Abstract
Gut microflora dysbiosis affects the majority of individuals after COVID-19, contributing to both gastro-intestinal (GI) and non-GI symptoms. Natural phytochemicals have reported anti-viral properties and favourable effects on inflammatory and oxidative pathways, both important for tissue damage post-viral pneumonia. This study involved 147 participants with symptomatic COVID-19, randomised to receive a placebo (P) or a phytochemical-rich concentrated food capsule (PC) in addition to a pre/probiotic lactobacillus capsule. Participants taking the PC had an almost two-fold reduction in mean fatigue scores compared to P [p = 0.02], a three-fold reduction in cough score and more than a double improvement in overall well-being scores [p = 0.02]. Two (1.5%) participants reported mild, increased bloating which they felt was attributable to the capsules, although GI symptoms improved in 25 of 31 participants (82%) who reported them at baseline. Sedentary, older, previously hospitalised men with GI symptoms had a statistically significantly improvement among those given the probiotic. Although some participants with early disease would have improved spontaneously, such a rapid improvement observed in the majority of participants, who had been suffering for an average of 108 days, was clinically relevant and welcomed, especially among those more likely to have pre-existing gut dysbiosis. We are now evaluating whether this blend could also enhance antibody titres post-COVID-19 vaccination.
1. Introduction
Following COVID-19 infection, genetic variabilities in both the host and virus, along with co-morbidities, reduce an individual’s ability to sustain the viral assault and influence the severity of disease [1,2,3]. In addition, emerging data is linking worse outcomes with factors associated with less-favourable gut microflora (dysbiosis) [1,2,3]. Gut dysbiosis can cause impaired gut wall integrity, reduced immunosurveillance, excessive inflammation and impaired oxidative pathways, all of which are very relevant following COVID-19 infection [1,4,5,6,7,8,9,10,11,12,13,14,15,16]. An exaggerated release of inflammatory cytokines triggers an influx of innate immune cells into tissues, causing collateral damage and excess pulmonary exudates which block pulmonary airways, both features of acute respiratory distress syndrome (ARDS) [4,5,13,17,18,19,20,21,22].
Dysbiosis has been reported in the majority of patients with COVID-19, particularly those expressing gastrointestinal (GI) symptoms. This is thought, in part, to be attributed to an increased susceptibility of individuals with dysbiosis to contract COVID-19, but also the ability of the virus in the gut to directly cause gut inflammation which can upset micro-floral balance [6,7,8,10,11,14,23,24,25,26]. Previous studies have highlighted that patients with GI symptoms at presentation had worse non-GI symptoms, particularly fatigue, during COVID-19 infection, and had a greater risk of chronic symptom burden [6,11,12,13,27]. The link between bowel dysbiosis and hyper-inflammation has also been well documented in other chronic respiratory diseases [19,25,26,27,28]. Factors which influence dysbiosis include recent use of antibiotics, steroids and antacids, increasing age, obesity, sedentary behaviour and smoking [29]. Dietary factors include a high intake of processed sugar and meat and a low intake of fermentable, soluble fibres and prebiotic polyphenols [1,30,31,32].
The role of nutritional supplements, as a way to enhance prebiotic and probiotic intake throughout the day, has been the subject of extensive investigation [33,34,35,36]. The most widely researched probiotics include lactic-acid-producing bacteria, such as the species Lactobacillus, the gut colonization of which is enhanced by concomitant intake with prebiotic soluble fibres such as inulin [30,37]. Interventional studies in humans and animals have shown them to improve microflora biodiversity, downregulate mucosal inflammation, correct GI symptoms, such as bloating and diarrhoea, and help to modify a range of chronic diseases [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48].
The reported mechanisms of action of probiotic supplement are multifactorial [38,40]. Probiotics encourage gut colonisation of anti-inflammatory bacterial strains which then outpace pro-inflammatory bacteria [30,49,50]. Furthermore, they also aid fermentation of otherwise poorly digestible dietary carbohydrates into short-chain fatty acids such as butyrate, which are an efficient energy source for gut mucosal and immune cells and so help improve gut wall growth, function and integrity [3]. As well as their positive influence on immune balance, probiotics have been found to augment intracellular oxidative enzyme capacity in both laboratory and clinical studies among patients with COVID-19, enhancing activity of scavenge tissue damaging superoxide anions [51,52]. Probiotics also help to increase vitamin D absorption and expression, which is important post-COVID-19 as suboptimal vitamin D levels have been associated with hyper-inflammatory cytokine production, and ultimately more severe respiratory COVID-19-related symptoms [52,53,54,55,56,57,58,59].
In addition to dampening excess inflammation, intervention studies have shown that diet enhancement with a lactic-acid probiotic supplement helps to reduce an age-related drop in natural killer cell activity and augments anti-viral immune surveillance [15,40,60,61,62,63,64,65,66,67,68,69,70,71]. A meta-analysis of small randomised controlled trials (RCTs) suggests they decreased the need for invasive mechanical ventilation in patients with ARDS [72].
Natural phytochemicals, especially the polyphenol group, found in fruit, herbs and vegetables, are demonstrating an increasing role in mitigating certain symptomatic effects associated with viral infections [12,18,31,32,73]. As well as their positive effects on gut health, polyphenols such as curminoids (found in turmeric), ellagic acid and quercetin and resveratrol (found in tea, grapes, polygonum cuspidatum root and pomegranate) downregulate excess tissue chronic inflammation by reducing COX–2 activation of prostaglandins via modulating the function of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) [31,32,74,75,76]. This mechanism explains why polyphenols have been shown to reduce inflammatory-associated pain in joints, a symptom common among people with long-COVID-19 [73,74,75,76]. However, unlike aspirin and ibuprofen, polyphenol-rich foods have actually been shown to protect gastric mucosa [77]. The polyphenol resveratrol influences the regulation of angiotensin-converting enzyme, another factor implicated in virus-induced exudate production and lung parenchyma injury [78,79,80]. Unlike steroids and other immune suppressants, the ability of phytochemicals to suppress pro-inflammatory cytokines has not led to a reduced viral immunosurveillance [81,82,83,84]. Resveratrol has been reported to increase anti-viral cytotoxic T lymphocytes and natural killer immune cells [78]. Likewise, apigenin, derived from chamomile, has been shown to induce anti-viral activity in T-cell lines transfected with HIV [83,84].
Phytochemicals have also been shown to promote a natural adaptive response to tissue oxidative stress via their ability to facilitate activation of the transcription factor NF-E2–related factor 2 [18]. This enhances an appropriate antioxidant response to damaging reactive oxidative species (ROS), which is relevant to COVID-19 as tissue damage post-COVID-19 has been linked to excess oxidative damage [85]. Yet, unlike direct anti-oxidants such as vitamins A and E, there is no evidence that phytochemicals over-deplete ROS levels causing a situation coined anti-oxidative stress, as they do not impair degradation of antioxidant enzymes after a tissue insult diminishes [86,87,88,89].
Pomegranates and other citrus fruits are also rich in nitrates, which in the presence of vitamin C, citrus bioflavonoids and other polyphenols, are converted to nitric oxide (NO) [90]. NO has smooth muscle vasodilatory properties which help to lower blood pressure and enhance tissue oxygenation, both relevant factors following COVID-19 pneumonia [91,92].
On top of these protective immune mechanisms, phytochemicals have recently been discovered to have direct anti-viral properties in laboratory studies [15,57,71,93]. These processes include inhibition of viral penetration and replication by inhibiting viral gene expression, viral protein synthesis and downregulation of cellular transcription and signaling pathways [81,83,94,95,96,97,98,99,100,101,102,103].
The optimal health benefits of a diverse phytochemical, prebiotic and probiotic-rich diet are likely to be the result of synergy between them and long-term intake [104,105,106,107,108,109,110,111,112,113]. There are some data, however, to suggest that even short-term intervention can rapidly alter the composition of the gut microbiota in healthy volunteers [1,114]. It is unknown whether the same impact could be achieved in people suffering from COVID-19, who may have a poor appetite along with bowel symptoms and dysfunction. Likewise, it is not known whether the multiple properties summarised above could be achieved in humans to levels which could reduce symptoms and shorten the time to recovery, hence the rationale for this clinical study.
2. Materials and Methods
This double-blind, randomised trial was co-ordinated by the Research Office within the Primrose Unit, Bedford Hospital. Participants were approached via the local post-COVID-19 clinic and from the daily COVID-19 ward round; others directly contacted the Trials Unit after hearing about the study via word of mouth. Participants were excluded if they had known sensitivities and allergies to the investigational foods, were immunosuppressed or were too ill to take oral capsules.
All participants were randomised (1:1) to take either a placebo (P) or an identical looking and weighted phytochemical-rich concentrated food capsule (PC). They took one capsule twice a day. At the start of the study, participants were initially also randomised (1:1) to receive a Lactobacillus probiotic and inulin prebiotic, but recruitment became slow as potential participants were deterred by the possibility of receiving a placebo without the benefit of a probiotic. It also became clear that participants would buy their own probiotics over-the-counter, negating any comparative data. Considering these factors and following feedback from patients, the trial committee decided, subject to a protocol amendment, to provide the probiotic to all remaining participants. The design change rapidly increased recruitment rate and allowed recruitment targets to be achieved. In total, 126 participants received the probiotic capsules (see Figure 1).
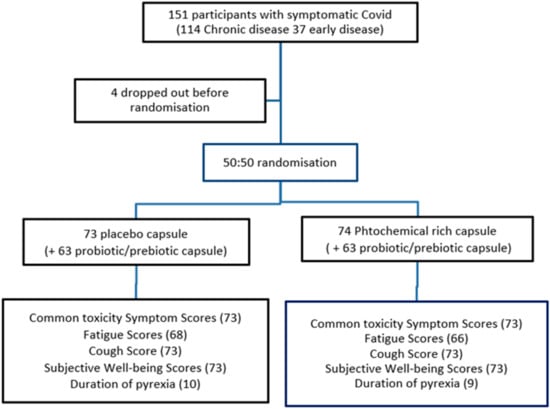
Figure 1.
Trial consort flow diagram.
The study was approved by the Yorkshire & The Humber (Sheffield) Research Ethics Committee (REC reference: 20/YH/0164) on 22 May 2020 and approved by the HRA and Health and Care Research Wales (HCRW) on 22 May 2020. IRAS project ID: 282517. EudraCT number 2020-001532-10.
Capsule contents and manufacture:
- Probiotic/prebiotic capsule (PP): The daily dose (from two capsules) was 200 mg of inulin and 10 billion colony forming units (CFU’s) of Lactobacillus plantarum, Lactobacillus rhamnosus, Lactobacillus bulgaricus, Lactococcus lactis and Lactobacillus paracasei. The delayed release vegan capsule was made of hypromellose and gellan.
- Phytochemical-rich whole food capsule (PC): Citrus Sinensis fruit (400 mg from 200 mg of 2:1 extract, standardised to contain 70 mg of Bioflavonoids). Chamomile, Matricaria recutita L. flower) (1000 mg from 22 mg of 10:1 extract and 65 mg of 12:1 extract). Curcuma Longa rhizome in Curcumin Complex (1600 mg of curcumin from 25 mg of 64:1 extract, standardised to contain 23.8 mg of curcuminoid). Pomegranate (Punica granatum L. rinds and seeds) (1000 mg from 25 mg of 40:1 extract, standardised to contain 10 mg of Ellagic Acid). Polygonum cuspidatum root containing 100 mg of resveratrol.
- Placebo (P): Inert microcrystalline cellulose, magnesium stearate and silicon dioxide.
- Capsule manufacture and quality assurance: The capsules and their contents were made specifically for this trial by ParkAcre, Lincoln, and The Oxford Health Company Ltd., Oxfordshire, UK, both certified by the Organic Food Federation to conform to Good Manufacturing Practice (GMP—FSSC 22000, ISO 22000), and UK and International food production laws. Their in-house Research and Development department tested each batch for biological contamination of yeast, mould, E. coli and salmonella, and measured lead, arsenic, cadmium, mercury and pesticides, to ensure that they met international thresholds. A unit of each supplement was securely stored by the Trust Secretary and can be sent to any regulatory body at their request.
Primary end points were the differences in mean symptom from days 1 to 30 between the two groups measured by the validated Chalder Fatigue Scale (CFS) (Figure A1 in Appendix A), the Cough Symptom Score (CSS) (Table A1 in Appendix A) and the Subjective Well-being Score (SWB) (Table A2 in Appendix A) [114,115]. Other symptoms were recorded with the NCI Common Toxicity Criteria. All 147 CFS and CSS forms were completed. A total of 134 CFS were completed (see consort diagram Figure 1).
The secondary end point was to evaluate which predetermined subgroups had a greater mean improvement between days 1 and 30—in particular, which subgroups were more likely to have suboptimal gut health at baseline, such as male versus (vs.) female; BMI (overweight or obese vs. normal weight); age (>60 years vs. <60 years); exercise levels (<3 h a week vs. >3 h a week); history of hospitalisation or not; history of bowel symptoms or not; history of GI symptoms at baseline or not.
Statistical analysis: The power calculation and analysis of results was performed by independent statisticians who were blind to the treatment arms with the aid of the IBM SPSS Statistical software (IBM Corp., Amonk, NY, USA). All dependent variables were checked for normal distribution using Quantile-Quantile (Q-Q) Plots and were deemed plausible in all instances. All data were presented as mean ± SD with 95% confidence intervals (95% CI). A dependent paired samples t-test was used to assess the mean differences in Cough, Subjective Well-being and Fatigue Scores at day 1 compared with day 30. An independent t-test was also used to determine the differences in these scores between the P and PC arm. Homogeneity of variance was checked and not violated for any variables. A predetermined subgroup analysis was used to determine an interaction between the arm (P vs. PC) with gender (male vs. female), COVID-19 status (early vs. long), or age (<60 years vs. >60 years) using a between-groups one-way analysis of variance (ANOVA). Assumptions of both tests were checked via the homogeneity of variance, which was not violated for any variables. In the instance of a significant interaction effect, a Bonferroni pairwise comparison was used to locate significant differences. The two-tailed alpha level was set as p < 0.05.
3. Results
All 151 participants, recruited between May 2020 and May 2021, had one or more symptoms related to their COVID-19 infection. Four withdrew before randomization, leaving a total of 147 study participants. The average time from COVID-19 diagnosis to trial entry was 108 days (range 2 to 467 days). Thirty-six participants (25%) entered the study within 30 days of diagnosis (average 14 days) and 112 (75%) after 30 days (average 120 days). The demographic of the trial cohort is summarised in Table 1, which also shows that both randomised groups were evenly balanced, apart from Subjective Well-being Score which was slightly worse at baseline in the PC group. There was no baseline bias. Details of the range of symptoms experienced by the trial participants at trial baseline are summarised in Table 2.

Table 1.
Demographics for whole cohort (n = 147) and each randomised group.

Table 2.
Self-reported symptoms at baseline.
The most commonly self-reported symptoms for all participants are listed in Table 2. The five most commonly experienced symptoms were fatigue, shortness of breath, pains, altered sense of smell and bowel symptoms.
The average change in symptoms from day 1 to 30 was compared between the two randomised groups, and the results are summarised in Table 3. Participants taking the PC had an almost two-fold reduction in mean fatigue compared to placebo [6.1 vs. 3.4 (95% CI −5 to −0.3, p = 0.02)]. Likewise, there was a three-fold reduction in cough (CSS) amongst those randomised to the PC group compared to placebo [1.1 vs. 0.4 (95% CI −1.3 to −0.1, p = 0.02)]. This improvement in symptoms resulted in an a more than doubly improved overall well-being measured by the SWS score [2.0 vs. 4.5 (95% CI 1.5 to −4, p = 0.02)]

Table 3.
Differences in the mean improvement in symptoms between the two randomised arms.
A second part of the evaluation measured symptoms among the 126 participants who received the probiotic supplement between day 1 to day 30 (Table 4). CSS significantly reduced over two-fold (1.4 to 0.6, CI 0.5 to 1.1, p < 0.001). Likewise, SWB scores significantly improved from 24.5 to 28.5 (CI −5.5 to −2.4, p < 0.001) and CFS significantly reduced from 21.2 to 16.5 (CI 3.5 to 5.8, p < 0.001). Table 3 summarised the changes in cough and fatigue as a whole and when subdivided into the early (n = 30) and chronic phase cohorts (n = 96).

Table 4.
Change in symptom severity in participants who received probiotic supplement.
With respect to subgroup effect on symptom scores, there was one or more statistically significant difference exhibited in the level of improvement in males (vs. females), over 60 years (vs. <60 years old), participants who exercised < 3 h a week (vs. 3 h/week), had previous hospitalisation (vs. no hospitalisation) and had prior or current indigestion (vs. no indigestion) (Table 5). BMI had no significant effect on symptom improvement.

Table 5.
Subgroup analysis highlighting who got the greatest benefit from the intervention.
No formal statistical analysis was performed comparing symptoms between the 11 participants who received PC without probiotic, with the 63 participants who received PC and probiotic, as the sample size difference between these two groups is not conducive to accurate statistical analysis. Symptoms were compared between the 126 who received probiotics with the 21 who did not. There was a trend in benefit for the probiotic group: cough (mean difference 0.48 vs. 0.83, p = 0.08); fatigue score (3 vs. 4.92 vs. 3, p = 0.09); and subjective well-being (4.34 vs. 1.52, p = 0.1). Likewise, the difference in the sample sizes were too large for these figures to be statistically meaningful, hence they have not been displayed in tables.
4. Discussion
This study highlights the wide variety of symptoms participants suffer following COVID-19, in particular symptoms of fatigue and those of a GI origin. The randomised element of this study demonstrated that intake of the phytochemical-rich food capsule, in addition to the probiotic and prebiotic capsule, resulted in a statistically significant improvement in symptoms. Compared to participants who took the placebo, those taking the additional PC had an almost two-fold improvement in mean CFS and a three-fold better improvement in CSS. This resulted in a significant improvement in overall well-being in the latter group. This does imply that the quantity of phytochemicals in these foods is sufficient to elicit effects on biological pathways. Nevertheless, further research measuring the cellular and biochemical responses which take place following phytochemical intake would also be beneficial. This would help to establish whether their mechanisms of action are as an additional prebiotic to support the probiotic, or whether they have a more substantive role in helping reduce tissue inflammation, improved oxidative balance, oxygenation or even have direct anti-viral properties [15,52,71,93].
Among the cohort (126) given the Lactobacillus probiotic and inulin, there was a clear improvement in CFS, CSS and SWB scores over the 30 days. For patients within the acute phase of an infection this result would have been expected, as most patients are likely to improve within this time frame [7,116,117,118]. The same, however, cannot be said for participants with chronic, persistent COVID-19-related symptoms. In this cohort, participants had fluctuating symptoms for an average of 120 days pre-entry, and therefore improvement in symptoms within 30 days of the intervention was unlikely to have happened spontaneously and the improvements were certainly clinically meaningful. A limitation of the study was, however, the non-randomisation design of the probiotic element, but as mentioned in the methods section above, the number of participants who would have bought their own probiotics would have negated any comparative data. Nevertheless, this data does support studies which have demonstrated that probiotic supplements, particularly in vulnerable groups, shortened the incidence, duration and severity of respiratory and systemic symptoms associated with upper respiratory tract infections [6,13,15,40,54,61,62,63,64,65,66,67,68,69,70,71,72,118,119,120]. It also supports two recent randomised trials involving patients and their relatives with COVID-19 [121,122]. Likewise, it supports the finding from the King’s College ZOE STUDY which established a link between people who took probiotics and a lower risk of symptomatic COVID-19 [123].
Another limitation of the study is the uncertainty of which elements of the food intervention had the biggest impact, or whether it is the combination of all, working in synergy, which provides the therapeutic effect [117]. There are some data which highlight that the combination of probiotic bacteria, inulin and phytochemicals appears particularly advantageous via a number of synergistic mechanisms. The phytochemical-rich foods in this study can act as prebiotics which help to support probiotic bacteria growth [74,75,108]. Resveratrol and emodin in Aloe vera and polygonum cuspidatum root also enhance the formation of a protective biofilm over bacteria such as Lactobacillus paracasei, facilitating adhesion, aggregation and colony formation [108]. Curcuminoids, which are poorly absorbed in the small bowel, pass into the colon where they promote local synthesis of anti-oxidant enzymes, protecting probiotic bacteria from oxidative damage [109]. In return, probiotic bacteria help the breakdown of polyphenols into more readily absorbed and more bioactive varieties [110,111,112]. Inulin, a soluble fermentable polysaccharide found in chicory root, also passes into the large bowel where it functions as a carbon source for growth favoured by anti-inflammatory commensal bacteria. A combination of curcuminoids and ellagic acid has demonstrated synergy in their ability to generate apoptosis in viral-infected cell lines [100].
In terms of subgroup differences, participants admitted to hospital had a greater improvement after this intervention compared with non-hospitalised participants. A possible explanation for this is that gut dysbiosis is aggravated by treatments such as dexamethasone and antibiotics, routinely administered to patients during their hospital management of COVID-19, so we postulate that an intervention to improve gut health has an even greater impact on these patients [1,123]. More detailed information on antibiotic use in the non-hospitalised patients, in retrospect, would have been useful. Likewise, the benefit was greater in the older participants and those who exercised less, also factors known to be linked to less favourable gut microflora profiles [3,124]. Males did better following probiotic consumption vs. females. It has previously been reported that males have worse outcomes after COVID-19 [125]; our data adds weight to the theory that underlying variance in gut microbiota could be an explanation for this gender difference [126,127].
The mechanism of action of the probiotics on symptoms was not addressed in this study, but previous literature suggests a number of contributory possibilities. The improvement in cough may be explained by augmentation of the gut-lung axis and a reduction in lung inflammation [4,5,19,20,21,28,29]. Some authors postulate that the cause of fatigue, common during viral infections, is part of an innate immune-related defense, as it reduces movement and hence interaction with other people [128]. It is well-known that poor gut health has a link to gut inflammation and chronic fatigue [129,130,131]. Previous interventions with lactobacillus supplements reduced fatigue-related behaviour in laboratory animals [132]. In humans, there have been reported improvements in fatigue and memory following probiotic interventions, alongside reductions in systemic inflammatory cytokines and improvements in gut integrity [129,133]. Some studies have shown a microbial-neuroendocrine relationship between certain dysbiotic flora species and a resultant adverse change in hormones and neurotransmitters such as acetylcholine and gamma-amino butyrate, serotonin and dopamine, and that administration of Lactobacillus probiotics helped restore these changes [128,129,130,131,132,133]. Further research is required to establish whether these biochemical changes are responsible for the profound fatigue in long-COVID-19 sufferers and the improvements seen following this intervention.
In terms of safety, only two participants discontinued their capsules because of an increase in bloating, which they attributed to the capsules. Both received probiotic capsules, one placebo and one PC. This high safety profile is in agreement with the thousands of studies which have reported a high safety profile involving millions of people who have consumed both phytochemical-rich whole foods and probiotic capsules [33,134,135,136,137,138,139,140]. In particular, the intake of Lactobacillus probiotics has shown to be especially safe and beneficial among patients with several different medical conditions, including immunocompromised patients such as those on chemotherapy and over a wide age spectrum, ranging from premature infants to the elderly [36,42,140,141,142]. We add to these data by reporting that this Lactobacillus blend was safe in patients suffering from COVID-19.
Although it is still not known whether the benefits seen in this study could have been achieved with determined dietary manipulation alone, in this current pandemic, it would be wise to emphasise food with higher phytochemical content and lifestyle and nutritional factors which are associated with a more favorable gut microbiome. Concentrating whole foods into supplements is a convenient way to boost intake of specific nutrients throughout the day, especially for individuals who have reduced appetite. It should be noted, however, that even though these supplements used sophisticated methods to standardize the concentration of phytochemicals within capsules, regulatory bodies class them as foods rather than medicines and so they cannot be prescribed by medical practitioners. Going forward, our research group has now designed and started a study to evaluate whether this blend could also enhance antibody titres post-COVID-19 vaccination.
5. Conclusions
In conclusion, intake of this blend of probiotics and prebiotics appears to influence the severity and persistence of symptoms post COVID-19 infection, a finding which supports other recently published studies that have also reported beneficial roles for similar Lactobacillus probiotics [121,122,123]. In addition, we believe our study is one of the first to report an additional benefit for patients with symptomatic post-COVID-19 infection for this safe, low-cost phytochemical rich nutritional intervention.
6. Patents
There are no intellectual patents issued on any of the investigational ingredients, as these are already freely available and can be purchased over-the-counter. Information generated by the trial is published in the public domain for any company or government body to, hopefully, act upon.
Author Contributions
Conceptualization, R.T., R.K., J.A. and A.C.; methodology, R.T., J.A., A.C. and M.W.; software, Not applicable; validation, R.T. and J.A.; formal analysis, J.A.; investigation, R.T. and M.W.; resources, R.T. and M.W.; data, M.W. and R.T.; writing—original draft preparation, R.T.; writing—review and editing, Y.Y., R.F. and M.W.; visualization, M.W. and Y.Y.; supervision, R.T.; project administration, M.W.; funding acquisition, Not applicable. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Yorkshire & The Humber (Sheffield) Research Ethics Committee (REC reference: 20/YH/0164) protocol number: Bedford PACCT-20 Study on 22 May 2020 and approved by the HRA and Health and Care Research Wales (HCRW) on 22 May 2020. This study was sponsored and approved by Bedfordshire Hospitals Research & Development on 1 June 2020. The Medicines and Health Regulatory Agency (MHRA) gave formal authority to proceed with the trial, as no medical products license is required for food products. IRAS project ID: 282517. EudraCT number 2020-001532-10.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data not available due to [ethical/legal/commercial] restrictions. Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
Acknowledgments
The probiotics were designed by the scientific committee specifically for this study, made by The Oxford Health Company Ltd. and ParkAcre Ltd., and donated free-of-charge by the Keep-healthy group, Isle of Man, (Keep-healthy.com (accessed on 16 March 2022) who we would like to thank. We are grateful to the staff of the post-COVID-19 clinic, Bedford Hospital, for their vital help in recruiting participants. It should be noted that even though these supplements, subsequently known as yourgutplus+ and Phyto-V, used sophisticated methods to standardise the concentration of phytochemicals within capsule, regulatory bodies class them as foods not medicines and so they cannot be prescribed by medical practitioners.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
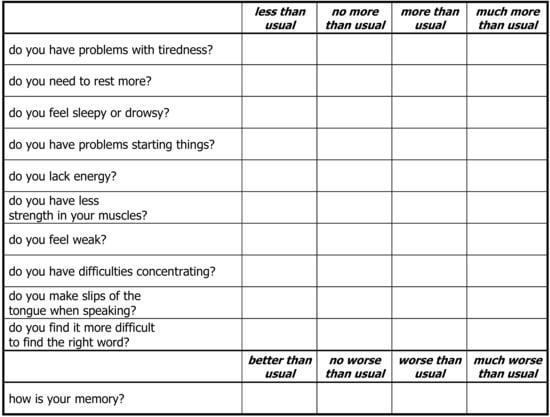
Figure A1.
Chalder Fatigue Scale.

Table A1.
Cough Symptom Score.
Table A1.
Cough Symptom Score.
| DAYTIME COUGH |
| 0 → No cough during the day |
| 1 → Cough for one short period |
| 2 → Cough for more than two short periods |
| 3 → Frequent coughing, which did not interfere with usual daytime activities |
| 4 → Frequent coughing, which did interfere with usual daytime activities |
| 5 → Distressing coughs most of the day |
| NIGHT TIME COUGH |
| 0 → No cough during the night |
| 1 → Cough on waking only |
| 2 → Wake once or early due to cough |
| 3 → Frequent waking due to choughs |
| 4 → Frequent coughs most of the night |
| 5 → Distressing coughs preventing any sleep |

Table A2.
Subjective Well-being questionnaire.
Table A2.
Subjective Well-being questionnaire.
| Subjective Well-Being (SWB) |
|---|
| Overall, how satisfied are you with your life nowadays? 0 → 1 → 2 → 3 → 4 → 5 → 6 → 7 → 8 → 9 → 10 Not at all Completely |
| Overall, to what extent do you feel that the things you do in your life are worthwhile? 0 → 1 → 2 → 3 → 4 → 5 → 6 → 7 → 8 → 9 → 10 Not at all Completely |
| Overall, how happy did you feel yesterday? 0 → 1 → 2 → 3 → 4 → 5 → 6 → 7 → 8 → 9 → 10 Not at all Completely |
| Overall, how anxious did you feel yesterday? 0 → 1 → 2 → 3 → 4 → 5 → 6 → 7 → 8 → 9 → 10 Not at all Completely |
References
- Conlon, M.A.; Bird, A.R. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients 2015, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Gu, J.; Hou, P.; Zhang, L.; Bai, Y.; Guo, Z.; Wu, H.; Zhang, B.; Li, P.; Zhao, X. Incidence, clinical characteristics and prognostic factor of patients with COVID-19: A systematic review and meta-analysis. MedRxIV 2020, 72. [Google Scholar] [CrossRef]
- Thomas, C.M.; Versalovic, J. Probiotics-host communication: Modulation of signaling pathways in the intestine. Gut Microbes 2010, 1, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Kurian, S.J.; Unnikrishnan, M.K.; Miraj, S.S.; Bagchi, D.; Banerjee, M.; Reddy, B.S.; Rodrigues, G.S.; Manu, M.K.; Saravu, K.; Mukopadhyay, C.; et al. Probiotics in Prevention and Treatment of COVID-19: Current Perspective and Future Prospects. Arch. Med. Res. 2021, 52, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Budden, K.F.; Gellatly, S.L.; Wood, D.L.A.; Cooper, M.A.; Morrison, M.; Hugenholtz, P.; Hansbro, P.M. Emerging pathogenic links between microbiota and the gut-lung axis. Nat. Rev. Microbiol. 2016, 15, 55–63. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.Y.; Zhang, F.; Liu, Q.; Li, A.Y.L.; Chung, A.C.K.; Chung, C.P.; Tso, E.Y.K.; Fung, K.S.C.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef]
- Xu, K.; Cai, H.; Shen, Y.; Ni, Q.; Chen, Y.; Hu, S.; Li, J.; Wang, H.; Yu, L.; Huang, H.; et al. Management of coronavirus disease-19 (COVID-19): The Zhejiang experience. J. Zhejiang Univ. Med. Sci. 2020, 49, 147–157. [Google Scholar]
- Chen, Y.; Gu, S.; Chen, Y.; Lu, H.; Shi, D.; Guo, J.; Wu, W.R.; Yang, Y.; Li, Y.; Xu, K.J.; et al. Six-month follow-up of gut microbiota richness in patients with COVID-19. Gut 2021, 71, 222–225. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-Month consequences of COVID-19 in patients discharged from Hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Gu, S.; Chen, Y.; Wu, Z.; Chen, Y.; Gao, H.; Lv, L.; Guo, F.; Zhang, X.; Luo, R.; Huang, C.; et al. Alterations of the Gut Microbiota in Patients with Coronavirus Disease or H1N1 Influenza. Clin. Infect. Dis. 2020, 71, 2669–2678. [Google Scholar] [CrossRef]
- Ng, S.C.; Tilg, H. COVID-19 and the gastrointestinal tract: More than meets the eye. Gut 2020, 69, 973–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, C.-L.; Smith, B.J.; Lo, D.-F.; Chyu, M.-C.; Dunn, D.M.; Chen, C.-H.; Kwun, I.-S. Dietary polyphenols and mechanisms of osteoarthritis. J. Nutr. Biochem. 2012, 23, 1367–1377. [Google Scholar] [CrossRef]
- Dhar, D.; Mohanty, A. Gut microbiota and Covid-19-possible link and implications. Virus Res. 2020, 285, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, L.; Qin, S. Gut microbiota modulation on intestinal mucosal adaptive immunity. J. Immunol. Res. 2019, 47, 47–54. [Google Scholar] [CrossRef]
- Lin, C.W.; Tsai, F.J.; Tsai, C.H.; Lai, C.C.; Wan, L.; Ho, T.Y.; Hsieh, C.C.; Chao, P.D.L. Anti-SARS coronavirus 3C-like protease effects of plant-derived phenolic compounds. Antivir. Res. 2005, 68, 36–42. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Aolymat, I.; Al-Holy, M.; Ayyash, M.; Abu Ghoush, M.; Al-Nabulsi, A.A.; Osaili, T.; Apostolopoulos, V.; Liu, S.Q.; Shah, N.P. The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19. Nature 2020, 4, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.P. The microbiome and critical illness. Lancet Respir. Med. 2016, 4, 59–72. [Google Scholar] [CrossRef] [Green Version]
- Uchide, N.; Toyoda, H. Antioxidant therapy as a potential approach to severe influenza-associated complications. Molecules 2011, 16, 2032–2052. [Google Scholar] [CrossRef] [Green Version]
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieërs, G.; Guery, B.; Delhaes, L. The gut-lung axis in health and respiratory diseases: A place for inter-organ and inter-kingdom crosstalks. Front. Cell. Infect. Microbiol. 2020, 10, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Namasivayam, S.; Sher, A.; Glickman, M.S.; Wipperman, M.F. The Microbiome and Tuberculosis: Early Evidence for Cross Talk. Am. Soc. Microbiol. 2018, 9, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Fanos, V.; Pintus, M.C.; Pintus, R.; Marcialis, M.A. Lung microbiota in the acute respiratory disease: From coronavirus to metabolomics. J. Paediatr. Neonatal Individ. Med. 2020, 9, 90–99. [Google Scholar]
- Cecchini, R.; Cecchini, A.L. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med. Hypotheses 2020, 11, 102–110. [Google Scholar] [CrossRef]
- Dumas, A.; Bernard, L.; Poquet, Y.; Lugo-Villarino, G.; Neyrolles, O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell. Microbiol. 2018, 20, 129–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, Y.; Lie, J.; Shen, L.; Zou, Y.; Hou, L.; Zhu, L.; Faden, H.S.; Tang, Z.; Shi, M.; Jiao, N.; et al. Enteric involvement in hospitalised patients with COVID-19 outside Wuhan. Lancet Gastroenterol. Hepatol. 2020, 5, 534–535. [Google Scholar] [CrossRef]
- Reinold, J.; Farahpour, F.; Fehring, C.; Dolff, S.; Konik, M.; Korth, J.; Van Baal, L.; Hoffmann, D.; Buer, J.; Witzke, O.; et al. A Pro-Inflammatory Gut Microbiome Characterizes SARS-CoV-2 Infected Patients and a Reduction in the Connectivity of an Anti-Inflammatory Bacterial Network Associates with Severe COVID-19. Front. Cell Infect. Microbiol. 2021, 17, 747816. [Google Scholar] [CrossRef]
- Anand, S.; Mande, S. Diet, Microbiota and Gut-Lung Connection. Front. Microbiol. 2018, 9, 2147–2154. [Google Scholar] [CrossRef]
- Van Heul, A.; Planer, J.; Kau, A.L. The human microbiota and asthma. Clin. Rev. Allergy Immunol. 2019, 57, 350–363. [Google Scholar] [CrossRef]
- Mammen, M.; Sethi, S. COPD and the microbiome. Respiratory 2016, 21, 590–599. [Google Scholar] [CrossRef] [Green Version]
- Martinez, J.E.; Kahana, D.D.; Ghuman, S.; Wilson, H.P.; Wilson, J.; Kim, S.C.J.; Lagishetty, V.; Jacobs, J.P.; Sinha-Hikim, A.P.; Friedman, T.C. Unhealthy Lifestyle and Gut Dysbiosis: A Better Understanding of the Effects of Poor Diet and Nicotine on the Intestinal Microbiome. Front. Endocrinol. 2021, 12, 66–74. [Google Scholar] [CrossRef]
- Carlson, J.L.; Erickson, J.M.; Lloyd, B.B.; Slavin, J.L. Health Effects and Sources of Prebiotic Dietary Fibre. Curr. Dev. Nutr. 2018, 2, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.; Yang, D.; Zollman, C.; Williams, M. Phytochemicals in Cancer Management. Curr. Res. Complement. Altern. Med. 2017, 105, 1–14. [Google Scholar]
- Thomas, R.; Aldous, J.; Forsyth, R.; Chater, A.; Williams, M. The Influence of a blend of Probiotic Lactobacillus and Prebiotic Inulin on the Duration and Severity of Symptoms among Individuals with COVID-19. Infect. Dis. Diagn. Treat. 2021, 5, 1–14. [Google Scholar]
- Nobaek, S.; Johansson, M.L.; Molin, G.; Ahrne, S.; Jeppsson, B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am. J. Gastroenterol. 2000, 95, 1231–1238. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Wan, D. Immunomodulatory Effects of Probiotics on Cytokine Profiles. BioMed Res. Int. 2018, 47, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morshedi, M.; Hashemi, R.; Moazzen, S.; Sahebkar, A.; Hosseinifard, E.S. Immunomodulatory and anti-inflammatory effects of probiotics in multiple sclerosis: A systematic review. J. Neuroinflamm. 2019, 16, 1611–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AlFaleh, K.; Anabrees, J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2014, 4, 584–671. [Google Scholar]
- Macfarlane, S.; Cleary, S.; Bahrami, B.; Reynolds, N.; Macfarlane, G.T. Synbiotic consumption changes the metabolism and composition of the gut microbiota in older people and modifies inflammatory processes: A randomised, double-blind, placebo-controlled crossover study. Aliment. Pharmacol. Ther. 2013, 38, 804–816. [Google Scholar] [CrossRef]
- Borruel, N.; Carol, M.; Casellas, F.; Antolin, M.; de Lara, F.; Epsin, E.; Naval, J.; Guarner, F.; Malagelda, J.R. Increased mucosal tumour necrosis factor alpha production in Crohn’s disease can be downregulated ex vivo by probiotic bacteria. Gut 2002, 51, 659–664. [Google Scholar] [CrossRef] [Green Version]
- Brito, M.B.; Diaz, J.P.; Muñoz-Quezada, S.; Llorente, C.G.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- Gill, H.S.; Rutherfurd, K.J.; Cross, M.L. Dietary probiotic supplementation enhances natural killer cell activity in the elderly: An investigation of age-related immunological changes. J. Clin. Immunol. 2001, 21, 264–271. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of action of probiotics. Adv. Nutr. 2019, 10, 49–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livingston, M.; Loach, D.; Wilson, M.; Tannock, G.W.; Baird, M. Gut commensal Lactobacillus reuteri 100-23 stimulates an immunoregulatory response. Immunol. Cell Biol. 2010, 88, 99–102. [Google Scholar] [CrossRef]
- Dehghan, P.; Gargari, B.P.; Jafarabadi, M.A.; Aliasgharzadeh, A. Inulin controls inflammation and metabolic endotoxemia in women with type 2 diabetes mellitus: A randomized-controlled clinical trial. Int. J. Food Sci. Nutr. 2014, 65, 65–117. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Totino, V.; Cacciotti, F.; Lebba, V.; Neroni, B.; Bonfiglio, G.; Trancassini, M.; Passariello, C.; Pantanella, F.; Schippa, S. Rebuilding the Gut Microbiota Ecosystem. Int. J. Environ. Res. Public Health 2018, 15, 1679–1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Meijnikman, A.S.; Gerdes, V.E.; Nieuwdorp, M.; Herrema, H. Evaluating causality of gut microbiota in obesity and diabetes in humans. Endocr. Rev. 2018, 39, 133–153. [Google Scholar] [CrossRef]
- Jiang, C.; Li, G.; Huang, P.; Liu, Z.; Zhao, B. The gut microbiota and Alzheimer’s disease. J. Alzheimers Dis. 2017, 58, 1–15. [Google Scholar] [CrossRef]
- Van Baarlen, P.; Troost, F.; van der Meer, C.; Hooiveld, G.; Boekschoten, M.; Brummer, R.J.M.; Kleerebezem, M. Human mucosal in vivo transcriptome responses to three Lactobacilli indicate how probiotics may modulate human cellular pathways. Proc. Natl. Acad. Sci. USA 2011, 108, 4562–4569. [Google Scholar] [CrossRef] [Green Version]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients 2018, 10, 988–1005. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.; Rao, A. Probiotics: A potential immunomodulator in COVID-19 infection management. Nutr. Res. 2021, 87, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Martoni, C.J.; Prakash, S. Oral supplementation with probiotic L. reuteri increases mean circulating 25-hydroxyvitamin D: A post hoc analysis of a randomized controlled trial. J. Clin. Endocrinol. Metab. 2013, 98, 2944–2951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.; Rawat, A.; Alwakeel, M.; Sharif, E.; Al Khodor, S. The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals. Sci. Rep. 2020, 10, 21–32. [Google Scholar] [CrossRef]
- Shang, M.; Sun, J. Vitamin D/VDR, Probiotics, and Gastrointestinal Diseases. Curr. Med. Chem. 2017, 24, 876–877. [Google Scholar] [CrossRef]
- Yoon, S.; Wu, S.; Zhang, Y.-G.; Lu, R.; Petrof, E.O.; Yuan, L.; Claud, E.; Sun, J. Probiotic regulation of vitamin D receptor in intestinal inflammation. Gastroenterology 2011, 140, 19. [Google Scholar] [CrossRef]
- Wu, S.; Yoon, S.; Zhang, Y.-G.; Lu, R.; Xia, Y.; Wan, J.; Petrof, E.O.; Claud, E.C.; Chen, D.; Sun, J. Vitamin D receptor pathway is required for probiotic protection in colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Jamilian, M.; Amirani, E.; Asemi, Z. The effects of vitamin D and probiotic co-supplementation on glucose homeostasis, inflammation, oxidative stress and pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2018, 38, 2098–2105. [Google Scholar] [CrossRef]
- Daneshkhah, A.; Agrawal, V.; Eshein, A.; Subramanian, H.; Roy, H.K.; Backman, V. The possible role of vitamin D in suppressing cytokine storm and associated mortality in COVID-19 patients. MedRxiv 2020, 78, 1–15. [Google Scholar]
- Demir, M.; Demir, F.; Aygun, H. Vitamin D deficiency is associated with COVID-19 positivity and severity of the disease. J. Med. Virol. 2021, 93, 2992–2999. [Google Scholar] [CrossRef]
- Al Kassaa, I.; Hober, D.; Hamze, M.; Chihib, N.E.; Drider, D. Antiviral potential of lactic acid bacteria and their bacteriocins. Probiotics Antimicrob. Proteins 2014, 6, 177–185. [Google Scholar] [CrossRef]
- Salaris, C.; Scarpa, M.; Elli, M.; Bertolini, A.; Guglielmetti, S.; Pregliasco, F.; Brun, P.; Catagliuolo, I. Lacticaseibacillus enhances the lactoferrin anti-SARS-CoV-2 response in Caco-2 cells. Gut Microbes 2021, 13, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Ng, T.B.; Sun, W.Z. Lactoferrin as potential preventative and adjunct treatment for COVID-19. Int. J. Antimicrob. Agents 2020, 56, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Kotwal, G. Natural Antivirals against Human Viruses. Virol. Mycol. 2014, 3, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Jassim, S.A.; Naji, M.A. Novel antiviral agents: A medicinal plant perspective. J. Appl. Microbiol. 2003, 95, 412–427. [Google Scholar] [CrossRef] [Green Version]
- Fujita, R.; Iimuro, S.; Shinozaki, T.; Sakamaki, K.; Uemura, Y.; Takeuchi, A.; Matsuyama, Y.; Ohashi, Y. Decreased duration of acute upper respiratory tract infections with daily intake of fermented milk: A multicenter, double-blinded, randomized comparative study in users of day care facilities for the elderly population. Am. J. Infect. Control 2013, 41, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Rerksuppaphol, S.; Rerksuppaphol, L. Randomized controlled trial of probiotics to reduce common cold in schoolchildren. Paediatr. Int. 2012, 54, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Waki, N.; Mastumoto, M.; Fuku, Y.; Suganuma, H. Effects of probiotic Lactobacillus brevis KB290 on incidence of influenza infection among schoolchildren: An open-label pilot study. Lett. Appl. Microbiol. 2014, 59, 565–571. [Google Scholar] [CrossRef] [Green Version]
- Kang, E.J.; Kim, S.Y.; Hwang, I.H.; Ji, Y.J. The effect of probiotics on prevention of common cold: A meta-analysis of randomized controlled trial studies. Korean J. Fam. Med. 2013, 34, 2–10. [Google Scholar] [CrossRef] [Green Version]
- Eguchi, K.; Fujitani, N.; Nakagawa, H.; Miyazaki, T. Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus. Sci. Rep. 2019, 9, 4812–4821. [Google Scholar] [CrossRef]
- Li, S.-W.; Yang, T.-C.; Lai, C.-C.; Huang, S.-H.; Liao, J.M.; Wan, L.; Lin, Y.-J.; Lin, C.-W. Antiviral activity of aloe-emodin against Influenza A virus via galectin-3 up-regulation. Eur. J. Pharmacol. 2014, 738, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Jia, Y.; Li, Y.; Zhou, D.; Jia, J. Probiotics for the Prevention of Ventilator-Associated Pneumonia: A Meta-Analysis of Randomized Controlled Trials. Respir. Care 2020, 65, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Powanda, M.; Whitehouse, M.W.; Rainsford, K.D. Celery Seed and Related Extracts with Antiarthritic, Antiulcer, and Antimicrobial Activities. Prog. Drug Res. 2015, 70, 133–153. [Google Scholar] [PubMed]
- Powanda, M.C.; Rainsford, K.D. A toxicological investigation of a celery seed extract having anti-inflammatory activity. Inflammopharmacology 2011, 19, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Karunaweera, N.; Raju, R.; Gyengesi, E.; Munch, G. Plant polyphenols as inhibitors of NF-κB induced cytokine production—A potential anti-inflammatory treatment for Alzheimer’s disease? Front. Mol. Neurosci. 2015, 8, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Funk, J.L.; Oyarzo, J.N.; Frye, J.B.; Chen, G.; Lantz, R.C.; Jolad, S.D.; Solyom, A.M.; Timmermann, B.N. Turmeric extracts containing curcuminoids prevent experimental rheumatoid arthritis. J. Nat. Prod. 2006, 69, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Al-Howiriny, T.; Alsheikh, A.; Alqasoumi, S.; Al-Yahya, M.; ElTahir, K.; Rafatullah, S. Protective Effect of Origanum majorana L. ‘Marjoram’ on various models of gastric mucosal injury in rats. Am. J. Chin. Med. 2009, 37, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.A.; Sacco, O.; Capizzi, A.; Mastromarino, P. Can Resveratrol-Inhaled Formulations Be Considered Potential Adjunct Treatments for COVID-19? Front. Immunol. 2021, 12, 670–679. [Google Scholar] [CrossRef]
- Martinez, J.; Moreno, J. Effect of Resveratrol, a Natural Polyphenolic Compound, on Reactive Oxygen Species and Prostaglandin Production. Biochem. Pharmacol. 2000, 59, 865–870. [Google Scholar] [CrossRef]
- Nencioni, L.; Iuvara, A.; Aquilano, K.; Ciriolo, M.R.; Cozzolino, F.; Rotilio, G.; Garaci, E.; Palamara, E.T. Influenza A Virus Replication is Dependent on an Antioxidant Pathway That Involves GSH and Bcl-2. J. Am. Soc. Exp. Biol. 2003, 17, 758–760. [Google Scholar] [CrossRef]
- Filardo, S.; di Pietro, M.; Mastromarino, P.; Sessa, R. Therapeutic Potential of Resveratrol Against Emerging Respiratory Viral Infections. Pharmacol. Ther. 2020, 214, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Campagna, M.; Rivas, C. Antiviral Activity of Resveratrol. Biochem. Soc. Trans. 2010, 38, 50–53. [Google Scholar] [CrossRef]
- Tang, R.; Cosentino, M.; Lee, K.H. Apigenin-7-O-β-D-glucopyranoside, an anti-HIV principle from Kummerowia striata. Bioorganic Med. Chem. Lett. 1994, 4, 455–458. [Google Scholar] [CrossRef]
- Critchfield, J.W.; Butera, S.T.; Folks, T.M. Inhibition of HIV Activation in Latently Infected Cells by Flavonoid Compounds. AIDS Res. Hum. Retrovir. 1996, 12, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-García, J.; Osca-Verdegal, R.; Pallardó, F.V.; Ferreres, J.; Rodríguez, M.; Mulet, S.; Sanchis-Gomar, F.; Carbonell, N.; Garcia-Gimenez, J.L. Oxidative Stress and Inflammation in COVID-19-Associated Sepsis: The Potential Role of Anti-Oxidant Therapy in Avoiding Disease Progression. Antioxidants 2020, 9, 936–949. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B. Strategies for reducing or preventing the generation of oxidative stress. Oxidative Med. Cell. Longev. 2011, 19, 194–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ristow, M.; Zarse, K. How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis). Exp. Gerontol. 2010, 45, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, V.H.; Valente, H.F.; Casal, S.I.; Marques, A.F.; Moreira, P.A. Antioxidant vitamins do not prevent post exercise peroxidation and may delay muscle recovery. Med. Sci. Sports Exerc. 2009, 41, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Avery, N.G.; Kaiser, J.L.; Sharman, M.J.; E Scheett, T.; Barnes, D.M.; Gómez, A.L.; Kramer, W.J.; Volek, J.S. Effects of vitamin E supplementation on recovery from repeated bouts of resistance exercise. J. Strength Cond. Res. 2003, 17, 801–809. [Google Scholar]
- Crow, J.P. Dichlorodihydrofluorescein and dihydrorhodamine 123 are sensitive indicators of peroxynitrite in vitro: Implications for intracellular measurement of reactive nitrogen and oxygen species. Nitric Oxide 1997, 1, 145–157. [Google Scholar] [CrossRef]
- Bailey, J.D.; Diotallevi, M.; Nicol, T.; McNeill, E.; Shaw, A.; Chuaiphichai, S.; Hale, A.; Starr, A.; Nandi, M.; Sylianou, E.; et al. Nitric Oxide Modulates Metabolic Remodeling in Inflammatory Macrophages through TCA Cycle Regulation and Itaconate Accumulation. Cell Rep. 2019, 28, 218–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, D.J.; Dawson, E.A.; Groenewoud, H.M.; Jones, H.; Thijssen, D.H. Is Flow-Mediated Dilation Nitric Oxide Mediated? Hypertension 2014, 63, 376–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.-Y.; Chen, C.; Zhang, H.-Q.; Guo, H.-Y.; Wang, H.; Wang, L.; Zhang, X.; Hua, S.-N.; Yu, J.; Xiao, P.-G.; et al. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir. Res. 2005, 67, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Kwon, M.J.; Yoo, J.Y.; Choi, H.J.; Ahn, Y.J. Antiviral activity and possible mode of action of ellagic acid identified in Lagerstroemia speciosa leaves toward human rhinoviruses. BMC Complementary Med. Ther. 2014, 14, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Tito, A.; Colantuono, A.; Pirone, L.; Pedone, E.; Intartaglia, D.; Giamundo, G.; Conte, I.; Vitaglione, P.; Apone, F. Pomegranate Peel Extract as an Inhibitor of SARS-CoV-2 Spike Binding to Human ACE2 Receptor (in vitro): A Promising Source of Novel Antiviral Drugs. Front. Chem. 2021, 28, 81–87. [Google Scholar] [CrossRef]
- Biancatelli, R.M.L.C.; Berrill, M.; Catravas, J.D.; Marik, P.E. Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19). Front. Immunol. 2020, 11, 145–151. [Google Scholar] [CrossRef]
- Palamara, A.T.; Nencioni, L.; Aquilano, K.; de Chiara, G.D.; Hernandez, L.; Cozzolino, F.; Ciriolo, M.R.; Garaci, E. Inhibition of Influenza A Virus Replication by Resveratrol. J. Infect. Dis. 2005, 191, 1719–1729. [Google Scholar] [CrossRef]
- Jennings, M.R.; Parks, J.R. Curcumin as an Antiviral Agent. Viruses 2020, 12, 1242–1262. [Google Scholar] [CrossRef]
- Rattis, B.A.C.; Ramos, S.G.; Celes, M.R.N. Curcumin as a Potential Treatment for COVID-19. Front. Pharmacol. 2021, 12, 673–675. [Google Scholar] [CrossRef]
- Kumar, D.; Basu, S.; Parija, L.; Rout, D.; Manna, S.; Dandapat, J.; Debata, P.R. Curcumin and Ellagic acid synergistically induce ROS generation, DNA damage, p53 accumulation and apoptosis in HeLa cervical carcinoma cells. Biomed. Pharmacol. 2016, 81, 31–37. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, H.J.; Lee, Y.S. A New Anti-HIV Flavonoid Glucuronide from Chrysanthemum Morifolium. Planta Medica 2003, 69, 859–861. [Google Scholar]
- Lv, X.; Qiu, M.; Chen, D.; Zheng, N.; Jin, Y.; Wu, Z. Apigenin Inhibits Enterovirus 71 Replication Through Suppressing Viral IRES Activity and Modulating Cellular JNK Pathway. Antivir. Res. 2014, 109, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Fan, W.; Qian, P.; Zhang, D.; Wei, Y.; Chen, H.; Li, X. Apigenin Restricts FMDV Infection and Inhibits Viral IRES Driven Translational Activity. Viruses 2015, 7, 1613–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising natural compounds against viral infections. Arch. Virol. 2017, 162, 2539–2551. [Google Scholar] [CrossRef] [PubMed]
- Alzaabi, M.M.; Hamdy, R.; Ashmawy, N.S.; Hamoda, A.M.; Alkhayat, F.; Khademi, N.N.; Abo Al Joud, S.M.; El-Keblawy, A.A.; Soliman, S.S.M. Flavonoids are promising safe therapy against COVID-19. Phytochem. Rev. Proc. Phytochem. Soc. Eur. 2021, 21, 291–312. [Google Scholar] [CrossRef]
- Horvat, M.; Avbelj, M.; Duran-Alonso, M.B.; Banjanac, M.; Petkovic, H.; Iskra, J. Antiviral Activities of Halogenated Emodin Derivatives against Human Coronavirus NL63. Molecules 2021, 26, 6825–6847. [Google Scholar] [CrossRef]
- Alves-Santos, A.M.; Sugizaki, C.S.A.; Lima, G.C.; Naves, M.M.V. Prebiotic effect of dietary polyphenols: A systematic review. J. Funct. Foods 2020, 74, 3–104. [Google Scholar] [CrossRef]
- Al Azzaz, J.; Al Tarraf, A.; Heumann, A.; da Silva Barreira, D.; Laurent, J.; Assifaoui, A.; Rieu, A.; Guzzo, J.; Lapaquette, P. Resveratrol Favors Adhesion and Biofilm Formation of Lacticaseibacillus paracasei subsp. paracasei Strain ATCC334. Int. J. Mol. Sci. 2020, 21, 5423–5446. [Google Scholar] [CrossRef]
- Arcanjo, N.O.; Andrade, M.J.; Padilla, P.; Rodríguez, A.; Madruga, M.S.; Estévez, M. Resveratrol protects Lactobacillus reuteri against H2O2-induced oxidative stress and stimulates antioxidant defenses through upregulation of the dhaT gene. Free. Radic. Biol. Med. 2019, 135, 38–45. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Koh, A.; de Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From dietary fibre to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, G.; Jacobs, D.M.; Peters, S.; Possemiers, S.; van Duynhoven, J.; Vaughan, E.E.; Van de Wiele, T. In vitro bioconversion of polyphenols from black tea and red wine/grape juice by human intestinal microbiota displays strong inter-individual variability. J. Agric. Food Chem. 2010, 58, 10236–10246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolte, L.A.; Vich Vila, A.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.L.; Campmans-Kuijpers, M.J.E.; Fu, J.; et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Develin, S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [Green Version]
- Pontin, E.; Schwannauer, M.; Tai, S.; Kinderman, P. A UK validation of a general measure of subjective well-being: The modified BBC subjective well-being scale (BBC-SWB). Health Qual. Life Outcomes 2013, 11, 150–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalder, T.; Berelowitz, G.; Pawlikowska, T.; Watts, L.; Wessely, S.; Wright, D.; Wallace, E.P. Development of a fatigue scale. J. Psychosom. Res. 1993, 37, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Jiang, X.; Zhang, Z.; Huang, S.; Zhang, Z.; Fang, Z.; Gu, Z.; Gao, L.; Shi, H.; Mai, L.; et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 2020, 69, 997–1001. [Google Scholar] [CrossRef]
- Baud, D.; Agri, V.D.; Gibson, G.R.; Reid, G.; Giannoni, E. Using Probiotics to Flatten the Curve of Coronavirus Disease COVID-2019 Pandemic. Front. Public Health 2020, 8, 186–194. [Google Scholar] [CrossRef]
- Pourhossein, M.; Moravejolahkami, A. Probiotics in viral infections, with a focus on COVID-19: A systematic review. Authorea 2020, 1–2. [Google Scholar] [CrossRef]
- Adnan, M.L.; Dewi, M.D. Potential Effects Immunomodulators on Probiotics in COVID-19 Preventing Infection in the Future. A Narrative Review. Int. J. Med. Stud. 2020, 8, 121–125. [Google Scholar] [CrossRef]
- Gutiérrez-Castrellón, P.; Gandara-Martí, T.; Abreu, A.T.A.Y.; Nieto-Rufino, C.D.; López-Orduña, E.; Jiménez-Escobar, I.; Jiménez-Gutiérrez, C.; López-Velazquez, G.; Espadaler-Mazo, J. Probiotic improves symptomatic and viral clearance in Covid19 outpatients: A randomized, quadruple-blinded, placebo-controlled trial. Gut Microbes 2022, 14, 2018899. [Google Scholar] [CrossRef] [PubMed]
- Wischmeyer, P.E.; Tang, H.; Ren, Y.; Bohannon, L.; Ramirez, Z.; Andermann, T.; Messina, J.A.; Sung, J.A.; Jensen, D.; Jung, S.-H.; et al. Daily Lactobacillus Probiotic versus Placebo in COVID-19-Exposed Household Contacts (PROTECT-EHC): A Randomized Clinical Trial. medRxiv 2022. [Google Scholar] [CrossRef]
- Louca, P.; Murray, B.; Klaser, K.; Graham, M.S.; Mazidi, M.; Leeming, E.R.; Thompson, E.; Bowyer, R.; Drew, D.A.; Nguyen, L.H.; et al. Modest effects of dietary supplements during the COVID-19 pandemic: Insights from 445 850 users of the COVID-19 Symptom Study app. BMJ Nutr. Prev. Health 2021, 4, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Meijvis, S.C.; Hardeman, H.; Remmelts, H.H.; Heijligenberg, R.; Rijkers, G.T.; van Velzen-Blad, H.; Voorn, G.P.; van de Garde, E.M.W.; Endeman, H.; Grutters, J.C.; et al. Dexamethasone and length of hospital stay in patients with community-acquired pneumonia: A randomised, double-blind, placebo-controlled trial. Lancet 2011, 377, 2023–2030. [Google Scholar] [CrossRef]
- Hassan, N.E.; Wahba, S.A.; El-Masry, S.A.; Elhamid, E.R.A.; Boseila, S.A.W.; Ahmed, N.H.; Ibrahim, T.S. Eating Habits and Lifestyles among a Sample of Obese Working Egyptian Women. Open Access Maced. J. Med. Sci. 2015, 3, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Bwire, G. Coronavirus: Why Men are More Vulnerable to Covid-19 Than Women? SN Compr. Clin. Med. 2020, 2, 874–876. [Google Scholar] [CrossRef]
- Yurkovetskiy, L.; Burrows, M.; Khan, A.A.; Graham, L.; Volchkov, P.; Becker, L.; Antonopoulos, D.; Umesaki, Y.; Chervonsky, A.V. Gender bias in autoimmunity is influenced by microbiota. Immunity 2013, 39, 400–412. [Google Scholar] [CrossRef] [Green Version]
- Dantzer, R.; Heijnen, C.J.; Kavelaars, A.; Laye, S.; Capuron, L. The neuroimmune basis of fatigue. Trends Neurosci. 2014, 37, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Giloteaux, L.; Goodrich, J.K.; Walters, W.A.; Levine, S.M.; Ley, R.E.; Hanson, M. R Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome 2016, 4, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Williamson, C.B.; Burns, C.M.; Gossard, C.M.; Pizano, J.M.; E Dolan, K.; Finley, H.J.; Gasta, M.G.; Parker, E.C.; Lipski, E.A. Probiotics and Disease: A Comprehensive Summary-Part 3, Cardiometabolic Disease and Fatigue Syndromes. Integr. Med. A Clin. J. 2017, 16, 30–41. [Google Scholar]
- Galland, L. The gut microbiome and the brain. J. Med. Food 2014, 17, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, A.; Nord, C.E.; Evengård, B. Effect of supplement with lactic-acid producing bacteria on fatigue and physical activity in patients with chronic fatigue syndrome. Nutr. J. 2009, 8, 4–11. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.K.; Chopra, K.; Kuhad, A.; Kaur, I.P. Role of Lactobacillus acidophilus loaded floating beads in chronic fatigue syndrome: Behavioural and biochemical evidences. Neurogastroenterol. Motil. 2012, 24, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Doron, S.; Snydman, D. Risk and safety of probiotics. Clin. Infect. Dis. 2015, 60, 129–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardeau, M.; Vernoux, J.P.; Henri-Dubernet, S.; Guéguen, M. Safety assessment of dairy microorganisms: The Lactobacillus genus. Int. J. Food Microbiol. 2008, 126, 278–285. [Google Scholar] [CrossRef]
- Preidis, G.; Weizman, A.V.; Kashyap, P.C.; Morgan, R.L. AGA Technical Review on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology 2020, 159, 708–738. [Google Scholar] [CrossRef]
- Hempel, S.; Newberry, S.; Ruelaz, A.; Wang, Z.; Miles, J.N.V.; Suttorp, M.J.; Johnsen, B.; Shanman, R.; Slusser, W.; Fu, N.; et al. Safety of probiotics used to reduce risk and prevent or treat disease. Evid. Rep. Technol. Assess. 2011, 200, 1–645. [Google Scholar]
- Sanders, M.E.; Akkermans, L.M.; Haller, D.; Hammerman, C.; Heimbach, J.T.; Hörmannsperger, G.; Huys, G.; Levy, D.D.; Lutgendorff, F.; Mack, D.; et al. Safety assessment of probiotics for human use. Gut Microbes 2010, 1, 164–185. [Google Scholar] [CrossRef]
- Borriello, S.P.; Hammes, W.P.; Holzapfel, W.; Marteau, P.; Schrezenmeir, J.; Vaara, M.; Valtonen, V. Safety of probiotics that contain lactobacilli or bifidobacteria. Clin. Infect. Dis. 2003, 36, 775–780. [Google Scholar] [CrossRef]
- Bernardeau, M.; Guguen, M.; Vernoux, J.P. Beneficial lactobacilli in food and feed: Long-term use, biodiversity and proposals for specific and realistic safety assessments. FEMS Microbiol. Rev. 2006, 30, 487–513. [Google Scholar] [CrossRef]
- Lu, D.; Yan, J.; Liu, F.; Ding, P.; Chen, B.; Lu, Y.; Sun, Z. Probiotics in preventing and treating chemotherapy-induced diarrhoea: A meta-analysis. Asia Pac. J. Clin. Nutr. 2019, 28, 701–710. [Google Scholar] [PubMed]
- Salminen, M.K.; Tynkkynen, S.; Rautelin, H.; Saxelin, M.; Vaara, M.; Ruutu, P.; Sarna, S.; Valtonen, V.; Jarvinen, A. Lactobacillus bacteremia during a rapid increase in probiotic use of Lactobacillus rhamnosus GG in Finland. Clin. Infect. Dis. 2002, 35, 1155–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).