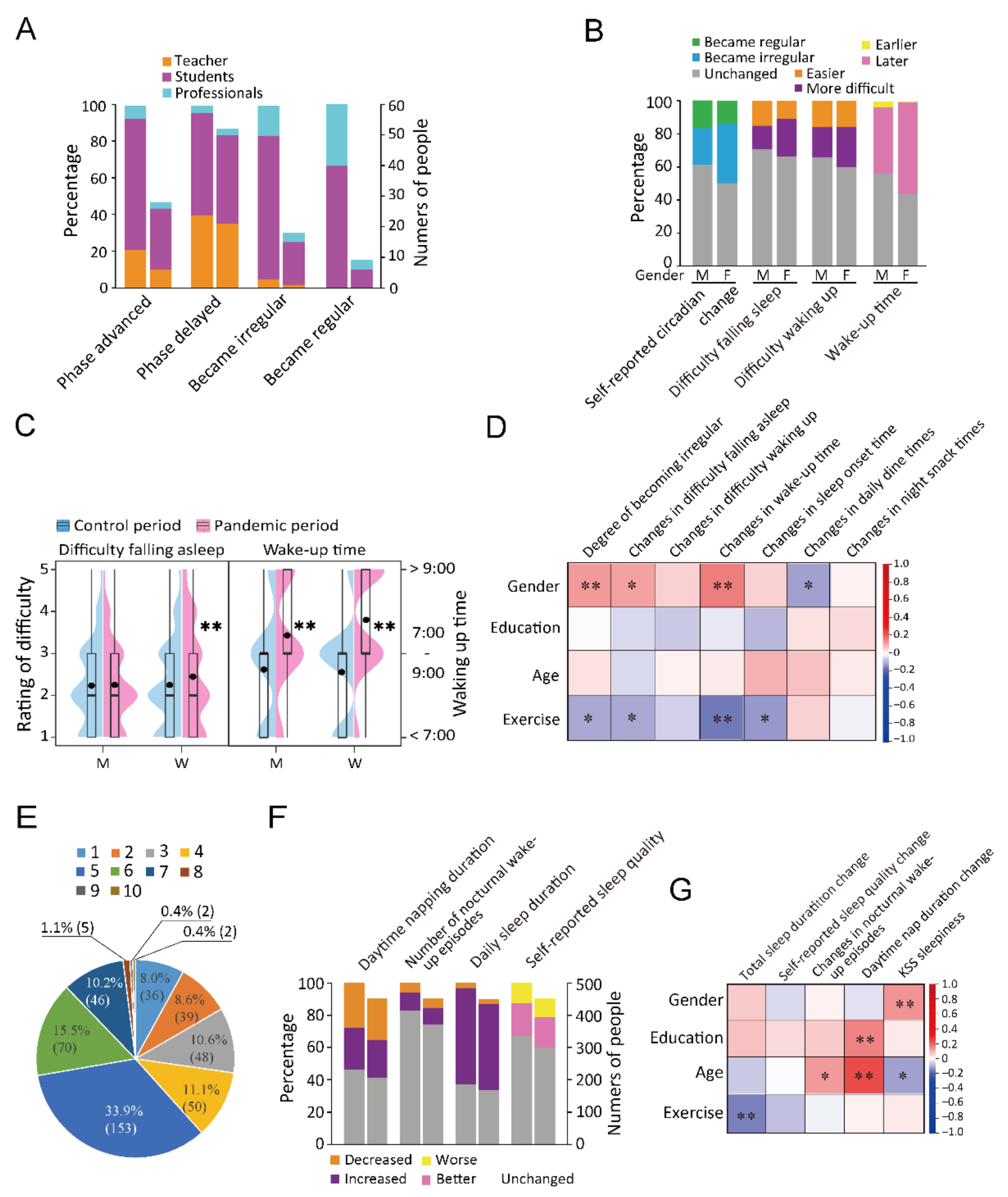
Among the teachers, 29.6% (21/71) of individuals showed a delayed sleep phase, which was a notable increase from the average (18.0%). Among the students, 9.5% (20/210) and 13.8% (29/210) reported an advanced and delayed sleep phase, respectively (Figure 2A). Among the participants, 102 (36.3%) females and 38 (22.4%) males reported more irregular time of sleeping. Increased difficulty waking up and falling asleep was reported by 18.8% and 14.7% of the males, respectively, and 24.9% and 23.5% of the females, respectively (Figure 2B,C). In addition, 26.7% (27/101) of individuals aged between 41 and 50 years showed a delayed sleep phase, which was approximately 9.0% more than the proportion in other age groups (Table S4). There was a positive correlation between gender and irregular rhythms (r = 0.130, p = 0.006), delayed waking up (r = 0.163, p = 0.001), and difficulty falling asleep (r = 0.115, p = 0.015). Furthermore, there was a positive correlation between age and increased nocturnal wake-up episodes (r = 0.122, p = 0.011) and prolonged daytime napping (r = 0.236, p < 0.001); moreover, age was negatively correlated with KSS-rated sleepiness (r = −0.115, p = 0.015). Education level was positively associated with increased exercise (r = 0.094, p = 0.046). Exercise was negatively associated with self-reported circadian change (r = −0.109, p = 0.021), difficulty falling asleep (r = −0.109, p = 0.021), delayed degree of waking up (r = −0.164, p < 0.001), and delayed degree of falling asleep (r = −0.125, p = 0.012) (Figure 2D and Table S5).
Figure 2.
Changes in sleep-related parameters. (A): The percentages and numbers of participants of each profession (teachers, students, and professionals) in phase changes of the four variables. (B): The percentages of people of different genders in four circadian rhythm variables. M presents male and F presents female. (C): Violin plot and box plot visualizing the between-gender comparison of difficulty falling asleep and wake-up time. M presents male and F presents female. ** p < 0.01. (D): Correlations between demographic factors and circadian rhythms. * p < 0.05, ** p < 0.01. (E): Distribution of 10 scores of KSS-rated sleepiness. (F): An accumulative bar diagram showing the percentages and numbers of people who reported changes in the four sleep variables. (G): Correlations between demographic factors and sleep. * p < 0.05, **p < 0.01. For stacked bar charts (A,F), in two parallel bars, the one on the left represents proportion and the one on the right represents number of people.
3.2. Analysis of Changes in Sleep Duration and Quality
We also assessed sleep quantity and quality by investigating daily total sleep duration, daytime nap duration, nocturnal wake-up times, and KSS-rated sleepiness, in which KSS referred to the average daytime sleepiness in the pandemic period. During the quarantine period, 59.7% (269), 3.1% (14), and 37.3% (168) of the participants reported an increase, decrease, and no change in the daily total sleep duration during the quarantine period (Figure 2E,F). Nocturnal wake-up episodes were associated with sleep quality and caused sleep fragmentation and sleep cycle disturbance. The female gender was positively associated with KSS-rated sleepiness (r = 0.133, p = 0.005). Education level was positively associated with prolonged daytime napping (r = 0.154, p = 0.001). Age was positively associated with increased waking episodes (r = 0.122, p = 0.011) and prolonged daytime napping (r = 0.236, p < 0.001) and negatively associated with KSS-rated sleepiness (r = −0.115, p = 0.015) (Figure 2G and Table S5). After 20 January 2020, female participants reported a later time of waking up and greater difficulty falling asleep than men did (Tables S6 and S7); moreover, women had higher ratios of delayed wake-up time and difficulty falling asleep (Figure 2C,G and Tables S8 and S9).
Compared with that in the control period, there was an increase in the incidence of nocturnal waking episodes (53, 11.8%) during the pandemic period. An assessment of KSS-rated sleepiness showed that most participants had a score of 5 (33.9%, 153). In addition, 123 (27.3%) participants had a score of 1–3, 319 (70.7%) had a score of 4–7, and 9 (2.0%) had a score of 8–10 (Figure 2E). Furthermore, 12.6% (57) reported a subjective decrease in sleep quality (Figure 2F). Before 20 January 2020, 27.9% (126) of the participants did not have a daytime napping habit; among them, 80.2% (101) maintained this habit after the emergency launch. Some of the participants with previous daytime napping did not report it during quarantine. The proportion of participants without daytime napping habits during the quarantine period was 49.0% (221) (Figure 2F). Exercise was negatively associated with prolonged total sleep duration (r = −0.157, p = 0.001) (Figure 2G and Table S5).
3.3. Analysis of Changes in Negative Emotions
The questionnaire assessed negative emotions, including worry, fear, downheartedness, anxiety, depression, and stupefaction, which might be associated with circadian rhythms and sleep. Among the 451 participants, 303 (67.2%) showed at least one negative emotion as follows: worry, 257 (57.0%); anxiety, 231 (51.2%); stupefaction, 222 (49.2%); downheartedness, 214 (47.5%); fear: 208 (46.1%); and depression: 166 (36.8%). Moreover, 148 individuals (32.8% of 451) reported no obvious negative emotions. Among the six negative emotions, participants scored the highest on worry (average 2.18), which 16.4% (74/451) of participants rated as being 4 (moderate negative emotion) or 5 (severe negative emotion). Furthermore, 7.1% (32/451) of the participants rated ≥4 on depression (Figure 3A,B and Table S10).
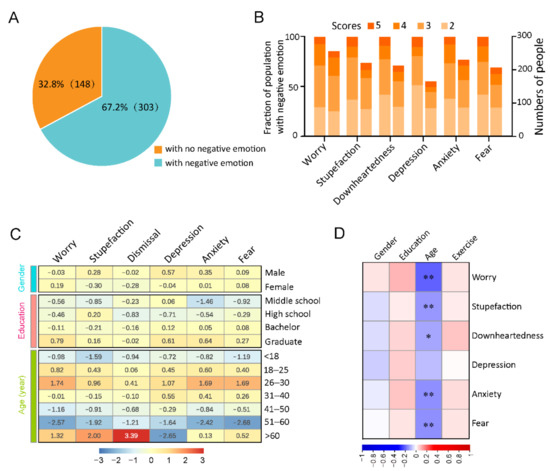
Figure 3.
Analysis of the negative emotion. (A): The percentages of participants who reported and did not report negative emotions. Participants “with negative emotion” is defined as those who reported at least one of the six negative emotions. (B): An accumulative bar diagram showing the fraction (left) and numbers (right) of participants with different grades of assessed negative emotion types. These negative emotions were assessed on a 1–5 scale indicating the different degrees of negative emotions as follows: 1, 2, 3, 4, and 5 indicated no, slight, mild, moderate, and severe negative emotions, respectively. (C): The self-assessed negative emotions were classified according to gender, age, and education. We calculated the average scores of each negative emotion. The numerals in the heat map are Z-score values after normalization by column. * p < 0.05, ** p < 0.01. (D): Correlations between negative emotions and gender, education, age, and exercise. * p < 0.05, ** p < 0.01.
Differentially reported negative emotions between men and women were also observed. For instance, men showed higher scores than women on stupefaction (men 2.05; women 1.89), downheartedness (men 1.97; women 1.85), depression (men 1.74; women 1.60), and anxiety (men 2.05; women 1.97). In addition, individuals with higher education levels reported more negative emotions (Figure 3C and Table S11). Age was negatively associated with worry, stupefaction, downheartedness, anxiety, and fear (Figure 3D and Table S5).
3.4. Correlation Coefficient Analysis among Changes in Circadian Rhythms, Sleep, and Negative Emotions
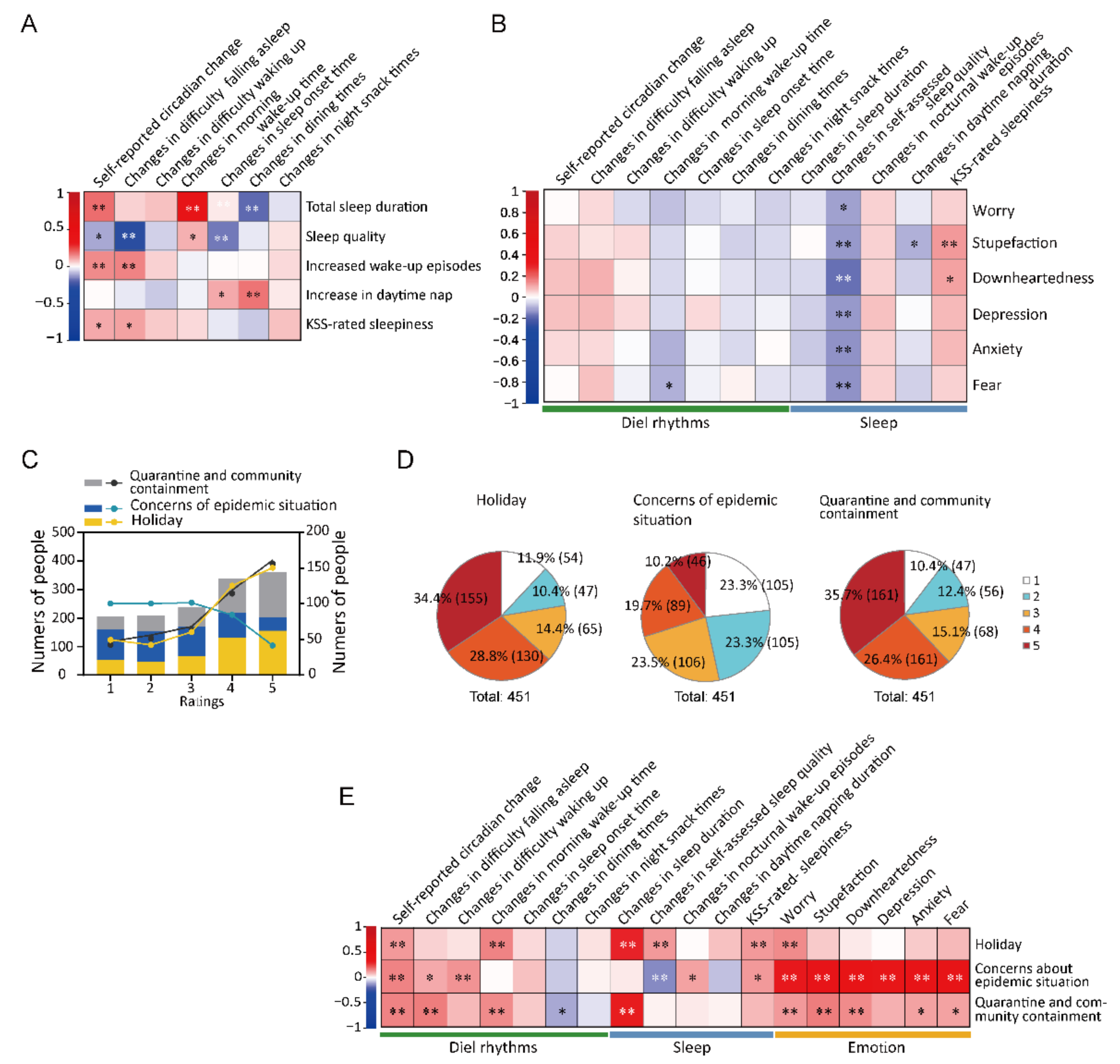
There was a positive correlation between total sleep duration and the frequency of irregular sleep–wake cycles (r = 0.180, p < 0.001) and changes in wake-up time (r = 0.393, p < 0.001), as well as a negative correlation between total sleep duration and changes in dining times (r = −0.187, p < 0.001). Changes in sleep quality were positively associated with changes in wake-up time (r = 0.094, p = 0.045) and negatively associated with the frequency of irregular sleep–wake cycles (r = −0.102, p = 0.030), difficulty falling asleep (r = −0.275, p < 0.001), daytime sleepiness (r = −0.252, p < 0.001), and changes in sleep onset time (r = −0.162, p = 0.001). Increased waking times were positively associated with the frequency of irregular sleep–wake cycles (r = 0.148, p = 0.002) and increased difficulty falling asleep (r = 0.160, p = 0.001). Increased daytime napping was positively associated with changes in sleep-onset time (r = 0.108, p = 0.029) and changes in dining times (r = 0.171, p < 0.001) (Figure 4A and Table S5).
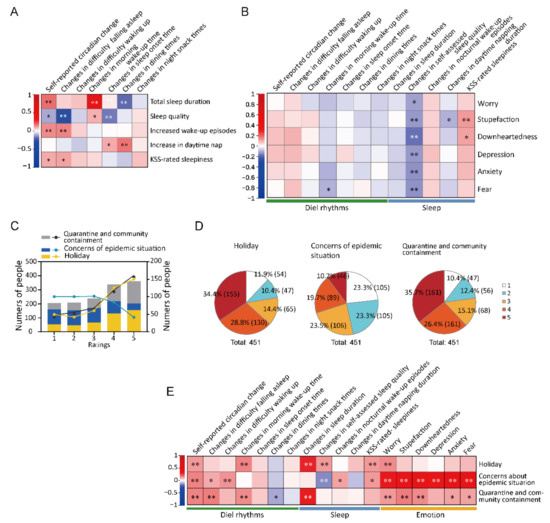
Figure 4.
Correlative analysis among the changes in circadian rhythms, sleep, and mood. (A): Spearman correlation between circadian rhythms and sleep. * p < 0.05, ** p < 0.01. (B): Spearman correlation coefficient analysis of circadian variables, sleep, and mood. * p < 0.05, ** p < 0.01. (C): Subjective self-evaluation of the effects of the three factors: holiday, concerns about the epidemic situation, and quarantine or community containment on circadian rhythms and sleep. Here, 1 indicates no effects while 2–5 indicate increased effects. Columns in the different colors represent the numbers and ratios of participants who rated the corresponding grades as indicated. Line graphs in the different colors represent the numbers of participants who rated the corresponding grades as indicated. (D): Pie charts showing the proportions of participants who reported disturbed circadian rhythms and sleep due to the following three factors: holiday, concerns about the pandemic situation, and quarantine or community containment. The ratings are the same as those in (C). (E): Spearman correlation coefficient analysis among circadian rhythms, sleep, and negative emotion. * p < 0.05, ** p < 0.01.
A negative correlation for changes in sleep quality was revealed with all the studied negative emotion types (worry, stupefaction, downheartedness, depression, anxiety, and fear), with the following correlation coefficients: −0.116 (p = 0.014), −0.130 (p = 0.006), −0.180 (p < 0.001), −0.127 (p = 0.007), −0.134 (p = 0.004), and −0.140 (p = 0.003), respectively. Among the sleep parameters, prolonged daytime napping was negatively associated with stupefaction (r = −0.093, p = 0.047); moreover, KSS-rated sleepiness was positively associated with stupefaction (r = 0.125, p = 0.008) and downheartedness (r = 0.111, p = 0.018). Regarding the circadian rhythm parameters, changes in daytime sleepiness were negatively associated with fear (r = −0.098, p = 0.037) and downheartedness (r = 0.111, p = 0.018). Changes in wake-up time were negatively associated with fear (r = −0.098, p = 0.037) (Figure 4B and Table S5).
Among the circadian rhythm variables, self-reported circadian changes were positively associated with difficulty falling asleep, difficulty waking up, changes in wake-up time, and changes in sleep onset time, with the following respective correlation coefficients: 0.337, 0.426, 0.356, and 0.367. Furthermore, circadian change was negatively associated with changes in dining times (r = −0.189). Difficulty falling asleep was positively associated with difficulty waking up (r = 0.252), changes in wake-up time (r = 0.131, p = 0.005), and changes in sleep onset time (r = 0.173). Additionally, difficulty waking up was positively correlated with wake-up time (r = 0.195) and changes in sleep onset time (r = 0.231). Changes in wake-up time were positively associated with changes in sleep onset time (r = 0.308) and negatively associated with changes in dining times (r = −0.212). Total sleep duration was positively associated with increased sleep quality (r = 0.229) and increased daytime napping (r = 0.141, p = 0.003). Sleep quality was negatively associated with increased nocturnal wake-up episodes (r = −0.174) (Table S5).
3.5. Subjective Assessments of the Effect of Quarantine on Circadian Rhythms and Sleep
We assessed three potential factors: the Spring Festival holiday, concerns regarding the epidemic situation and constant quarantine and community containment. In this subjective assessment, the effects on circadian rhythms and sleep were considered to be one entity. The participants reported the subjective assessments of these factors on circadian rhythms and sleep using the corresponding scales 1–5. Among the 451 participants, 89.6% (404), 76.7% (346), and 88.0% (397) of the participants considered constant quarantine, holiday, and community containment as the causative factors, respectively (rating ≥ 2), for circadian rhythms and sleep disturbance (Figure 4C,D and Table S12).
There was a positive correlation of the Spring Festival holiday with daily sleep duration (r = 0.292, p < 0.001), sleep quality (r = 0.123, p = 0.009), delayed waking up time (r = 0.160, p = 0.001), irregularity in circadian rhythms (r = 0.130, p = 0.006), KSS-rated sleepiness (r = 0.127, p = 0.007), and worry (r = 0.150, p = 0.001). Concerns regarding the pandemic situation were positively correlated with nocturnal wake-up episodes (r = 0.112, p = 0.020) and negatively related to sleep quality (r = −0.146, p = 0.002) and the components of circadian rhythms and negative emotions. Constant quarantine and community containment were positively related to total sleep duration, delayed waking up time, difficulty falling asleep, and the degree of rhythms becoming irregular. There were positive relationships of constant quarantine, community containment, and five negative emotions, i.e., worry, stupefaction, depression, anxiety, and fear (Figure 4E and Table S5).
Moreover, 80 participants (17.7%) listed that other factors could affect circadian rhythms and sleep including family (38.8%, 31/80), work or schoolwork (30%, 24/80), environmental comfort (17.5%, 14/80), and economic pressure (2.5%, 2/80). Regarding environmental comfort, we found that noise (3.8%, 3/80) and ambient temperature (2.5%, 2/80) could contribute to effects on circadian rhythms and sleep (Table S13).
The regression analysis was conducted to explore the potential casualty, and the results reveal significant correlations between negative emotions (worry, stupefaction, anxiety, and fear) and sleep variables, but not between negative emotions and circadian alterations, suggesting that the negative emotions may contribute more to the sleep disorders. Significant correlations between many variables of circadian rhythm and sleep were also observed. The factor of “Spring Festival Holiday” influenced several variables of sleep, including changes in sleep duration (p = 0.005), changes in self-assessed sleep quality (p = 0.012), changes in daytime napping duration (p = 0.015), and KSS-rated sleepiness (p = 0.012), and the factor of “constant quarantine and community containment” may account for changes in sleep duration (p = 0.012). In contrast, there was no significant correlation between these three factors and circadian rhythm. Moreover, changed time of exercise may account for the changes in morning wake-up time (p = 0.029) and evening sleep onset time (p = 0.031). The results also showed that changes in difficulty falling asleep contributed to KSS-rated sleepiness (p = 0.025), which support the validity of this analysis (Table S14).
4. Discussion
In this study, we surveyed the changes in circadian rhythms and sleep of 451 Chinese people during the traditional Spring Festival who had experienced quarantine and community containment for approximately two weeks during the outbreak of COVID-19. We show that from 20 January 2020 to 31 January 2020, the circadian rhythms, sleep, and negative emotions of a substantial part of the population were subject to change, owing to at least three factors: holiday, quarantine, and concerns regarding the pandemic situation. In this study, we performed correlation coefficient analysis to address the possible associations among demographic characteristics, environmental factors, variables related to circadian rhythms and sleep, and negative emotions.
In different countries, accumulating data have demonstrated that a substantial proportion of populations underwent sleep problems during COVID-19, including infected patients, health care staff, and general populations [26,27,28,29,30,31,32]. However, by far, alteration of circadian rhythm during the pandemic resulted in fewer concerns relative to sleep. In this study, we revealed that there were changes in the circadian rhythms of 85.6% of the participants, with 36.3% of these individuals developing irregular circadian rhythms and sleep disturbance. Approximately 49.7% of the participants showed a delayed wake-up time (Figure 1C). Sleep changes were found during the quarantine period, which was indicated by several parameters. First, 59.6% of the participants showed increased total sleep duration (Figure 2F). Nocturnal wake-up episodes are indicative of sleep disturbance; in our study, 10.4% participants reported increased nocturnal wake-up episodes, which was negatively associated with subjectively self-assessed sleep quality (Table S5).
Cain et al. analyzed circadian changes in serum melatonin and core body temperature and reported that compared with men, women displayed more advanced phases of these variables [33]. Women have a shorter free-running period than men, which indicates gender-based differences in circadian patterns [34]. In this survey, female participants reported an earlier time of waking up and less difficulty falling asleep than men did before 20 January 2020. In consistence, in this study the female participants reported a later phase of waking up and lower sleep quality relative to men (Tables S6–S9). Consistently, surveys in other countries, e.g., Italy and India, revealed that females reported poorer sleep quality and more disturbed sleep–wake patterns compared with males [28,35]. Together, these facts suggest that the circadian rhythm and sleep of women could be more susceptible to the stresses.
It has been noticed that U.S. women are more emotionally affected by the pandemic, which could be explained by the social roles of women, who are affected by chronic stress due to their lack of social support and due to their parental and caregiving responsibilities, which are likely exacerbated by the pandemic [18,36,37]. However, in this study, we found that overall, most of the scores of negative emotions were slightly lower in women than those in men (Table S11), which may be attributed to the different culture in China [38], although direct evidence is lacking.
Circadian misalignment causes emotional deterioration [39,40,41] and extensive alterations in circadian rhythm and sleep during the pandemic period, which were correlated with the six types of negative emotions (Figure 4B). In this study, we found that the majority of the participants (67.2%) reported at least one negative emotion type, with worry being the most prevalent (57.0%). Across all negative emotions, 7.1–16.4% of participants reported very high scores (scales ≥ 4) (Figure 3A,B and Table S10). Similarly, Lenzo et al. reported that about a third of the Italian population reported moderate-to-extremely severe depression, anxiety, and stress and that the severity was partly dependent on resilience dimensions [42]. In this study, we observed a negative relationship between fear and delayed wake-up time, which is an indicator of disturbed sleep–wake rhythm (Figure 4B). Among the possible causes captured in the survey, the negative emotions should be more attributed to the pandemic situation than to the Spring Festival holiday.
In this study, we also analyzed the effects of age and exercise on circadian rhythm, sleep, and emotion. As for different age groups, older individuals reported fewer negative emotions, which could be attributed to increased ability to regulate emotions [37,43,44]. Consistent with these previous studies, we observed a negative correlation between age and worry, stupefaction, downheartedness, anxiety, and fear (Figure 3D and Tables S5 and S14). It may be explained that the older people possess superior emotion regulation and coping strategies [37]. Exercise was negatively related to the degree of circadian rhythms and sleep components (Figure 2D,G and Tables S5 and S14), suggesting that exercise may serve as a countermeasure for improving the circadian rhythm and sleep.
Night work, shift work, and transmeridian travel cause social jet lag, which leads to circadian misalignment [45]. During holidays or weekends, people usually display a delayed circadian phase. The traditional Spring Festival holiday lasted between 24 January 2020 and 2 February 2020, while the level 1 emergency was launched on 23 January 2020. Therefore, the periods for the three factors (Spring Festival holiday, quarantine, and concerns regarding the pandemic situation) might overlap. To discriminate the effects of these three factors on circadian rhythms and sleep, we analyzed their relationships with subjective self-assessed variables. We found that more than half of the participants reported the holiday as a causative factor for circadian rhythm and sleep disturbance. Moreover, a coefficient analysis revealed positive correlations between the Spring Festival and total sleep duration, sleep quality, KSS-rated sleepiness, delayed wake-up time, and self-reported changes in circadian rhythms. However, among the six negative emotions, only worry was positively correlated with the Spring Festival holiday (Figure 4E and Table S5), which suggests that the holiday was not associated with negative emotions.
Compared with the holiday, quarantine was correlated with more negative emotions, including worry, stupefaction, downheartedness, anxiety, and fear. Furthermore, quarantine was reported by participants to be related to a number of circadian rhythm components, including the growing irregularity of circadian rhythms, difficulty falling asleep, delayed wake-up time, and dining times, suggesting that quarantine also contributes to disturbance of circadian rhythms (Figure 4E and Table S5). In addition, most of the participants reported that concern about the pandemic situation was significantly correlated with several sleep and circadian rhythm variables, as well as all six negative emotions (Figure 4E and Table S5). The results of regression analysis further reveal that circadian rhythm and sleep were tightly linked; however, the negative emotions were associated with some variables of sleep disturbance but not circadian changes (Table S14). The factors of holiday and quarantine and community containment were associated with sleep changes but not circadian changes (Table S14). The data suggest that negative emotions may affect sleep, which further cause disturbance in circadian rhythm. Moreover, insufficient indoor lighting may also contribute to circadian misalignment during the pandemic period. Room light is usually lower than 500 lux, which is not effective in entraining circadian rhythms [46,47].
This study was conducted immediately after the quarantine; thus, bias may occur due to the limited quantity of the participants. Therefore, some of the findings should be interpreted with caution. For instance, the negative emotions of participants who were <20 years or >60 years old remain unclear because the number of participants was very small (Table S11). In this study, we used simplified indicators to represent sleep quality, chronotype, KSS, and emotions to avoid a lengthy questionnaire with too many questions that might have brought about unwillingness to participate and our inability to obtain valid feedback. However, it is noteworthy to remember that such simplified indicators might also cause bias for further evaluation. In addition, although correlational analysis can suggest a potential relationship between two variables, it cannot prove that one variable causes a change in another variable.
5. Conclusions and Perspectives
Our findings show that quarantine and concerns regarding the pandemic situation are associated with sleep, circadian rhythms, and negative emotions. Moreover, some factors, e.g., gender and age, might be causes of the difference in some of the changes. These findings suggest that the general population may also be subject to extensive influence.
Over 474 million cases of COVID-19 have been reported worldwide as of 24 March 2022, and pandemic surveillance may last for additional months or even years [48]. Our findings could contribute to improvements in circadian rhythms, sleep, and emotion during the pandemic period. Due to the dramatically increased transmissibility of the new Omicron mutant of COVID-19, mandatory quarantine has been reimplemented in many countries or regions [49]. According to our findings, measures for improving the circadian rhythms—e.g., optimization of the living schedule, enhancement of the indoor daylight and appropriate exercise—may be applied to alleviate the negative emotions and promote the public health.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/covid2040032/s1, Table S1: Demographic characteristics of the participants; Table S2: Correlation coefficient analysis of sleep onset time and wake-up time in control and pandemic period; Table S3: Changes in the morning wake-up time of responders; Table S4: Changes in the circadian variables at different ages; Table S5: Correlation coefficients between changes of all variables; Table S6: Difference in circadian rhythm and sleep between control and pandemic periods for both genders; Table S7: Gender difference in difficulty falling asleep and wake-up time; Table S8: Descriptive statistics of changes in circadian rhythms and sleep of both genders; Table S9: Comparison of changes in circadian rhythms and sleep between genders; Table S10: Numbers and proportions of responders who scored the corresponding degrees; Table S11: Negative emotions classified into different gender, education level, and age group; Table S12: Degrees of three potential factors affecting circadian rhythms and sleep; Table S13: Other factors affecting circadian rhythms and sleep; Table S14: Regression analysis summary. Supplemental File S1: Questionnaire.
Author Contributions
Conceptualization, J.G.; methodology, S.C., T.H. and Y.H.; formal analysis, S.C., T.H., Y.H., J.G., X.L. and H.M.; data curation, S.C., T.H., Y.H., C.N. and J.L.; writing—original draft preparation, J.G.; writing—review and editing, X.L. and Y.X.; funding acquisition, J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Open Fund of the National Key Laboratory of Human Factors Engineering in the Astronaut Center of China, grant number SYFD180051809K; and the National Natural Science Foundation of China, grant number 31871188.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the study was approved by the Institutional Review Board of School of Life Sciences, Sun Yat-sen University (May 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the study participants for their contribution to making this study possible.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Jin, Y.H.; Cai, L.; Cheng, Z.S.; Cheng, H.; Deng, T.; Fan, Y.P.; Fang, C.; Huang, D.; Huang, L.Q.; Huang, Q.; et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil. Med. Res. 2020, 7, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, S.Q.; Peng, H.A.O. Characteristics of and Public Health Responses to the Coronavirus Disease 2019 Outbreak in China. J. Clin. Med. 2020, 9, 575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilder-Smith, A.; Freedman, D.O. Isolation, quarantine, social distancing and community containment: Pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. J. Travel Med. 2020, 27, taaa020. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, J.C.; Loros, J.J. Yes, circadian rhythms actually do affect almost everything. Cell Res. 2016, 26, 759–760. [Google Scholar] [CrossRef] [Green Version]
- Honma, S. The mammalian circadian system: A hierarchical multi-oscillator structure for generating circadian rhythm. J. Physiol. Sci. 2018, 68, 207–219. [Google Scholar] [CrossRef]
- Arnold, W.; Ruf, T.; Loe, L.E.; Irvine, R.J.; Ropstad, E.; Veiberg, V.; Albon, S. Circadian rhythmicity persists through the Polar night and midnight sun in Svalbard reindeer. Sci. Rep. 2018, 8, 14466. [Google Scholar] [CrossRef] [Green Version]
- Bechtold, D.A.; Gibbs, J.E.; Loudon, A.S. Circadian dysfunction in disease. Trends Pharmacol. Sci. 2010, 31, 191–198. [Google Scholar] [CrossRef]
- Antúnez, J.M. Circadian typology is related to emotion regulation, metacognitive beliefs and assertiveness in healthy adults. PLoS ONE 2020, 15, e0230169. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Morris, C.J.; Scheer, F.A.J.L. Circadian misalignment increases mood vulnerability in simulated shift work. Sci. Rep. 2020, 10, 18614. [Google Scholar] [CrossRef]
- Friborg, O.; Rosenvinge, J.H.; Wynn, R.; Gradisar, M. Sleep timing, chronotype, mood, and behavior at an Arctic latitude (69° N). Sleep Med. 2014, 15, 798–807. [Google Scholar] [CrossRef]
- Jensen, H.I.; Larsen, J.W.; Thomsen, T.D. The impact of shift work on intensive care nurses’ lives outside work: A cross-sectional study. J. Clin. Nurs. 2018, 27, e703–e709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luik, A.I.; Zuurbier, L.A.; Direk, N.; Hofman, A.; Van Someren, E.J.; Tiemeier, H. 24-hour activity and rhythm and sleep disturbances in depression and anxiety: A population-based sutdy of middle aged and older persons. Depress. Anxiety 2015, 32, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.C.A.; Abreu, R.L.C.; Linhares Neto, V.B.; de Bruin, P.F.C.; de Bruin, V.M.S. Chronotype and circadian rhythm in bipolar disorder: A systematic review. Sleep Med. Rev. 2017, 34, 46–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, K.J.; McGee-Koch, L.L.; Zee, P.C. Cognition in circadian rhythm sleep disorders. Prog. Brain Res. 2011, 190, 3–20. [Google Scholar] [PubMed]
- Vandekerckhove, M.; Wang, Y.L. Emotion, emotion regulation and sleep: An intimate relationship. AIMS Neurosci. 2017, 5, 1–17. [Google Scholar] [CrossRef]
- Hilditch, C.J.; Short, M.; Van Dongen, H.P.; Centofanti, S.A.; Dorrian, J.; Kohler, M.; Banks, S. Sleep inertia during a simulated 6-h on/6-h off fixed split duty schedule. Chronobiol. Int. 2016, 33, 685–696. [Google Scholar] [CrossRef]
- Basner, M.; Dinges, D.F.; Mollicone, D.; Ecker, A.; Jones, C.W.; Hyder, E.C.; Di Antonio, A.; Savelev, I.; Kan, K.; Goel, N.; et al. Mars 520-d mission simulation reveals protracted crew hypokinesis and alterations of sleep duration and timing. Proc. Natl. Acad. Sci. USA 2013, 110, 2635–2640. [Google Scholar] [CrossRef] [Green Version]
- Connor, J.; Madhavan, S.; Mokashi, M.; Amanuel, H.; Johnson, N.R.; Pace, L.E.; Bartz, D. Health risks and outcomes that disproportionately affect women during the COVID-19 pandemic: A review. Soc. Sci. Med. 2020, 266, 113364. [Google Scholar] [CrossRef]
- Akerstedt, T.; Gillberg, M. Subjective and objective sleepiness in the active individual. Int. J. Neurosci. 1990, 52, 29–37. [Google Scholar] [CrossRef]
- Steer, R.A.; Clark, D.A.; Beck, A.T.; Ranieri, W.F. Common and specific dimensions of self-reported anxiety and depression: The BDI-II versus the BDI-IA. Behav. Res. Ther. 1999, 37, 183–190. [Google Scholar] [CrossRef]
- Horne, J.A.; Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar] [PubMed]
- Antoneli, F.; Passos, F.M.; Lopes, L.R.; Briones, M.R.S. A Kolmogorov-Smirnov test for the molecular clock based on Bayesian ensembles of phylogenies. PLoS ONE 2018, 13, e0190826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosner, B.; Glynn, R.J.; Lee, M.L. The Wilcoxon signed rank test for paired comparisons of clustered data. Biometrics 2006, 62, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Rosner, B.; Grove, D. Use of the Mann-Whitney U-test for clustered data. Stat. Med. 1999, 18, 1387–1400. [Google Scholar] [CrossRef]
- Prematunga, R.K. Correlational analysis. Aust. Crit. Care 2012, 25, 195–199. [Google Scholar] [CrossRef]
- Jahrami, H.; BaHammam, A.S.; Bragazzi, N.L.; Saif, Z.; Faris, M.; Vitiello, M.V. Sleep problems during the COVID-19 pandemic by population: A systematic review and meta-analysis. J. Clin. Sleep Med. 2021, 17, 299–313. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, Y.; Kong, D.; Li, S.; Yang, N. Social Capital and Sleep Quality in Individuals Who Self-Isolated for 14 Days During the Coronavirus Disease 2019 (COVID-19) Outbreak in January 2020 in China. Med. Sci. Monit. 2020, 26, e923921. [Google Scholar] [CrossRef]
- Innocenti, P.; Puzella, A.; Mogavero, M.P.; Bruni, O.; Ferri, R. Letter to editor: COVID-19 pandemic and sleep disorders-a web survey in Italy. Neurol. Sci. 2020, 41, 2021–2022. [Google Scholar] [CrossRef]
- Beck, F.; Léger, D.; Fressard, L.; Peretti-Watel, P.; Verger, P. COVID-19 health crisis and lockdown associated with high level of sleep complaints and hypnotic uptake at the population level. J. Sleep Res. 2021, 30, e13119. [Google Scholar] [CrossRef]
- Killgore, W.D.S.; Cloonan, S.A.; Taylor, E.C.; Fernandez, F.; Grandner, M.A.; Dailey, N.S. Suicidal ideation during the COVID-19 pandemic: The role of insomnia. Psychiatry Res. 2020, 290, 113134. [Google Scholar] [CrossRef]
- Zhao, X.; Lan, M.; Li, H.; Yang, J. Perceived stress and sleep quality among the non-diseased general public in China during the 2019 coronavirus disease: A moderated mediation model. Sleep Med. 2021, 77, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Ingram, J.; Maciejewski, G.; Hand, C.J. Changes in Diet, Sleep, and Physical Activity Are Associated With Differences in Negative Mood During COVID-19 Lockdown. Front. Psychol. 2020, 11, 588604. [Google Scholar]
- Cain, S.W.; Dennison, C.F.; Zeitzer, J.M.; Guzik, A.M.; Khalsa, S.B.; Santhi, N.; Schoen, M.; Czeisler, C.A.; Duffy, J.F. Sex differences in phase angle of entrainment and melatonin amplitude in humans. J. Biol. Rhythms. 2010, 25, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Duffy, J.F.; Cain, S.W.; Chang, A.-M.; Phillips, A.J.K.; Münch, M.Y.; Gronfier, C.; Wyatt, J.K.; Dijk, D.-J.; Wright, K.P.; Czeisler, C.A. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S3), 15602–15608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, M.; Pande, B.; Sinha, R. Impact of COVID-19 lockdown on sleep-wake schedule and associated lifestyle related behavior: A national survey. J. Public Health Res. 2020, 9, 1826. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, B.; Gomez, I.; Salganicoff, A.; Ranji, U. Coronavirus: A Look at Gender Differences in Awareness and Actions. 2020. Available online: https://www.kff.org/coronavirus-covid-19/issue-brief/coronavirus-a-look-at-gender-differences-in-awareness-and-actions (accessed on 30 October 2021).
- Barber, S.J.; Kim, H. COVID-19 Worries and Behavior Changes in Older and Younger Men and Women. J. Gerontol. B Psychol. Sci. Soc. Sci. 2021, 76, e17–e23. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q. Division of domestic labor and fertility behaviors in China: The impact of extended family traditions on gender equity theory. China Popul. Dev. Stud. 2021, 5, 41–60. [Google Scholar] [CrossRef]
- Albrecht, U. Molecular Mechanisms in Mood Regulation Involving the Circadian Clock. Front. Neurol. 2017, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Heyde, I.; Kiehn, J.T.; Oster, H. Mutual influence of sleep and circadian clocks on physiology and cognition. Free Radic. Biol. Med. 2018, 119, 8–16. [Google Scholar] [CrossRef]
- Peterson, M.J.; Benca, R.M. Sleep in mood disorders. Psychiatr. Clin. N. Am. 2006, 29, 1009–1032. [Google Scholar] [CrossRef]
- Lenzo, V.; Quattropani, M.C.; Musetti, A.; Zenesini, C.; Freda, M.F.; Lemmo, D.; Vegni, E.; Borghi, L.; Plazzi, G.; Castelnuovo, G.; et al. Resilience Contributes to Low Emotional Impact of the COVID-19 Outbreak Among the General Population in Italy. Front. Psychol. 2020, 11, 576485. [Google Scholar] [CrossRef] [PubMed]
- Carstensen, L.L. Motivation for social contact across the life span: A theory of socioemotional selectivity. Nebr. Symp. Motiv. 1992, 40, 209–254. [Google Scholar] [PubMed]
- Gross, J.J.; Carstensen, L.L.; Pasupathi, M.; Tsai, J.; Skorpen, C.G.; Hsu, A.Y. Emotion and aging: Experience, expression, and control. Psychol. Aging 1997, 12, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Merrow, M. The Circadian Clock and Human Health. Curr. Biol. 2016, 26, R432–R443. [Google Scholar] [CrossRef]
- Turner, P.L.; Mainster, M.A. Circadian photoreception: Ageing and the eye’s important role in systemic health. Br. J. Ophthalmol. 2008, 92, 1439–1444. [Google Scholar] [CrossRef]
- Lewy, A.J.; Wehr, T.A.; Goodwin, F.K.; Newsome, D.A.; Markey, S.P. Light suppresses melatonin secretion in humans. Science 1980, 210, 1267–1269. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://covid19.who.int (accessed on 24 March 2022).
- Gu, H.; Krishnan, P.; Ng, D.Y.; Chang, L.D.; Liu, G.Y.; Cheng, S.S.; Hui, M.M.; Fan, M.C.; Wan, J.H.; Lau, L.H.; et al. Probable Transmission of SARS-CoV-2 Omicron Variant in Quarantine Hotel, Hong Kong, China, November 2021. Emerg. Infect. Dis. 2022, 28, 460–462. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).