Fabrication and Analysis of Carboxylic Acid-Functionalized SWCNT/PDMS-Based Electrodes for ECG Monitoring via IoT
Abstract
1. Introduction
2. Materials and Methods
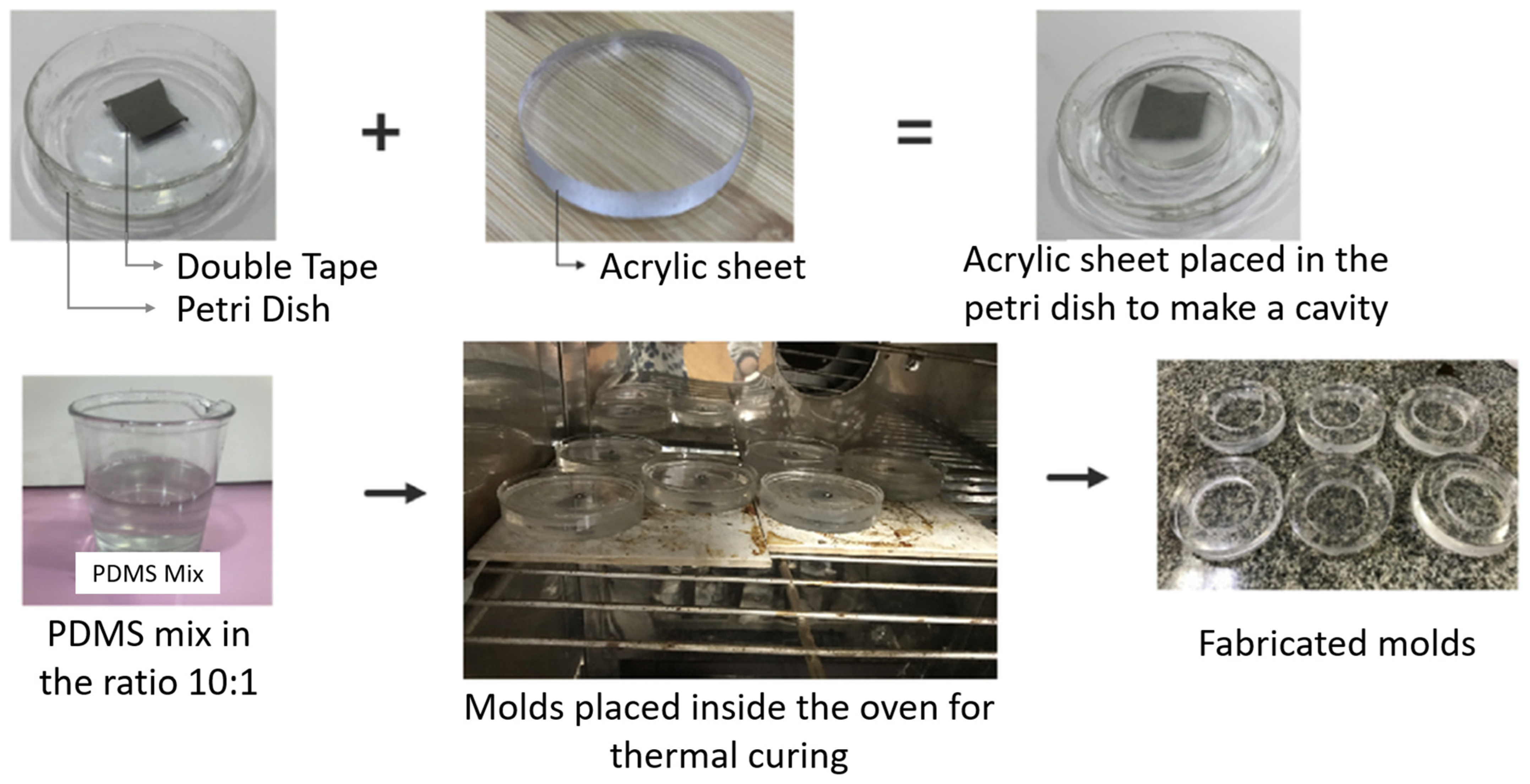
2.1. Mold Formation
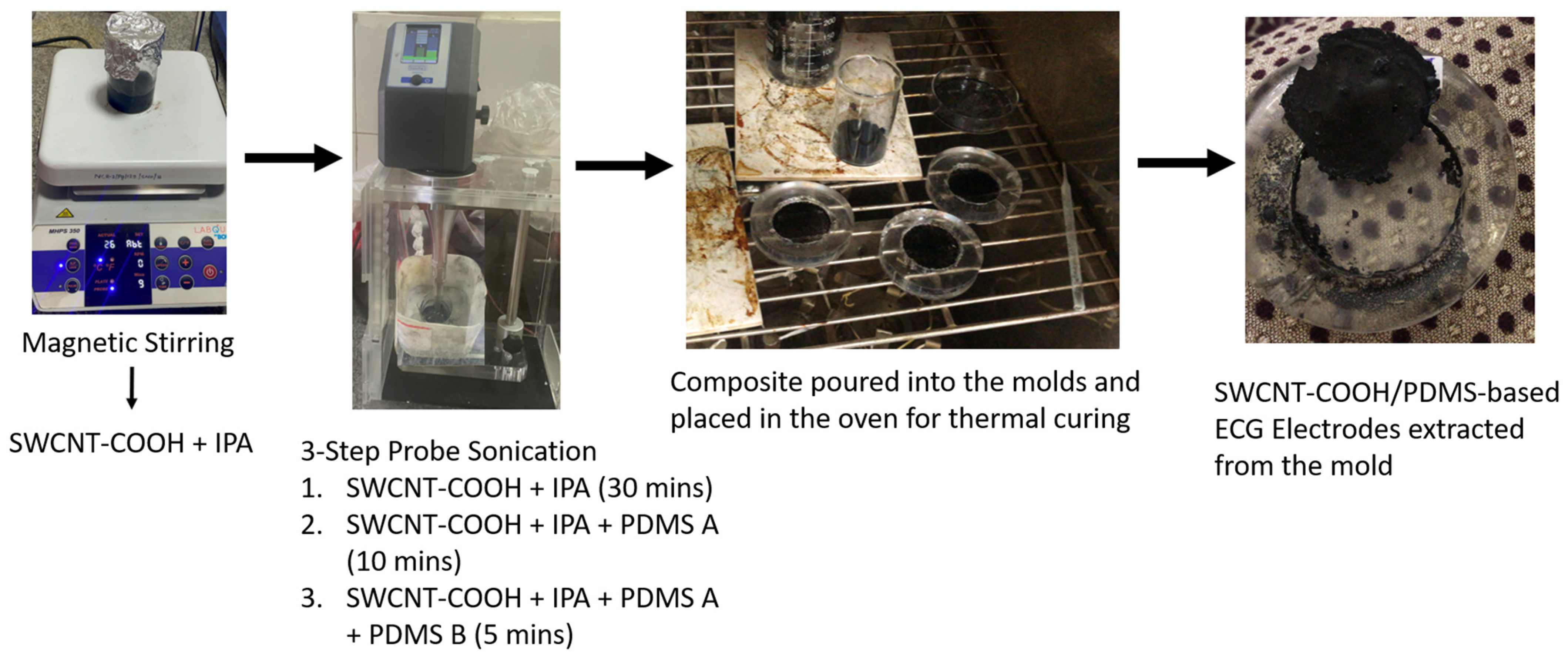
2.2. CNT/PDMS Composite Preparation
2.2.1. Materials
2.2.2. Distribution of SWCNT-COOHs
2.2.3. Dispersion Process:
SWCNT-COOHs with IPA
SWCNT-COOH Solution with PDMS
2.2.4. Fabrication of Final Composite
3. Results
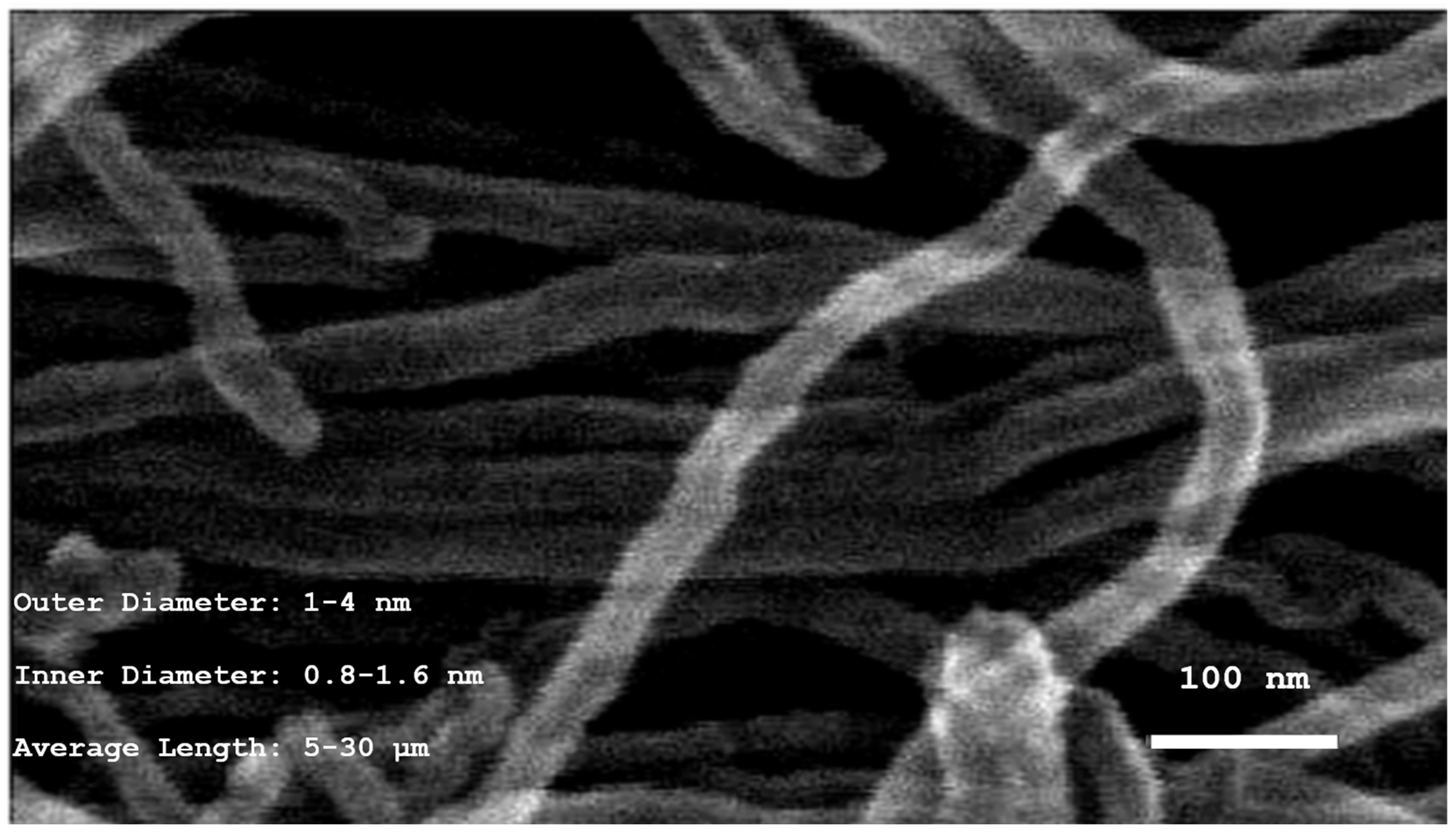
3.1. Physical Characterization of SWCNT-COOHs
3.2. Scanning Electron Microscopy (SEM)
3.3. Transmission Electron Microscopy (TEM)
3.4. Fourier Transform Infrared Spectroscopy (FTIR)
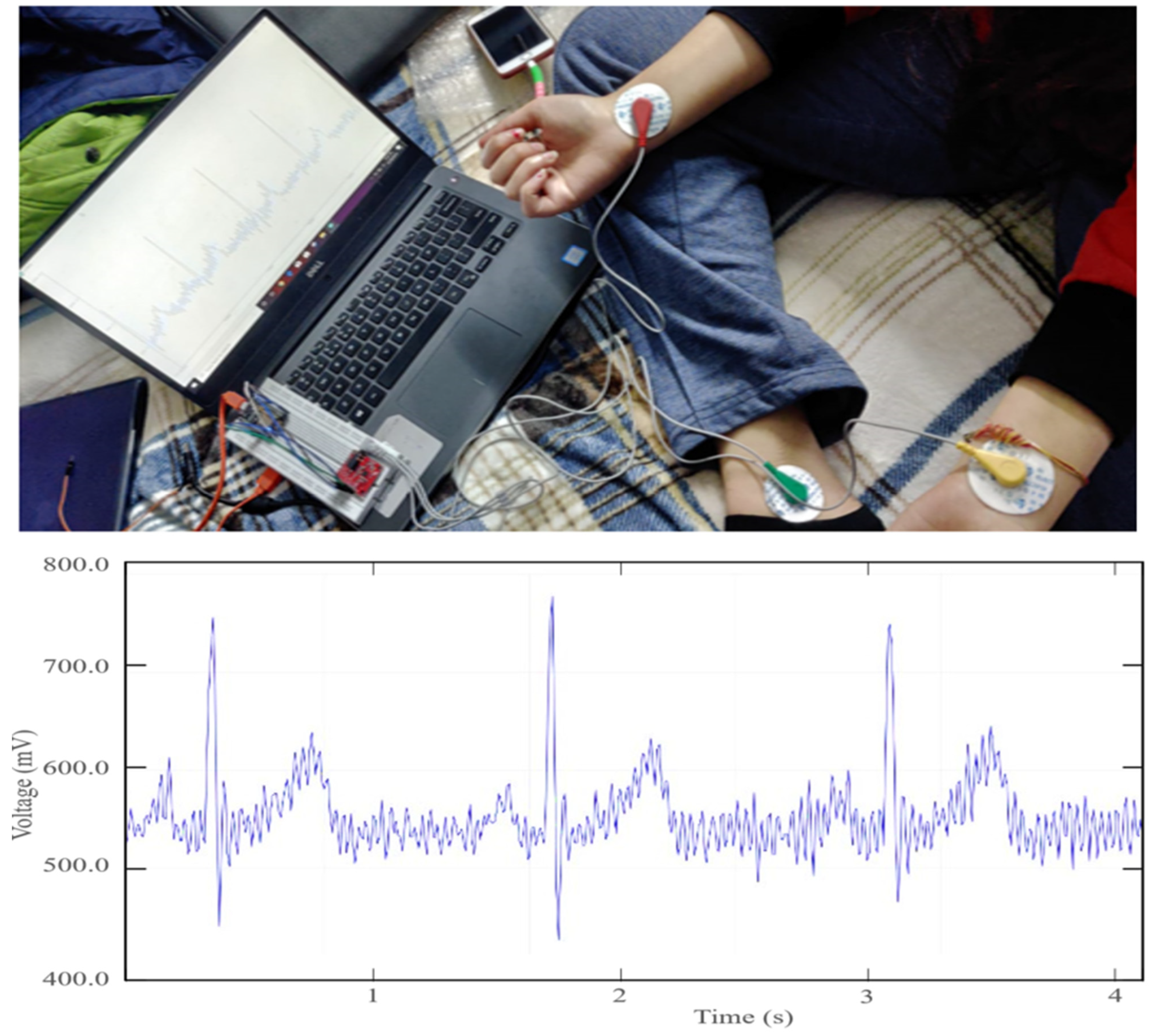
3.5. ECG Experimental Setup
4. ECG Measurements and Analysis
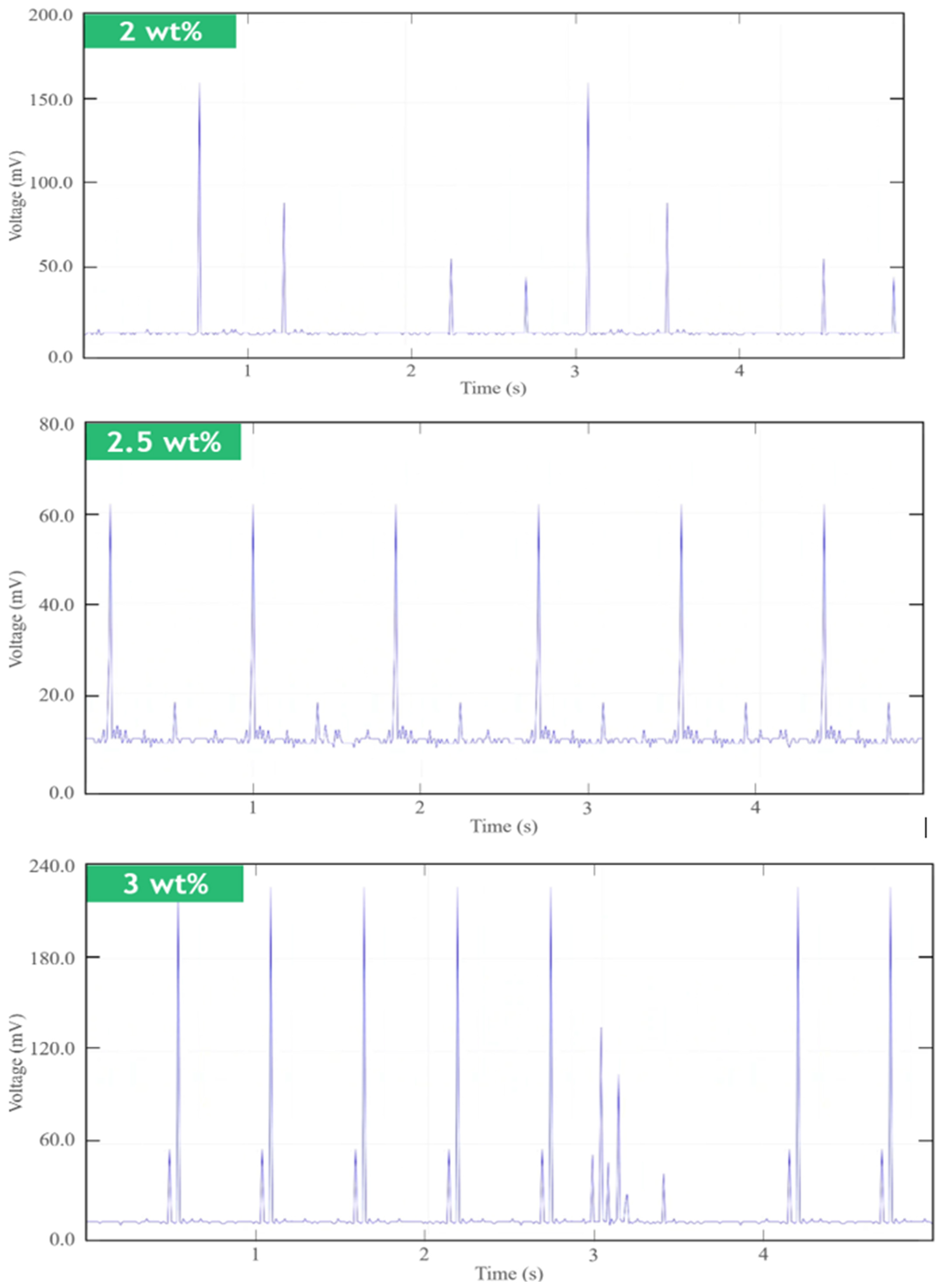
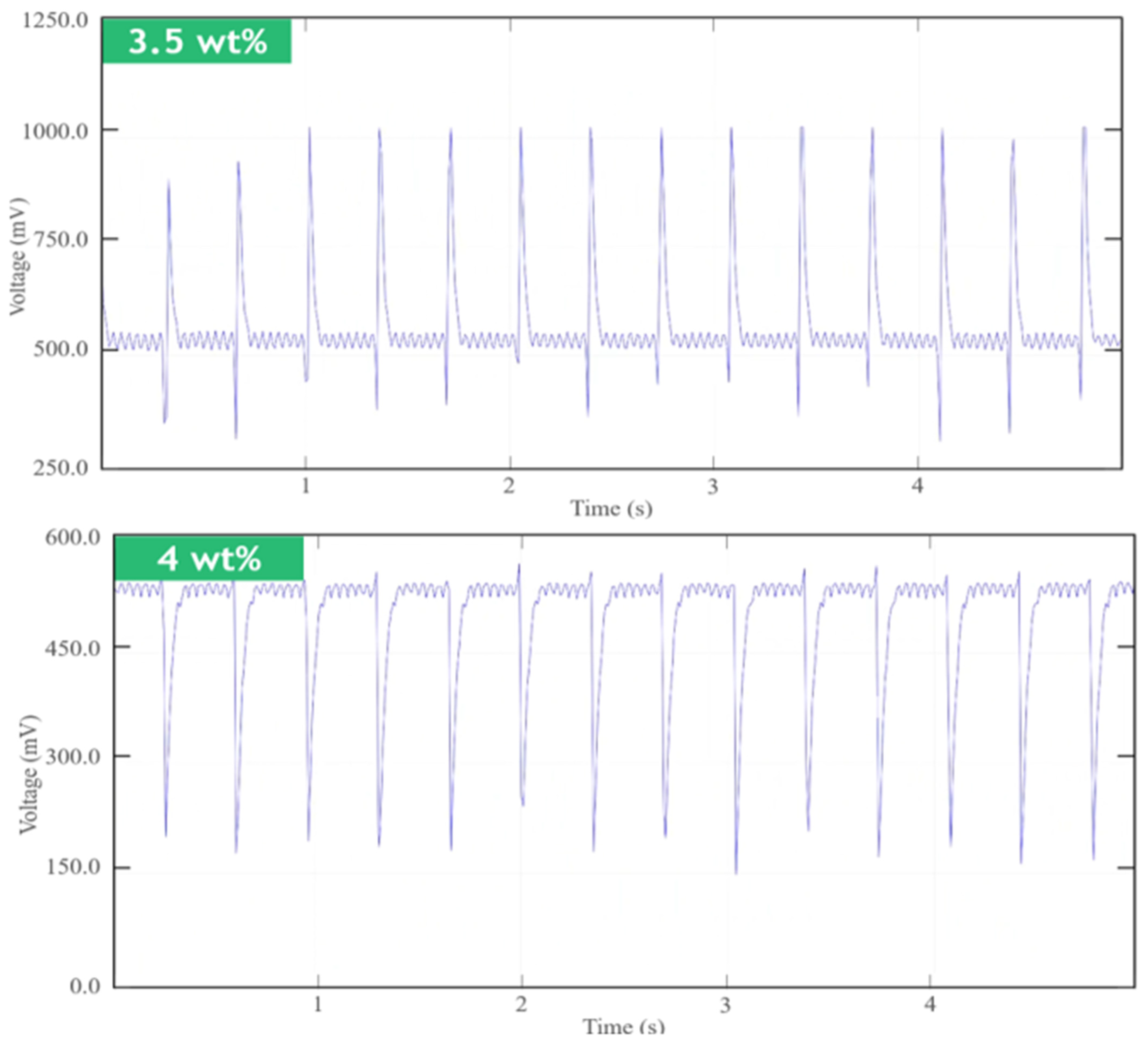
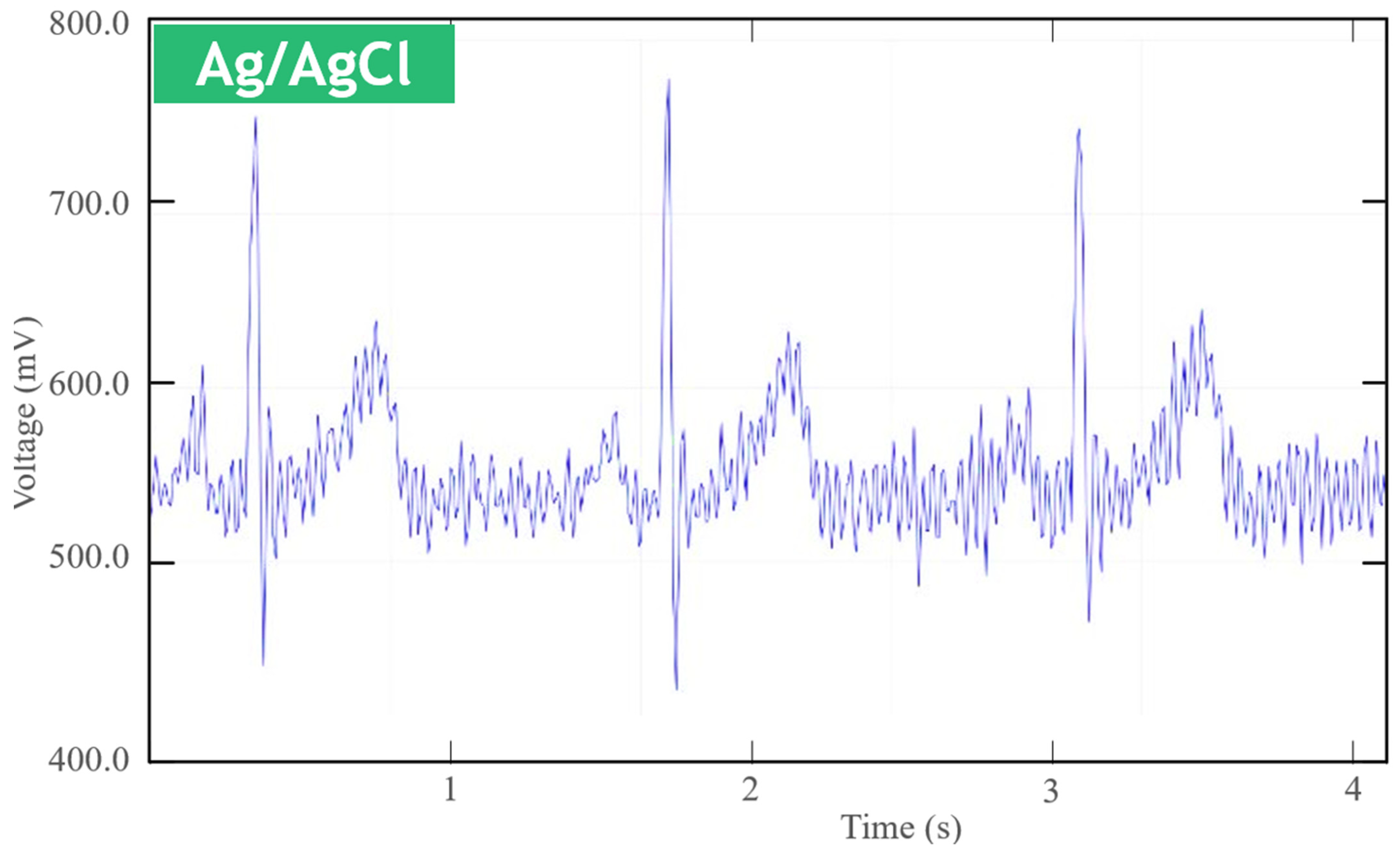
4.1. Short-Term Measurements
4.2. Long-Term Measurements
4.3. Capacitance of the Fabricated Electrodes
4.4. Surface Resistance of the Fabricated Electrodes
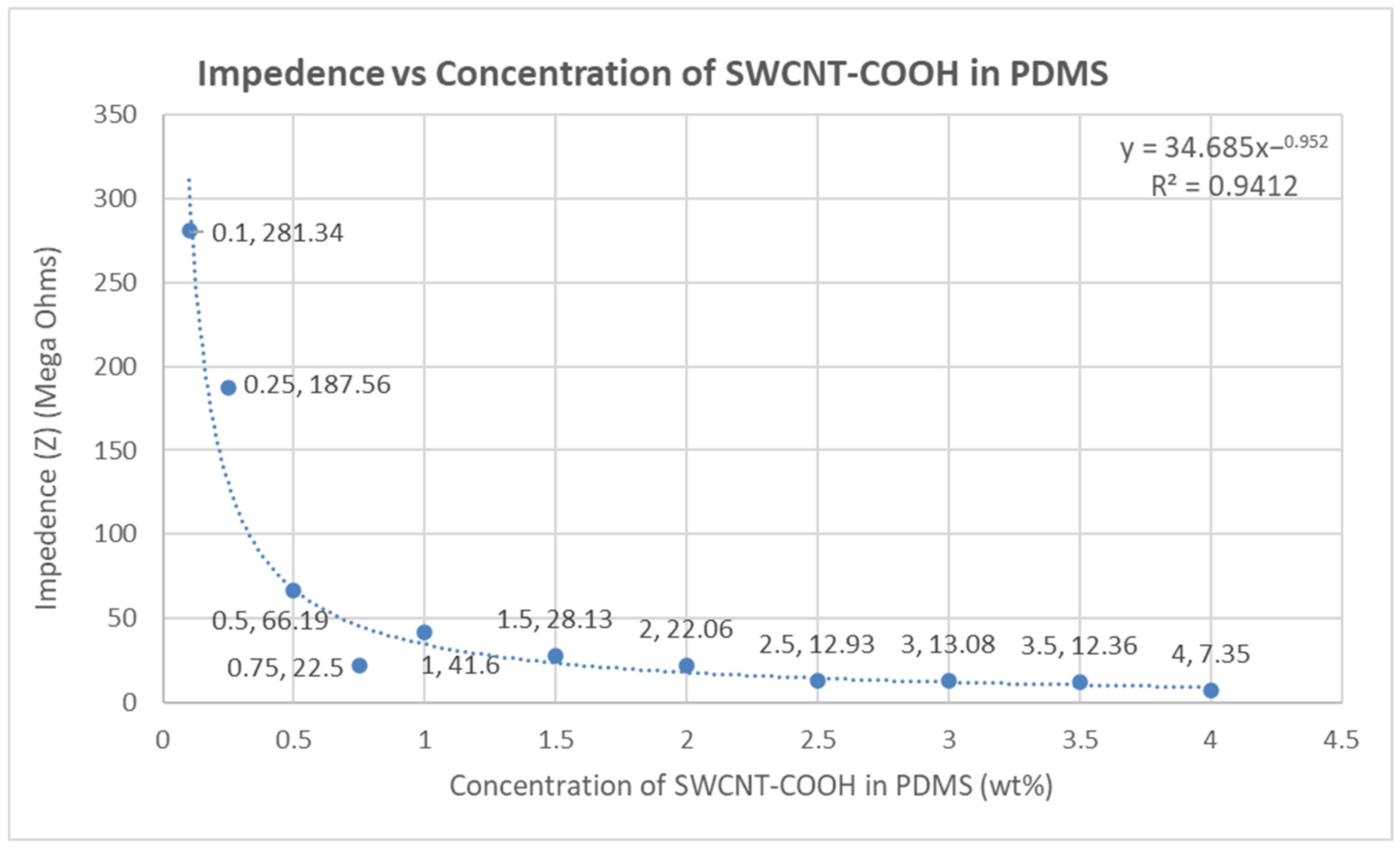
4.5. Impedance of the Fabricated Electrodes
4.6. Comparison with Ag/AgCl Electrodes
4.6.1. Signal-to-Noise Ratio (SNR)
4.6.2. Sensitivity
4.6.3. Durability
4.6.4. User Testing and Anticipated Usability Challenges
5. Discussion
5.1. Overview of Key Findings
5.2. Comparison with Previous Work
5.3. Ethical Implications and Data Privacy Measures
5.4. Strengths of Work Performed
5.5. Limitations of Work Performed and Improvement Areas
5.6. Risks and Hazards of SWCNT-COOH in Human Contact
5.7. Ethics and Safety
5.8. Future Scope
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoo, J.; Jin, Y.; Ko, B.; Kim, M.S. K-Labelsets Method for Multi-Label ECG Signal Classification Based on SE-ResNet. Appl. Sci. 2021, 11, 7758. [Google Scholar] [CrossRef]
- Iqbal, S.M.A.; Leavitt, M.A.; Mahgoub, I.; Asghar, W. Advances in Cardiovascular Wearable Devices. Biosensors 2024, 14, 525. [Google Scholar] [CrossRef] [PubMed]
- Moshawrab, M.; Fatani, B.; Zaki, S.; Tayeb, H.; Meccawy, M.; Rashid, M. Smart Wearables for the Detection of Cardiovascular Diseases: A Systematic Literature Review. Sensors 2023, 23, 828. [Google Scholar] [CrossRef] [PubMed]
- Nigusse, A.B.; Malengier, B.; Mengistie, D.A.; Van Langenhove, L. A Washable Silver-Printed Textile Electrode for ECG Monitoring. Eng. Proc. 2021, 6, 63. [Google Scholar] [CrossRef]
- Rafie, N.; Kashou, A.H.; Noseworthy, P.A. ECG Interpretation: Clinical Relevance, Challenges, and Advances. Hearts 2021, 2, 39. [Google Scholar] [CrossRef]
- An, X.; Wang, H.; Li, Z.; Zhao, Y.; Feng, Z.; Song, S. Research on a Lightweight Arrhythmia Classification Model for Wearable Single-Lead ECG. Sensors 2024, 24, 7896. [Google Scholar] [CrossRef]
- Nigusse, A.B.; Mengistie, D.A.; Malengier, B.; Tseghai, G.B.; Van Langenhove, L. Wearable Smart Textiles for Long-Term Electrocardiography Monitoring—A Review. Sensors 2021, 21, 4174. [Google Scholar] [CrossRef]
- Avanu, A.E.; Dodi, G. Wear Your Heart on Your Sleeve: Smart Textile ECG Wearables for Comfort, Integration, Signal Quality, and Continuous Monitoring in Paroxysmal Atrial Fibrillation. Sensors 2025, 25, 676. [Google Scholar] [CrossRef]
- Chi, M.; Li, X.; Li, Z.; Liu, H.; Zhao, J.; Wu, Y. Flexible Carbon Nanotube-Based Polymer Electrode for Long-Term Electrocardiographic Recording. Materials 2019, 12, 971. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, J.; Dong, Y.; Wang, X. Dry Electrodes for Human Bioelectrical Signal Monitoring. Sensors 2020, 20, 3651. [Google Scholar] [CrossRef]
- Rauf, S.; Hassan, M.; Liu, H.; Tariq, A.; Zhang, X.; Zhu, J. A Wearable ECG System with Printed Electrodes for Heart Health Monitoring and Diagnosis. In Proceedings of the 2024 IEEE SENSORS, Kobe, Japan, 20–23 October 2024; pp. 1–4. [Google Scholar]
- Demirel, B.U.; Bayoumy, I.A.; Faruque, M.A.A. Energy-Efficient Real-Time Heart Monitoring on Edge–Fog–Cloud Internet of Medical Things. IEEE Internet Things J. 2022, 9, 12472–12481. [Google Scholar] [CrossRef]
- Bushnag, A. A Wireless ECG Monitoring and Analysis System Using the IoT Cloud. Intell. Automat. Soft Comput. 2022, 33, 51–70. [Google Scholar] [CrossRef]
- Alimbayeva, Z.; Kim, D.; Jeong, S.; Park, J.; Lee, J. Wearable ECG Device and Machine Learning for Heart Monitoring. Sensors 2024, 24, 4201. [Google Scholar] [CrossRef]
- Tasneem, N.T.; Rahman, M.S.; Hossain, A. A Low-Power On-Chip ECG Monitoring System Based on MWCNT/PDMS Dry Electrodes. IEEE Sens. J. 2020, 20, 12799–12806. [Google Scholar] [CrossRef]
- Oh, J.; Kim, H.; Park, S.; Lee, J.; Choi, H. Flexible Dry Electrode Based on a Wrinkled Surface That Uses Carbon Nanotube/Polymer Composites for Recording Electroencephalograms. Materials 2024, 17, 668. [Google Scholar] [CrossRef]
- Shao, M.; Zhang, J.; Wang, L.; Liu, Z.; Wang, X.; Li, H. A Wearable Electrocardiogram Telemonitoring System for Atrial Fibrillation Detection. Sensors 2020, 20, 606. [Google Scholar] [CrossRef]
- Bhattarai, A.; Xu, T.; Lee, S.; Park, H. Adaptive Partition of ECG Diagnosis Between Cloud and Wearable Sensor Net Using Open-Loop and Closed-Loop Switch Mode. IEEE Access 2022, 10, 63684–63697. [Google Scholar] [CrossRef]
- Technical Datasheet. 1995. Available online: https://www.dow.com/en-us/document-viewer.html?randomVar=4328647061587729765&docPath=/content/dam/dcc/documents/11/11-3184-01-sylgard-184-elastomer.pdf (accessed on 30 July 2017).
- Bani, G.; Raghava, N.S. Fabrication Techniques for Carbon Nanotubes Based ECG Electrodes: A Review. IETE J. Res. 2020, 71, 1. [Google Scholar] [CrossRef]
- Jung, H.C.; Moon, J.H.; Baek, D.H.; Lee, J.H.; Choi, Y.Y.; Hong, J.S.; Lee, S.H. CNT/PDMS Composite Flexible Dry Electrodes for Long-Term ECG Monitoring. IEEE Trans. Biomed. Eng. 2021, 59, 1472–1479. [Google Scholar] [CrossRef]
- Liu, B.; Chen, Y.; Luo, Z.; Zhang, W.; Tu, Q.; Jin, X. A Novel Method of Fabricating Carbon Nanotubes Polydimethylsiloxane Composite Electrodes for Electrocardiography. Biomater. Sci. Polym. Ed. 2015, 26, 1229–1235. [Google Scholar] [CrossRef]
- Shadpour, M.; Soltaniana, S. Surface Functionalization of Carbon Nanotubes: Fabrication and Applications. RSC Adv. 2016, 6, 109916–109935. [Google Scholar] [CrossRef]
- Yan, Z.; Yi, F.; Ramaraja, P.R. Non-Covalent Functionalization of Carbon Nanotubes for Electrochemical Biosensor Development. Sensors 2019, 19, 392. [Google Scholar] [CrossRef]
- Philip, G.W.; Adrian, A.G.; Geoffrey, M.S.; Kerry, J.G.; Gordon, G. Free Standing Carbon Nanotube Composite Bio-Electrodes. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 37, 43. [Google Scholar] [CrossRef]
- Kim, J.H.; Hwang, J.Y.; Hwang, H.R.; Kim, H.S.; Lee, J.H.; Seo, J.W.; Shin, U.S.; Lee, S.H. Simple and Cost-Effective Method of Highly Conductive and Elastic Carbon Nanotube/Polydimethylsiloxane Composite for Wearable Electronics. Sci. Rep. 2018, 8, 1375. [Google Scholar] [CrossRef]
- Nidhi, P.; Asad, N.; Ashok, M.B.; Ashok, M.; Rajesh, K. Conducting Polymer Functionalized Single-Walled Carbon Nanotube-Based Chemiresistive Biosensor for the Detection of Human Cardiac Myoglobin. Appl. Phys. Lett. 2014, 105, 153701. [Google Scholar] [CrossRef]
- Santosh, A.; Bertram, R.; Mace, Z.S.; Sclabassi, R.J.; Boyle, C.; Whiting, D.M.; Saadyah, A.; Nel, T.L. Novel Organic Conductive Fibers as Nonmetallic Electrodes and Neural Interconnects. Ind. Eng. Chem. Res. 2018, 57, 7866–7871. [Google Scholar] [CrossRef]
- Gilshteyn, E.P.; Lin, S.; Kozlov, V.A.; Kuzin, D.S.; Timonina, A.P.; Anisimov, A.S.; Hart, A.J.; Zhao, X.; Nasibulin, A.G. A One-Step Method of Hydrogel Modification by Single-Walled Carbon Nanotubes for Highly Stretchable and Transparent Electronics. ACS Appl. Mater. Interfaces 2018, 10, 28069–28075. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, Y.; Liu, J.; Zheng, Z.; Chen, X.; Lin, W.J. Single-Wall Carbon Nanotube-Coated Cotton Yarn for Electrocardiography Transmission. Micromachines 2018, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Cho, G. PU Nanoweb-Based Textile Electrode Treated with Single-Walled Carbon Nanotube/Silver Nanowire and Its Application to ECG Monitoring. Smart Mater. Struct. 2019, 28, 045004. [Google Scholar] [CrossRef]
- Rao, M.S.G. ECG Electrode (Sensor) Printing with Ion Sensitive Membrane on Flexible Substrate. IOSR J. Electr. Electron. Eng. 2015, 10, 13–18. [Google Scholar]
- Sun, P.; Rizkalla, M. Temperature, Gas, and Pressure ECG Sensor by Using Carbon Nanotube. In Proceedings of the 2013 IEEE 56th International Midwest Symposium on Circuits and Systems (MWSCAS), Columbus, OH, USA, 4–7 August 2013; pp. 721–723. [Google Scholar] [CrossRef]
- Hwang, D.H.; Choi, S.; Han, P.; Park, J.H.; Seol, J.W.; Kim, D.H.; Hong, J.T.; Choi, J.H. Multifunctional Smart Textronics with Blow-Spun Nonwoven Fabrics. Adv. Funct. Mater. 2019, 29, 1900025. [Google Scholar] [CrossRef]
- Young, G.O. Synthetic Structure of Industrial Plastics. In Plastics, 2nd ed.; Peters, J., Ed.; McGraw-Hill: New York, NY, USA, 1964; Volume 3, pp. 15–64. [Google Scholar]



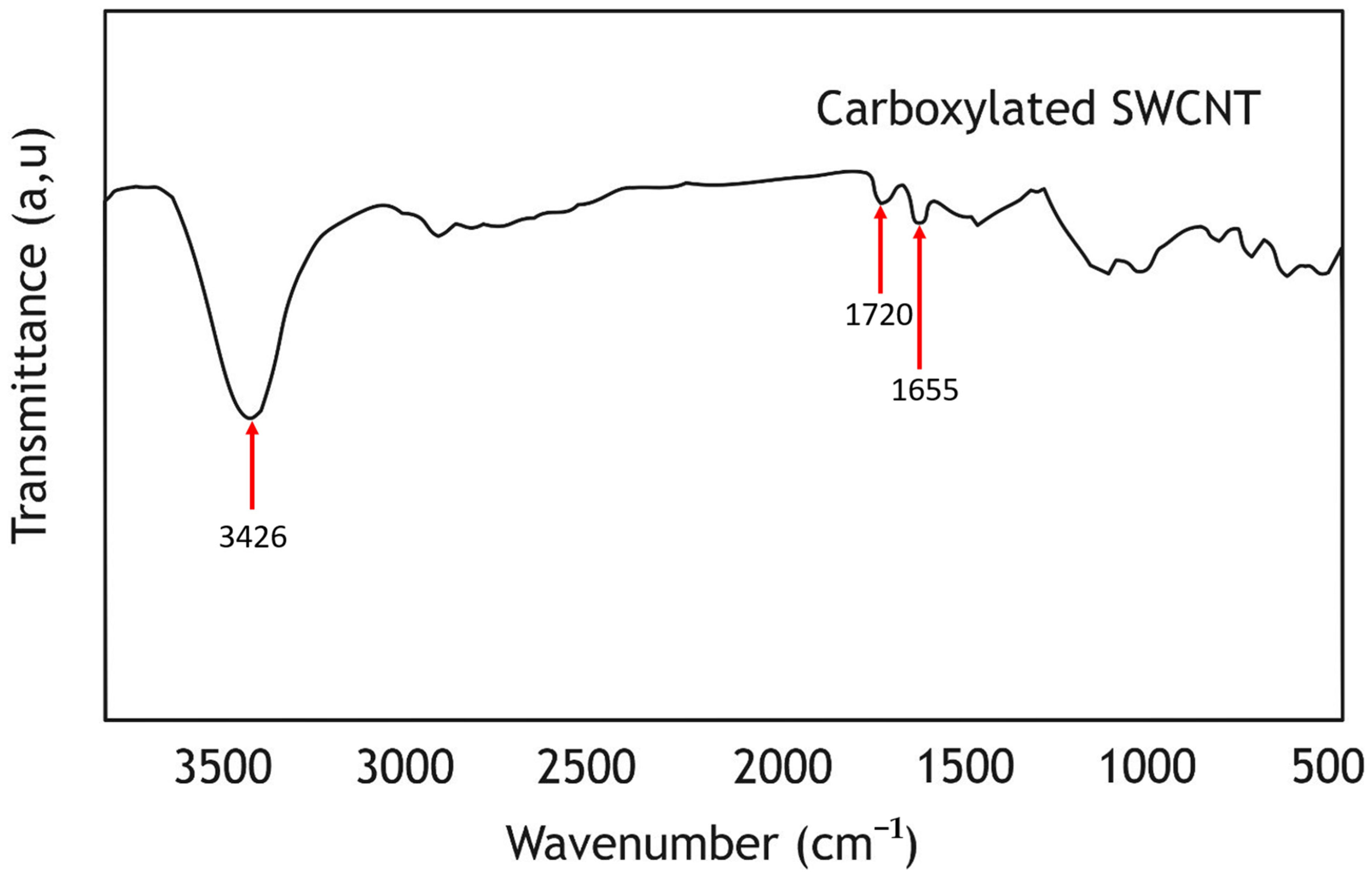
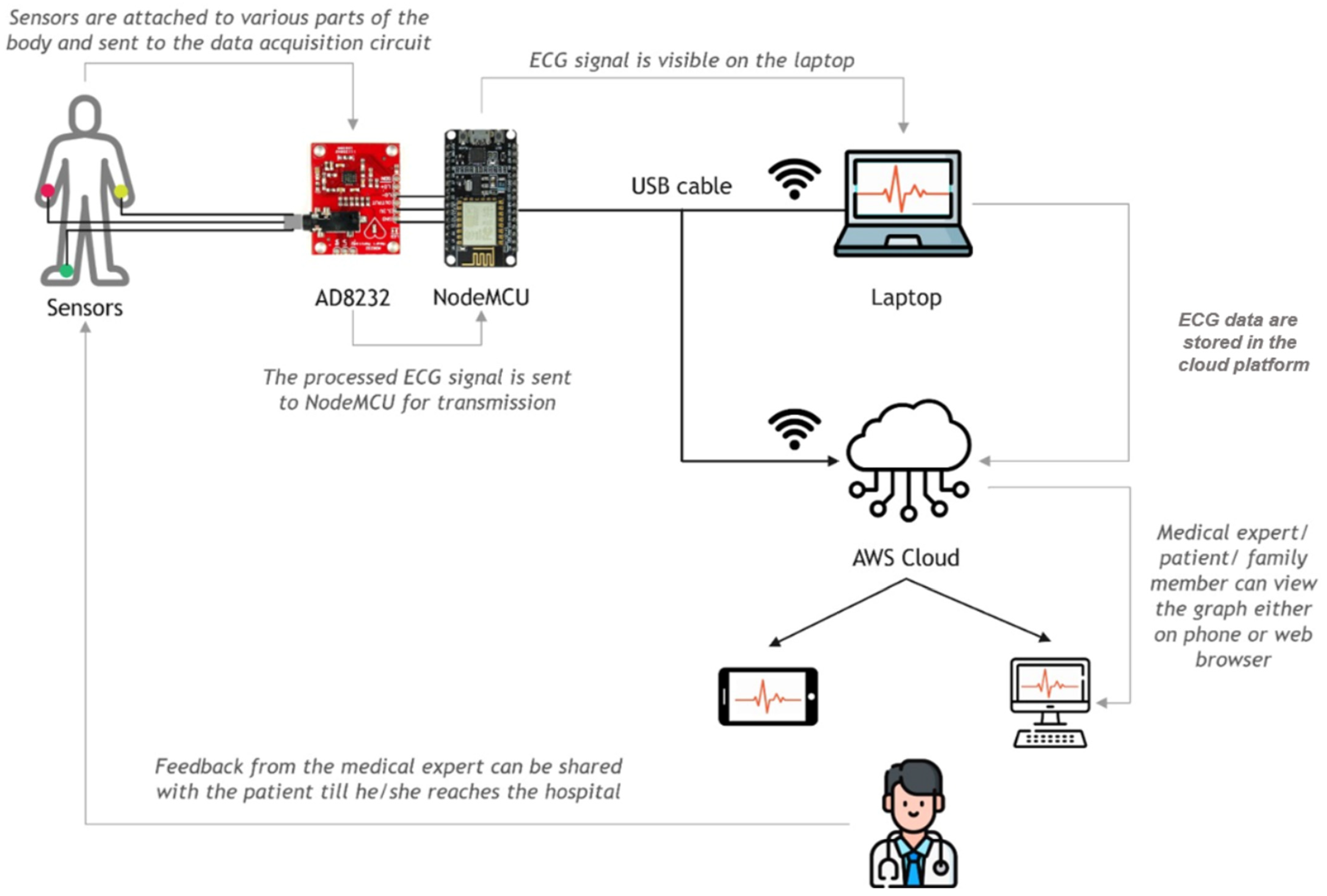
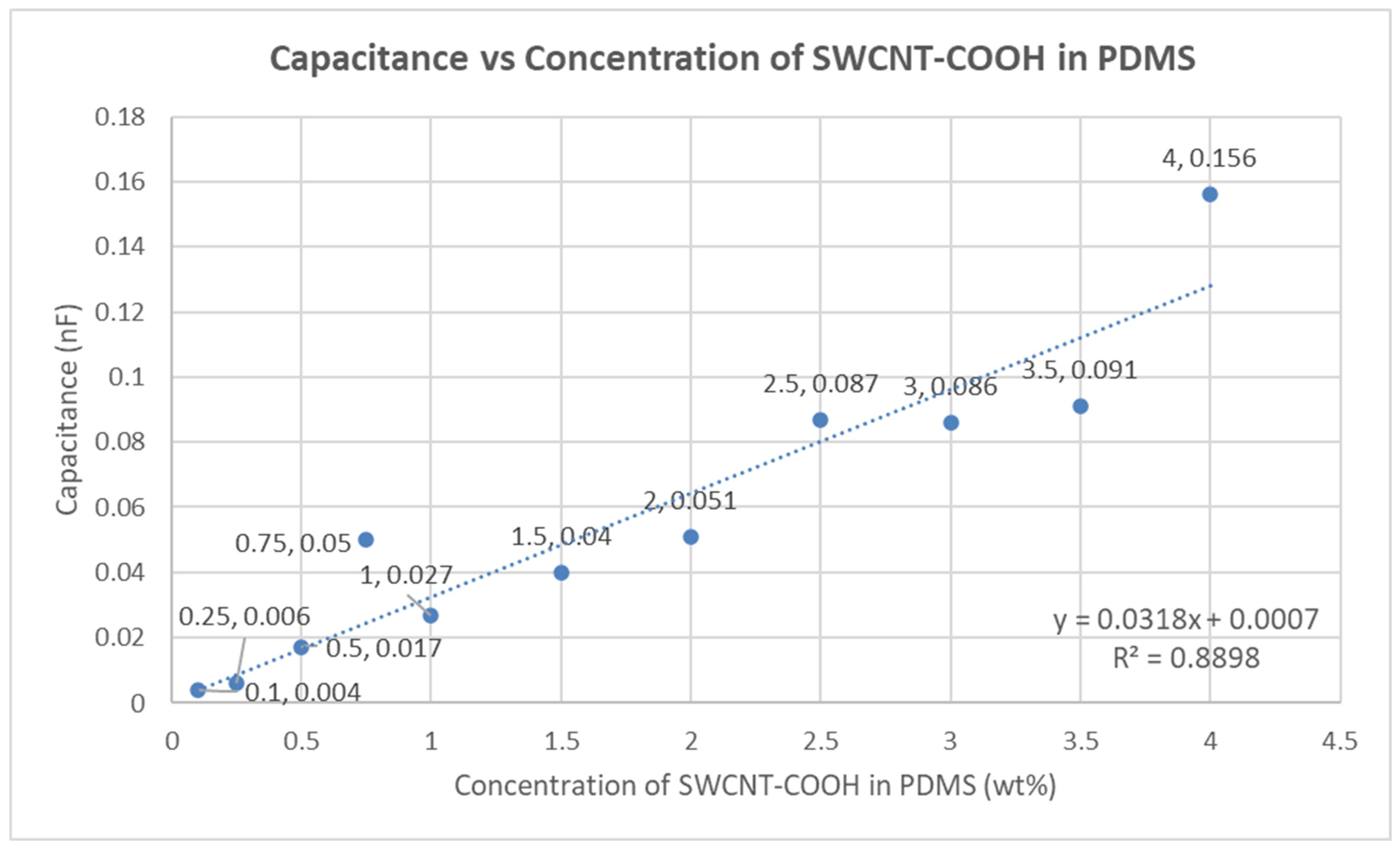
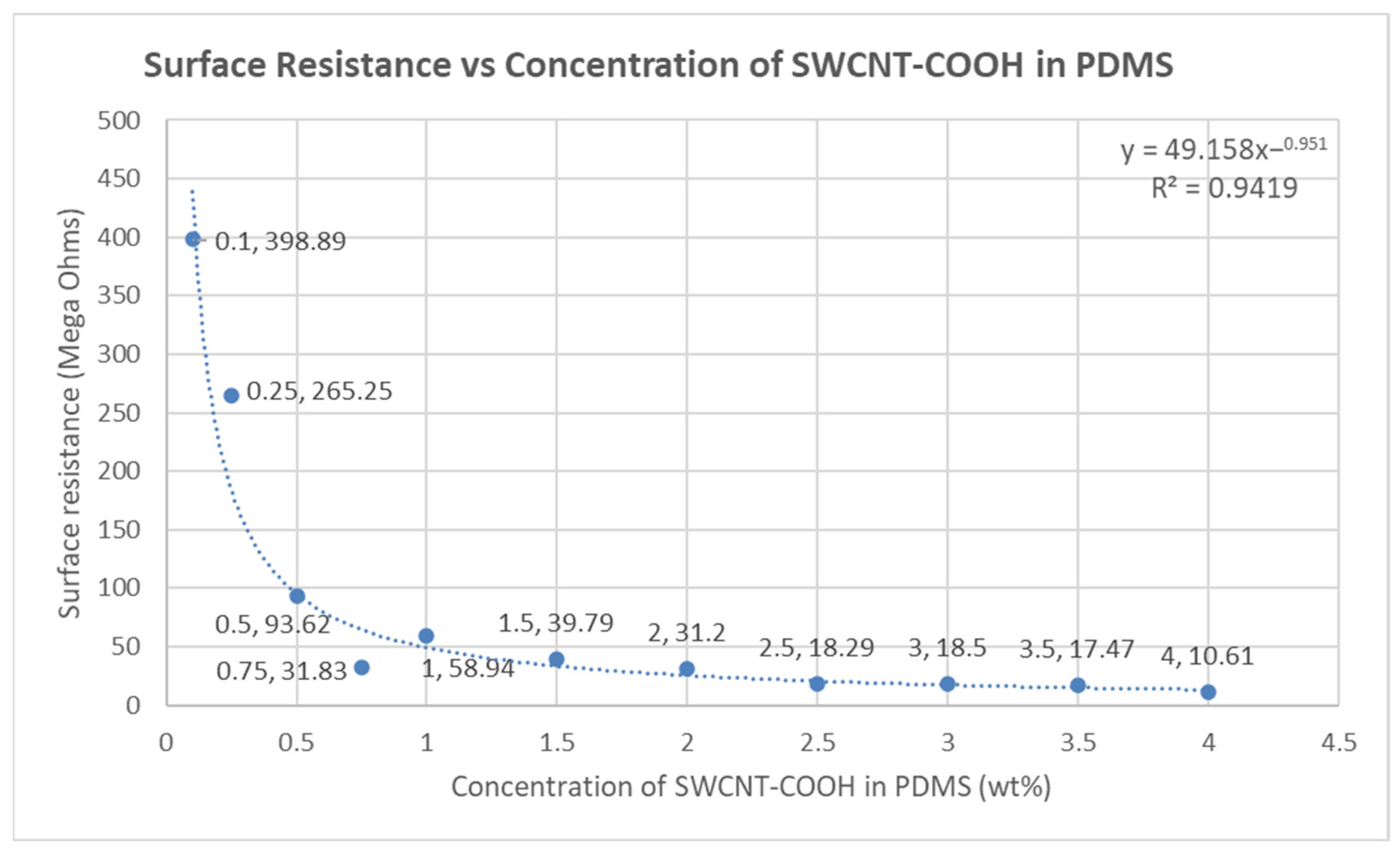



| Parameter | SWCNT-COOHs | PDMS | IPA |
|---|---|---|---|
| Manufacturer | Nano Research Elements | Dow Corning | Loba Chemie |
| Outer Diameter | 1–4 nm | - | - |
| Inner Diameter | 0.8–1.6 nm | - | - |
| Length | 5–30 μm | - | - |
| Specific Surface Area | 690 m2/g | - | - |
| Electrical Conductivity | >100 S/cm | - | - |
| One or Two Part | - | Two | - |
| Color | - | Colorless | Clear, Colorless |
| Viscosity (Base) | - | 5100 cP/5.1 Pa·s | - |
| Viscosity (Mixed) | - | 3500 cP/3.5 Pa·s | - |
| Thermal Conductivity | - | 0.15 btu/hr·ft·°F/0.27 W/m·K | - |
| Specific Gravity (Cured) | - | 1.03 | - |
| Working Time at 25 °C (Pot Life) | - | 1.5 h | - |
| Cure Time at 25 °C | - | 48 h | - |
| Heat Cure Time (100 °C/125 °C/150 °C) | - | 35 min/20 min/10 min | - |
| Durometer Shore | - | 43 | - |
| Dielectric Strength | - | 500 volts/mil/19 kV/mm | - |
| Volume Resistivity | - | 2.9 × 1014 ohm·cm | - |
| Dissipation Factor (100 Hz/100 kHz) | - | 0.00257/0.00133 | - |
| Dielectric Constant (100 Hz) | - | 2.72 | - |
| Linear CTE (by DMA) | - | 340 ppm/°C | - |
| Tensile Strength | - | 980 PSI/6.7 MPa/69 kg/cm2 | - |
| Refractive Index (589 nm/632.8 nm/1321 nm/1554 nm) | - | 1.4118/1.4225/1.4028/1.3997 | - |
| UL RTI Rating | - | 150 °C | - |
| Physical State | - | - | Liquid |
| Molecular Mass | - | - | 60.1 g/mol |
| Odor | - | - | Alcoholic Odor |
| Relative Evaporation Rate | - | - | 2.83 (butyl acetate = 1) |
| Melting Point | - | - | −89 °C |
| Boiling Point | - | - | 82 °C |
| Flash Point | - | - | 12 °C |
| Auto-Ignition Temperature | - | - | 399 °C |
| Flammability | - | - | Highly Flammable Liquid and Vapor |
| Vapor Pressure (20 °C) | - | - | 43.2 hPa |
| Relative Vapor Density (20 °C) | - | - | 2.1 |
| Density | - | - | 0.79 g/cm3 |
| Solubility | - | - | Miscible in Water |
| Log Pow | - | - | 0.00257 |
| Explosive Limits | - | - | 0.02–0.127 vol % |
| Parameter | SEM | TEM |
|---|---|---|
| Accelerating Voltage | 5–20 kV | 200 kV (approx.) |
| Beam Current | nanoamperes (nA) | nanoamperes (nA) |
| Working Distance | 5–15 nm | - |
| Magnification | Between 10,000 x–2000 x | Between 10,000 x–2000 x |
| Resolution | 3–5 nm (approx.) | 0.1–0.23 nm (approx.) |
| Imaging Mode (s) | Secondary Electron | Bright Field TEMs |
| (a) | |
| Parameter | AD8232 |
| Brand | NexGen Gadgets |
| Manufacturer | Generic |
| Item Weight | 150 g |
| Number of Memory Sticks | 1 |
| Electrode Interface | 3.5 mm |
| (b) | |
| Parameter | NodeMCU |
| Brand | Generic |
| Model Name | ESP8266 |
| Item Weight | 150 g |
| Memory Storage Capacity | 64 KB |
| Connectivity | Wi-Fi, USB |
| Processor Count | 2 |
| Included Components | Generic ESP8266 NodeMCU, ESP8266 Lua Amica Wifi, Internet of Things Development Board Cp2102 IoT |
| Type of Electrode | SNR | Sensitivity |
|---|---|---|
| Ag/AgCl | −9.99 dB | 1 |
| SWCNT-COOH/PDMS | −10.51 dB | 1 |
| Ref. | Materials Used | Fabrication Technique | Nature of Sensor | Comparative Results | IoT-Enabled |
|---|---|---|---|---|---|
| [27] | SWCNT, poly(pyrrole-co-pyrrolepropylic acid) with pendant carboxyl groups | Electrochemical process | Electrode | ECG was not measured. | No |
| [25] | SWCNT, DNA, and Chitosan | Membrane filtering technique | Freestanding films | ECG was not measured but it was mentioned that these will work well as ECG pads. | No |
| [28] | SWCNT, Electrically Conductive Fibers, regioregular poly(3-hexylthiophene) | Dipping and drying | Electrode | Moderate ECG was achieved. | No |
| [29] | SWCNT, Hydrogel | Doctor blade technique | Film | ECG results were better than the conventional electrodes. | No |
| [30] | SWCNT, Cotton Yarn (CY) | Dipping and drying | Textile-based electrode | ECG waveforms measured by four-strand and six-strand SWCNT/CY composites are comparable to the standard ECG waveforms measured by conventional lead wires. | No |
| [31] | SWCNT, Silver Nanowires, and Polyurethane nanoweb | Bar coating | Film | There was no substantial alteration in the quality of the ECG signal. | No |
| [32] | SWCNT and TFT (flexible substrate) | Inkjet printing | Film | ECG was not measured, but the authors mentioned there is a scope for online ECG measurements. | No |
| [33] | SWCNT, SSDNA | CMOS technology | Chip | Appropriate ECG signals were not visible. | No |
| [34] | SWCNT, Poly (vinylidene fluoride-cohexafluoropropylene) | Blow spinning technique, inkjet printing | Fabric-based sensor | Decent ECG signals were achieved. | No |
| This work | SWCNT-COOH, PDMS | Mold formation | Film (circular) | Composite with 3.5 wt% has the closest peaks to Ag/AgCl electrodes. | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandhi, B.; Nallanthighal, R.S. Fabrication and Analysis of Carboxylic Acid-Functionalized SWCNT/PDMS-Based Electrodes for ECG Monitoring via IoT. Micro 2025, 5, 16. https://doi.org/10.3390/micro5020016
Gandhi B, Nallanthighal RS. Fabrication and Analysis of Carboxylic Acid-Functionalized SWCNT/PDMS-Based Electrodes for ECG Monitoring via IoT. Micro. 2025; 5(2):16. https://doi.org/10.3390/micro5020016
Chicago/Turabian StyleGandhi, Bani, and Raghava Srinivasa Nallanthighal. 2025. "Fabrication and Analysis of Carboxylic Acid-Functionalized SWCNT/PDMS-Based Electrodes for ECG Monitoring via IoT" Micro 5, no. 2: 16. https://doi.org/10.3390/micro5020016
APA StyleGandhi, B., & Nallanthighal, R. S. (2025). Fabrication and Analysis of Carboxylic Acid-Functionalized SWCNT/PDMS-Based Electrodes for ECG Monitoring via IoT. Micro, 5(2), 16. https://doi.org/10.3390/micro5020016








