Electrodeposition of Zn and Cu Nanoparticles into TiO2 Nanotubes on Ti6Al4V: Antimicrobial Effect against S. Epidermidis and Cytotoxicity Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Ti6Al4V Specimen Preparation
2.2. Antimicrobial Decoration/Functionalization
2.3. Surface Analysis
2.4. Antimicrobial Tests
2.4.1. Bacterial Strain and Medium
2.4.2. In Vitro Antibacterial Assay
2.5. Mammalian Cell Cytotoxicity Assays
2.5.1. Cell Culture
2.5.2. Tests on Extracts
2.5.3. Resazurin Assay
2.5.4. Fluorescence Labeling and Microscopy
2.6. Ion Release
2.7. Statistical Analysis
3. Results
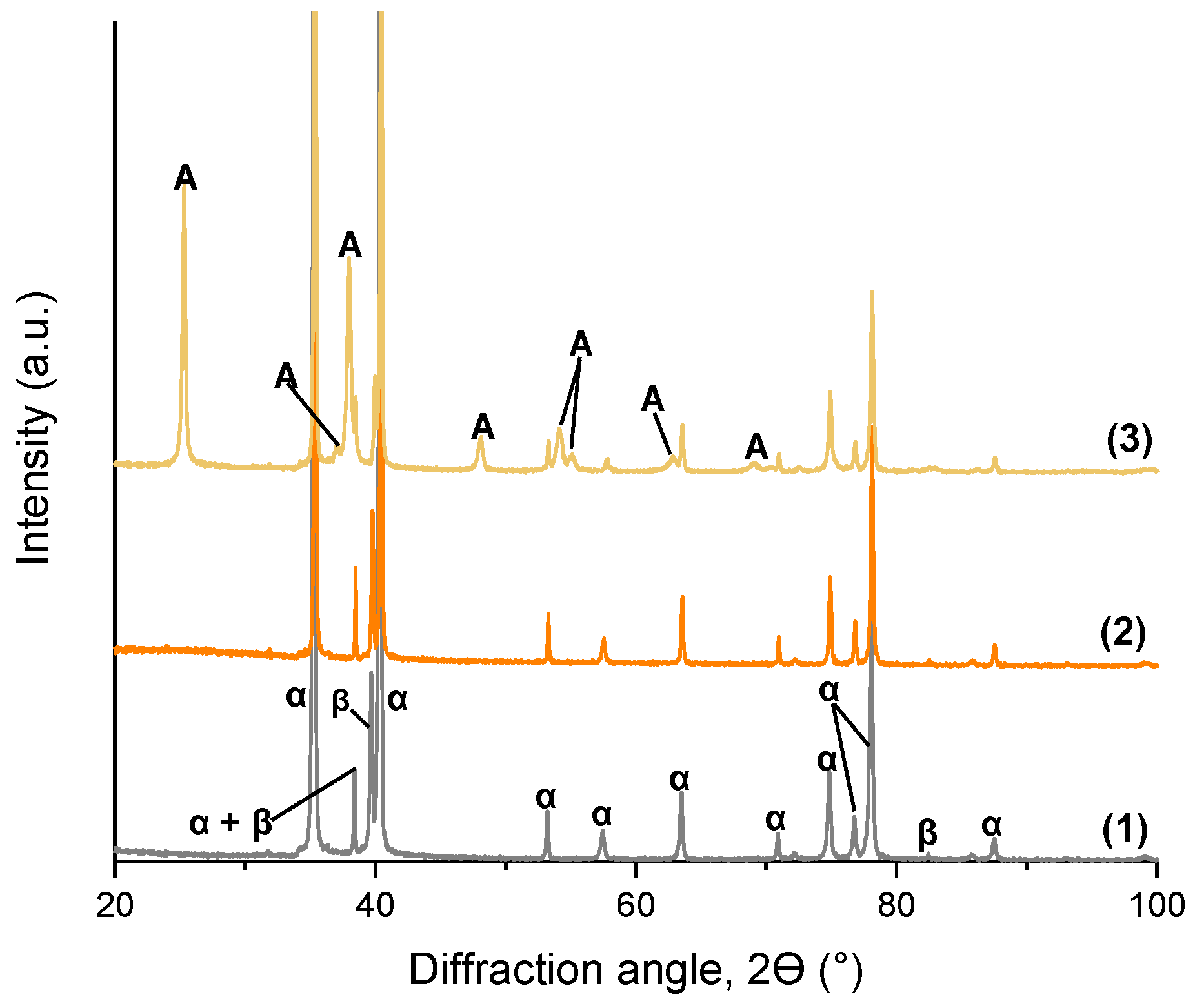
3.1. TiO2 Nanotube Morphology and Structure
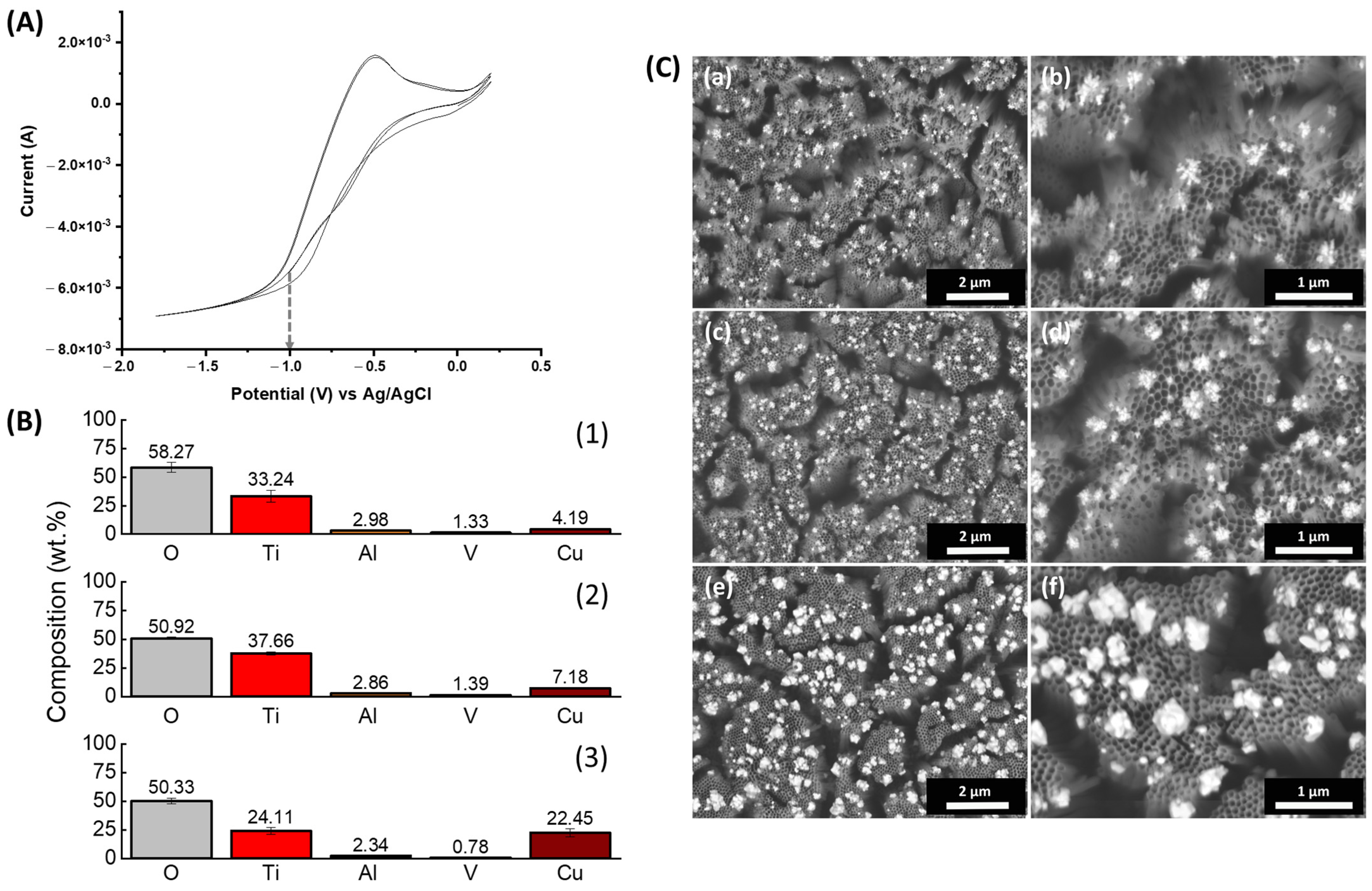
3.2. Electrodepostion of ZnNPs
3.3. Electrodeposition of CuNPs
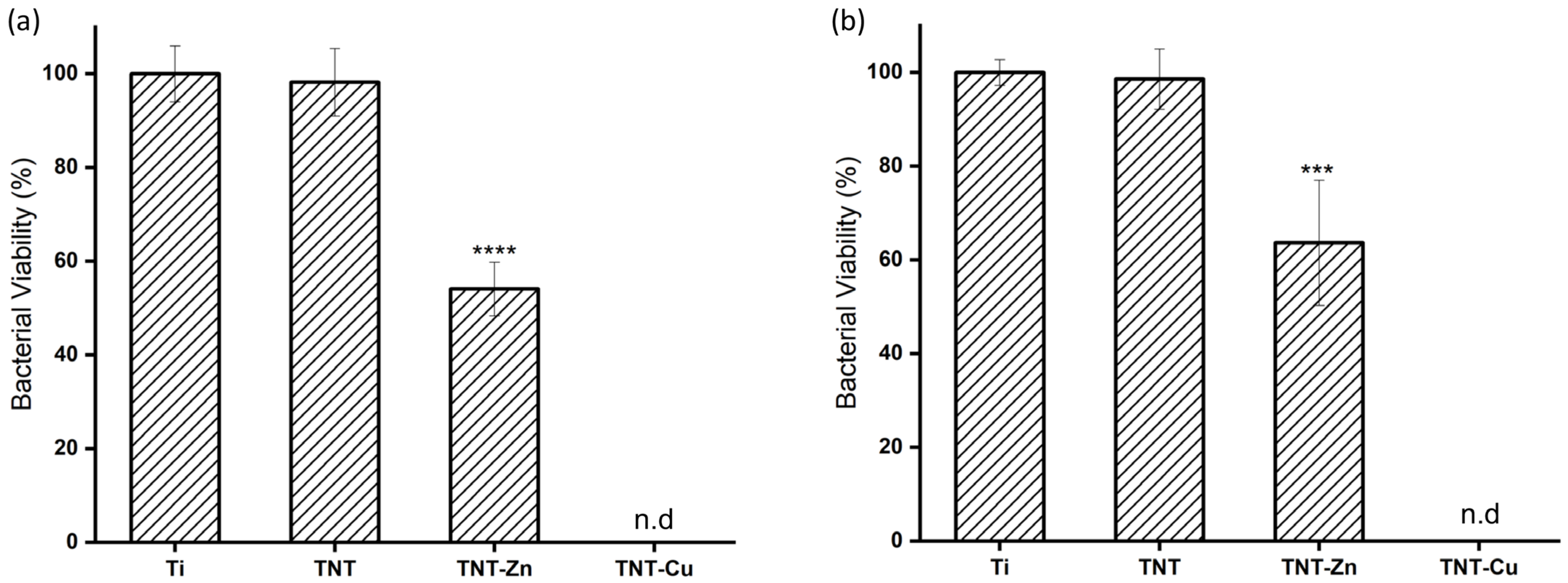
3.4. Antibacterial Activity of Zn- and Cu-Decorated TNTs
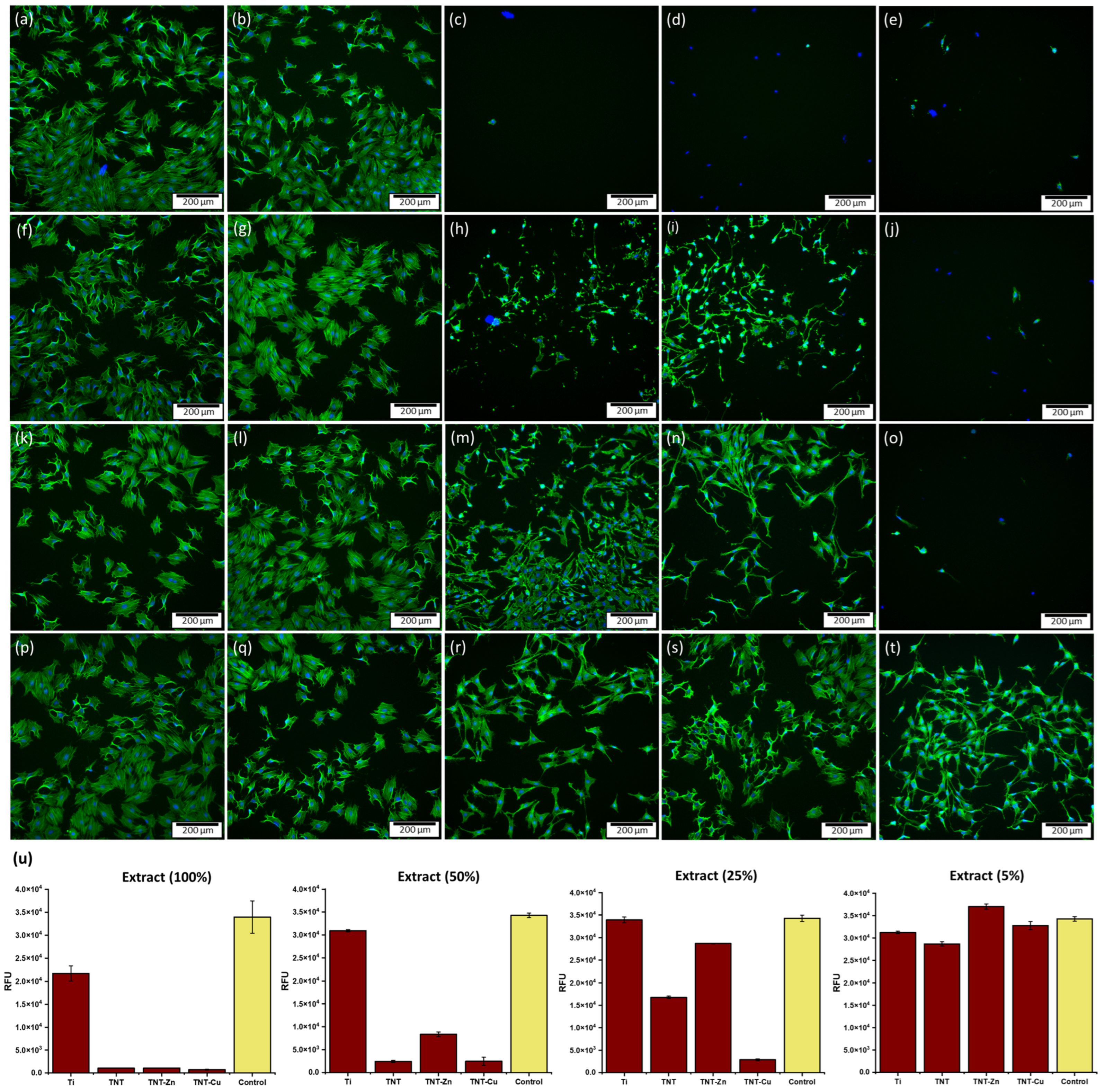
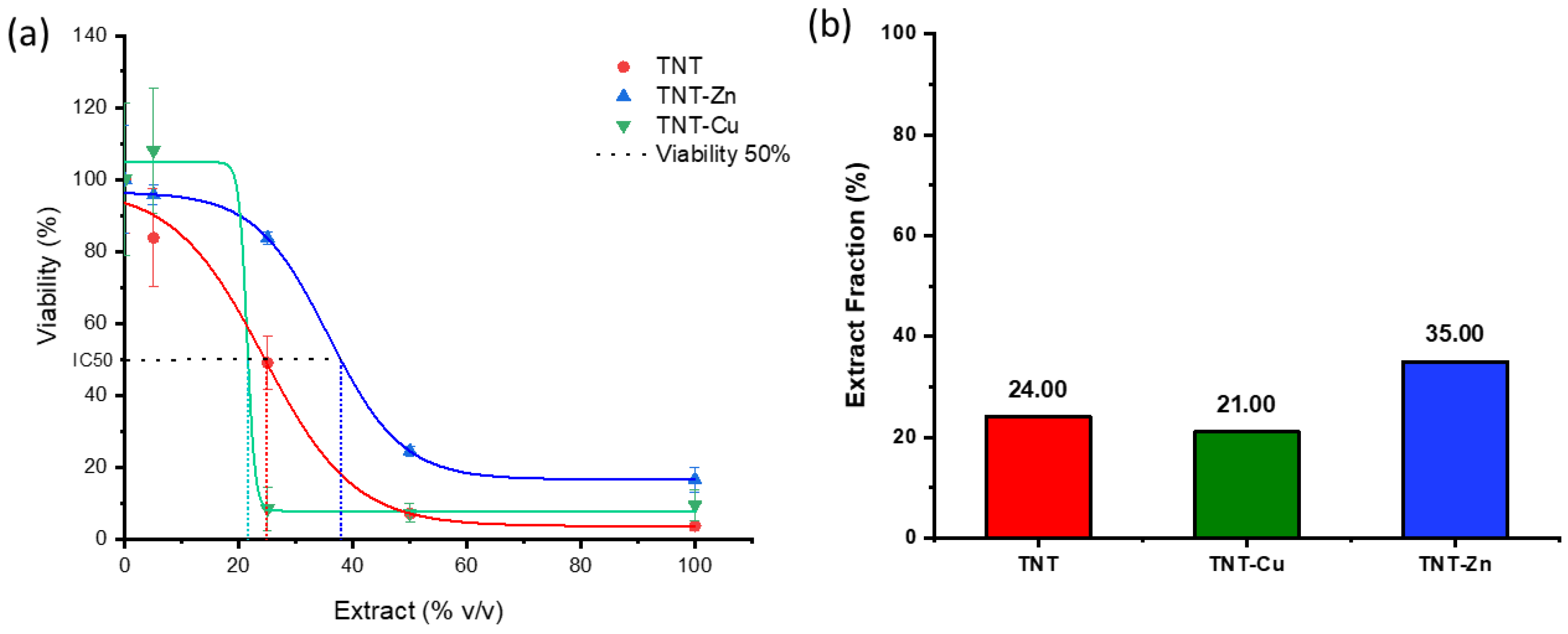
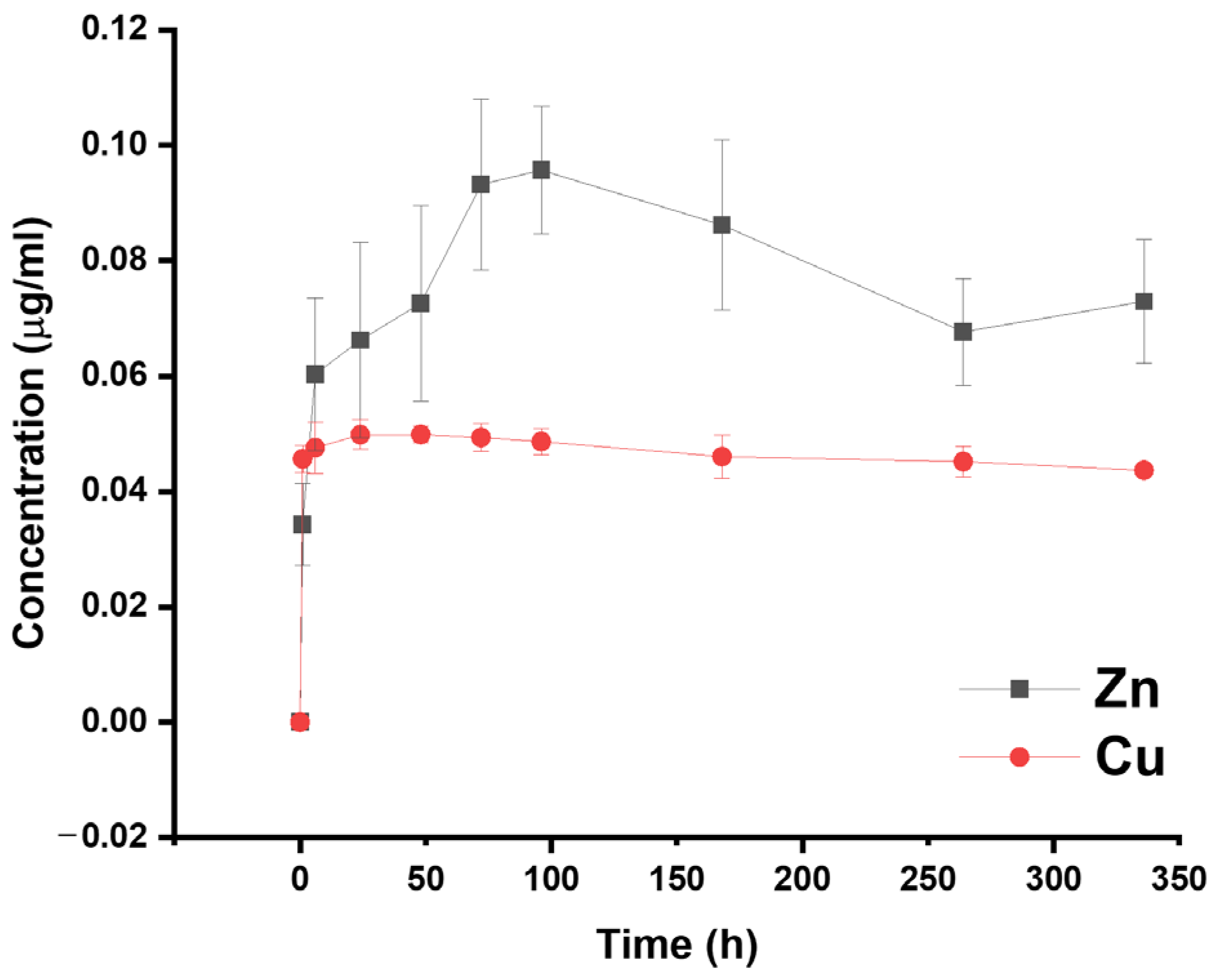
3.5. Cytocompatibility Evaluation and Ion Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Navarro, M.; Michiardi, A.; Castaño, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Kapadia, B.H.; Berg, R.A.; Daley, J.A.; Fritz, J.; Bhave, A.; Mont, M.A. Periprosthetic joint infection. Lancet 2016, 387, 386–394. [Google Scholar] [CrossRef]
- Pirisi, L.; Pennestrì, F.; Viganò, M.; Banfi, G. Prevalence and burden of orthopaedic implantable-device infections in Italy: A hospital-based national study. BMC Infect. Dis. 2020, 20, 337. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Sui, J. Surface Bio-Functionalization of Anti-Bacterial Titanium. Coatings 2022, 12, 1125. [Google Scholar] [CrossRef]
- Esteban, J.; Vallet-Regí, M.; Aguilera-Correa, J.J. Antibiotics-and heavy metals-based titanium alloy surface modifications for local prosthetic joint infections. Antibiotics 2021, 10, 1270. [Google Scholar] [CrossRef]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Rams, T.E.; Degener, J.E.; Van Winkelhoff, A.J. Antibiotic resistance in human peri-implantitis microbiota. Clin. Oral Implant. Res. 2014, 25, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; De Vecchi, E.; Bortolin, M.; Zagra, L.; Romanò, C.L.; Cappelletti, L. Epidemiology and Antibiotic Resistance of Late Prosthetic Knee and Hip Infections. J. Arthroplast. 2017, 32, 2496–2500. [Google Scholar] [CrossRef]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2009, 91, 470–480. [Google Scholar] [CrossRef]
- Romanò, C.L.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Antibacterial coating of implants in orthopaedics and trauma: A classification proposal in an evolving panorama. J. Orthop. Surg. Res. 2015, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Milošev, I. Surface Treatments of Titanium with Antibacterial Agents for Implant Applications. In Biomedical and Pharmaceutical Applications of Electrochemistry; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–87. ISBN 9783319318479. [Google Scholar]
- Moraes, M.N.; da Silveira, W.C.; Teixeira, L.E.M.; Araújo, I.D. Mechanisms of bacterial adhesion to biomaterials. Rev. Médica Minas Gerais 2013, 23, 99–104. [Google Scholar] [CrossRef]
- Civantos, A.; Martínez-Campos, E.; Ramos, V.; Elvira, C.; Gallardo, A.; Abarrategi, A. Titanium Coatings and Surface Modifications: Toward Clinically Useful Bioactive Implants. ACS Biomater. Sci. Eng. 2017, 3, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Ellinas, K.; Kefallinou, D.; Stamatakis, K.; Gogolides, E.; Tserepi, A. Is There a Threshold in the Antibacterial Action of Superhydrophobic Surfaces? ACS Appl. Mater. Interfaces 2017, 9, 39781–39789. [Google Scholar] [CrossRef] [PubMed]
- Lüdecke, C.; Roth, M.; Yu, W.; Horn, U.; Bossert, J.; Jandt, K.D. Nanorough titanium surfaces reduce adhesion of Escherichia coli and Staphylococcus aureus via nano adhesion points. Colloids Surf. B Biointerfaces 2016, 145, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Podporska-Carroll, J.; Panaitescu, E.; Quilty, B.; Wang, L.; Menon, L.; Pillai, S.C. Antimicrobial properties of highly efficient photocatalytic TiO2 nanotubes. Appl. Catal. B Environ. 2015, 176–177, 70–75. [Google Scholar] [CrossRef]
- Roy, P.; Kim, D.; Lee, K.; Spiecker, E.; Schmuki, P. TiO2 nanotubes and their application in dye-sensitized solar cells. Nanoscale 2010, 2, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Hetrick, E.M.; Schoenfisch, M.H. Reducing implant-related infections: Active release strategies. Chem. Soc. Rev. 2006, 35, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Spriano, S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C 2016, 61, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Brammer, K.S.; Frandsen, C.J.; Jin, S. TiO2 nanotubes for bone regeneration. Trends Biotechnol. 2012, 30, 315–322. [Google Scholar] [CrossRef]
- Hamlekhan, A.; Sinha-Ray, S.; Takoudis, C.; Mathew, M.T.; Sukotjo, C.; Yarin, A.L.; Shokuhfar, T. Fabrication of drug eluting implants: Study of drug release mechanism from titanium dioxide nanotubes. J. Phys. D Appl. Phys. 2015, 48, 275401. [Google Scholar] [CrossRef]
- Kazemzadeh-Narbat, M.; Lai, B.F.L.; Ding, C.; Kizhakkedathu, J.N.; Hancock, R.E.W.; Wang, R. Multilayered coating on titanium for controlled release of antimicrobial peptides for the prevention of implant-associated infections. Biomaterials 2013, 34, 5969–5977. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef]
- Haleem, A.A.; Rouse, M.S.; Lewallen, D.G.; Hanssen, A.D.; Steckelberg, J.M.; Patel, R. Gentamicin and vancomycin do not impair experimental fracture healing. Clin. Orthop. Relat. Res. 2004, 427, 22–24. [Google Scholar] [CrossRef]
- Hake, M.E.; Young, H.; Hak, D.J.; Stahel, P.F.; Hammerberg, E.M.; Mauffrey, C. Local antibiotic therapy strategies in orthopaedic trauma: Practical tips and tricks and review of the literature. Injury 2015, 46, 1447–1456. [Google Scholar] [CrossRef]
- Tarzimoghadam, Z.; Sandlöbes, S.; Pradeep, K.G.; Raabe, D. Microstructure design and mechanical properties in a near-α Ti-4Mo alloy. Acta Mater. 2015, 97, 291–304. [Google Scholar] [CrossRef]
- Rathbone, C.R.; Cross, J.D.; Brown, K.V.; Murray, C.K.; Wenke, J.C. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. J. Orthop. Res. 2011, 29, 1070–1074. [Google Scholar] [CrossRef]
- Gallo, J.; Panacek, A.; Prucek, R.; Kriegova, E.; Hradilova, S.; Hobza, M.; Holinka, M. Silver nanocoating technology in the prevention of prosthetic joint infection. Materials 2016, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B.G. Silver in Health Care: Antimicrobial Effects and Safety in Use. In Current Problems in Dermatology; Karger: Basel, Switzerland, 2006; Volume 33, pp. 17–34. [Google Scholar]
- Mehtar, S.; Wiid, I.; Todorov, S.D. The antimicrobial activity of copper and copper alloys against nosocomial pathogens and Mycobacterium tuberculosis isolated from healthcare facilities in the Western Cape: An in-vitro study. J. Hosp. Infect. 2008, 68, 45–51. [Google Scholar] [CrossRef]
- Ingle, A.P.; Duran, N.; Rai, M. Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: A review. Appl. Microbiol. Biotechnol. 2014, 98, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Noyce, J.O.; Michels, H.; Keevil, C.W. Potential use of copper surfaces to reduce survival of epidemic meticillin-resistant Staphylococcus aureus in the healthcare environment. J. Hosp. Infect. 2006, 63, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, R. Zinc oxide based Inorganic Antimicrobial agents. Int. J. Sci. Res. 2012, 1, 35–46. [Google Scholar]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B.G. A pharmacological and toxicological profile of silver as an antimicrobial agent in medical devices. Adv. Pharmacol. Sci. 2010, 2010, 910686. [Google Scholar] [CrossRef]
- Ribeiro, B.; Vázquez-López, A.; Vazquez-Pufleau, M.; Llamosí, M.; Sempere, J.; Yuste, J.; Domenech, M.; Wang, D.-Y.; Vilatela, J.J.; Llorca, J.; et al. Control of microbial agents by functionalization of commercial air filters with metal oxide particles. Mater. Chem. Phys. 2024, 313, 128684. [Google Scholar] [CrossRef]
- Gao, C.; Li, C.; Wang, C.; Qin, Y.; Wang, Z.; Yang, F.; Liu, H.; Chang, F.; Wang, J. Advances in the induction of osteogenesis by zinc surface modification based on titanium alloy substrates for medical implants. J. Alloys Compd. 2017, 726, 1072–1084. [Google Scholar] [CrossRef]
- Lowe, N.M.; Fraser, W.D.; Jackson, M.J. Is there a potential therapeutic value of copper and zinc for osteoporosis? Proc. Nutr. Soc. 2002, 61, 181–185. [Google Scholar] [CrossRef]
- Sadowska, J.M.; Ginebra, M.P. Inflammation and biomaterials: Role of the immune response in bone regeneration by inorganic scaffolds. J. Mater. Chem. B 2020, 8, 9404–9427. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.; Offoiach, R.; Rahimi, E.; Salatin, E.; Lekka, M.; Fedrizzi, L. On growth and morphology of TiO2 nanotubes on Ti6Al4V by anodic oxidation in ethylene glycol electrolyte: Influence of microstructure and anodization parameters. Materials 2021, 14, 2540. [Google Scholar] [CrossRef]
- Tan, A.W.; Pingguan-Murphy, B.; Ahmad, R.; Akbar, S.A. Review of titania nanotubes: Fabrication and cellular response. Ceram. Int. 2012, 38, 4421–4435. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Chauhan, N.S.; Mittal, A.; Sharma, S. TiO2 and its composites as promising biomaterials: A review. BioMetals 2018, 31, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liao, X.; Fok, A.; Ning, C.; Ng, P.; Wang, Y. Effect of crystalline phase changes in titania (TiO2) nanotube coatings on platelet adhesion and activation. Mater. Sci. Eng. C 2018, 82, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.Q.; Jiang, X.Q.; Zhang, F.Q.; Xu, L. The effect of anatase TiO2 nanotube layers on MC3T3-E1 preosteoblast adhesion, proliferation, and differentiation. J. Biomed. Mater. Res. Part A 2010, 94, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Regonini, D.; Jaroenworaluck, A.; Stevens, R.; Bowen, C.R. Effect of heat treatment on the properties and structure of TiO2 nanotubes: Phase composition and chemical composition. Surf. Interface Anal. 2010, 42, 139–144. [Google Scholar] [CrossRef]
- Jordanovová, V.; Losertová, M.; Štencek, M.; Lukášová, T.; Martynková, G.S.; Peikertová, P. Microstructure and properties of nanostructured coating on Ti6Al4V. Materials 2020, 13, 708. [Google Scholar] [CrossRef]
- Rossi, S.; Volgare, L.; Perrin-Pellegrino, C.; Chassigneux, C.; Dousset, E.; Eyraud, M. Dual electrochemical treatments to improve properties of Ti6Al4V alloy. Materials 2020, 13, 2479. [Google Scholar] [CrossRef]
- Regonini, D.; Bowen, C.R.; Jaroenworaluck, A.; Stevens, R. A review of growth mechanism, structure and crystallinity of anodized TiO2 nanotubes. Mater. Sci. Eng. R Rep. 2013, 74, 377–406. [Google Scholar] [CrossRef]
- Hilario, F.; Roche, V.; Nogueira, R.P.; Junior, A.M.J. Influence of morphology and crystalline structure of TiO2 nanotubes on their electrochemical properties and apatite-forming ability. Electrochim. Acta 2017, 245, 337–349. [Google Scholar] [CrossRef]
- Bai, Y.; Park, I.S.; Park, H.H.; Lee, M.H.; Bae, T.S.; Duncan, W.; Swain, M. The effect of annealing temperatures on surface properties, hydroxyapatite growth and cell behaviors of TiO2 nanotubes. Surf. Interface Anal. 2011, 43, 998–1005. [Google Scholar] [CrossRef]
- Das, K.; Bose, S.; Bandyopadhyay, A. TiO2 nanotubes on Ti: Influence of nanoscale morphology on bone cell-materials interaction. J. Biomed. Mater. Res. Part A 2009, 90, 225–237. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Test for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Jäger, M.; Jennissen, H.P.; Dittrich, F.; Fischer, A.; Köhling, H.L. Antimicrobial and osseointegration properties of nanostructured titanium orthopaedic implans. Materials 2017, 10, 1302. [Google Scholar] [CrossRef]
- Regonini, D.; Clemens, F.J. Anodized TiO2 nanotubes: Effect of anodizing time on film length, morphology and photoelectrochemical properties. Mater. Lett. 2015, 142, 97–101. [Google Scholar] [CrossRef]
- Kowalski, D.; Kim, D.; Schmuki, P. TiO2 nanotubes, nanochannels and mesosponge: Self-organized formation and applications. Nano Today 2013, 8, 235–264. [Google Scholar] [CrossRef]
- Indira, K.; Kamachi Mudali, U.; Rajendran, N. Corrosion behavior of electrochemically assembled nanoporous titania for biomedical applications. Ceram. Int. 2013, 39, 959–967. [Google Scholar] [CrossRef]
- Bayata, F.; Ürgen, M. Role of aluminum doping on phase transformations in nanoporous titania anodic oxides. J. Alloys Compd. 2015, 646, 719–726. [Google Scholar] [CrossRef]
- Khrunyk, Y.Y.; Belikov, S.V.; Tsurkan, M.V.; Vyalykh, I.V.; Markaryan, A.Y.; Karabanalov, M.S.; Popov, A.A.; Wysokowski, M. Surface-Dependent Osteoblasts Response to TiO2 Nanotubes of Different Crystallinity. Nanomaterials 2020, 10, 320. [Google Scholar] [CrossRef] [PubMed]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Gallaway, J.W.; Gaikwad, A.M.; Hertzberg, B.; Erdonmez, C.K.; Chen-Wiegart, Y.K.; Sviridov, L.A.; Evans-Lutterodt, K.; Wang, J.; Banerjee, S.; Steingart, D.A. An In Situ Synchrotron Study of Zinc Anode Planarization by a Bismuth Additive. J. Electrochem. Soc. 2014, 161, A275–A284. [Google Scholar] [CrossRef]
- Shin, S.; Park, C.; Kim, C.; Kim, Y.; Park, S.; Lee, J.H. Cyclic voltammetry studies of copper, tin and zinc electrodeposition in a citrate complex system for CZTS solar cell application. Curr. Appl. Phys. 2016, 16, 207–210. [Google Scholar] [CrossRef]
- Zoolfakar, A.S.; Rani, R.A.; Morfa, A.J.; Balendhran, S.; O’Mullane, A.P.; Zhuiykov, S.; Kalantar-Zadeh, K. Enhancing the current density of electrodeposited ZnO-Cu2O solar cells by engineering their heterointerfaces. J. Mater. Chem. 2012, 22, 21767–21775. [Google Scholar] [CrossRef]
- Roguska, A.; Belcarz, A.; Pisarek, M.; Ginalska, G.; Lewandowska, M. TiO2 nanotube composite layers as delivery system for ZnO and Ag nanoparticles—An unexpected overdose effect decreasing their antibacterial efficacy. Mater. Sci. Eng. C 2015, 51, 158–166. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Li, R.; Tang, X.; Guo, D.; Qing, Y.; Qin, Y. Enhanced antibacterial properties of orthopedic implants by titanium nanotube surface modification: A review of current techniques. Int. J. Nanomed. 2019, 14, 7217–7236. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.T.; Tan, H.L.; Duan, Z.L.; Yue, B.; Ma, R.; He, G.; Tang, T.T. Inhibited bacterial biofilm formation and improved osteogenic activity on gentamicin-loaded titania nanotubes with various diameters. Int. J. Nanomed. 2014, 9, 1215–1230. [Google Scholar]
- Peng, Z.; Ni, J.; Zheng, K.; Shen, Y.; Wang, X.; He, G.; Jin, S.; Tang, T. Dual effects and mechanism of TiO2 nanotube arrays in reducing bacterial colonization and enhancing C3H10T1/2 cell adhesion. Int. J. Nanomed. 2013, 8, 3093–3105. [Google Scholar] [CrossRef]
- Yang, Y.; Ao, H.Y.; Yang, S.B.; Wang, Y.G.; Lin, W.T.; Yu, Z.F.; Tang, T.T. In vivo evaluation of the anti-infection potential of gentamicin-loaded nanotubes on titania implants. Int. J. Nanomed. 2016, 11, 2223–2234. [Google Scholar] [CrossRef][Green Version]
- Raja, F.N.S.; Worthington, T.; Martin, R.A. The antimicrobial efficacy of copper, cobalt, zinc and silver nanoparticles: Alone and in combination. Biomed. Mater. 2023, 18, 045003. [Google Scholar] [CrossRef]
- Allizond, V.; Comini, S.; Cuffini, A.M.; Banche, G. Current Knowledge on Biomaterials for Orthopedic Applications Modified to Reduce Bacterial Adhesive Ability. Antibiotics 2022, 11, 529. [Google Scholar] [CrossRef]
- Akshaya, S.; Rowlo, P.K.; Dukle, A.; Nathanael, A.J. Antibacterial Coatings for Titanium Implants: Recent Trends and Future Perspectives. Antibiotics 2022, 11, 1719. [Google Scholar] [CrossRef]
- Alves, S.A.; Ribeiro, A.R.; Gemini-Piperni, S.; Silva, R.C.; Saraiva, A.M.; Leite, P.E.; Perez, G.; Oliveira, S.M.; Araujo, J.R.; Archanjo, B.S.; et al. TiO2 nanotubes enriched with calcium, phosphorous and zinc: Promising bio-selective functional surfaces for osseointegrated titanium implants. RSC Adv. 2017, 7, 49720–49738. [Google Scholar] [CrossRef]
- Huo, K.; Zhang, X.; Wang, H.; Zhao, L.; Liu, X.; Chu, P.K. Osteogenic activity and antibacterial effects on titanium surfaces modified with Zn-incorporated nanotube arrays. Biomaterials 2013, 34, 3467–3478. [Google Scholar] [CrossRef]
- Filova, E.; Fojt, J.; Kryslova, M.; Moravec, H.; Joska, L.; Bacakova, L. The diameter of nanotubes formed on Ti-6Al-4V alloy controls the adhesion and differentiation of Saos-2 cells. Int. J. Nanomed. 2015, 10, 7145–7163. [Google Scholar] [CrossRef]
- Saha, S.; Pramanik, K.; Biswas, A. Antibacterial activity and biocompatibility of curcumin/TiO2 nanotube array system on Ti6Al4V bone implants. Mater. Technol. 2021, 36, 221–232. [Google Scholar] [CrossRef]
- Sarraf, M.; Dabbagh, A.; Abdul Razak, B.; Mahmoodian, R.; Nasiri-Tabrizi, B.; Hosseini, H.R.M.; Saber-Samandari, S.; Abu Kasim, N.H.; Abdullah, H.; Sukiman, N.L. Highly-ordered TiO2 nanotubes decorated with Ag2O nanoparticles for improved biofunctionality of Ti6Al4V. Surf. Coat. Technol. 2018, 349, 1008–1017. [Google Scholar] [CrossRef]
- Swain, S.; Misra, R.D.K.; You, C.K.; Rautray, T.R. TiO2 nanotubes synthesised on Ti-6Al-4V ELI exhibits enhanced osteogenic activity: A potential next-generation material to be used as medical implants. Mater. Technol. 2021, 36, 393–399. [Google Scholar] [CrossRef]
- Barrio, D.A.; Etcheverry, S.B. Vanadium and bone development: Putative signaling pathways. Can. J. Physiol. Pharmacol. 2006, 84, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.P.; Videira, A.; Soares, P.; Máximo, V. Orthovanadate-induced cell death in RET/PTC1-harboring cancer cells involves the activation of caspases and altered signaling through PI3K/Akt/mTOR. Life Sci. 2011, 89, 371–377. [Google Scholar] [CrossRef]
- Montiel-Dávalos, A.; Gonzalez-Villava, A.; Rodriguez-Lara, V.; Montaño, L.F.; Fortoul, T.I.; López-Marure, R. Vanadium pentoxide induces activation and death of endothelial cells. J. Appl. Toxicol. 2012, 32, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ye, L.; Liu, H.; Xia, Q.; Zhang, Y.; Yang, X.; Wang, K. Vanadium compounds induced mitochondria permeability transition pore (PTP) opening related to oxidative stress. J. Inorg. Biochem. 2010, 104, 371–378. [Google Scholar] [CrossRef]
- Gomes, C.C.; Moreira, L.M.; JSV Santos, V.; Ramos, A.S.; Lyon, J.P.; Soares, C.P.; Santos, F.V.; Bosco, D.; João Del Rei, S. Assessment of the genetic risks of a metallic alloy used in medical implants. Genet. Mol. Biol. 2011, 34, 116–121. [Google Scholar] [CrossRef]
- Challa, V.S.A.; Mali, S.; Misra, R.D.K. Reduced toxicity and superior cellular response of preosteoblasts to Ti-6Al-7Nb alloy and comparison with Ti-6Al-4V. J. Biomed. Mater. Res.-Part A 2013, 101 Pt A, 2083–2089. [Google Scholar] [CrossRef]
- Barrio, D.A.; Cattáneo, E.R.; Apezteguía, M.C.; Etcheverry, S.B. Vanadyl(IV) complexes with saccharides. Bioactivity in osteoblast-like cells in culture. Can. J. Physiol. Pharmacol. 2006, 84, 765–775. [Google Scholar] [CrossRef]
- Costa, B.C.; Tokuhara, C.K.; Rocha, L.A.; Oliveira, R.C.; Lisboa-Filho, P.N.; Costa Pessoa, J. Vanadium ionic species from degradation of Ti-6Al-4V metallic implants: In vitro cytotoxicity and speciation evaluation. Mater. Sci. Eng. C 2019, 96, 730–739. [Google Scholar] [CrossRef]
- Hierro-Oliva, M.; Gallardo-Moreno, A.M.; González-Martín, M.L. XPS Analysis of Ti6Al4V Oxidation Under UHV Conditions. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2014, 45, 6285–6290. [Google Scholar] [CrossRef]
- Lewandowska, M.; Pisarek, M.; Rozniatowski, K.; Gradzka-Dahlke, M.; Janik-Czachor, M.; Kurzydłowski, K.J. Nanoscale characterization of anodic oxide films on Ti-6Al-4V alloy. Thin Solid Films 2007, 515, 6460–6464. [Google Scholar] [CrossRef]
- Chávez-Díaz, M.P.; Luna-Sánchez, R.M.; Vazquez-Arenas, J.; Lartundo-Rojas, L.; Hallen, J.M.; Cabrera-Sierra, R. XPS and EIS studies to account for the passive behavior of the alloy Ti-6Al-4V in Hank’s solution. J. Solid State Electrochem. 2019, 23, 3187–3196. [Google Scholar] [CrossRef]
- Ocampo, R.A.; Bedoya Ochoa, N.; Tamayo, J.A.; Botero, C.; Vargas, C.A.; Gómez, M.; Castaño, J.G.; Zuleta Gil, A.A. Formation of highly ordered TiO2 nanotubes on Ti6Al4V alloys manufactured by electron beam powder bed fusion (E-PBF). Int. J. Adv. Manuf. Technol. 2023, 128, 257–266. [Google Scholar] [CrossRef]
- Brammer, K.S.; Oh, S.; Cobb, C.J.; Bjursten, L.M.; van der Heyde, H.; Jin, S. Improved bone-forming functionality on diameter-controlled TiO2 nanotube surface. Acta Biomater. 2009, 5, 3215–3223. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.Q.; Zhang, Y.L.; Jiang, X.Q.; Zhang, F.Q. In vitro behavior of MC3T3-E1 preosteoblast with different annealing temperature titania nanotubes. Oral Dis. 2010, 16, 624–630. [Google Scholar] [CrossRef]
- Salou, L.; Hoornaert, A.; Louarn, G.; Layrolle, P. Enhanced osseointegration of titanium implants with nanostructured surfaces: An experimental study in rabbits. Acta Biomater. 2015, 11, 494–502. [Google Scholar] [CrossRef]
- Byeon, S.M.; Kim, H.J.; Lee, M.H.; Bae, T.S. Enhancement of bioactivity and osseointegration in Ti-6Al-4V orthodontic mini-screws coated with calcium phosphate on the TiO2 nanotube layer. Korean J. Orthod. 2022, 52, 412–419. [Google Scholar] [CrossRef]
- Byeon, S.M.; Jeon, J.; Jang, Y.S.; Jeon, W.Y.; Lee, M.H.; Jeon, Y.M.; Kim, J.G.; Bae, T.S. Evaluation of osseointegration of Ti-6Al-4V alloy orthodontic mini-screws with ibandronate-loaded TiO2 nanotube layer. Dent. Mater. J. 2023, 42, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Manivasagam, G.; Dhinasekaran, D.; Rajamanickam, A. Biomedical Implants: Corrosion and its Prevention—A Review. Recent Pat. Corros. Sci. 2010, 2, 40–54. [Google Scholar] [CrossRef]
- Manam, N.S.; Harun, W.S.W.; Shri, D.N.A.; Ghani, S.A.C.; Kurniawan, T.; Ismail, M.H.; Ibrahim, M.H.I. Study of corrosion in biocompatible metals for implants: A review. J. Alloys Compd. 2017, 701, 698–715. [Google Scholar] [CrossRef]



| Element | Concentration (µg/mL) |
|---|---|
| Ti | 0 |
| Al | 0 |
| V | 0.59 |
| Na | 4151.00 |
| P | 365.3 |
| K | 0 |
| Zn | 0 |
| Cu | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, B.; Offoiach, R.; Monteiro, C.; Morais, M.R.G.; Martins, M.C.L.; Pêgo, A.P.; Salatin, E.; Fedrizzi, L.; Lekka, M. Electrodeposition of Zn and Cu Nanoparticles into TiO2 Nanotubes on Ti6Al4V: Antimicrobial Effect against S. Epidermidis and Cytotoxicity Assessment. Micro 2024, 4, 97-116. https://doi.org/10.3390/micro4010007
Ribeiro B, Offoiach R, Monteiro C, Morais MRG, Martins MCL, Pêgo AP, Salatin E, Fedrizzi L, Lekka M. Electrodeposition of Zn and Cu Nanoparticles into TiO2 Nanotubes on Ti6Al4V: Antimicrobial Effect against S. Epidermidis and Cytotoxicity Assessment. Micro. 2024; 4(1):97-116. https://doi.org/10.3390/micro4010007
Chicago/Turabian StyleRibeiro, Bruno, Ruben Offoiach, Claudia Monteiro, Miguel R. G. Morais, M. Cristina L. Martins, Ana Paula Pêgo, Elisa Salatin, Lorenzo Fedrizzi, and Maria Lekka. 2024. "Electrodeposition of Zn and Cu Nanoparticles into TiO2 Nanotubes on Ti6Al4V: Antimicrobial Effect against S. Epidermidis and Cytotoxicity Assessment" Micro 4, no. 1: 97-116. https://doi.org/10.3390/micro4010007
APA StyleRibeiro, B., Offoiach, R., Monteiro, C., Morais, M. R. G., Martins, M. C. L., Pêgo, A. P., Salatin, E., Fedrizzi, L., & Lekka, M. (2024). Electrodeposition of Zn and Cu Nanoparticles into TiO2 Nanotubes on Ti6Al4V: Antimicrobial Effect against S. Epidermidis and Cytotoxicity Assessment. Micro, 4(1), 97-116. https://doi.org/10.3390/micro4010007







