Biogenic TiO2–ZnO Nanocoatings: A Sustainable Strategy for Visible-Light Self-Sterilizing Surfaces in Healthcare
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Culture Conditions
2.2. Biosynthesis of TiO2 Nanoparticles by Bacillus subtilis
2.3. Biosynthesis of ZnO Nanoparticles by Bacillus subtilis
2.4. Nanoparticle Characterization
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. Transmission Electron Microscopy (TEM)
2.4.3. X-Ray Diffraction (XRD)
2.4.4. UV-Visible Spectroscopy
2.4.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.6. Zeta Potential
2.5. Fabrication of Multilayer Photonic Nanocoatings
2.5.1. Layer-by-Layer Deposition
2.5.2. Photonic/Optical Characterization of Coatings
2.5.3. Stability Assessment (Preliminary)
2.6. Antibacterial Activity Assay
2.6.1. Preparation of Inoculum
2.6.2. Sample Disks
2.6.3. Visible Light Exposure
2.6.4. Zone of Inhibition Measurement
2.6.5. Radical Scavenging Assay
2.6.6. Direct Contact-Killing Assay
2.6.7. Quantification of Hydrogen Peroxide (H2O2) Under Visible Light
2.6.8. Statistical Analysis
3. Results
3.1. Biosynthesis and Characterization of TiO2 and ZnO Nanoparticles
3.1.1. Nanoparticle Formation
3.1.2. Morphology (SEM)
3.1.3. Crystalline Phase (XRD)
3.1.4. Optical Properties (UV-Vis Absorption and Band Gap)
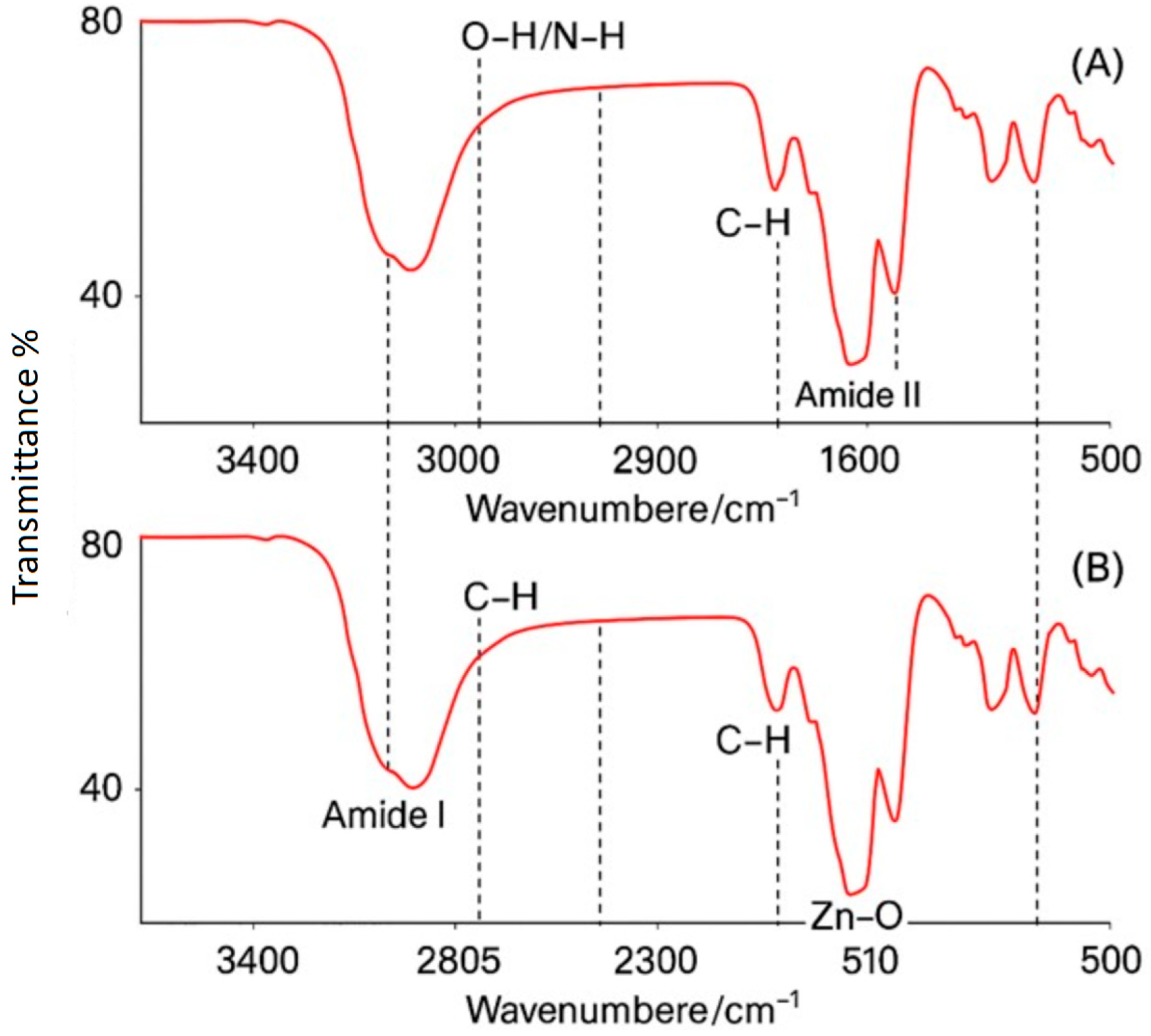
3.1.5. FTIR Analysis
3.2. Properties of Multilayer TiO2/ZnO Nanocoatings
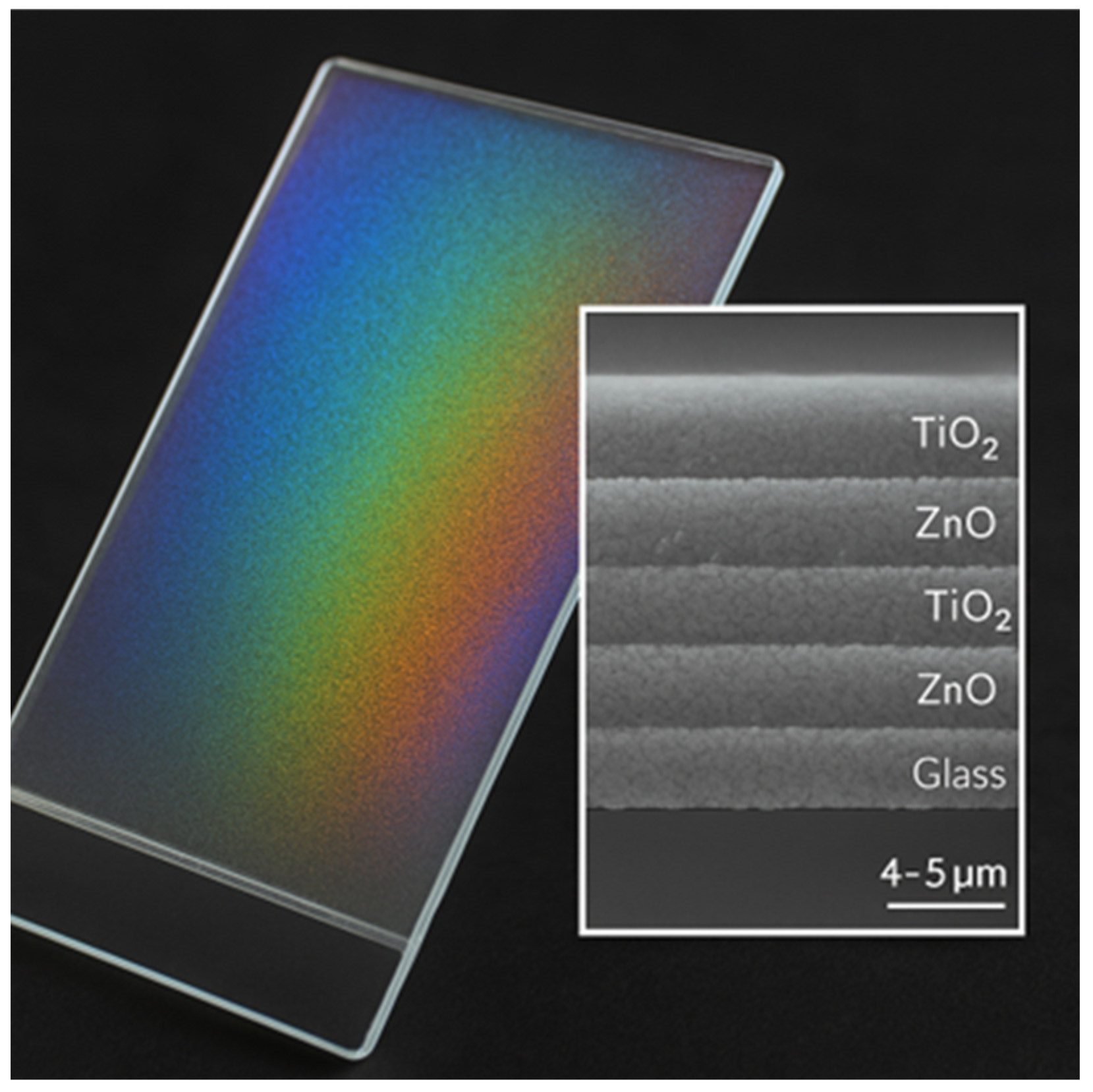
Coating Structure and Appearance
3.3. Antimicrobial Efficacy Under Visible Light
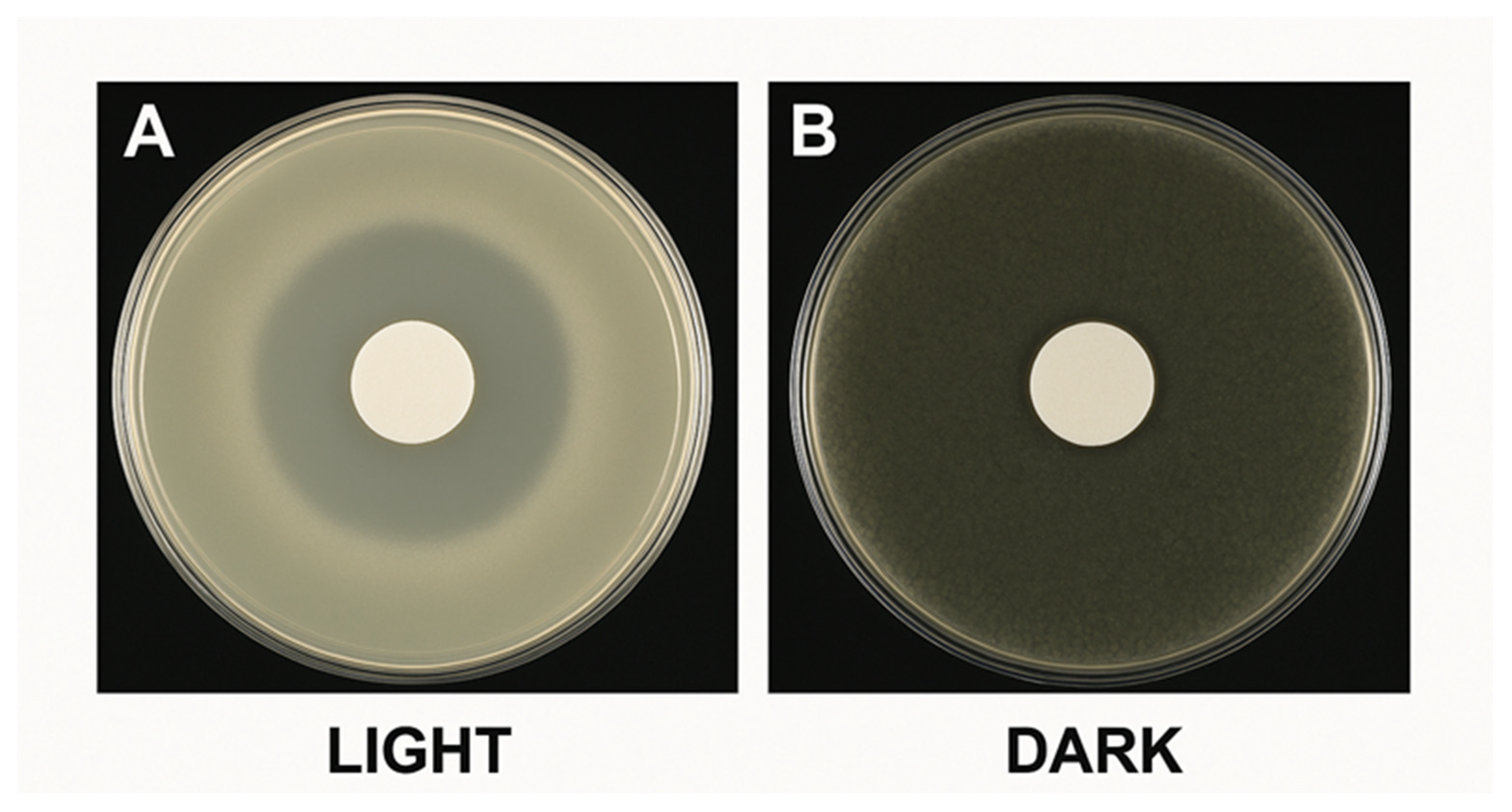
3.3.1. Visible Light vs. Dark Performance
3.3.2. Comparison of Coating Types
3.3.3. Species Susceptibility
3.3.4. Direct Contact-Killing Activity of Glass-Slide Coatings
3.3.5. Effect of ROS Scavengers on Antibacterial Activity
3.3.6. Visible-Light Generation of H2O2
3.3.7. Stability and Durability Observations
4. Discussion
4.1. Green Synthesis of Photocatalytic Nanoparticles
4.2. Visible-Light Photocatalysis
4.3. Optical Evidence of Light Trapping
4.4. Mechanistic Confirmation by Radical Scavenging
4.5. Role of ROS Generation
4.6. Validation of Contact-Killing Ability
4.7. Antibacterial Activity Compared to Literature
4.8. Stability and Durability Considerations
4.9. Implications for Hospital Surfaces
4.10. Safety and Efficacy
4.11. Role of Zn2+ Release and Bacterial Tolerance
4.12. Comparison with Other Approaches
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tabah, A.; Buetti, N.; Staiquly, Q.; Ruckly, S.; Akova, M.; Aslan, A.T.; Leone, M.; Morris, A.C.; Bassetti, M.; Arvaniti, K.; et al. Epidemiology and outcomes of hospital-acquired bloodstream infections in intensive care unit patients: The EUROBACT-2 international cohort study. Intensive Care Med. 2023, 49, 178–190. [Google Scholar] [CrossRef]
- Porter, L.; Sultan, O.; Mitchell, B.; Jenney, A.; Kiernan, M.; Brewster, D.; Russo, P. How long do nosocomial pathogens persist on inanimate surfaces? A scoping review. J. Hosp. Infect. 2024, 147, 25–31. [Google Scholar] [CrossRef]
- Coza, H. Evaluation of antimicrobial properties in coatings for operating room surfaces. J. Sustain. Constr. Mater. Technol. 2024, 9, 138–143. [Google Scholar] [CrossRef]
- Elgohary, E.A.; Mohamed, Y.M.A.; El Nazer, H.A.; Baaloudj, O.; Alyami, M.S.S.; El Jery, A.; Assadi, A.A.; Amrane, A. A review of the use of semiconductors as catalysts in the photocatalytic inactivation of microorganisms. Catalysts 2021, 11, 1498. [Google Scholar] [CrossRef]
- Domyati, D. Dielectric and optoelectronics properties of thin films based on chitosan containing titanium oxide/hematite and their potential for optoelectronics devices. J. Mater. Sci. Mater. Electron. 2024, 35, 406. [Google Scholar] [CrossRef]
- Schutte-Smith, M.; Erasmus, E.; Mogale, R.; Marogoa, N.; Jayiya, A.; Visser, H.G. Using visible light to activate antiviral and antimicrobial properties of TiO2 nanoparticles in paints and coatings: Focus on new developments for frequent-touch surfaces in hospitals. J. Coat. Technol. Res. 2023, 20, 789–817. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-E.; Jin, H.-E. Antimicrobial activity of zinc oxide nano/microparticles and their combinations against pathogenic microorganisms for biomedical applications: From physicochemical characteristics to pharmacological aspects. Nanomaterials 2021, 11, 263. [Google Scholar] [CrossRef]
- Shahed, C.A.; Ahmad, F.; Günister, E.; Foudzi, F.M.; Ali, S.; Malik, K.; Harun, W.S.W. Antibacterial mechanism with consequent cytotoxicity of different reinforcements in biodegradable magnesium and zinc alloys: A review. J. Magnes. Alloys 2023, 11, 3038–3058. [Google Scholar] [CrossRef]
- Puri, N.; Gupta, A. Water remediation using titanium and zinc oxide nanomaterials through disinfection and photocatalysis process: A review. Environ. Res. 2023, 227, 115786. [Google Scholar] [CrossRef]
- Khan, M.M.; Abdulwahab, K.O. Metals- and non-metals-doped ZnS for various photocatalytic applications. Mater. Sci. Semicond. Process. 2024, 181, 108634. [Google Scholar] [CrossRef]
- de Maria, V.P.K.; de Paiva, F.F.G.; Tamashiro, J.R.; Silva, L.H.P.; Pinho, G.d.S.; Rubio-Marcos, F.; Kinoshita, A. Advances in ZnO nanoparticles in building material: Antimicrobial and photocatalytic applications—A systematic literature review. Constr. Build. Mater. 2024, 417, 135337. [Google Scholar] [CrossRef]
- Ghamarpoor, R.; Fallah, A.; Jamshidi, M. A review of synthesis methods, modifications, and mechanisms of ZnO/TiO2-based photocatalysts for photodegradation of contaminants. ACS Omega 2024, 9, 25457–25492. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Z.L.; Daneshjou, S. Bacillus megaterium as TiO2 nano-factory: Biosynthesis, characterization, and antibacterial activity. Waste Biomass Valorization 2025, 16, 4295–4308. [Google Scholar] [CrossRef]
- Abo-Shanab, W.A.; Elsilk, S.E.; Afifi, S.S.; El-Shenody, R.A. Ecofriendly biosynthesis of zinc oxide nanoparticles from Arthrospira platensis and their assessment for antimicrobial, antibiofilm, anticancer potency, and alleviation of copper stress in Vicia faba (L.) plants. J. Soil Sci. Plant Nutr. 2025, 25, 2742–2762. [Google Scholar] [CrossRef]
- Roy, T.; Basak, N.; Mainak, S.; Das, S.; Ali, S.I.; Islam, E. Burkholderia sp. EIKU21-mediated synthesis of biogenic ZnO nanoparticle–based pigment for development of antibacterial cotton fabric through nanocoating. Biomass Convers. Biorefinery 2024, 15, 6927–6942. [Google Scholar] [CrossRef]
- Leistikow, K.R.; May, D.S.; Suh, W.S.; Asensio, G.V.; Schaenzer, A.J.; Currie, C.R.; Hristova, K.R. Bacillus subtilis-derived peptides disrupt quorum sensing and biofilm assembly in multidrug-resistant Staphylococcus aureus. mSystems 2024, 9, e00712-24. [Google Scholar] [CrossRef]
- Thajuddin, F.; Palanivel, P.; Chithirai, A.; Thajuddin, S.F.; Arivalagan, P.; Saravanan, C.; Nooruddin, T.; Dharumadurai, D. Green synthesis of titanium oxide nanoparticles using aqueous extracts of Limnospira fusiformis and their multifunctional applications in biomedical and biodiesel production. Algal Res. 2025, 89, 104082. [Google Scholar] [CrossRef]
- Quispe Cohaila, A.B.; Fora Quispe, G.d.L.; Lanchipa Ramos, W.O.; Cáceda Quiroz, C.J.; Tamayo Calderón, R.M.; Medina Salas, J.P.; Rajendran, S.; Sacari Sacari, E.J. Synthesis of ZnO nanoparticles by Bacillus subtilis for efficient photocatalytic degradation of cyanide. Nanomaterials 2025, 15, 501. [Google Scholar] [CrossRef]
- Leslie, H.A.; Van Velzen, M.J.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Bibi, H.; Mansoor, M.A.; Asghar, M.A.; Ahmad, Z.; Numan, A.; Haider, A. Facile hydrothermal synthesis of highly durable binary and ternary cobalt–nickel–copper oxides for high-performance oxygen evolution reaction. Int. J. Hydrogen Energy 2025, 50, 369–377. [Google Scholar] [CrossRef]
- Bouzidi, I.; Khazri, A.; Mougin, K.; Bendhafer, W.; Abu-Elsaoud, A.M.; Plavan, O.A.; Ali, M.A.; Plavan, G.; Özdemir, S.; Beyrem, H.; et al. Doping zinc oxide and titanium dioxide nanoparticles with gold induces additional oxidative stress, membrane damage, and neurotoxicity in Mytilus galloprovincialis: Results from a laboratory bioassay. J. Trace Elements Med. Biol. 2024, 83, 127401. [Google Scholar] [CrossRef]
- Heryanto, H.; Tahir, D.; Abdullah, B.; Sayyed, M.I.; Yunas, J.; Masrour, R.; Veeravelan, K. Fast Fourier transform implementation for determining band gap energy from UV–Vis spectra as a fresh methodology. Arab. J. Sci. Eng. 2025, 50, 533–539. [Google Scholar] [CrossRef]
- Liang, Y.P.; Chan, Y.B.; Aminuzzaman, M.; Shahinuzzaman, M.; Djearamane, S.; Thiagarajah, K.; Leong, S.-Y.; Wong, L.-S.; Tey, L.-H. Green synthesis and characterization of copper oxide nanoparticles from durian (Durio zibethinus) husk for environmental applications. Catalysts 2025, 15, 275. [Google Scholar] [CrossRef]
- Benamer Oudih, S.; Tahtat, D.; Nacer Khodja, A.; Mahlous, M.; Hammache, Y.; Guittoum, A.E.; Gana, S.K. Chitosan nanoparticles with controlled size and zeta potential. Polym. Eng. Sci. 2023, 63, 1011–1021. [Google Scholar] [CrossRef]
- Joseph, S.; Nallaswamy, D.; Rajeshkumar, S.; Dathan, P.; Jose, L. A comprehensive review of synthesis, properties, and applications of TiO2 and ZnO nanoparticles. J. Clin. Diagn. Res. 2024, 18, 5–10. [Google Scholar] [CrossRef]
- Sharif Bakhsh, E.; Tavakoli Dare, M.; Jafari, A.; Shahi, F. Light-activated nanofibers: Advances in photo-responsive electrospun polymer technologies. Polym.-Plast. Technol. Mater. 2025, 64, 397–438. [Google Scholar] [CrossRef]
- Mashaly, K.B. Optimal design of multilayer optical thin-film structure for smart energy-saving applications using a needle optimization approach. Phys. Scr. 2024, 99, 075530. [Google Scholar] [CrossRef]
- Butler, J.; Handy, R.D.; Upton, M.; Besinis, A. Review of antimicrobial nanocoatings in medicine and dentistry: Mechanisms of action, biocompatibility performance, safety, and benefits compared to antibiotics. ACS Nano 2023, 17, 7064–7092. [Google Scholar] [CrossRef]
- Sunarno; Nursofiah, S.; Hartoyo, Y.; Amalia, N.; Febrianti, T.; Febriyana, D.; Saraswati, R.D.; Puspandari, N.; Sariadji, K.; Khariri; et al. Long-term storage of bacterial isolates by using tryptic soy broth with 15% glycerol in the deep freezer (−70 to −80 °C). IOP Conf. Ser. Earth Environ. Sci. 2021, 913, 012070. [Google Scholar] [CrossRef]
- Nakashima, D.; Fujioka, Y.; Katayama, K.; Morita, T.; Kikuiri, M.; Amano, K.; Kagawa, E.; Nakamura, T. Development of preparation methods of polished sections of returned samples from asteroid Ryugu by the Hayabusa2 spacecraft. Meteorit. Planet. Sci. 2024, 59, 1829–1844. [Google Scholar] [CrossRef]
- Ni, M.; Jiang, C.; Cheng, W.; Yang, K.; Dai, L.; Zeng, Y.; Su, J.; Lu, Z.; Zou, S.; Su, X. A solid strategy to realize efficient antibacterial activity on the shade surface of bulk silicon under natural or indoor lighting. Chem. Eng. J. 2024, 479, 147734. [Google Scholar] [CrossRef]
- Ameen, F.; Al-Maary, K.S.; Almansob, A.; AlNadhari, S. Antioxidant, antibacterial and anticancer efficacy of Alternaria chlamydospora-mediated gold nanoparticles. Appl. Nanosci. 2023, 13, 2233–2240. [Google Scholar] [CrossRef]
- Kwak, S. Are only p-values less than 0.05 significant? A p-value greater than 0.05 is also significant! J. Lipid Atheroscler. 2023, 12, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Ragavendran, C.; Kamaraj, C.; Jothimani, K.; Priyadharsan, A.; Kumar, D.A.; Natarajan, D.; Malafaia, G. Eco-friendly approach for ZnO nanoparticle synthesis and evaluation of its possible antimicrobial, larvicidal and photocatalytic applications. Sustain. Mater. Technol. 2023, 36, e00597. [Google Scholar] [CrossRef]
- Sagadevan, S.; Imteyaz, S.; Murugan, B.; Anita Lett, J.; Sridewi, N.; Weldegebrieal, G.K.; Fatimah, I.; Oh, W.C. A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications. Green Process. Synth. 2022, 11, 44–63. [Google Scholar] [CrossRef]
- Kawakami, R.; Matsumoto, T.; Yanagiya, S.I.; Shirai, A.; Nakano, Y.; Niibe, M. Enhanced photocatalytic activity of anatase/rutile mixed phase titanium dioxide nanoparticles annealed with polyethylene glycol at low temperatures in aluminum foil covered combustion boats. Phys. Status Solidi A 2025, 222, 2400478. [Google Scholar] [CrossRef]
- Nargund, V.B.; Patil, R.R.; Vanti, G.L. Bacillus sp. extract used to fabricate ZnO nanoparticles for their antagonist effect against phytopathogens. Biometals 2022, 35, 1255–1269. [Google Scholar] [CrossRef]
- Chauke, N.M.; Ngqalakwezi, A.; Raphulu, M. Transformative advancements in visible-light-activated titanium dioxide for industrial wastewater remediation. Int. J. Environ. Sci. Technol. 2025, 22, 8521–8552. [Google Scholar] [CrossRef]
- Diniz, R.R.; de Pádula, M.; de Souza, A.M.T. Let’s shed light on photogenotoxicity. Sci. Total Environ. 2024, 954, 176354. [Google Scholar] [CrossRef]
- Popova, A.; Advakhova, D.Y.; Sheveyko, A.N.; Kuptsov, K.A.; Slukin, P.; Ignatov, S.G.; Ilnitskaya, A.; Timoshenko, R.V.; Erofeev, A.S.; Kuchmizhak, A.A.; et al. Synergistic bactericidal effect of Zn2+ ions and reactive oxygen species generated in response to either UV or X-ray irradiation of Zn-doped plasma electrolytic oxidation TiO2 coatings. ACS Appl. Bio Mater. 2024, 7, 5579–5596. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, V.; Yadav, N.; Dagar, R.; Kalyankar, R.; Gupta, K. Enhanced antimicrobial activity of biogenic zinc oxide (ZnO) nanoparticles. Chem. Sel. 2025, 10, e01732. [Google Scholar] [CrossRef]
- Lettieri, S.; Pavone, M.; Fioravanti, A.; Santamaria Amato, L.; Maddalena, P. Charge carrier processes and optical properties in TiO2 and TiO2-based heterojunction photocatalysts: A review. Materials 2021, 14, 1645. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, Z.; Wang, X.; Guo, Y.; Xie, Y.; Yao, W.; Kawasaki, H. Piezoelectric nanomaterials for antibacterial strategies. Appl. Mater. Today 2024, 40, 102419. [Google Scholar] [CrossRef]
- Goyal, M.R.; Mishra, S.K.; Dasarahalli-Huligowda, L.K. Nanotechnology Applications in Agricultural and Bioprocess Engineering: Farm to Table; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Al-Saeedi, S.I. Photoelectrochemical green hydrogen production utilizing ZnO nanostructured photoelectrodes. Micromachines 2023, 14, 1047. [Google Scholar] [CrossRef]
- Kirubakaran, D.; Bupesh, G.; Wahid, J.B.A.; Murugeswaran, R.; Ramalingam, J.; Arokiyaraj, S.; Sivasakthi, V.; Panigrahi, J. Green synthesis of zinc oxide nanoparticles using Acmella caulirhiza leaf extract: Characterization and assessment of antibacterial, antioxidant, anti-inflammatory and hemolytic properties. Biomed. Mater Devices 2025, 4, 552–573. [Google Scholar] [CrossRef]
- Ismail, A.A.; Al-Hajji, L.; Azad, I.S.; Al-Yaqoot, A.; Habibi, N.; Alseidi, M.; Ahmed, S. Self-cleaning application of mesoporous ZnO, TiO2 and Fe2O3 films with the accommodation of silver nanoparticles for antibacterial activity. J. Taiwan Inst. Chem. Eng. 2023, 142, 104627. [Google Scholar] [CrossRef]
- Khames, K.; Ahmed, S.; Thanoon, S. Effect of chemically synthesis compared to biosynthesized zinc oxide nanoparticles using extract of Vitex agnus on the expression of MexAB-OprM efflux pump genes of multi-drug-resistant Pseudomonas aeruginosa. Baghdad Sci. J. 2024, 21, 50. [Google Scholar] [CrossRef]
- Fayyadh, A.A.; Essa, A.F.; Batros, S.S.; Shallal, Z.S. Studying the crystal structure, topography, and anti-bacterial of a novel titania (TiO2 NPs) prepared by a sol-gel manner. Baghdad Sci. J. 2019, 16, 16. [Google Scholar] [CrossRef]
- Faiq, N.H.; Ahmed, M.E. Effect of biosynthesized zinc oxide nanoparticles on phenotypic and genotypic biofilm formation of Proteus mirabilis. Baghdad Sci. J. 2024, 21, 8. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Zhong, B.J.; Su, C.; Lu, Z.C.; Yu, H. Enhanced tooth bleaching with a hydrogen peroxide/titanium dioxide gel. BMC Oral Health 2024, 24, 923. [Google Scholar] [CrossRef]
- Kadapure, A.J.; Dalbanjan, N.P.; SK, P.K. Characterization of heat, salt, acid, alkaline, and antibiotic stress responses in soil isolate Bacillus subtilis strain PSK.A2. Int. Microbiol. 2025, 28, 315–332. [Google Scholar] [CrossRef]
- Campano, M.Á.; García-Martín, G.; Acosta, I.; Bustamante, P. Designing intensive care unit windows in a Mediterranean climate: Efficiency, daylighting, and circadian response. Appl. Sci. 2024, 14, 9798. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef]
- Gaballa, A.; Wang, T.; Ye, R.W.; Helmann, J.D. Functional analysis of the Bacillus subtilis Zur regulon. J. Bacteriol. 2002, 184, 6508–6514. [Google Scholar] [CrossRef]
- Maret, W.; Li, Y. Coordination dynamics of zinc in proteins. Chem. Rev. 2009, 109, 4682–4707. [Google Scholar] [CrossRef]
- Maret, W.; Li, Y. Coordination dynamics of zinc in proteins: Regulatory zinc sites and buffering. Chem. Rev. 2008, 108, 4610–4627. [Google Scholar] [CrossRef]





| Bacterial Strain | Light (mm) | Dark (mm) |
|---|---|---|
| S. aureus | 14.8 | 6.2 |
| E. coli | 12.3 | 5.5 |
| Coating Sample | S. aureus (Light) | S. aureus (Dark) | E. coli (Light) | E. coli (Dark) |
|---|---|---|---|---|
| Uncoated control | 6.0 ± 0.0 (no zone) | 6.0 ± 0.0 (no zone) | 6.0 ± 0.0 (no zone) | 6.0 ± 0.0 (no zone) |
| TiO2-only coating | 8.9 ± 0.3 | 6.1 ± 0.1 | 6.5 ± 0.5 | 6.0 ± 0.0 |
| ZnO-only coating | 9.7 ± 0.6 | 7.0 ± 0.5 | 7.8 ± 0.3 | 6.3 ± 0.3 |
| TiO2 + ZnO multilayer | 14.8 ± 0.6 | 6.2 ± 0.4 | 12.3 ± 0.5 | 5.5 ± 0.3 |
| Coating Sample | S. aureus (Light) | S. aureus (Dark) | E. coli (Light) | E. coli (Dark) |
|---|---|---|---|---|
| Uncoated control | 0.0 (reference) | 0.0 (reference) | 0.0 (reference) | 0.0 (reference) |
| TiO2-only coating | 0.4 ± 0.1 | 0.1 ± 0.0 | 0.3 ± 0.1 | 0.1 ± 0.0 |
| ZnO-only coating | 1.5 ± 0.2 | 0.5 ± 0.1 | 1.0 ± 0.2 | 0.3 ± 0.1 |
| TiO2/ZnO multilayer | 3.5 ± 0.3 | 0.4 ± 0.1 | 2.8 ± 0.3 | 0.2 ± 0.1 |
| Coating Sample | Condition | S. aureus | E. coli |
|---|---|---|---|
| TiO2/ZnO multilayer | No scavenger | 14.8 ± 0.6 | 12.3 ± 0.5 |
| TiO2/ZnO multilayer | +Mannitol | 9.2 ± 0.5 | 7.4 ± 0.3 |
| TiO2/ZnO multilayer | +Catalase | 8.7 ± 0.4 | 7.0 ± 0.4 |
| TiO2/ZnO multilayer | +p-benzoquinone | 9.5 ± 0.6 | 7.6 ± 0.5 |
| TiO2-only | No scavenger | 8.9 ± 0.3 | 6.5 ± 0.5 |
| TiO2-only | +Mannitol | 6.2 ± 0.3 | 5.9 ± 0.2 |
| ZnO-only | No scavenger | 9.7 ± 0.6 | 7.8 ± 0.3 |
| ZnO-only | +Catalase | 6.8 ± 0.4 | 6.1 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkawaz, A.J.A.A.-H.; Naser, M.S.; Obaid, A.J. Biogenic TiO2–ZnO Nanocoatings: A Sustainable Strategy for Visible-Light Self-Sterilizing Surfaces in Healthcare. Micro 2025, 5, 45. https://doi.org/10.3390/micro5040045
Alkawaz AJAA-H, Naser MS, Obaid AJ. Biogenic TiO2–ZnO Nanocoatings: A Sustainable Strategy for Visible-Light Self-Sterilizing Surfaces in Healthcare. Micro. 2025; 5(4):45. https://doi.org/10.3390/micro5040045
Chicago/Turabian StyleAlkawaz, Ali Jabbar Abd Al-Hussain, Maryam Sabah Naser, and Ali Jalil Obaid. 2025. "Biogenic TiO2–ZnO Nanocoatings: A Sustainable Strategy for Visible-Light Self-Sterilizing Surfaces in Healthcare" Micro 5, no. 4: 45. https://doi.org/10.3390/micro5040045
APA StyleAlkawaz, A. J. A. A.-H., Naser, M. S., & Obaid, A. J. (2025). Biogenic TiO2–ZnO Nanocoatings: A Sustainable Strategy for Visible-Light Self-Sterilizing Surfaces in Healthcare. Micro, 5(4), 45. https://doi.org/10.3390/micro5040045








