Abstract
For the first time AFM (atomic-force microscopy) was used to record significant changes in the geometric parameters of the image of erythrocytes in vitro under conditions of glycolytic starvation (ATP (Adenosine triphosphate) deficiency). The difference in the action of antioxidants, phenosan K, and Ihfan-10 on erythrocytes that we detected with AFM seems to be mainly due to their difference in hydrophobicity. We used the AFM method to research the self-organization of the components of the active center of P450 (Porphyrin-450) metalloenzymes that are part of a class of hemoproteins with functions of affinity to molecular oxygen O2. Stable supramolecular nanostructures in the form of triangular prisms based on the iron porphyrin complex with amino acids due to self-assembly involving intermolecular hydrogen bonds were received. A possible scheme for the formation of such structures is proposed.
Keywords:
AFM; erythrocytes; glucose; ATP deficiency; phenolic antioxidants; nanostructures; H-bonds; Hemin; L-Histidine; L-Tyrosine 1. Introduction
The main function of erythrocytes is the transport of oxygen and other substances from the lungs to the tissues of the body, participation in the regulation of acid-base balance, and maintenance of isotonicity of blood and tissues. An insufficient supply of oxygen to the tissues of the bodies of animals and humans, as well as a violation of its utilization in the process of biological oxidation, leads to hypoxia [1,2]. Favism uniquely arises from a genetic defect of the Glucose-6 Phosphate Dehydrogenase (G6PD) enzyme and results in a severe reduction of erythrocytes (RBCs), reducing power that impairs the cells’ ability to respond to oxidative stresses [3]. Atomic-force microscopy (AFM) methods are increasingly being used in biology, medicine, and pharmacology. AFM has advantages compared with optical and electron microscopy in that the atomic-force microscopy method gives a truly three-dimensional relief of the investigated surface, in order to make measurements in various media, which opens up wide opportunities for studying biomolecules and living cells [2]. Flavin and redox-active disulfide domains of ferredoxin-dependent flavin thioredoxin reductase (FFTR) homodimers should pivot between flavin-oxidizing (FO) and flavin-reducing (FR) conformations during catalysis, but only FR conformations have been detected by X-ray diffraction and scattering techniques. Atomic-force microscopy (AFM) is a single-molecule technique that allows the observation of individual biomolecules with sub-nm resolution in near-native conditions in real-time, providing sampling of molecular property distributions and identification of existing subpopulations. In [4] the authors show that AFM is suitable to evaluate FR and FO conformations.
For registration of erythrocytes in the AFM method, various fixation methods are used, which have a number of disadvantages [5,6]. It was noted that when scanning on AFM, erythrocytes fixed with methanol lose their native shape, and membrane distortion occurs; at the same time, scanning air-dried erythrocyte preparations gave clear images of undeformed cells. The preliminary fixation with glutaraldehyde, which was used in AFM studies of erythrocytes, allows fixing of the shape of the erythrocyte.
Currently, the study of phenolic antioxidants and their use for the prevention and treatment of cancer and neurodegenerative and other diseases is relevant in vivo [7]. In this regard, the effect of the antioxidants phenosan K and Ihfan-10 on the erythrocytes of mice was studied for the first time by the AFM statistic method. All experiments in this research were carried out in vitro (in contrast to previous in vivo studies on erythrocytes).
Supramolecular chemistry is a field of research that has grown significantly in recent years. A concept has been developed for research of intermolecular bonds, including coordination bonds, halogen bonds, and hydrogen bonds at molecular organization on surfaces [8]. The formation of supramolecular complexes and the role of hydrogen bonds in the formation of these structures based on metal complexes, which are catalysts for oxidation processes and models of active centers of enzymes, were first studied by AFM [9]. Here we used the AFM method to research the self-organization of the components of the active center of Hem proteins and of the P450 metalloenzymes that are part of a class of hemoproteins, with functions to bind molecular oxygen O2 and transport it in the form of oxyhemoglobin throughout the body to organs and tissues, or to activate O2.
2. Material and Methods
2.1. AFM Research of Erythrocytes
Sample Preparation of Erythrocytes and Instrumentation
When carrying out the in vitro experiments, the studied heparin erythrocytes of mice were incubated in PBS buffer with 10 mM glucose for 1 h with various concentrations of antioxidant at 37 °C. Then the erythrocytes were fixed on a substrate with 2% glutaraldehyde for 30 min and washed three times with distilled water.
In the AFM study, we used the scanning probe AFM microscope SOLVER P47 SMENA10 (Adm. Distr. Zelenograd of Moscow, Russia) at a frequency of 150 kHz, using an NSG30 cantilever with a radius of curvature of 10 nm, a resonance frequency of 300 kHz, and a force constant of 22–100 N/m in the tapping mode. Polished quartz with a roughness Ra = 6 nm was used as a substrate.
When this cantilever passes over the surface of fixed erythrocytes in the tapping mode, it does not leave any traces of passage on the surface. The survey scan range is 30 × 30 μm (the step was 117 nm); a range of 15 × 15 μm was used to obtain the parameters of individual erythrocytes (the step was 59 nm). To obtain the parameters of 100 erythrocytes, several scans were performed in different areas of the substrate.
The resulting scans in the form of mdb files were processed using an Image Analysis program (NT MDT) to obtain the necessary parameters (area, volume, and average height).
2.2. AFM Studies of Model Porphyrin Complexes
AFM studies of supramolecular structures were carried out on similar equipment. We used an NSG30_SS cantilever (NanosensorsTM Advanced TecTM AFM probes, CH-2000 Neuchatel, Switzerland) with a radius of curvature of 2 nm, a resonance frequency of 300 kHz, and a force constant of 22–100 N/m in the tapping mode. To form nanostructures on the surface of a silicon substrate, the sample was prepared using a spin-coating process from an aqueous-alcohol solution of the mixture {Hem + Tyr + His} (Hem = hemin, Tyr = L-tyrosine, His = L-histidine) with a molar ratio of 1:1:1. Self-assembly of supramolecular structures is due to intermolecular interactions with a certain coordination of these interactions, which may be a consequence of the properties of the components themselves, the participation of hydrogen bonds and other non-covalent interactions, as well as the balance of the interaction of these components with the surface.
3. Results and Discussion
3.1. Imaging Erythrocyte Shape Using the AFM Method
It was noted in [10] that fixation of erythrocytes with glutaraldehyde in the AFM method is best suited for fine topographical imaging. In addition, the work notes that the presence of adenosine triphosphate (ATP) in the buffer is very important for maintaining the shape of the erythrocyte in the form of a discocyte and also has a significant effect on the deformability of erythrocytes. Without ATP, erythrocytes lose their biconcave shape and become precursors of echinocytes. Membrane fluctuations are observed that change the mechanical properties of erythrocytes, which suggests metabolic remodeling of the lipid part of the membranes and the structure of the spectrinal network.
Depending on the environmental conditions, the shape of the erythrocyte can be distorted, and certain parameters in the architectonics of erythrocytes change. Moreover, although the volume of an erythrocyte is a dynamic value, depending on the ratio of the concentrations of many substances on both sides of the membrane, it is strictly controlled by a complex ion-transport system of ion pumps and channels in the membrane, which allows maintaining the shape of the erythrocyte [11].
In Figure 1, we show the AFM image of a mouse erythrocyte, which in blood usually has the shape of a discocyte (a), and a typical scanning area of 30 × 30 μm2 (b).
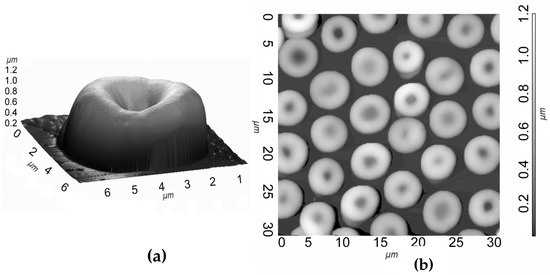
Figure 1.
Three-dimensional AFM image of a single erythrocyte on a silicon substrate fixed with glutaraldehyde (a) and two-dimensional area (30 × 30 μm2) AFM image of erythrocytes (b).
Erythrocytes adsorb glucose, proteins, lipids, and amino acids from blood plasma and transport them to tissues. Since glucose is the main indicator of carbohydrate metabolism, i.e., the body’s primary source of energy, red blood cells are completely dependent on glucose levels for their consumers. The concentration of glucose in the body plays a leading role in energy metabolism, and its maintenance at the proper level is essential and decisive for vitality [1].
We used the AFM method for detection of changes in the functional state of erythrocytes. Previously, a number of important structural changes that occur with the participation of ATP were indicated. Therefore, in our work, the task was set to check the possibility of the AFM method and the technique of fixation with glutaraldehyde in the study of morphological changes that occur in erythrocytes in the presence and absence of a substrate necessary for glycolysis, the main source of ATP in erythrocytes in the in vitro system. For this, erythrocytes were incubated in a standard phosphate-buffered saline for 1–4 h in the presence of 10 mM glucose and without it, i.e., with glycolytic starvation of erythrocytes.
Statistical processing of the AFM images of these preparations showed that the distribution of the volume of erythrocytes was close to normal, both in the presence of glucose and without it. Incubation of erythrocytes for an hour in a buffer with and without glucose insignificantly affected the size of erythrocytes. However, incubation in a buffer containing no glucose for 4 h led to a significant decrease in the dimensional parameters of the AFM image of erythrocytes. The results are shown in Figure 2 and Figure 3.
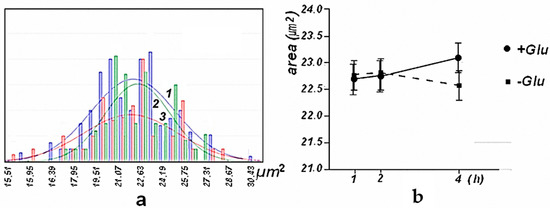
Figure 2.
Histogram of the distribution of the area of the AFM image of the erythrocytes of mice after their incubation 1 h (“1”-blue), 2 h (“2”-green), and 3 h (“3”-red) in the absence of glucose and approximation by a normal distribution–(a) Changes in the area of the AFM image of erythrocytes (mean) of mice during their incubation in the presence of glucose ● (+) and glucose absence ■ (−). A 95% confidence interval is shown-(b).
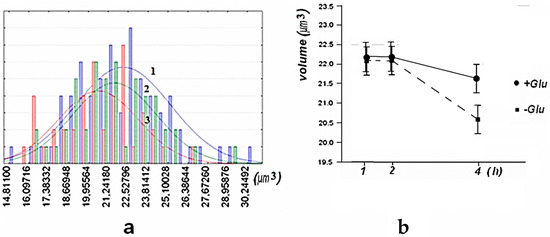
Figure 3.
Histograms of the distribution of the volumes of the AFM image of the erythrocytes of mice after their incubation 1 h: blue–“1”, 2 h: green–“2” and 3 h: red–“3” in the absence of glucose and approximation by normal distribution-(a) Changes in the volume of the AFM image of erythrocytes (average) of mice during their incubation in the presence of glucose ● (+) and its absence ■ (−). A 95% confidence interval is shown-(b).
Histograms of the distribution of the average height of the AFM image and the perimeter of the AFM image of mouse erythrocytes, under conditions similar to those shown in Figure 3, have similar distribution characteristics, and the estimate of the change in mean height has a 95% confidence interval.
From these results, it follows that AFM is used to record significant changes in the geometric parameters of the image of erythrocytes under conditions of glycolytic starvation (ATP deficiency). The data on the effect of glucose concentration on the state of the erythrocyte (SEM method) [12] do not contradict our results.
Indeed, the lack of ATP leads to changes in the operation of ATP-dependent ion pumps and a change in the osmotic state of the cell and a decrease in all measured parameters of the AFM image of the erythrocyte.
Taking into account the obtained above results, experiments on the influence of biologically active compounds on morphology erythrocytes were carried out in a buffer containing 10 mM glucose when incubating erythrocytes for 1 h.
3.2. AFM Research of the Effects of Phenolic Antioxidants
The effect of the antioxidants phenosan K and Ihfan-10 on the erythrocytes of mice were studied using the tapping mode of the AFM method (Figure 4).
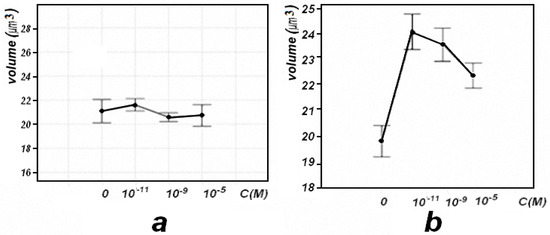
Figure 4.
Changes in the volume of AFM images of mouse red blood cells when phenosan K–(a), and Ihfan-10–(b), were injected into a suspension of red blood cells in a phosphate-salt buffer (in vitro), depending on its concentration. A 95% confidence interval is shown.
With the introduction of phenosan K into the suspension of erythrocytes in vitro, insignificant changes in the volumetric parameters of AFM images of erythrocytes were observed (Figure 4a). These facts indicate that there are no effects on the osmotic state of erythrocytes. With the introduction of Ihfan-10 into the suspension of erythrocytes in the in vitro system, starting from a low concentration of 10−11 M, an increase in the volume of erythrocytes was observed (Figure 4b). The data on changes in the architectonics of erythrocytes with the introduction of Ihfan-10 in vitro do not contradict the data obtained by electron microscopy and spin labels. For example, it was previously shown that Ihfan-10 in vitro, at a concentration of 10−4 M, causes a change in the shape of discocytes and, within a certain time (hours), converts them into the form of stomatocytes and echinocytes [13,14].
Such a difference in the action of phenosan K and Ihfan-10 may be explained next. Infan-10 can penetrate the lipid components of the membrane and affect the membrane-bound enzymes that regulate water metabolism. It is possible that the effect of Ihfan-10 on these enzymes in low concentrations is due to exposure through lipid or peptide rafts. Ihfan-10 is capable of being fixed on the surface of the erythrocyte membrane and partially penetrates into the lipid part of the membrane [13], changing the osmotic state and volume of the cell.
3.3. AFM Research of Model Porphyrin Complexes
Iron porphyrins are in hemoglobin, which is part of red blood cells and in plasma in free form. The main function of hemoglobin is to bind molecular oxygen and transport it in the form of oxyhemoglobin throughout the body to organs and tissues.
The family of Cytochrome P450-dependent monooxygenases is part of a class of hemoproteins with extremely diverse functions. The importance of H-bonds for coordination of molecular oxygen O2 and activation of O2 by P450 metalloenzymes has been well-studied [15].
We propose to use the AFM method [9] to study the possibility of the formation of supramolecular structures, as well as the role of intermolecular hydrogen bonds (and other non-covalent interactions) in the mechanisms of enzymatic catalysis by heteroligand complexes of nickel and iron, simulating the active centers of enzymes [15].
The coordination sphere of the active centers of proteins plays a considerable role in determining the properties of metallic cofactors. The tyrosine residues adjacent to the C termini of the hemoglobin (Hb) subunits, αY140 and βY145, are expected to play important structural roles, because the C termini are the loci of T-state quaternary salt-bridges, and because the tyrosine side-chains bridge the H and F helices due to H bonds to the αV93 and βV98 carbonyl groups. These roles have been investigated via measurements of oxygen binding, 1H NMR spectra, resonance Raman (RR) spectra, and time-resolved resonance Raman (TR3) spectra on site mutants in which the H• • •F• • •H bonds are eliminated by replacing the tyrosine residues with phenylalanine. The TR3 spectra confirm the hypothesis, based on TR3 studies of wild-type Hb, that the H• • •F• • •H bonds break and then re-form during the sub-microsecond phase of the R–T quaternary transition [16].
In nature, various types of binding of amino acids to metalloporphyrins are observed, and Tyr (tyrosine) is able to form hydrogen bonds with His (histidine) [17,18,19].
Based on published data on the regulatory role of His and Tyr fragments, as well as porphyrins in the functioning of enzymes (Hem proteins) of the P450 family [20,21,22], one could expect self-organization of nanostructures of these complexes. Porphyrin linkage through H-bonds is the binding type generally observed in nature. One of the simplest artificial self-assembling supramolecular porphyrin systems is the formation of a dimer based on carboxylic acid functionality [17]. The fact of the formation of such supramolecular structures can be used to analyze non-covalent molecular interactions [20,21] involved in the mechanism of enzymatic catalysis.
The AFM method was used for the first time to study the formation of supramolecular structures, based on model porphyrin metal complexes with amino acids tyrosine and histidine, which are part of the active centers of enzymes: in particular, the Hem proteins family [9].
Figure 5a,b shows that the AFM image of stable nanostructures of model systems {Hem + Tyr + His} (Hem = Hemin, Tyr = L-tyrosine, and His = Histidine) has a form of triangular prisms. These structures are apparently formed due to the coordination of intermolecular interactions of the components of the {Hem·Tyr·His} complex during spontaneous self-organization with the participation of mainly hydrogen bonds and possibly other intermolecular non-covalent interactions.
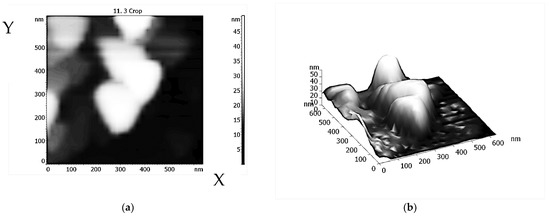
Figure 5.
AFM two-(a) and three-dimensional (b) image of stable nanostructures based on model {Hem·Tyr·His} complexes.
One of the possible schemes for the formation of triangular complexes based on the {Hem + Tyr + His} system (and then prisms) due to H-bonds (NH•••O or N•••HO) [15,16,17,18] can be seen in Figure 6a,b. The combination of individual triangles ({Hem·Tyr·His} complex, Figure 6a) into triangular structures like Sierpinski triangular motifs [23] (Figure 6b) due to H-bonds, and then π–π intermolecular interplanar interactions, can lead to the formation of triangular prisms (Figure 5).
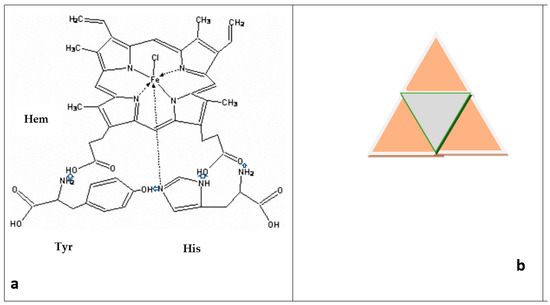
Figure 6.
One of the possible triangular structures of {Hem·Tyr·His} complex that is formed as result of intermolecular interaction due to H-bonds NH•••O or N•••HO (marked with blue arrows) (a). The combination of individual triangles into structures like Sierpinski triangular motifs (b).
4. Conclusions
We used AFM to record significant changes in the geometric parameters of the image of erythrocytes under conditions of glycolytic starvation (ATP deficiency). We established that the difference in the action of antioxidants phenosan K and Ihfan-10 on erythrocytes might be mainly due to their difference in hydrophobicity. Ihfan-10 is capable not only of being fixed on the surface of the erythrocyte membrane, but also partially penetrating into the lipid part of the membrane, changing the osmotic state and volume of the cell.
Using the more sensitive AFM method, it is possible to observe the self-organization of systems {Hem·Tyr·His} (Hem = Hemin, Tyr = L-Tyrosine, His = L-Histidine) into stable nanostructures in a form of triangular prisms due to H-bonds and possibly other non-covalent interactions. A possible scheme for the formation of such structures is proposed. The received data are considered as a step towards understanding the regulator activity of Tyr and His in relation to the active sites of Hem proteins that have affinity to molecular oxygen O2—to bind O2 and transport O2 in the form of oxyhemoglobin to organs and to tissues of the body, or to activate O2.
Author Contributions
L.M.: conceptualization, methodology, experiences investigation, writing—original draft preparation, review, and editing; V.B.: conceptualization, methodology, experiences investigation, writing, resources, validation, and formal analysis; E.M.: conceptualization, methodology, writing, software, and investigation; A.A.: investigation resources and visualization; A.G.: data curation and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
Our research “Using new approaches (Atomic Force Microscopy, AFM) to study the role of supramolecular structures in enzymatic catalysis” received financial support from the Russian Federation, and the Presidium of the Russian Academy of Sciences RAS 14P, State Register Number: AAAA-A17-117121920169-0.
Institutional Review Board Statement
The study was performed in accordance with the Rules of laboratory practice in the Russian Federation, in accordance with the rules adopted by the European Convention for the protection of vertebrate animals used for experimental and other scientific purposes (European Convention for the Protection of Vertebrate Animals Used for Experimental and other Scientific Purposes (ETS 123), Strasburg, 1986). The research was performed in accordance with the approved protocol and standard operating procedures of the researcher (SOPR), as well as with the Guidelines at laboratory animals and alternative models in biomedical researches on laboratory animals [Karkishchenko N.N.; Grachevoy S.V. A guide to laboratory animals and alternative models in biomedical research. Profile: Moscow, 2010]. No animals/humans were used for studies that are the basis of this research.
Informed Consent Statement
Not applicable.
Data Availability Statement
All of the experimental data presented belong to the authors of this manuscript and are available.
Acknowledgments
To A.V. Lobanov for the reagents provided and participation in the discussions.
Conflicts of Interest
The authors declare no conflict of interest, financial or otherwise.
Abbreviations
| AFM method | Atomic-force microscopy method |
| ATP | Adenosine triphosphate |
| Glu | glutamic acid residue |
| Hem | Hemin |
| His | L-Histidine |
| Ihfan-10 | 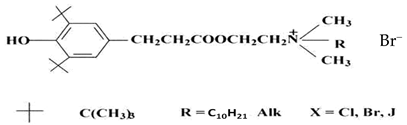 |
| PBS | Phosphate buffered saline–buffer solution of salts, NaCl, Na2HPO4, KCl, and KH2PO4, used in biological research. The osmolarity and concentrations of ions in the solution usually correspond to concentrations in the human body (i.e., this buffer solution is isotonic). |
| Phenosan K | Phenozan potassium–potassium compound based on synthetic phenolic antioxidant β-(4-hydroxy-3,5-di-tert-butylphenyl) propionic acid |
| P450 | Porphyrin-450 |
| Tyr | L-Tyrosine |
References
- Kankozha, M.K. Erythrocyte glucose transport during experimental hypoxia. In Proceedings of the Materials of the International Scientific and Practical Conference “Fundamental Biomedical Sciences and Practical Health Care”, St. Petersburg, Russia, 22–23 April 2010; Available online: http://www.rusnauka.com/12_ENXXI_2010/Medecine/64619.doc.htm (accessed on 1 January 2019).
- Skorkina, M.Y.; Fedorova, M.Z.; Muravyov, A.V.; Sladkova, E.A. The use of nanomechanical sensor for studying the morphofunctional properties of lymphocytes from healthy donors and patients with chronic lymphoblastic leukemia. Cell Technol. Biol. Med. 2012, 154, 172–176. (In Russian) [Google Scholar]
- Dinarelli, S.; Longo, G.; Germanova-Taneva, S.; Todinova, S.; Krumova, S.; Girasole, M. Surprising Structural and Functional Properties of Favism Erythrocytes Are Linked to Special Metabolic Regulation: A Cell Aging Study. Int. J. Mol. Sci. 2022, 24, 637. [Google Scholar] [CrossRef] [PubMed]
- Marcuello, C.; Frempong, G.A.; Balsera, M.; Medina, M.; Lostao, A. Atomic Force Microscopy to Elicit Conformational Transitions of Ferredoxin-Dependent Flavin Thioredoxin Reductases. Antioxidants 2021, 10, 1437. [Google Scholar] [CrossRef] [PubMed]
- Panyusheva, E.S.; Bodryagina, A.M.; Sonina, M.V.; Ivanova, I.A.; Stolbovskaya, O.V. Investigation of the Structural and Functional State of Erythrocytes by Atomic Force Spectroscopy. Available online: www.scienceforum.ru/2013/pdf/3697.pdf (accessed on 1 January 2019).
- Sabanova, R.K. Seasonal changes in hematological parameters in rodents, reflecting their adaptive capabilities. Agric. Biol. 2008, 43, 117–119. [Google Scholar]
- Binyukov, V.I.; Alekseeva, O.M.; Mil, E.M.; Albantova, A.A.; Fattakhov, S.G.; Goloshchapov, A.N.; Burlakova, E.B.; Konovalov, A.I. Research of influence of PHENOSAN, Ihfan-10, MELAPHEN on erythrocytes (in vivo) by Atomic-Force Microscopy. Dokl. Biochem. Biophys. 2011, 441, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Slater, A.G.; Perdigão, L.M.A.; Beton, P.H.; Champness, N.R. Surface-Based Supramolecular Chemistry Using Hydrogen Bonds. Accounts Chem. Res. 2014, 47, 3417–3427. [Google Scholar] [CrossRef] [PubMed]
- Matienko, L.I.; Mil, E.M.; Binyukov, V.I. AFM Research of Supramolecular Structures. Russ. J. Phys. Chem. B 2020, 14, 559–563. [Google Scholar] [CrossRef]
- Zachee, P.; Snauwaert, J.; Vandenberghe, P.; Hellemans, L.; Boogaerts, M. Imaging red blood cells with the atomic force microscope. Br. J. Haematol. 1996, 95, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Ataullakhanov, F.I.; Korunova, N.O.; Spiridonov, I.S.; Pivovarov, I.O.; Kalyagina, N.V.; Martynov, M.V. How erythrocyte volume is regulated, or what mathematical models can and cannot do for biology. Biochem. Moscow Suppl. Ser. A 2009, 3, 101–115. [Google Scholar] [CrossRef]
- Loyola-Leyva, A.; Alcántara-Quintana, L.E.; Terán-Figueroa, Y.; González, F.J. In vitro effect of high glucose concentrations on erythrocyte morphology assessed by scanning electron microscopy. Micron 2022, 154, 103179. [Google Scholar] [CrossRef] [PubMed]
- Parshina, E.Y.; Gendel, L.Y.; Rubin, A.B. Effect of Hybrid Antioxidants—IHFAN-10-on the Surface Architectonics of Eritrocytes. In Chemical and Biochemical Reactions; Nova Science Publ. Inc.: New York, NY, USA, 2011; pp. 71–78. [Google Scholar]
- Panin, L.E.; Mokrushnikov, P.V.; Kunitsyn, V.G.; Zaitsev, B.N. Interaction Mechanism of Cortisol and Catecholamines with Structural Components of Erythrocyte Membranes. J. Phys. Chem. B 2010, 114, 9462–9473. [Google Scholar] [CrossRef] [PubMed]
- Matienko, L.I.; Mosolova, L.A.; Zaikov, G.E. Selective Catalytic Hydrocarbons Oxidation: New Perspectives; Nova Science Publ. Inc.: New York, NY, USA, 2010; 150p. [Google Scholar]
- Kneipp, J.; Balakrishnan, G.; Chen, R.; Shen, T.J.; Sahu, S.C.; Ho, N.T.; Giovannelli, J.L.; Simplaceanu, V.; Ho, C.; Spiro, T. Dynamics of allostery in hemoglobin: Roles of the penultimate tyrosine H bonds. J. Mol. Biol. 2005, 356, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Beletskaya, I.; Tyurin, V.S.; Tsivadze, A.Y.; Guilard, R.R.; Stem, C. Supramolecular Chemistry of Metalloporphyrins. Chem. Rev. 2009, 109, 1659–1713. [Google Scholar] [CrossRef] [PubMed]
- Murry, D.T.; Tysko, R. Side Chain Hydrogen-Bonding Interactions within Amiloid-like Fibrils Formed by the Low-Complexity Domain of FUS: Evidence from Solid State Nuclear Magnetic Resonance Spectroscopy. Biochemistry 2020, 59, 304–378. [Google Scholar]
- Matienko, L.I.; Binyukov, V.I.; Mil, E.M.; Mosolova, L.A.; Zaikov, G.E. Application of the AFM method to studying the role of Supramolecular structures and Tyr-fragment in the mechanism of Ni(Fe)ARD action on model systems. Oxid. Commun. 2018, 41, 429–440. [Google Scholar]
- Biedermann, F.; Schneider, H.-J. Experimental Binding Energies in Supramolecular Complexes. Chem. Rev. 2016, 116, 5216–5300. [Google Scholar] [CrossRef] [PubMed]
- Basom, E.J.; Bryce, A.M.; Thielges, M.C. Conformational Heterogeneity and the Affinity of Substrate Molecular Recognition by Cytochrome P450cam. Biochemistry 2017, 56, 3248–3256. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R. Molecular level structural studies of metalloproteins/metalloenzymes by scanning tunneling microscopy; Scopes and promises. Curr. Sci. 2003, 84, 1202–1210. [Google Scholar]
- Sarkar, R.; Xie, T.-Z.; Endres, K.J.; Wang, Z.; Moorefield, C.N.; Saunders, M.J.; Ghorai, S.; Patri, A.K.; Wesdemiotis, C.; Dobrynin, A.V.; et al. Sierpinski Pyramids by Molecular Entanglement. J. Am. Chem. Soc. 2020, 142, 5526–5530. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).