Toward Remote Detection of Chemical Warfare Simulants Using a Miniature Potentiostat
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Electrochemical Measurements
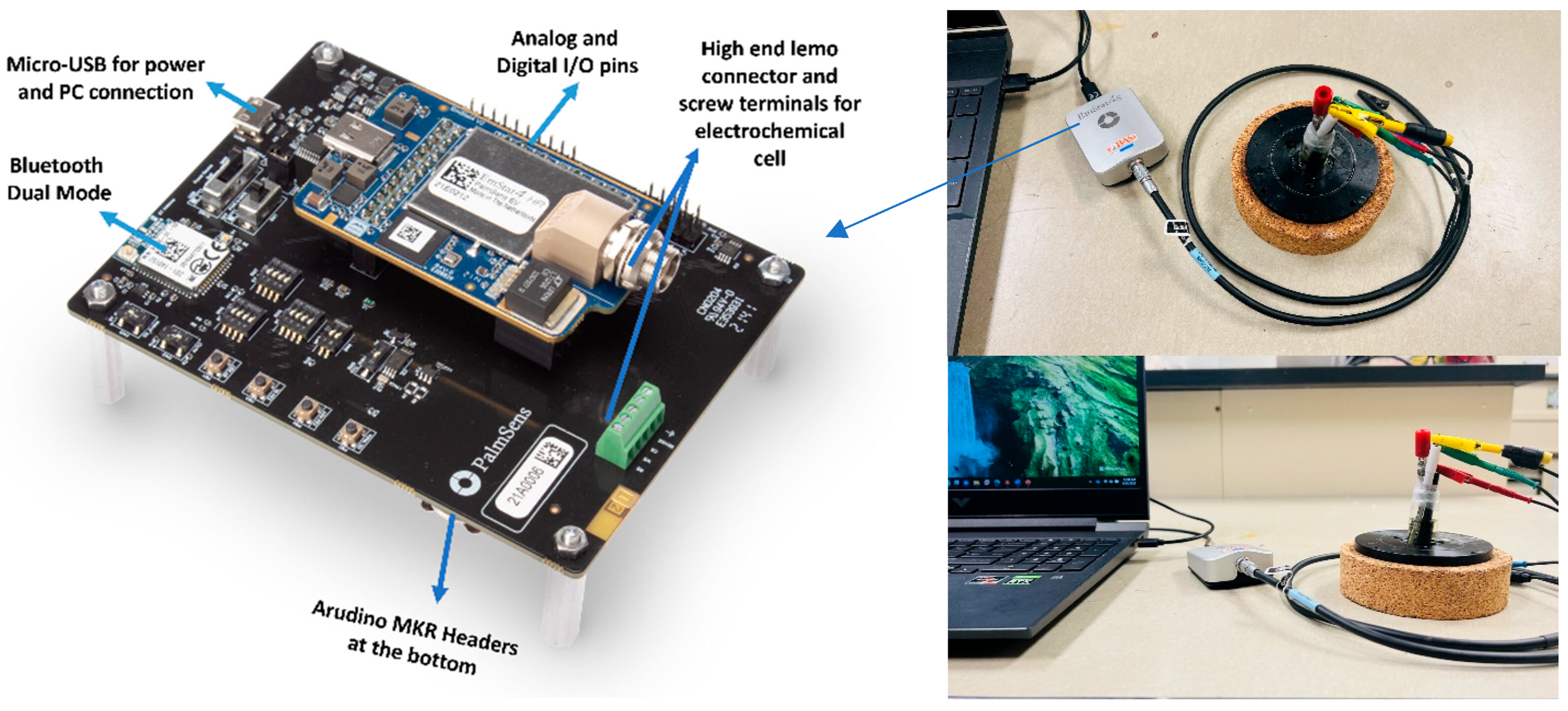
2.3. EmStat4s HR Postentiostat
2.4. Electrochemical Logic Gate Operation
3. Results and Discussion
3.1. EmStat4s HR Postentiostat Validation
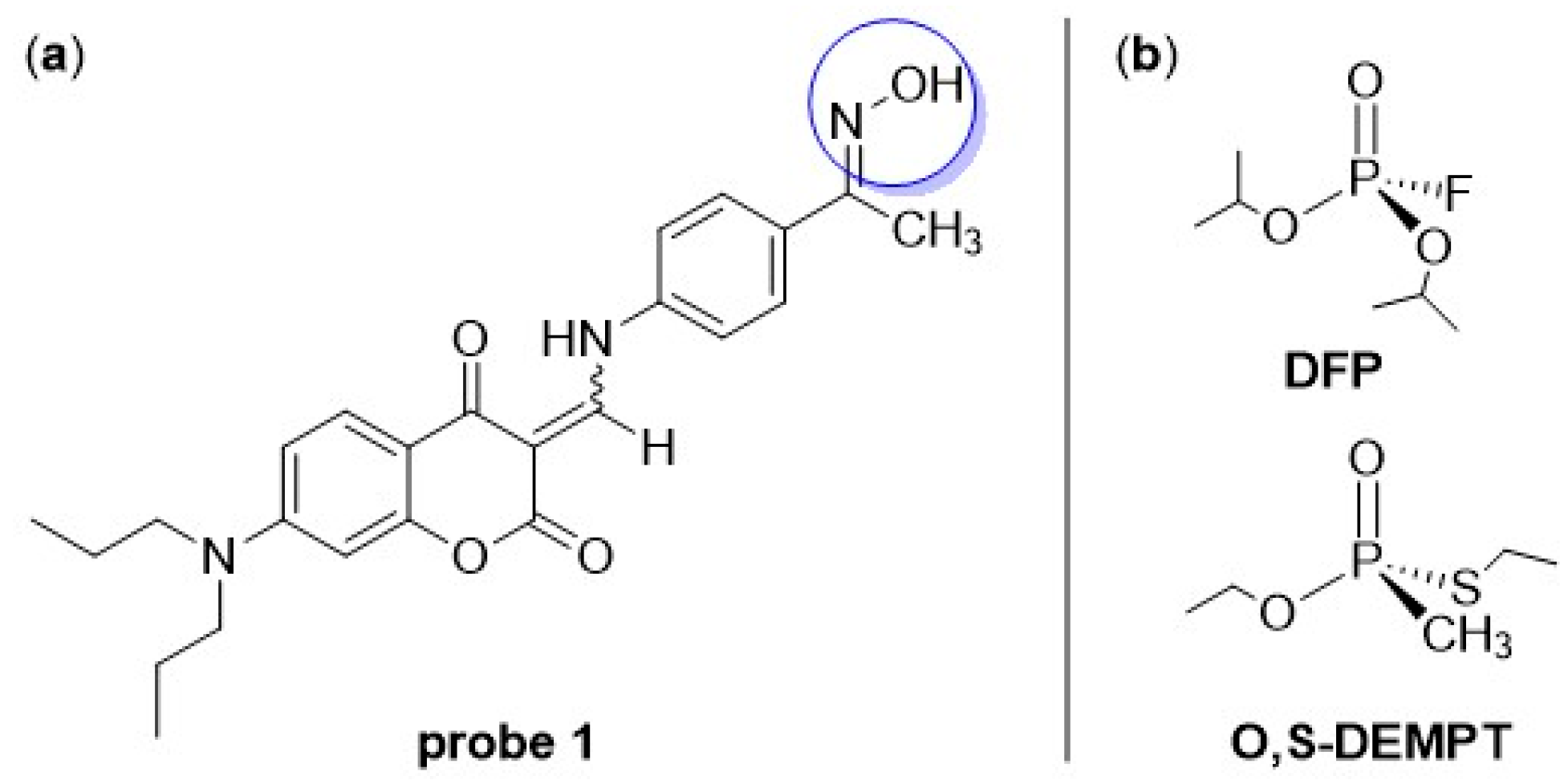
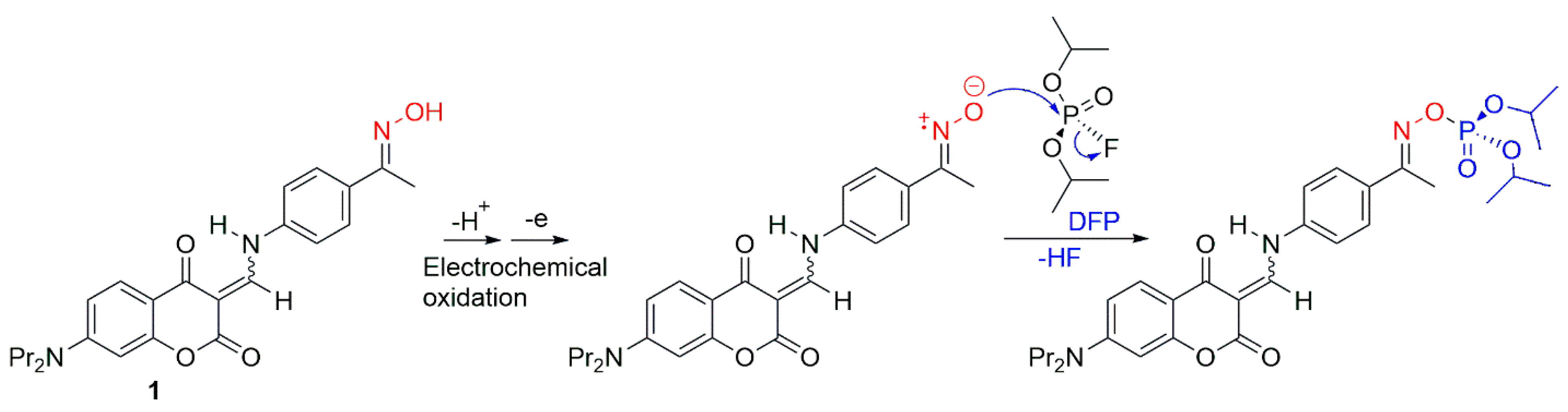
3.2. Electrochemical Detection of CWAs
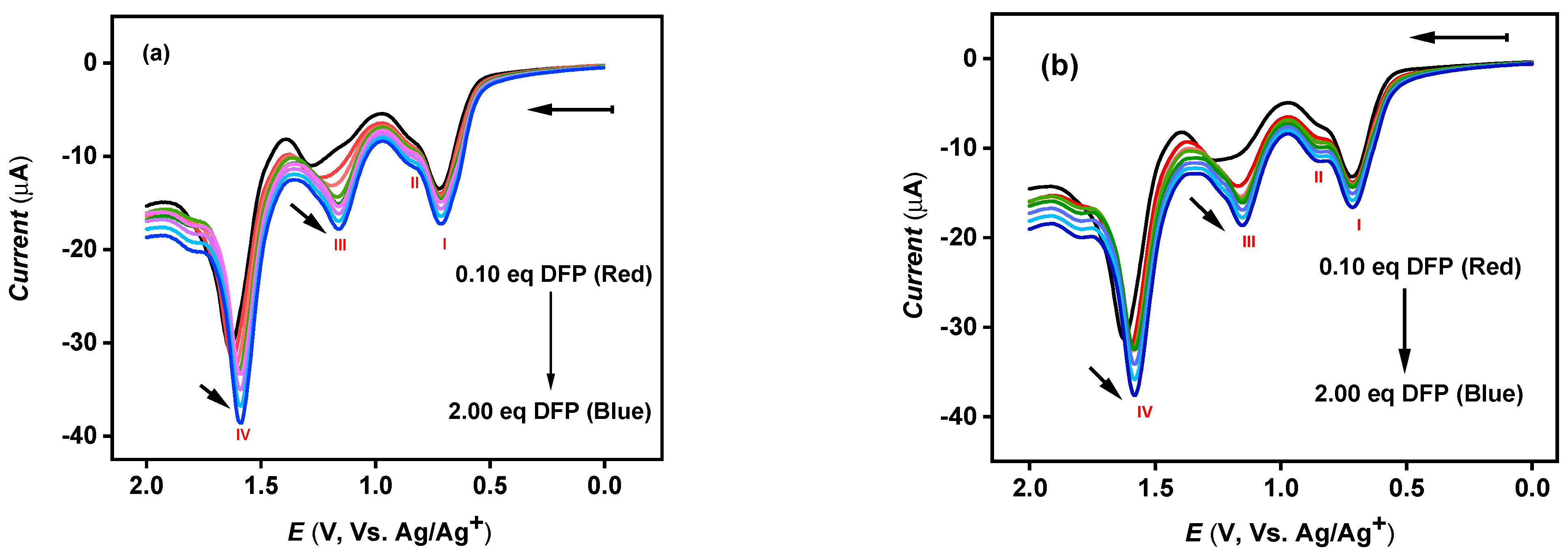
3.3. Sensor Sensitivity
3.4. Logic Gate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Picard, B.; Chataigner, I.; Maddaluno, J.; Legros, J. Introduction to chemical warfare agents, relevant simulants and modern neutralisation methods. Org. Biomol. Chem. 2019, 17, 6528–6537. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Tsay, O.G.; Atwood, D.A.; Churchill, D.G. Destruction and Detection of Chemical Warfare Agents. Chem. Rev. 2011, 111, 5345–5403. [Google Scholar] [CrossRef] [PubMed]
- Fazili, Y.U.S. Meets Milestone in Chemical Weapons Stockpile Destruction; U.S. Department of Defense: Washington, DC, USA, 2022. [Google Scholar]
- Che Sulaiman, I.S.; Chieng, B.W.; Pojol, F.E.; Ong, K.K.; Abdul Rashid, J.I.; Wan Yunus, W.M.Z.; Mohd Kasim, N.A.; Abdul Halim, N.; Mohd Noor, S.A.; Knight, V.F. A review on analysis methods for nerve agent hydrolysis products. Forensic Toxicol. 2020, 38, 297–313. [Google Scholar] [CrossRef]
- Valdez, C.A.; Leif, R.N.; Hok, S.; Hart, B.R. Analysis of chemical warfare agents by gas chromatography-mass spectrometry: Methods for their direct detection and derivatization approaches for the analysis of their degradation products. Rev. Anal. Chem. 2017, 37, 20170007. [Google Scholar] [CrossRef]
- Seto, Y.; Hashimoto, R.; Taniguchi, T.; Ohrui, Y.; Nagoya, T.; Iwamatsu, T.; Komaru, S.; Usui, D.; Morimoto, S.; Sakamoto, Y. Development of ion mobility spectrometry with novel atmospheric electron emission ionization for field detection of gaseous and blister chemical warfare agents. Anal. Chem. 2019, 91, 5403–5414. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.E.; Dixon, M.M.; Williams, B.R.; Kilper, G.K.; Lim, S.H.; Martino, R.A.; Rhodes, P.; Hulet, M.S.; Miles, R.W.; Samuels, A.C.; et al. Detection of chemical warfare agents by colorimetric sensor arrays. ACS Sens. 2020, 5, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Kangas, M.J.; Ernest, A.; Lukowicz, R.; Mora, A.V.; Quossi, A.; Perez, M.; Kyes, N.; Holmes, A.E. The identification of seven chemical warfare mimics using a colorimetric array. Sensors 2018, 18, 4291. [Google Scholar] [CrossRef]
- Seo, H.S.; Koh, Y.J.; Nam, H.; Kim, J.-S. Development of a rapid and accurate vapor generation system for real-time monitoring of a chemical warfare agent (CWA) by coupling fourier transform infrared (FT-IR) spectroscopy. ACS Omega 2023, 8, 18058–18063. [Google Scholar] [CrossRef]
- Dang, M.; Liu, R.; Dong, F.; Liu, B.; Hou, K. Vacuum ultraviolet photoionization on-line mass spectrometry: Instrumentation developments and applications. TrAC Trends Anal. Chem. 2022, 149, 116542. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, D.; Li, H. UV photoionization ion mobility spectrometry: Fundamentals and applications. Anal. Chim. Acta 2019, 1077, 1–13. [Google Scholar] [CrossRef]
- Huang, J.; Shu, J.; Yang, B.; Guo, Y.; Zhang, Z.; Jiang, K.; Li, Z. Ultrasensitive detection of trace chemical warfare agent-related compounds by thermal desorption associative ionization time-of-flight mass spectrometry. Talanta 2021, 235, 122788. [Google Scholar] [CrossRef] [PubMed]
- Sayago, I.; Matatagui, D.; Fernández, M.J.; Fontecha, J.L.; Jurewicz, I.; Garriga, R.; Muñoz, E. Graphene oxide as sensitive layer in Love-wave surface acoustic wave sensors for the detection of chemical warfare agent simulants. Talanta 2016, 148, 393–400. [Google Scholar] [CrossRef]
- Chen, A.; Shah, B. Electrochemical sensing and biosensing based on square wave voltammetry. Anal. Methods 2013, 5, 2158–2173. [Google Scholar] [CrossRef]
- Demirhan, A.; Eksin, E.; Kilic, Y.; Erdem, A. Low-cost high-resolution potentiostat for electrochemical detection of nucleic acids and biomolecular interactions. Micromachines 2022, 13, 1610. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.D.; Doeven, E.H.; Quayle, K.; Kouzani, A.Z. MiniStat: Development and evaluation of a mini-potentiostat for electrochemical measurements. IEEE Access 2019, 7, 31903–31912. [Google Scholar] [CrossRef]
- Prinith, N.S.; Manjunatha, J.G. Surfactant modified electrochemical sensor for determination of Anthrone—A cyclic voltammetry. Mater. Sci. Energy Technol. 2019, 2, 408–416. [Google Scholar] [CrossRef]
- García-Miranda Ferrari, A.; Foster, C.W.; Kelly, P.J.; Brownson, D.A.; Banks, C.E. Determination of the electrochemical area of screen-printed electrochemical sensing platforms. Biosensors 2018, 8, 53. [Google Scholar] [CrossRef]
- Baluta, S.; Meloni, F.; Halicka, K.; Szyszka, A.; Zucca, A.; Pilo, M.I.; Cabaj, J. Differential pulse voltammetry and chronoamperometry as analytical tools for epinephrine detection using a tyrosinase-based electrochemical biosensor. RSC Adv. 2022, 12, 25342–25353. [Google Scholar] [CrossRef]
- Mohan, J.M.; Amreen, K.; Javed, A.; Dubey, S.K.; Goel, S. Emerging trends in miniaturized and microfluidic electrochemical sensing platforms. Curr. Opin. Electrochem. 2022, 33, 100930. [Google Scholar] [CrossRef]
- Hoilett, O.S.; Walker, J.F.; Balash, B.M.; Jaras, N.J.; Boppana, S.; Linnes, J.C. KickStat: A coin-sized potentiostat for high-resolution electrochemical analysis. Sensors 2020, 20, 2407. [Google Scholar] [CrossRef]
- Schneeweiss, W.G. Boolean Functions: With Engineering Applications and Computer Programs; Springer: Berlin/Heidelberg, Germany, 1989. [Google Scholar]
- de Silvia, A.; Uchiyama, S. Molecular logic gates and luminescent sensors based on photoinduced electron transfer. In Luminescence Applied in Sensor Science. Topics in Current Chemistry; Prodi, L., Montalti, M., Zacceroni, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 300, pp. 1–28. [Google Scholar]
- Bazzicalupi, C.; Bianchi, A.; García-España, E.; Delgado-Pinar, E. Metals in supramolecular chemistry. Inorg. Chimi. Acta 2014, 417, 3–26. [Google Scholar] [CrossRef]
- Liu, Q.; Ren, X.; Hu, Y.; Zhou, J. Versatile electrochemical platform for GSH detection and its boolean logic application in related biological pathways. J. Electrochem. Soc. 2022, 169, 127516. [Google Scholar] [CrossRef]
- Lu, J.Y.; Zhang, X.X.; Huang, W.T.; Zhu, Q.Y.; Ding, X.Z.; Xia, L.Q.; Luo, H.Q.; Li, N.B. Boolean logic tree of label-free dual-signal electrochemical aptasensor system for biosensing, three-state logic computation, and keypad lock security operation. Anal. Chem. 2017, 89, 9734–9741. [Google Scholar] [CrossRef] [PubMed]
- Amatore, C.; Brown, A.R.; Thouin, L.; Warkocz, J.-S. Mimicking neuronal synaptic behavior: Processing of information with ‘AND’or ‘OR’Boolean logic via paired-band microelectrode assemblies. Comptes Rendus L’académie Sci.-Ser. IIC-Chem. 1998, 1, 509–515. [Google Scholar] [CrossRef]
- Johnson, A.D.; Curtis, R.M.; Wallace, K.J. Low molecular weight fluorescent probes (LMFPs) to detect the group 12 metal triad. Chemosensors 2019, 7, 22. [Google Scholar] [CrossRef]
- Mia, R.; Cragg, P.J.; Fronczek, F.R.; Wallace, K.J. Killing two birds with one stone: Phosphorylation by a tabun mimic and subsequent capture of cyanide using a single fluorescent chemodosimeter. New J. Chem. 2022, 46, 21278–21286. [Google Scholar] [CrossRef]
- Mia, R.; Cragg, P.J.; Wallace, K.J. Low molecular weight fluorescent probes for the detection of organophosphates. J. Lumin. 2021, 235, 118053. [Google Scholar] [CrossRef]
- Shim, N.Y.; Bernards, D.A.; Macaya, D.J.; DeFranco, J.A.; Nikolou, M.; Owens, R.M.; Malliaras, G.G. All-plastic electrochemical transistor for glucose sensing using a ferrocene mediator. Sensors 2009, 9, 9896–9902. [Google Scholar] [CrossRef]
- De Silva, A.P.; Moody, T.S.; Wright, G.D. Fluorescent PET (Photoinduced Electron Transfer) sensors as potent analytical tools. Analyst 2009, 134, 2385–2393. [Google Scholar] [CrossRef]
- Program, N.T. NTP toxicology and carcinogenesis studies of dimethyl methylphosphonate (CAS No. 756-79-6) in F344/N Rats and B6C3F1 Mice (Gavage Studies). Natl. Toxicol. Program Tech. Rep. Ser. 1987, 323, 1–172. [Google Scholar]
- Song, X.; Yang, C.; Yuan, R.; Xiang, Y. Electrochemical label-free biomolecular logic gates regulated by distinct inputs. Biosens. Bioelectron. 2022, 202, 114000. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Guo, Q.; Jiang, W.; Zhang, H.; Cai, C. Dual-aptamer-assisted and logic gate for cyclic enzymatic signal amplification electrochemical detection of tumor-derived small extracellular vesicles. Anal. Chem. 2021, 93, 11298–11304. [Google Scholar] [CrossRef]
- Ge, L.; Wang, W.; Sun, X.; Hou, T.; Li, F. Versatile and programmable DNA logic gates on universal and label-free homogeneous electrochemical platform. Anal. Chem. 2016, 88, 9691–9698. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, H.; Hu, N. pH-, sugar-, and temperature-sensitive electrochemical switch amplified by enzymatic reaction and controlled by logic gates based on semi-interpenetrating polymer networks. J. Phys. Chem. B 2012, 116, 1700–1708. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Zuo, X.; Yang, R.; White, R.J.; Xiao, Y.; Kang, D.; Gong, X.; Lubin, A.A.; Vallée-Bélisle, A.; Yuen, J.D. Label-free, dual-analyte electrochemical biosensors: A new class of molecular-electronic logic gates. J. Am. Chem. Soc. 2010, 132, 8557–8559. [Google Scholar] [CrossRef]
- Andrianova, M.; Kuznetsov, A. Logic Gates Based on DNA Aptamers. Pharmaceuticals 2020, 13, 417. [Google Scholar] [CrossRef]
- Goggins, S.; Stark, O.P.; Naz, C.; Marsh, B.J.; Frost, C.G. Ratiometric electrochemical detection of Pd...π interactions: Application towards electrochemical molecular logic gates. Supramol. Chem. 2017, 29, 749–757. [Google Scholar] [CrossRef]
- Jońca, J.; Pawnuk, M.; Bezyk, Y.; Arsen, A.; Sówka, I. Drone-assisted monitoring of atmospheric pollution—A comprehensive review. Sustainability 2022, 14, 11516. [Google Scholar] [CrossRef]
- Beryozkina, S.; Al-Shakhs, N. Real-life application of the emission monitoring system by using a drone. In Proceedings of the 2020 IEEE International Conference on Environment and Electrical Engineering and 2020 IEEE Industrial and Commercial Power Systems Europe (EEEIC/I&CPS Europe), Jammu, India, 17–18 April 2020; pp. 1–6. [Google Scholar]
- Rejeb, A.; Abdollahi, A.; Rejeb, K.; Treiblmaier, H. Drones in agriculture: A review and bibliometric analysis. Comput. Electron. Agric. 2022, 198, 107017. [Google Scholar] [CrossRef]
- Bukin, O.; Proschenko, D.; Korovetskiy, D.; Chekhlenok, A.; Yurchik, V.; Bukin, I. Development of the artificial intelligence and optical sensing methods for oil pollution monitoring of the sea by drones. Appl. Sci. 2021, 11, 3642. [Google Scholar] [CrossRef]
- Chen, W.; Zou, Y.; Mo, W.; Di, D.; Wang, B.; Wu, M.; Huang, Z.; Hu, B. Onsite identification and spatial distribution of air pollutants using a drone-based solid-phase microextraction array coupled with portable gas chromatography-mass spectrometry via continuous-airflow sampling. Environ. Sci. Technol. 2022, 56, 17100–17107. [Google Scholar] [CrossRef] [PubMed]
- Rutkauskas, M.; Asenov, M.; Ramamoorthy, S.; Reid, D.T. Autonomous multi-species environmental gas sensing using drone-based Fourier-transform infrared spectroscopy. Opt. Express 2019, 27, 9578–9587. [Google Scholar] [CrossRef] [PubMed]


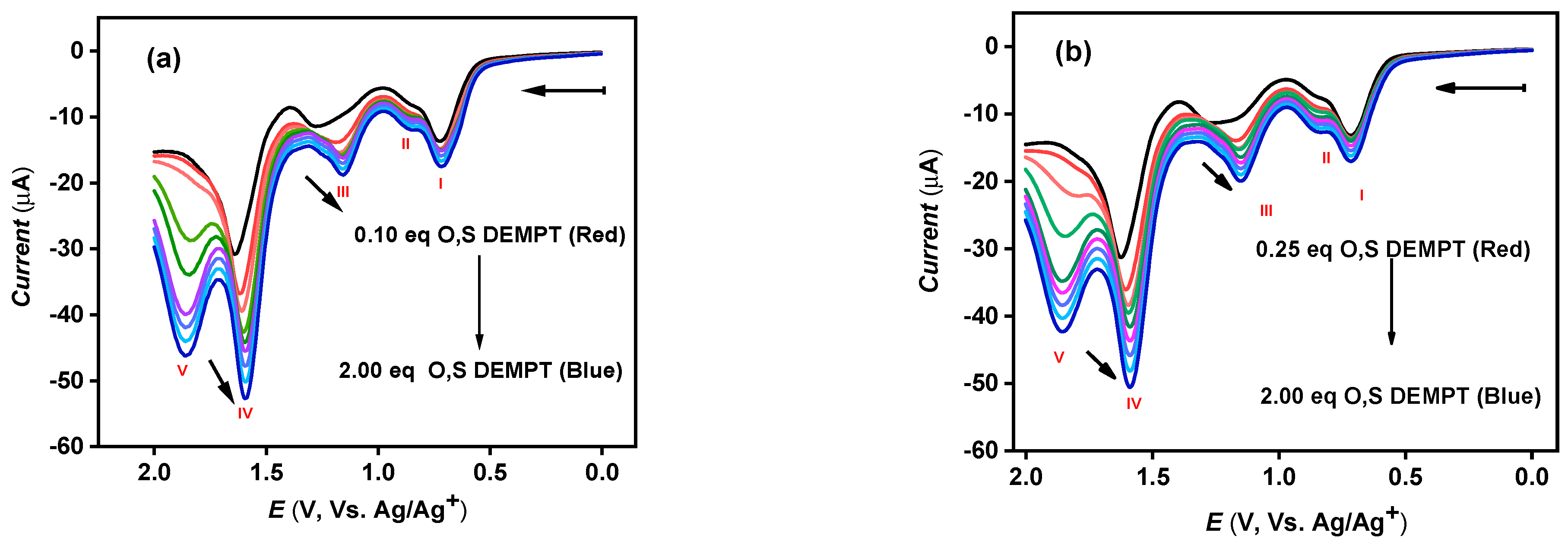
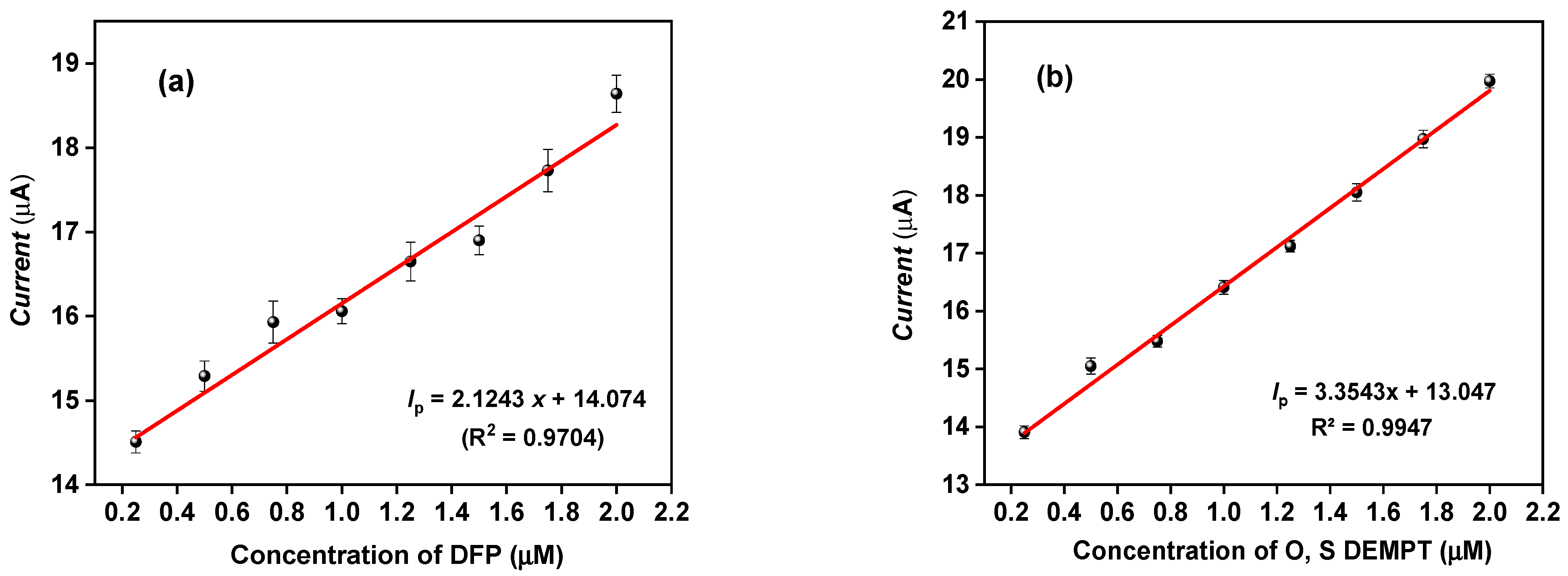
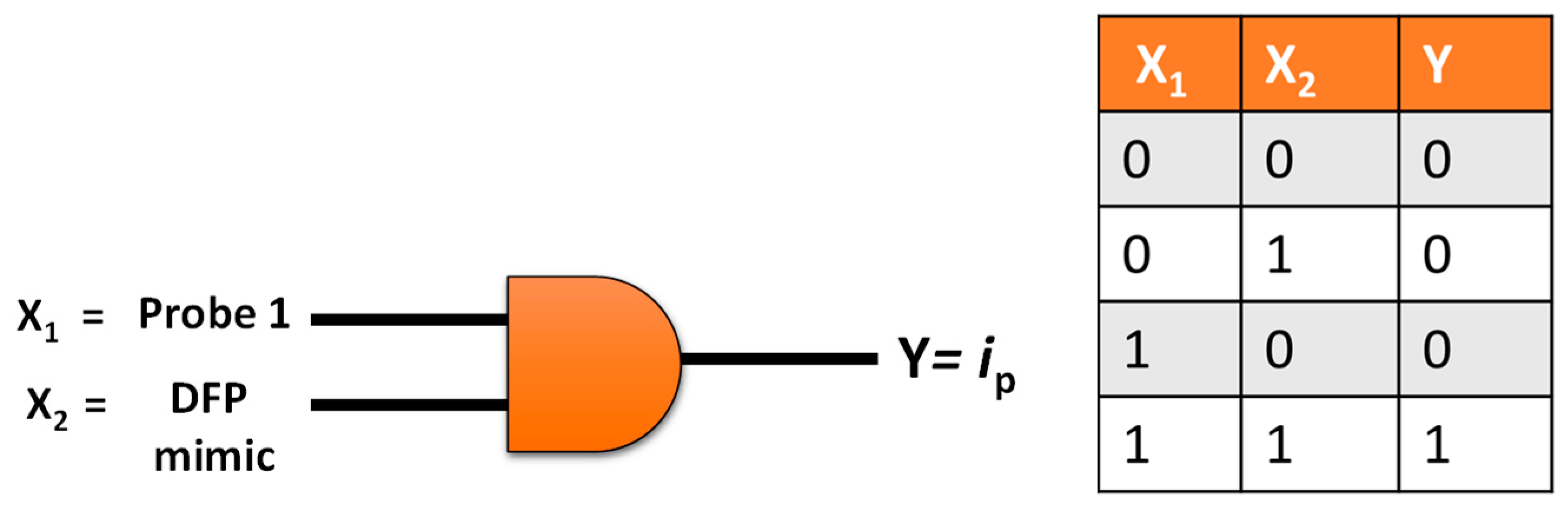
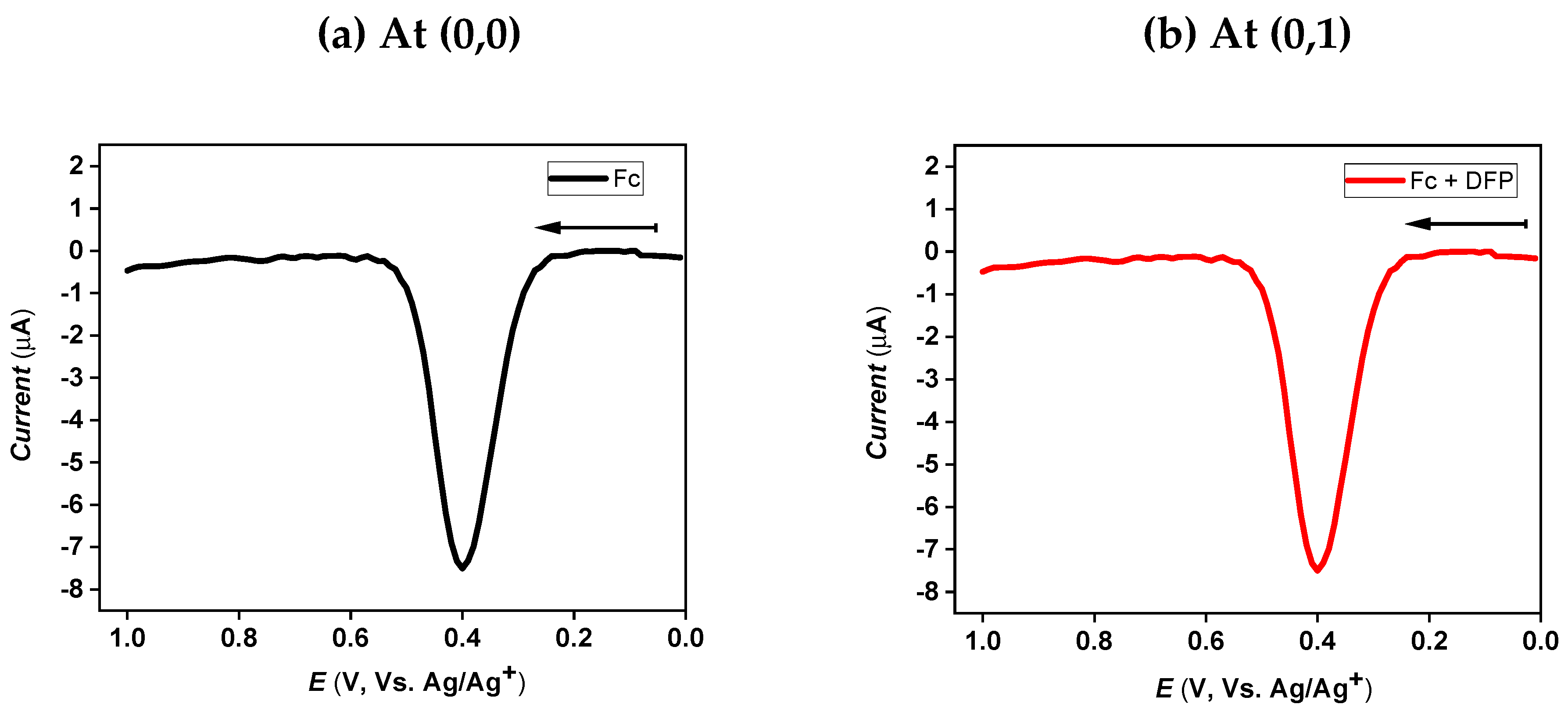

| Instrument | Compound | Eox (V vs. Ag/Ag+) | ||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | ||
| CHI 660A | Probe 1 | 0.72 | 0.86 | 1.29 | 1.64 | |
| DFP | 0.72 | 0.86 | 1.16 | 1.59 | ||
| O,S-DEMPT | 0.72 | 0.86 | 1.16 | 1.60 | 1.86 | |
| EmStat4s HR | Probe 1 | 0.72 | 0.86 | 1.28 | 1.63 | |
| DFP | 0.72 | 0.86 | 1.15 | 1.58 | ||
| O,S-DEMPT | 0.72 | 0.86 | 1.15 | 1.59 | 1.86 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawoud, A.; Mia, R.; Motchaalangaram, J.A.; Miao, W.; Wallace, K. Toward Remote Detection of Chemical Warfare Simulants Using a Miniature Potentiostat. Micro 2024, 4, 49-60. https://doi.org/10.3390/micro4010004
Dawoud A, Mia R, Motchaalangaram JA, Miao W, Wallace K. Toward Remote Detection of Chemical Warfare Simulants Using a Miniature Potentiostat. Micro. 2024; 4(1):49-60. https://doi.org/10.3390/micro4010004
Chicago/Turabian StyleDawoud, Amer, Rashid Mia, Jesy Alka Motchaalangaram, Wujian Miao, and Karl Wallace. 2024. "Toward Remote Detection of Chemical Warfare Simulants Using a Miniature Potentiostat" Micro 4, no. 1: 49-60. https://doi.org/10.3390/micro4010004
APA StyleDawoud, A., Mia, R., Motchaalangaram, J. A., Miao, W., & Wallace, K. (2024). Toward Remote Detection of Chemical Warfare Simulants Using a Miniature Potentiostat. Micro, 4(1), 49-60. https://doi.org/10.3390/micro4010004








