Clove Essential Oil–Hydroxypropyl-β-Cyclodextrin Inclusion Complexes: Preparation, Characterization and Incorporation in Biodegradable Chitosan Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Clove Essential Oil (CEO)
2.3. Preparation of HP-β-CD-CEO Inclusion Complexes
2.4. Preparation of Chitosan Films
2.5. GC-MS Analysis of Clove Oil
2.6. CEO Inclusion Efficiency and Drug Loading
2.7. Characterization
2.7.1. FT-IR Spectroscopy
2.7.2. Dynamic Light Scattering (DLS)
2.7.3. Thermogravimetric Analysis (TGA)
2.7.4. Mechanical Properties of Films
2.8. Release Studies
2.9. DPPH Radical Scavenging Ability
3. Results and Discussion
3.1. Composition of the CEO
3.2. Size, Size Distribution and Zeta-Potential (ζ-Potential)
3.3. Inclusion Efficiency and Drug Loading of CEO in the CEO-HP-β-CD Complexes
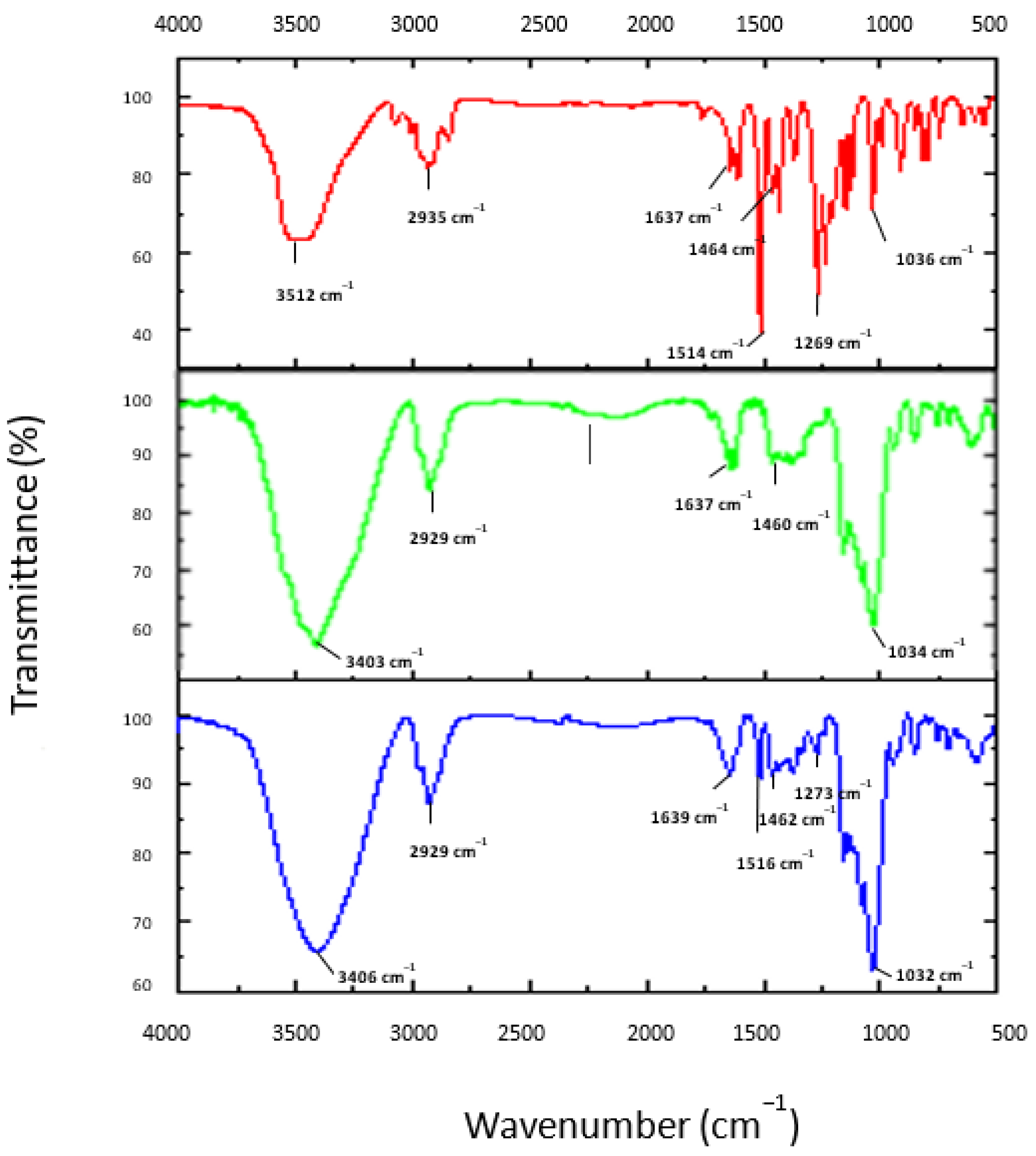
3.4. FT-IR Analysis
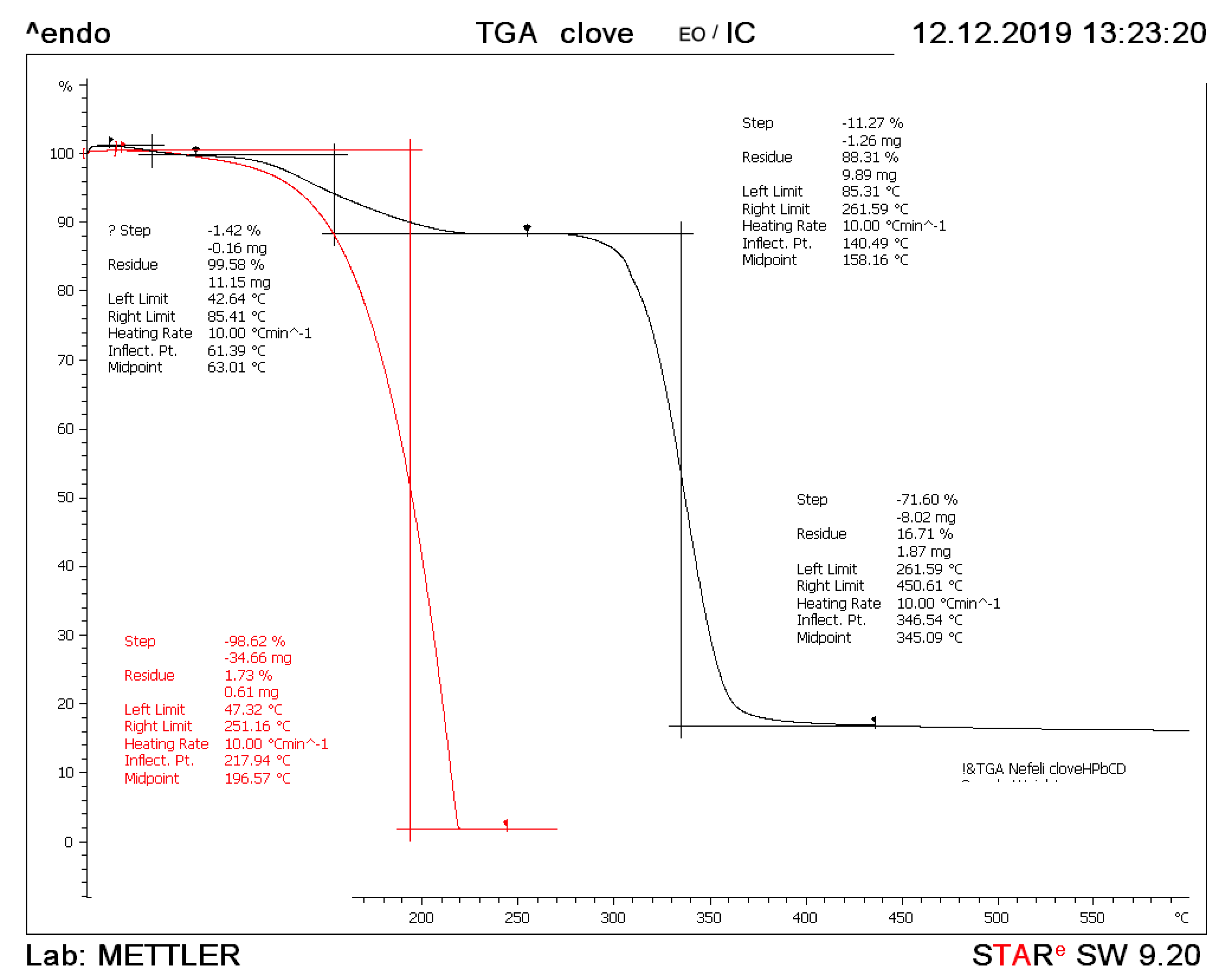
3.5. Thermogravimetric Analysis
3.6. Film Characterization
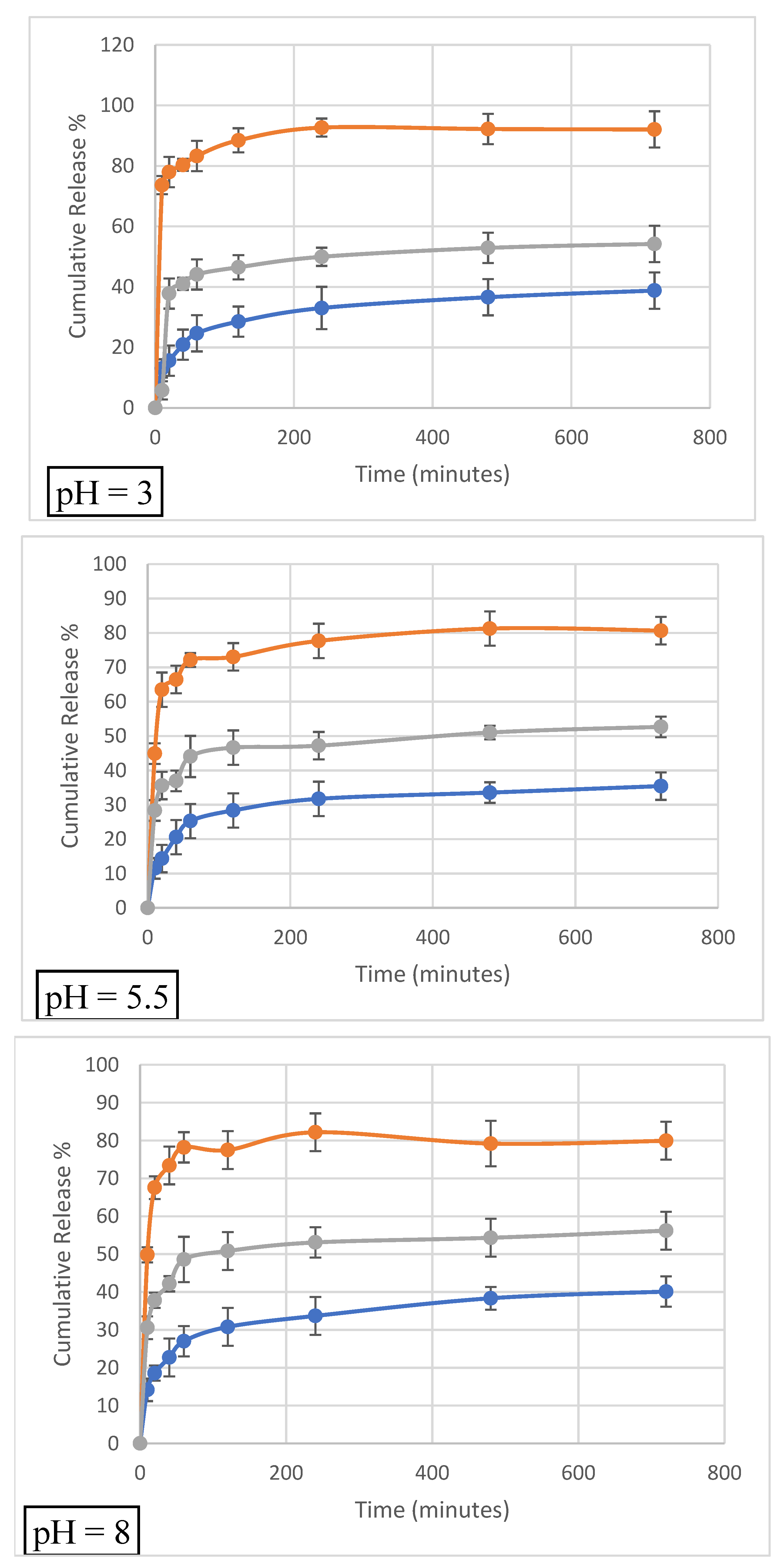
3.7. Release Studies
3.8. DPPH Radical Scavenging Ability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A short review. Phytother. Res. 2007, 21, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.S.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jirovetz, L.; Buchbauer, G.; Stoilova, I.; Stoyanova, A.; Krastanov, A.; Schmidt, E. Chemical composition and antioxidant properties of clove leaf essential oil. J. Agric. Food Chem. 2006, 54, 6303–6307. [Google Scholar] [CrossRef] [PubMed]
- Errafiy, N.; Ammar, E.; Soukri, A. Protective effect of some essential oils against oxidative and nitrosative stress on Tetrahymena thermophila growth. J. Essent. Oil Res. 2013, 25, 339–347. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Abdel-Samie, M.A.; Cui, H.; Lin, L. Unraveling the inhibitory mechanism of clove essential oil against Listeria monocytogenes biofilm and applying it to vegetable surfaces. LWT 2020, 134, 110210. [Google Scholar] [CrossRef]
- Kovács, J.K.; Felső, P.; Makszin, L.; Pápai, Z.; Horváth, G.; Ábrahám, H.; Palkovics, T.; Böszörményi, A.; Emődy, L.; Schneider, G. Antimicrobial and virulence-modulating effects of clove essential oil on the foodborne pathogen Campylobacter jejuni. Appl. Environ. Microbiol. 2016, 82, 6158–6166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqua, S.; Anusha, B.A.; Ashwini, L.S.; Negi, P.S. Antibacterial activity of cinnamaldehyde and clove oil: Effect on selected foodborne pathogens in model food systems and watermelon juice. J. Food Sci. Technol. 2015, 52, 5834–5841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purkait, S.; Bhattacharya, A.; Bag, A.; Chattopadhyay, R.R. TLC bioautography–guided isolation of essential oil components of cinnamon and clove and assessment of their antimicrobial and antioxidant potential in combination. Environ. Sci. Pollut. Res. 2021, 28, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Aboelhadid, S.M.; Youssef, I.M. Control of red flour beetle (Tribolium castaneum) in feeds and commercial poultry diets via using a blend of clove and lemongrass extracts. Environ. Sci. Pollut. Res. 2021, 28, 30111–30120. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Madhyastha, H.; Sandur, V.R.; Manikandanath, N.T.; Thiagarajan, N.; Thiagarajan, P. Anti-inflammatory and wound healing potential of a clove oil emulsion. Colloids Surf. B Biointerfaces 2020, 193, 111102. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Parker, T.L. Anti-inflammatory activity of clove (Eugenia caryophyllata) essential oil in human dermal fibroblasts. Pharm. Biol. 2017, 55, 1619–1622. [Google Scholar] [CrossRef] [Green Version]
- Katopodi, A.; Detsi, A. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers of natural products as promising systems for their bioactivity enhancement: The case of essential oils and flavonoids. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127529. [Google Scholar] [CrossRef]
- Kavetsou, E.; Koutsoukos, S.; Daferera, D.; Polissiou, M.G.; Karagiannis, D.; Perdikis, D.C.; Detsi, A. Encapsulation of Mentha pulegium essential oil in yeast cell microcarriers: An approach to environmentally friendly pesticides. J. Agric. Food Chem. 2019, 67, 4746–4753. [Google Scholar] [CrossRef]
- Pontillo, A.R.N.; Konstanteli, E.; Bairaktari, M.M.; Detsi, A. Encapsulation of the Natural Product Tyrosol in Carbohydrate Nanosystems and Study of Their Binding with ctDNA. Polymers 2021, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Kringel, D.H.; Antunes, M.D.; Klein, B.; Crizel, R.L.; Wagner, R.; de Oliveira, R.P.; Zavareze, E.D.R. Production, characterization, and stability of orange or eucalyptus essential oil/β-cyclodextrin inclusion complex. J. Food Sci. 2017, 82, 2598–2605. [Google Scholar] [CrossRef]
- Kotronia, M.; Kavetsou, E.; Loupassaki, S.; Kikionis, S.; Vouyiouka, S.; Detsi, A. Encapsulation of Oregano (Origanum onites L.) essential oil in β-cyclodextrin (β-CD): Synthesis and characterization of the inclusion complexes. Bioengineering 2017, 4, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Detsi, A.; Kavetsou, E.; Kostopoulou, I.; Pitterou, I.; Pontillo, A.R.N.; Tzani, A.; Christodoulou, P.; Siliachli, A.; Zoumpoulakis, P. Nanosystems for the encapsulation of natural products: The case of chitosan biopolymer as a matrix. Pharmaceutics 2020, 12, 669. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Preparation and incorporation of functional ingredients in edible films and coatings. Food Bioprocess Technol. 2021, 14, 209–231. [Google Scholar] [CrossRef]
- Alparslan, Y.; Baygar, T. Effect of chitosan film coating combined with orange peel essential oil on the shelf life of deepwater pink shrimp. Food Bioprocess Technol. 2017, 10, 842–853. [Google Scholar] [CrossRef]
- Molamohammadi, H.; Pakkish, Z.; Akhavan, H.R.; Saffari, V.R. Effect of salicylic acid incorporated chitosan coating on shelf life extension of fresh in-hull pistachio fruit. Food Bioprocess Technol. 2020, 13, 121–131. [Google Scholar] [CrossRef]
- Reyes-Avalos, M.C.; Femenia, A.; Minjares-Fuentes, R.; Contreras-Esquivel, J.C.; Aguilar-González, C.N.; Esparza-Rivera, J.R.; Meza-Velázquez, J.A. Improvement of the quality and the shelf life of figs (Ficus carica) using an alginate–chitosan edible film. Food Bioprocess Technol. 2016, 9, 2114–2124. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Yang, Z.; He, Q. Effects of incorporation with clove (Eugenia caryophyllata) essential oil (CEO) on overall performance of chitosan as active coating. Int. J. Biol. Macromol. 2021, 166, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Mendiratta, S.K.; Zende, R.J.; Agrawal, R.K.; Kumar Jaiswal, R. Effects of chitosan coating enriched with Syzygium aromaticum essential oil on quality and shelf-life of chicken patties. J. Food Processing Preserv. 2020, 44, e14870. [Google Scholar] [CrossRef]
- Dos Santos, E.P.; Nicácio, P.H.M.; Barbosa, F.C.; Da Silva, H.N.; Andrade, A.L.S.; Fook, M.V.L.; Silva, S.M.D.L.; Leite, I.F. Chitosan/essential oils formulations for potential use as wound dressing: Physical and antimicrobial properties. Materials 2019, 12, 2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.H.; Kim, S.Y.; Park, H.J. Effect of halloysite nanoclay on the physical, mechanical, and antioxidant properties of chitosan films incorporated with clove essential oil. Food Hydrocoll. 2018, 84, 58–67. [Google Scholar] [CrossRef]
- Xu, P.; Wang, H.M. Study on thermal decomposition behavior of survived β-cyclodextrin in its inclusion complex of clove oil by nonisothermal thermogravimetry and gas chromatography coupled to time-of-flight mass spectrometry analyses. Thermochim. Acta 2008, 469, 36–42. [Google Scholar] [CrossRef]
- Kfoury, M.; Landy, D.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Effect of cyclodextrin complexation on phenylpropanoids’ solubility and antioxidant activity. Beilstein J. Org. Chem. 2014, 10, 2322–2331. [Google Scholar] [CrossRef] [Green Version]
- Cetin Babaoglu, H.; Bayrak, A.; Ozdemir, N.; Ozgun, N. Encapsulation of clove essential oil in hydroxypropyl beta-cyclodextrin for characterization, controlled release, and antioxidant activity. J. Food Process. Preserv. 2017, 41, e13202. [Google Scholar] [CrossRef]
- Smirnov, M.A.; Nikolaeva, A.L.; Vorobiov, V.K.; Bobrova, N.V.; Abalov, I.V.; Smirnov, A.V.; Sokolova, M.P. Ionic conductivity and structure of chitosan films modified with lactic acid-choline chloride NADES. Polymers 2020, 12, 350. [Google Scholar] [CrossRef] [Green Version]
- Hill, L.E.; Gomes, C.; Taylor, T.M. Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT Food Sci. Technol. 2013, 51, 86–93. [Google Scholar] [CrossRef]
- Hadian, Z.; Maleki, M.; Abdi, K.; Atyabi, F.; Mohammadi, A.; Khaksar, R. Preparation and characterization of nanoparticle β-cyclodextrin: Geraniol inclusion complexes. Iran. J. Pharm. Res. IJPR 2018, 17, 39. [Google Scholar] [PubMed]
- Kavetsou, E.; Pitterou, I.; Katopodi, A.; Petridou, G.; Adjali, A.; Grigorakis, S.; Detsi, A. Preparation, Characterization, and Acetylcholinesterase Inhibitory Ability of the Inclusion Complex of β-Cyclodextrin–Cedar (Juniperus phoenicea) Essential Oil. Micro 2021, 1, 250–266. [Google Scholar] [CrossRef]
- Halahlah, A.; Kavetsou, E.; Pitterou, I.; Grigorakis, S.; Loupassaki, S.; Tziveleka, L.A.; Kikionis, S.; Ioannou, E.; Detsi, A. Synthesis and characterization of inclusion complexes of rosemary essential oil with various β-cyclodextrins and evaluation of their antibacterial activity against Staphylococcus aureus. J. Drug Deliv. Sci. Technol. 2021, 65, 102660. [Google Scholar] [CrossRef]
- Martins, J.T.; Cerqueira, M.A.; Vicente, A.A. Influence of α-tocopherol on physicochemical properties of chitosan-based films. Food Hydrocoll. 2012, 27, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Sui, S.; Ference, C.; Zhang, Y.; Sun, S.; Zhou, N.; Zhu, W.; Zhou, K. Antimicrobial and mechanical properties of β-cyclodextrin inclusion with essential oils in chitosan films. J. Agric. Food Chem. 2014, 62, 8914–8918. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Gao, C.; Yang, Y.; Shen, X.; Huang, M.; Liu, S.; Tang, X. Retention and release properties of cinnamon essential oil in antimicrobial films based on chitosan and gum arabic. Food Hydrocoll. 2018, 84, 84–92. [Google Scholar] [CrossRef]
- Merchán Arenas, D.R.; Muñoz Acevedo, A.; Vargas Méndez, L.Y.; Kouznetsov, V.V. Scavenger activity evaluation of the clove bud essential oil (Eugenia caryophyllus) and eugenol derivatives employing ABTS+• decolorization. Sci. Pharm. 2011, 79, 779–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
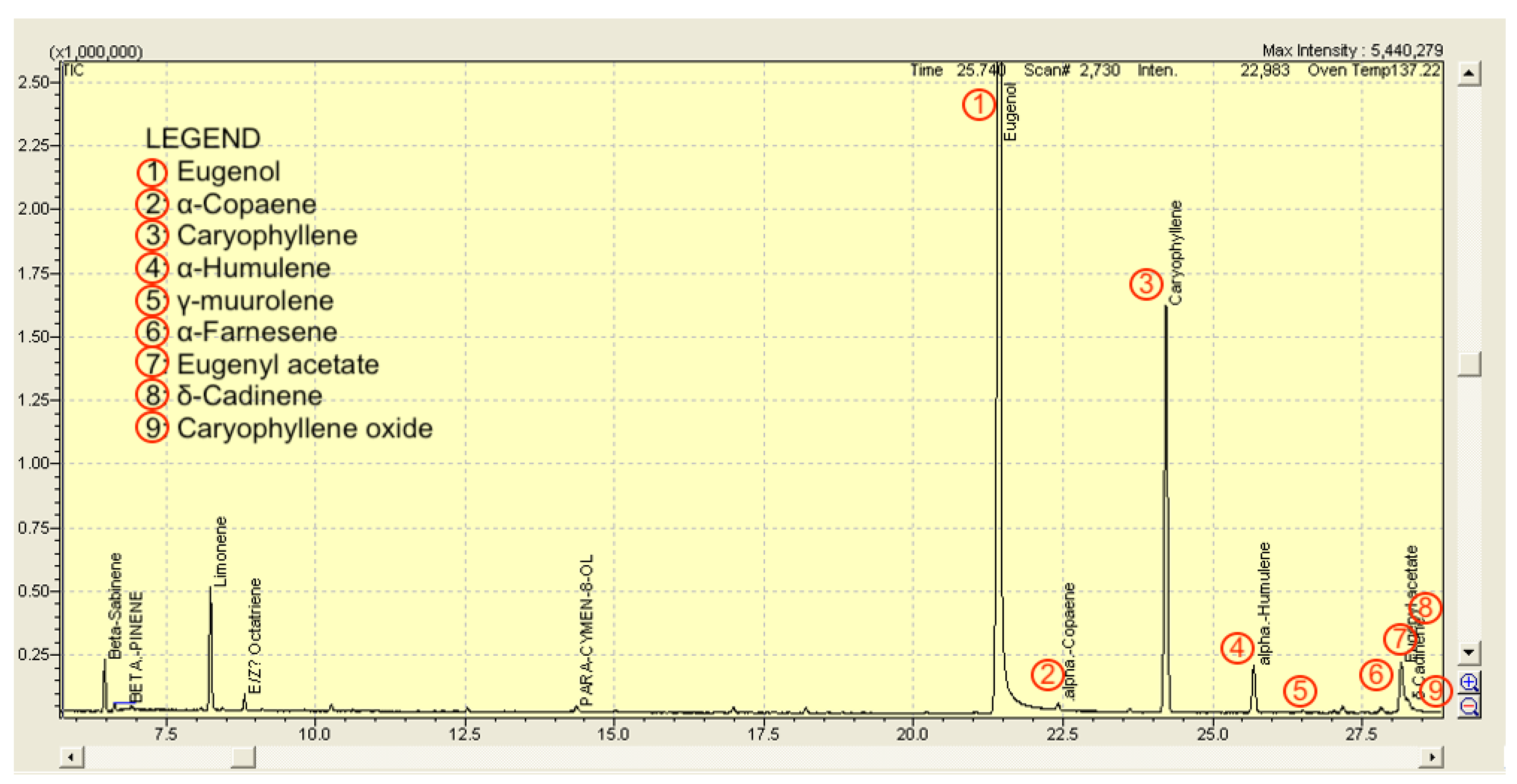
| No. | Compound | RT (min) | Area (%) |
|---|---|---|---|
| 1 | Eugenol | 21.450 | 71.3 |
| 2 | α-Copaene | 22.383 | 0.4 |
| 3 | Caryophyllene | 24.200 | 22.6 |
| 4 | α-Humulene | 25.650 | 2.1 |
| 5 | γ-muurolene | 26.483 | 0.1 |
| 6 | α -Farnesene | 27.783 | 0.3 |
| 7 | Eugenyl acetate | 28.117 | 2.5 |
| 8 | δ-Cadinene | 28.233 | 0.4 |
| 9 | Caryophyllene oxide | 30.667 | 0.2 |
| Characteristic Absorption Bands (cm−1) | |||||||
|---|---|---|---|---|---|---|---|
| OH Stretching | C-H Stretching | C-H Asymmetric Stretching of CH2 | C=C Stretching (Aromatic) | O-H Bending (Alcohol) | C-O Stretching (Aryl-Alkyl Ether) | C-O Stretching (Secondary Alcohols) | |
| CEO | 3512 | 2935 | 1637 | 1514 | 1464 | 1269 | 1036 |
| HP-β-CD | 3403 | 2929 | 1637 | - | 1462 | - | 1032 |
| HP-β-CD-CEO inclusion complex | 3406 | 2929 | 1639 | 1516 | 1460 | 1273 | 1034 |
| Sample | Thickness (mm) | Burst Strength, BS (N) | Distance at Burst, DB (mm) |
|---|---|---|---|
| Chitosan film (CS) | 0.0584 ± 0.018 | 10.66 ± 0.09 | 25.09 ± 0.06 |
| Chitosan film incorporating HP-β-CD (CS-HP-β-CD) | 0.0660 ± 0.018 | 8.24 ± 0.05 | 27.17 ± 0.04 |
| Chitosan film incorporating CEO (CS-CEO) | 0.0618 ± 0.008 | 5.64 ± 0.03 | 23.40 ± 0.03 |
| Chitosan film incorporating CEO-HP-β-CD inclusion complex (CS-CEO-HP-β-CD) | 0.0648 ± 0.012 | 8.41 ± 0.03 | 27.55 ± 0.05 |
| DPPH Radical Scavenging Ability | ||
|---|---|---|
| Sample | (%) 100 μg/mL/30 min | (%) 100 μg/mL/60 min |
| CEO | 71.2 ± 1.0 | 71.4 ± 1.2 |
| HP-β-CD | no | no |
| CEO-HP-β-CD (H2O) | 85.8 ± 0.8 | 87.8 ± 0.8 |
| CEO-HP-β-CD (DMSO) | 84.4 ± 0.4 | 87.5 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adjali, A.; Pontillo, A.R.N.; Kavetsou, E.; Katopodi, A.; Tzani, A.; Grigorakis, S.; Loupassaki, S.; Detsi, A. Clove Essential Oil–Hydroxypropyl-β-Cyclodextrin Inclusion Complexes: Preparation, Characterization and Incorporation in Biodegradable Chitosan Films. Micro 2022, 2, 212-224. https://doi.org/10.3390/micro2010014
Adjali A, Pontillo ARN, Kavetsou E, Katopodi A, Tzani A, Grigorakis S, Loupassaki S, Detsi A. Clove Essential Oil–Hydroxypropyl-β-Cyclodextrin Inclusion Complexes: Preparation, Characterization and Incorporation in Biodegradable Chitosan Films. Micro. 2022; 2(1):212-224. https://doi.org/10.3390/micro2010014
Chicago/Turabian StyleAdjali, Abdelaziz, Antonella Rosaria Nefeli Pontillo, Eleni Kavetsou, Annita Katopodi, Andromachi Tzani, Spyros Grigorakis, Sofia Loupassaki, and Anastasia Detsi. 2022. "Clove Essential Oil–Hydroxypropyl-β-Cyclodextrin Inclusion Complexes: Preparation, Characterization and Incorporation in Biodegradable Chitosan Films" Micro 2, no. 1: 212-224. https://doi.org/10.3390/micro2010014
APA StyleAdjali, A., Pontillo, A. R. N., Kavetsou, E., Katopodi, A., Tzani, A., Grigorakis, S., Loupassaki, S., & Detsi, A. (2022). Clove Essential Oil–Hydroxypropyl-β-Cyclodextrin Inclusion Complexes: Preparation, Characterization and Incorporation in Biodegradable Chitosan Films. Micro, 2(1), 212-224. https://doi.org/10.3390/micro2010014










