Assessment of Pharmaco-Technological Parameters of Solid Lipid Nanoparticles as Carriers for Sinapic Acid
Abstract
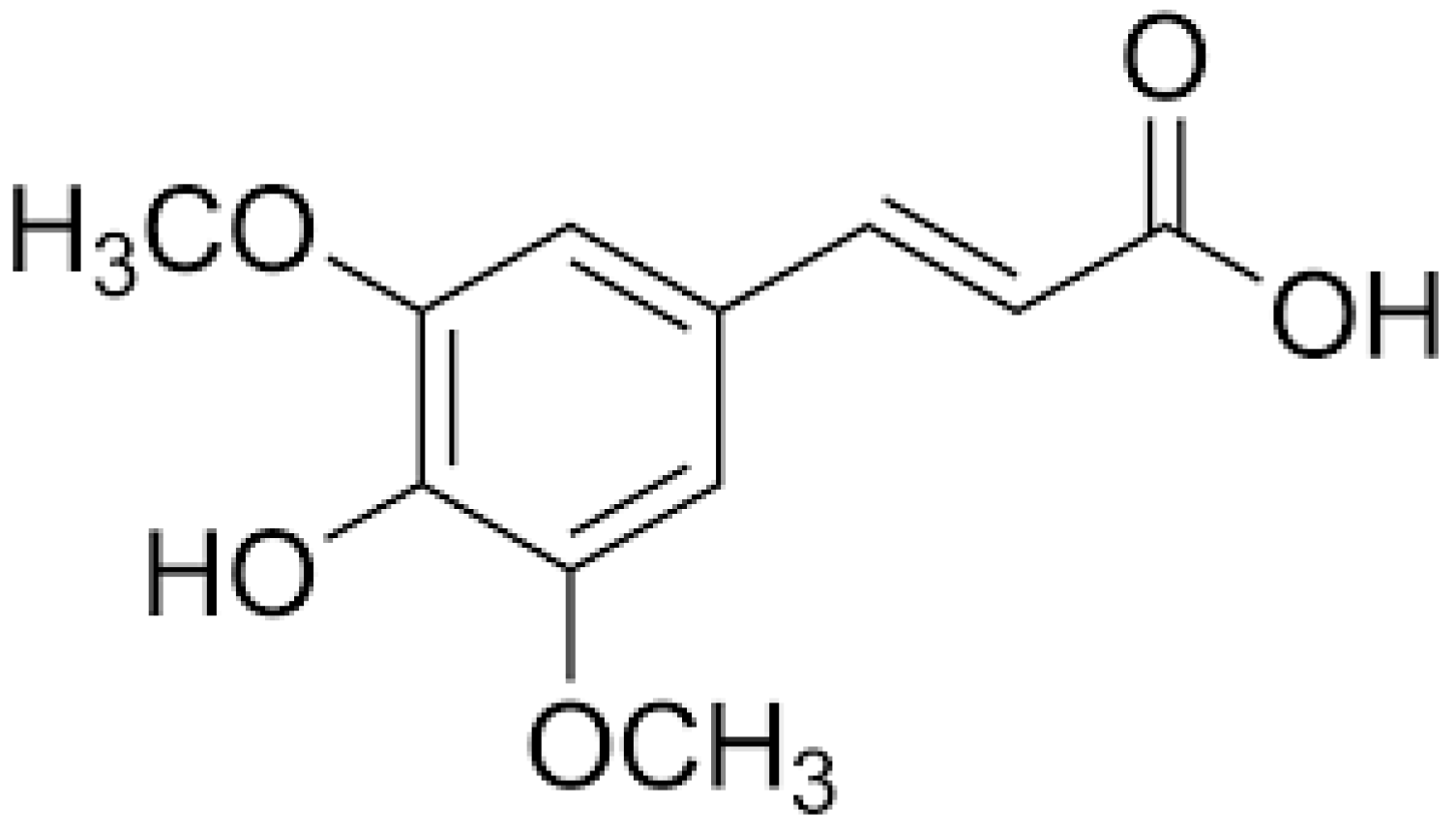
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SLNs
2.3. Characterization of SLNs
2.4. Encapsulation Efficiency
2.5. In Vitro Drug Release
2.6. Preparation of MLV
2.7. DSC Analysis
2.7.1. Calorimetric Analysis of SLNs and MLVs
- Heating from 5 °C to 70 °C, at 2 °C/min.
- Cooling from 70 °C to 5 °C, at 4 °C/min.
2.7.2. SLN/MLV Interaction
2.8. Statistical Analysis
3. Results
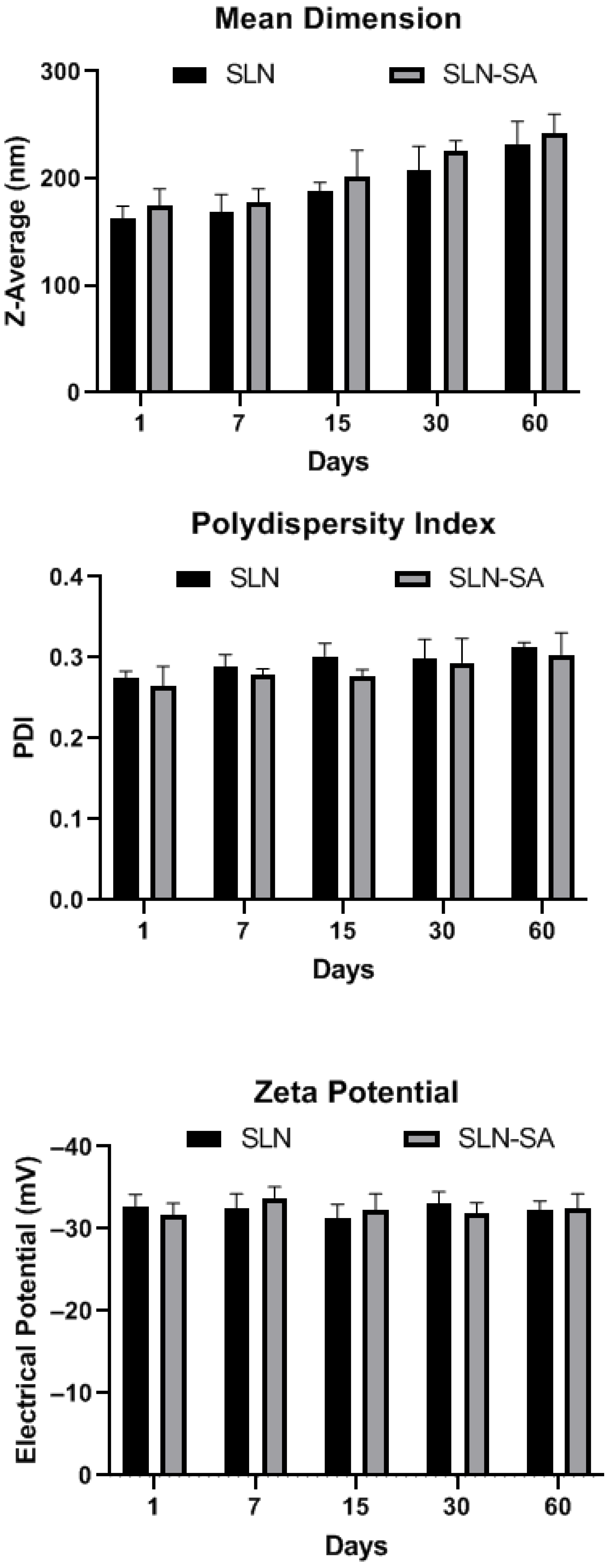
3.1. SLN Characterization
3.2. Entrapment Efficiency
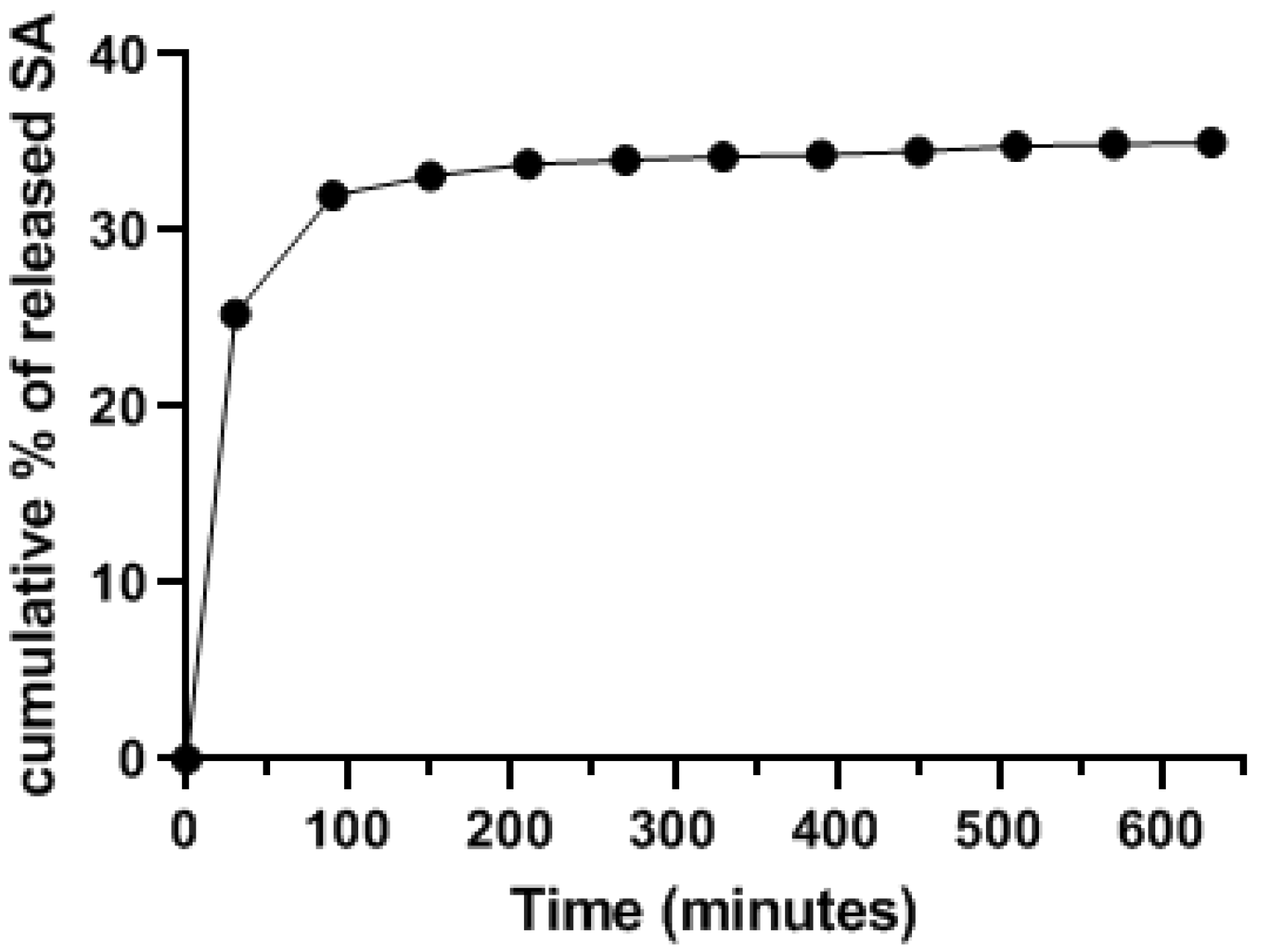
3.3. In Vitro Release Study
3.4. DSC Analysis
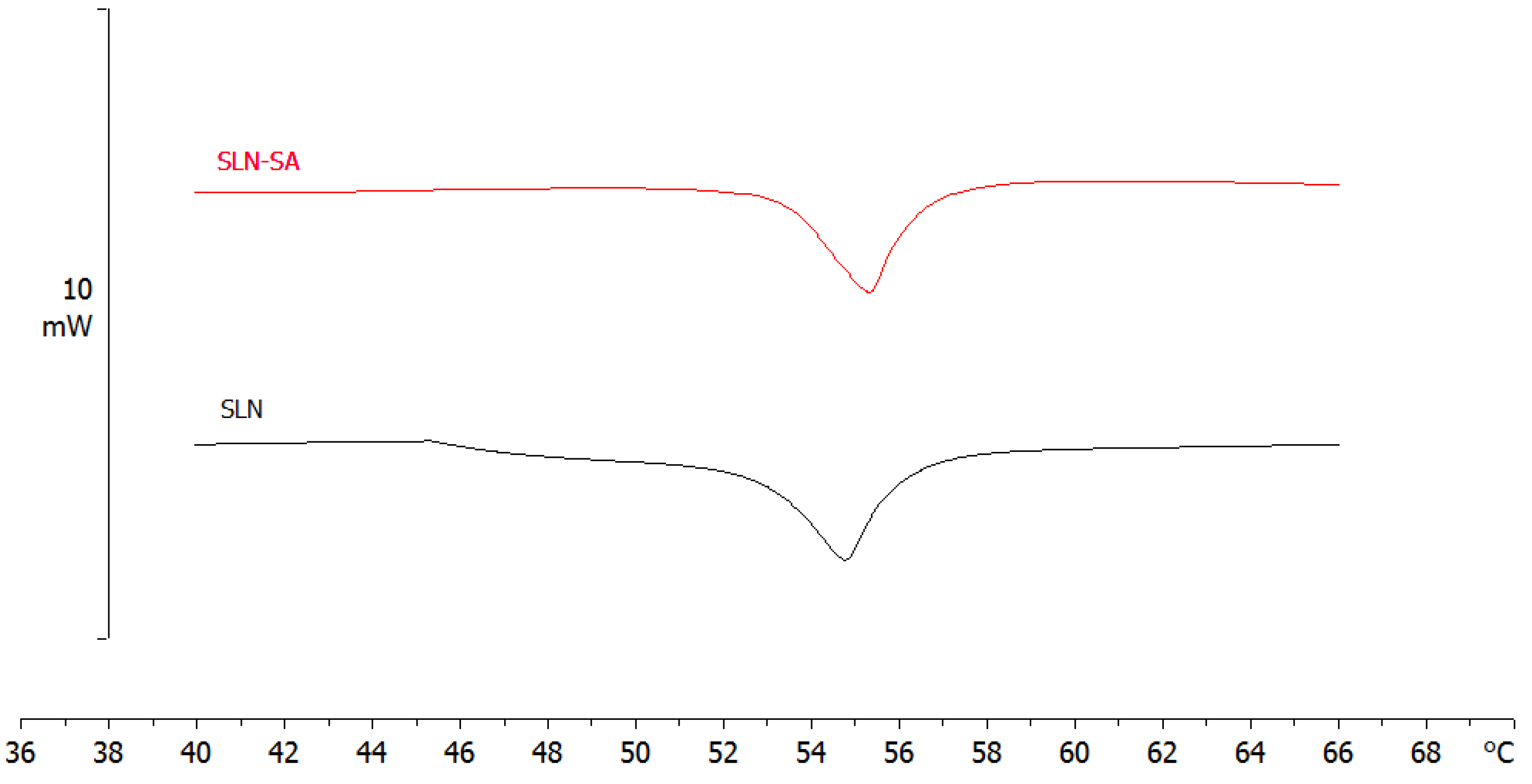
3.4.1. SLN Calorimetric Analysis
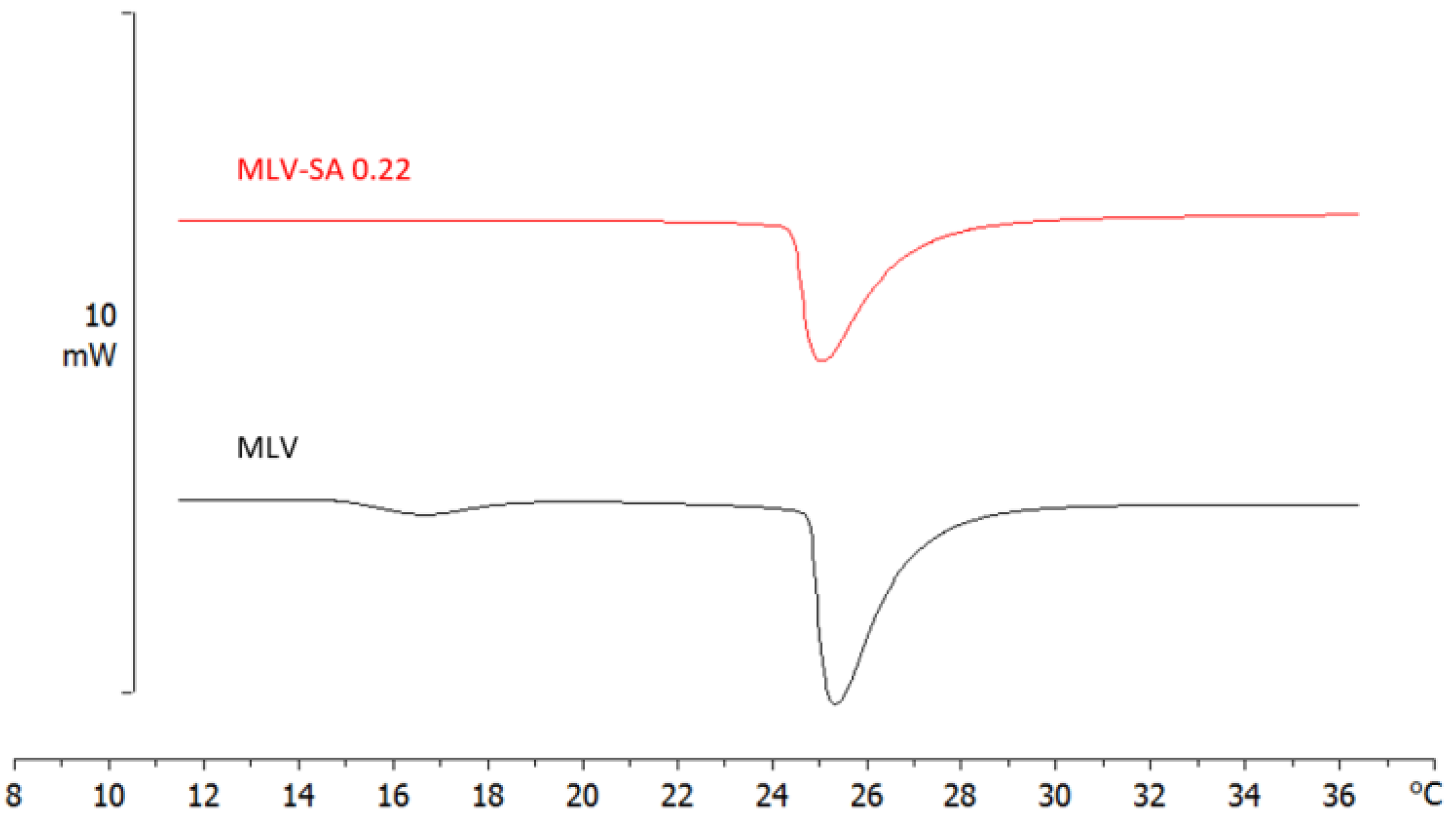
3.4.2. Calorimetric Analysis of MLVs
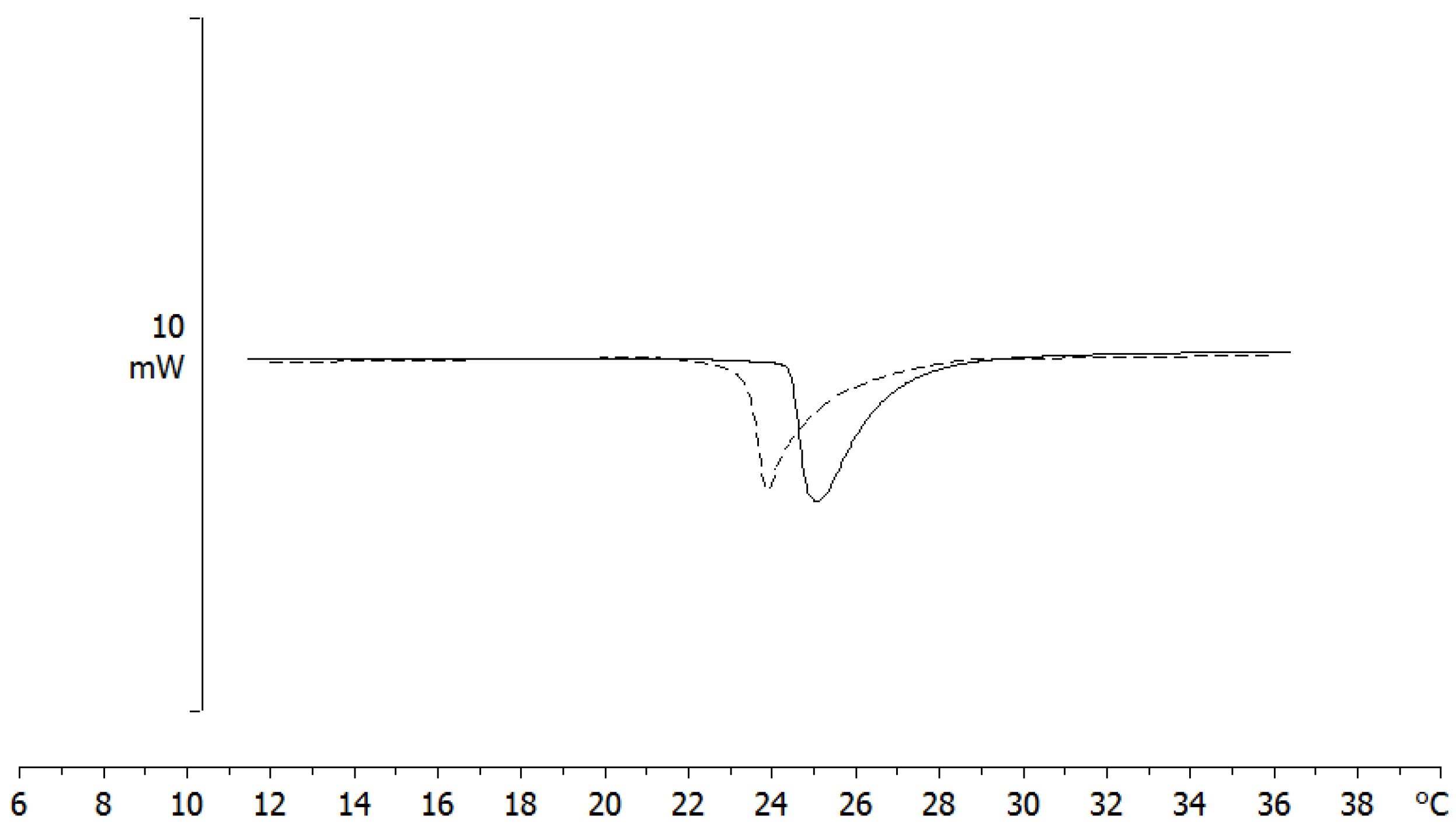
3.4.3. SLN/MLV Interaction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teixeira, J.; Gaspar, A.; Garrido, E.M.; Garrido, J.; Borges, F. Hydroxycinnamic Acid Antioxidants: An Electrochemical Overview. BioMed Res. Int. 2013, 2013, 251754. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant Properties of Ferulic Acid and Its Related Compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef]
- Cos, P.; Rajan, P.; Vedernikova, I.; Calomme, M.; Pieters, L.; Vlietinck, A.J.; Augustyns, K.; Haemers, A.; Berghe, D.V. In Vitro Antioxidant Profile of Phenolic Acid Derivatives. Free. Radic. Res. 2002, 36, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Anticancer Activity of Sinapic Acid by Inducing Apoptosis in HT-29 Human Colon Cancer Cell Line. Available online: https://cdnsciencepub.com/doi/10.1139/cjpp-2022-0523 (accessed on 19 April 2023).
- Rostami, A.; Baluchnejadmojarad, T.; Roghani, M. Sinapic Acid Ameliorates Paracetamol-Induced Acute Liver Injury through Targeting Oxidative Stress and Inflammation. Mol. Biol. Rep. 2022, 49, 4179–4191. [Google Scholar] [CrossRef]
- Stanely Mainzen Prince, P.; Dey, P.; Roy, S.J. Sinapic Acid Safeguards Cardiac Mitochondria from Damage in Isoproterenol-Induced Myocardial Infarcted Rats. J. Biochem. Mol. Toxicol. 2020, 34, e22556. [Google Scholar] [CrossRef] [PubMed]
- Tungalag, T.; Yang, D.K. Sinapic Acid Protects SH-SY5Y Human Neuroblastoma Cells against 6-Hydroxydopamine-Induced Neurotoxicity. Biomedicines 2021, 9, 295. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhao, D.; Lee, S.; Keum, G.; Yang, H.O. Sinapic Acid Attenuates the Neuroinflammatory Response by Targeting AKT and MAPK in LPS-Activated Microglial Models. Biomol. Ther. 2023, 31, 276–284. [Google Scholar] [CrossRef]
- Yoon, B.H.; Jung, J.W.; Lee, J.-J.; Cho, Y.-W.; Jang, C.-G.; Jin, C.; Oh, T.H.; Ryu, J.H. Anxiolytic-like Effects of Sinapic Acid in Mice. Life Sci. 2007, 81, 234–240. [Google Scholar] [CrossRef]
- Maddox, C.E.; Laur, L.M.; Tian, L. Antibacterial Activity of Phenolic Compounds against the Phytopathogen Xylella fastidiosa. Curr. Microbiol. 2010, 60, 53–58. [Google Scholar] [CrossRef]
- Pandi, A.; Kalappan, V.M. Pharmacological and Therapeutic Applications of Sinapic Acid—An Updated Review. Mol. Biol. Rep. 2021, 48, 3733–3745. [Google Scholar] [CrossRef] [PubMed]
- Shivashankara, K.S.; Acharya, S.N. Bioavailability of Dietary Polyphenols and the Cardiovascular Diseases. TONUTRAJ 2010, 3, 227–241. [Google Scholar] [CrossRef]
- Piazzon, A.; Forte, M.; Nardini, M. Characterization of Phenolics Content and Antioxidant Activity of Different Beer Types. J. Agric. Food Chem. 2010, 58, 10677–10683. [Google Scholar] [CrossRef]
- da Silveira, T.F.F.; Cajaíba, L.M.; Valentin, L.; Baréa, B.; Villeneuve, P.; Castro, I.A. Effect of Sinapic Acid Ester Derivatives on the Oxidative Stability of Omega-3 Fatty Acids Rich Oil-in-Water Emulsions. Food Chem. 2020, 309, 125586. [Google Scholar] [CrossRef] [PubMed]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef]
- Wang, J.; Cao, Y.; Sun, B.; Wang, C. Characterisation of Inclusion Complex of Trans-Ferulic Acid and Hydroxypropyl-β-Cyclodextrin. Food Chem. 2011, 124, 1069–1075. [Google Scholar] [CrossRef]
- Demurtas, M.; Onnis, V.; Zucca, P.; Rescigno, A.; Lachowicz, J.I.; De Villiers Engelbrecht, L.; Nieddu, M.; Ennas, G.; Scano, A.; Mocci, F.; et al. Cholinium-Based Ionic Liquids from Hydroxycinnamic Acids as New Promising Bioactive Agents: A Combined Experimental and Theoretical Investigation. ACS Sustain. Chem. Eng. 2021, 9, 2975–2986. [Google Scholar] [CrossRef]
- Sinha, A.S.; Khandavilli, U.B.R.; O’Connor, E.L.; Deadman, B.J.; Maguire, A.R.; Lawrence, S.E. Novel Co-Crystals of the Nutraceutical Sinapic Acid. CrystEngComm 2015, 17, 4832–4841. [Google Scholar] [CrossRef]
- Ahad, A.; Bin Jardan, Y.A.; Raish, M.; Al-Mohizea, A.M.; Al-Jenoobi, F.I. Hydroxypropyl-β-Cyclodextrin for Delivery of Sinapic Acid via Inclusion Complex Prepared by Solvent Evaporation Method. Processes 2022, 10, 2046. [Google Scholar] [CrossRef]
- Kishida, K.; Matsumoto, H. Urinary Excretion Rate and Bioavailability of Chlorogenic Acid, Caffeic Acid, p-Coumaric Acid, and Ferulic Acid in Non-Fasted Rats Maintained under Physiological Conditions. Heliyon 2019, 5, e02708. [Google Scholar] [CrossRef] [PubMed]
- Castelli, F.; Puglia, C.; Sarpietro, M.G.; Rizza, L.; Bonina, F. Characterization of Indomethacin-Loaded Lipid Nanoparticles by Differential Scanning Calorimetry. Int. J. Pharm. 2005, 304, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Lingayat, V.J.; Zarekar, N.S.; Shendge, R.S. Solid Lipid Nanoparticles: A Review. Nanosci. Nanotechnol. Res. 2017, 4, 67–72. [Google Scholar]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid Lipid Nanoparticles: A Modern Formulation Approach in Drug Delivery System. Ind. J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) in Cosmetic and Dermatological Preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef]
- Russo, S.; Torrisi, C.; Cardullo, N.; Muccilli, V.; Mantia, A.L.; Castelli, F.; Acquaviva, R.; Sarpietro, M.G. Ethyl Protocatechuate Encapsulation in Solid Lipid Nanoparticles: Assessment of Pharmacotechnical Parameters and Preliminary In Vitro Evaluation for Colorectal Cancer Treatment. Pharmaceutics 2023, 15, 394. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, C.; Cardullo, N.; Muccilli, V.; Tringali, C.; Castelli, F.; Sarpietro, M.G. Characterization and Interaction with Biomembrane Model of Benzo[k,l]Xanthene Lignan Loaded Solid Lipid Nanoparticles. Membranes 2022, 12, 615. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.P.; Faria, V.; Gonçalves, L.M.D.; Taboada, P.; Remuñán-López, C.; Almeida, A.J. Rifabutin-Loaded Solid Lipid Nanoparticles for Inhaled Antitubercular Therapy: Physicochemical and in Vitro Studies. Int. J. Pharm. 2016, 497, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-X.; Sun, X.; Zhang, Z.-R. Enhanced Brain Targeting by Synthesis of 3′,5′-Dioctanoyl-5-Fluoro-2′-Deoxyuridine and Incorporation into Solid Lipid Nanoparticles. Eur. J. Pharm. Biopharm. 2002, 54, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Venkateswarlu, V.; Manjunath, K. Preparation, Characterization and in Vitro Release Kinetics of Clozapine Solid Lipid Nanoparticles. J. Control. Release 2004, 95, 627–638. [Google Scholar] [CrossRef]
- Greening, D.W.; Simpson, R.J. A Centrifugal Ultrafiltration Strategy for Isolating the Low-Molecular Weight (<or=25K) Component of Human Plasma Proteome. J. Proteom. 2010, 73, 637–648. [Google Scholar] [CrossRef]
- Brito Raj, S.; Chandrasekhar, K.B.; Reddy, K.B. Formulation, in-Vitro and in-Vivo Pharmacokinetic Evaluation of Simvastatin Nanostructured Lipid Carrier Loaded Transdermal Drug Delivery System. Future J. Pharm. Sci. 2019, 5, 9. [Google Scholar] [CrossRef]
- Yadav, S.; Gupta, S. Development and in Vitro Characterization of Docetaxel-Loaded Ligand Appended Solid Fat Nanoemulsions for Potential Use in Breast Cancer Therapy. Artif. Cells Nanomed. Biotechnol. 2015, 43, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, C.; Cardullo, N.; Russo, S.; La Mantia, A.; Acquaviva, R.; Muccilli, V.; Castelli, F.; Sarpietro, M.G. Benzo[k,l]Xanthene Lignan-Loaded Solid Lipid Nanoparticles for Topical Application: A Preliminary Study. Molecules 2022, 27, 5887. [Google Scholar] [CrossRef] [PubMed]
- Walde, P. Preparation of Vesicles (Liposomes). In Encyclopedia of Nanoscience and Nanotechnology; Nalwa, H.S., Ed.; American Scientific Publishers: Los Angeles, CA, USA, 2004; Volume 9. [Google Scholar]
- Ladbrooke, B.D.; Chapman, D. Thermal Analysis of Lipids, Proteins and Biological Membranes. A Review and Summary of Some Recent Studies. Chem. Phys. Lipids 1969, 3, 304–356. [Google Scholar] [CrossRef] [PubMed]
- Cevc, G. Polymorphism of the Bilayer Membranes in the Ordered Phase and the Molecular Origin of the Lipid Pretransition and Rippled Lamellae. Biochim. Biophys. Acta 1991, 1062, 59–69. [Google Scholar] [CrossRef]
- Gardikis, K.; Hatziantoniou, S.; Viras, K.; Wagner, M.; Demetzos, C. A DSC and Raman Spectroscopy Study on the Effect of PAMAM Dendrimer on DPPC Model Lipid Membranes. Int. J. Pharm. 2006, 318, 118–123. [Google Scholar] [CrossRef]
- Basso, L.G.M.; Rodrigues, R.Z.; Naal, R.M.Z.G.; Costa-Filho, A.J. Effects of the Antimalarial Drug Primaquine on the Dynamic Structure of Lipid Model Membranes. Biochim. Biophys. Acta BBA—Biomembr. 2011, 1808, 55–64. [Google Scholar] [CrossRef]
- Lambros, M.P.; Rahman, Y.E. Effects of Cyclosporin A on Model Lipid Membranes. Chem. Phys. Lipids 2004, 131, 63–69. [Google Scholar] [CrossRef]
- Alves, I.D.; Goasdoué, N.; Correia, I.; Aubry, S.; Galanth, C.; Sagan, S.; Lavielle, S.; Chassaing, G. Membrane Interaction and Perturbation Mechanisms Induced by Two Cationic Cell Penetrating Peptides with Distinct Charge Distribution. Biochim. Biophys. Acta 2008, 1780, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Interaction of Dopamine with Zwitterionic DMPC and Anionic DMPS Multilamellar Vesicle Membranes | Langmuir. Available online: https://pubs.acs.org/doi/full/10.1021/acs.langmuir.1c02184 (accessed on 21 March 2023).
- Ezer, N.; Sahin, I.; Kazanci, N. Alliin Interacts with DMPC Model Membranes to Modify the Membrane Dynamics: FTIR and DSC Studies. Vib. Spectrosc. 2017, 89, 1–8. [Google Scholar] [CrossRef]
- Cherng, Y.-G.; Tsai, C.-C.; Chung, H.-H.; Lai, Y.-W.; Kuo, S.-C.; Cheng, J.-T. Antihyperglycemic Action of Sinapic Acid in Diabetic Rats. J. Agric. Food Chem. 2013, 61, 12053–12059. [Google Scholar] [CrossRef]
- Yang, C.; Deng, Q.; Xu, J.; Wang, X.; Hu, C.; Tang, H.; Huang, F. Sinapic Acid and Resveratrol Alleviate Oxidative Stress with Modulation of Gut Microbiota in High-Fat Diet-Fed Rats. Food Res. Int. 2019, 116, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Cassano, R.; Ferrarelli, T.; Barone, E.; Picci, N.; Mancuso, C. Trans-Ferulic Acid-Based Solid Lipid Nanoparticles and Their Antioxidant Effect in Rat Brain Microsomes. Colloids Surf. B Biointerfaces 2013, 109, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Hallan, S.S.; Sguizzato, M.; Drechsler, M.; Mariani, P.; Montesi, L.; Cortesi, R.; Björklund, S.; Ruzgas, T.; Esposito, E. The Potential of Caffeic Acid Lipid Nanoparticulate Systems for Skin Application: In Vitro Assays to Assess Delivery and Antioxidant Effect. Nanomaterials 2021, 11, 171. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, S.; Greco, G.; Sarpietro, M.G. Assessment of Pharmaco-Technological Parameters of Solid Lipid Nanoparticles as Carriers for Sinapic Acid. Micro 2023, 3, 510-520. https://doi.org/10.3390/micro3020034
Russo S, Greco G, Sarpietro MG. Assessment of Pharmaco-Technological Parameters of Solid Lipid Nanoparticles as Carriers for Sinapic Acid. Micro. 2023; 3(2):510-520. https://doi.org/10.3390/micro3020034
Chicago/Turabian StyleRusso, Stefano, Giuliana Greco, and Maria Grazia Sarpietro. 2023. "Assessment of Pharmaco-Technological Parameters of Solid Lipid Nanoparticles as Carriers for Sinapic Acid" Micro 3, no. 2: 510-520. https://doi.org/10.3390/micro3020034
APA StyleRusso, S., Greco, G., & Sarpietro, M. G. (2023). Assessment of Pharmaco-Technological Parameters of Solid Lipid Nanoparticles as Carriers for Sinapic Acid. Micro, 3(2), 510-520. https://doi.org/10.3390/micro3020034





