Design and Modeling of MEMS Microgrippers for Laser-Based Additive Manufacturing
Abstract
:1. Introduction
2. Microgrippers Design Criteria
3. Laser-Based AM Processes
3.1. Micro-Stereolithography (μ-SLA)
3.2. Mask–Image–Projection Stereolithography (MIP-SLA)
3.3. Continuous Liquid Interphase Printing (CLIP)
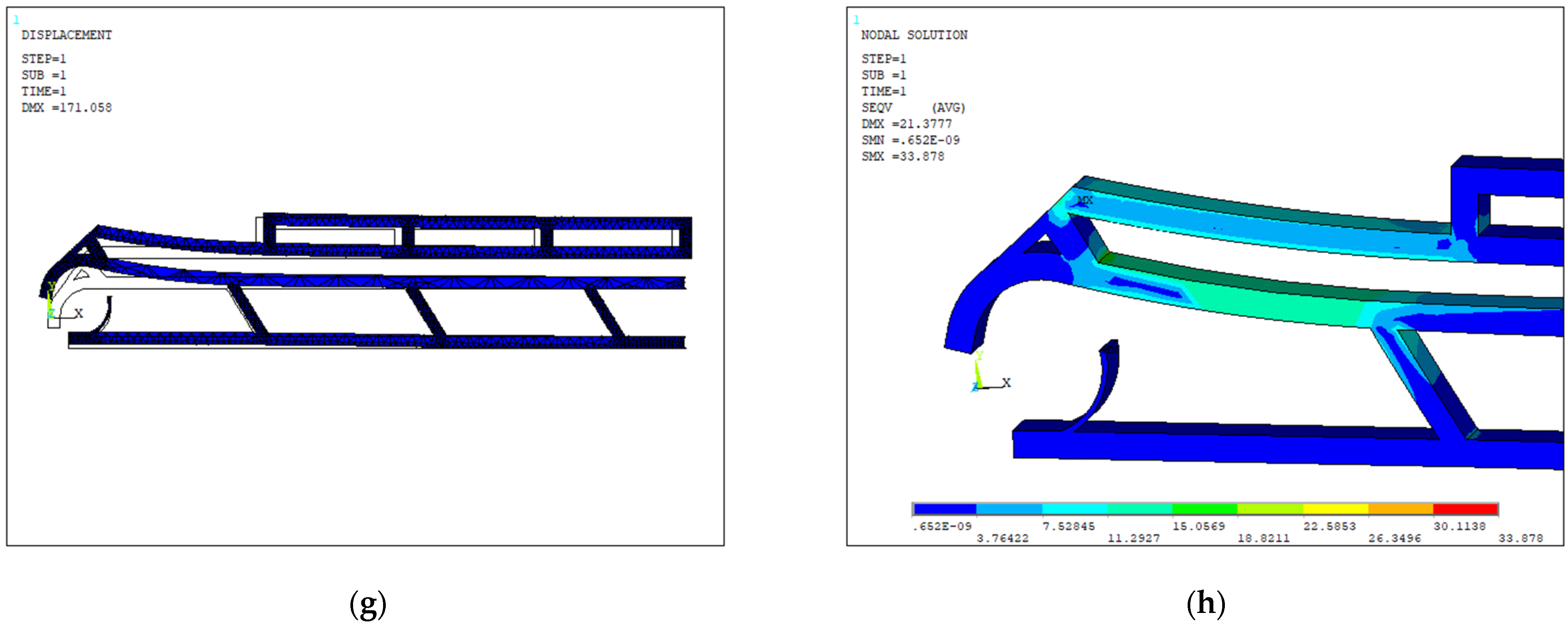
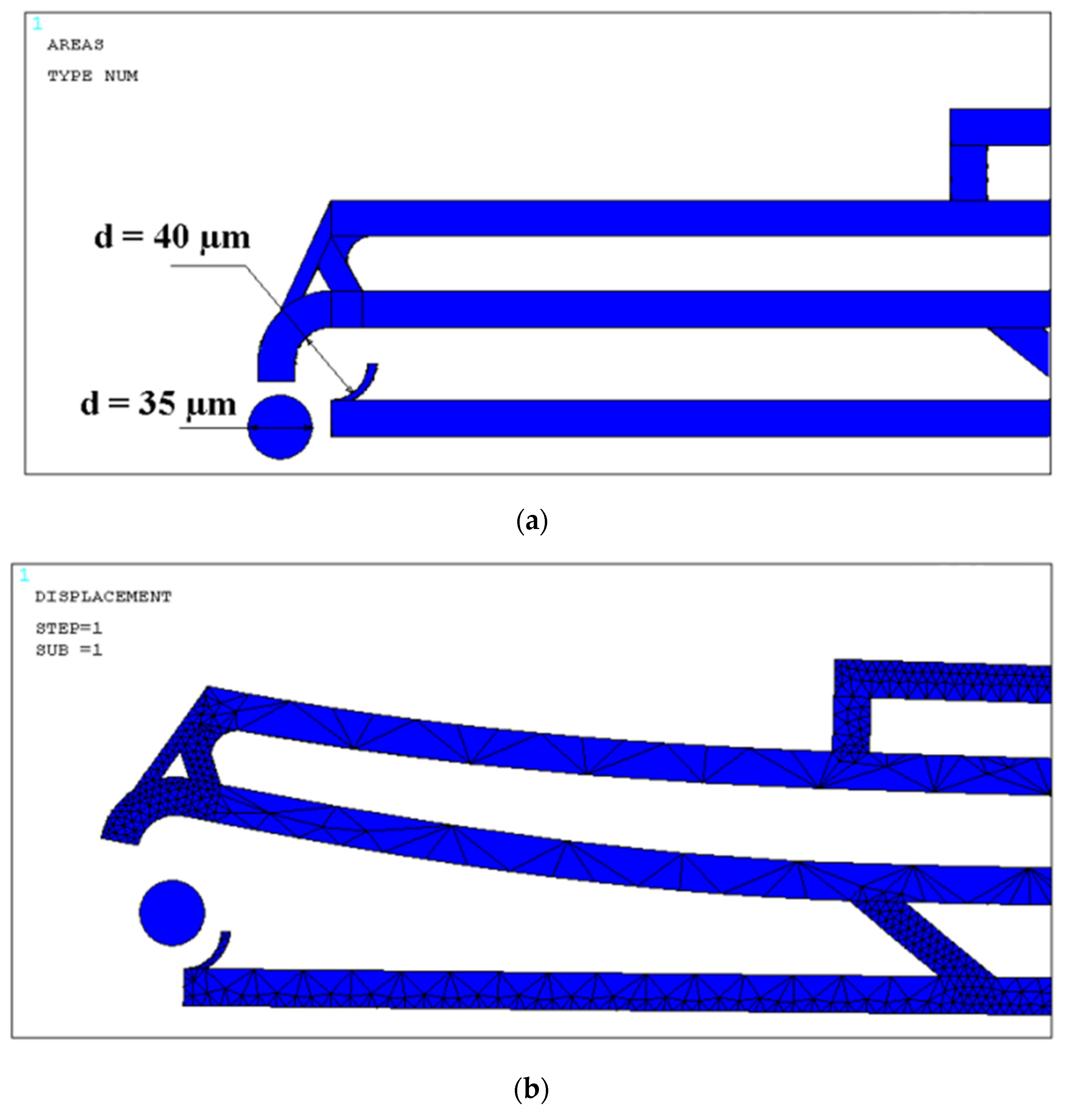
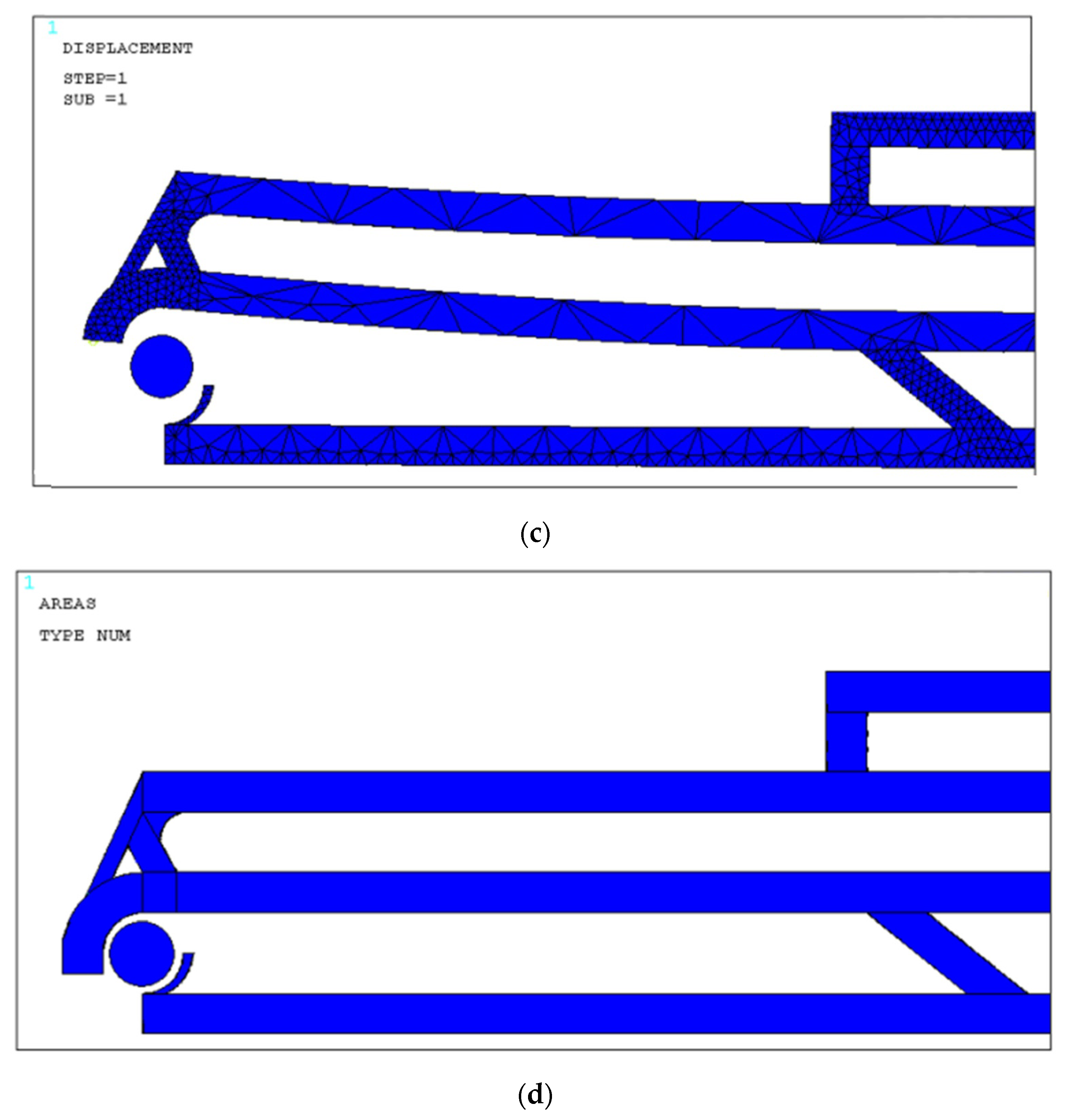
4. Design and Modeling
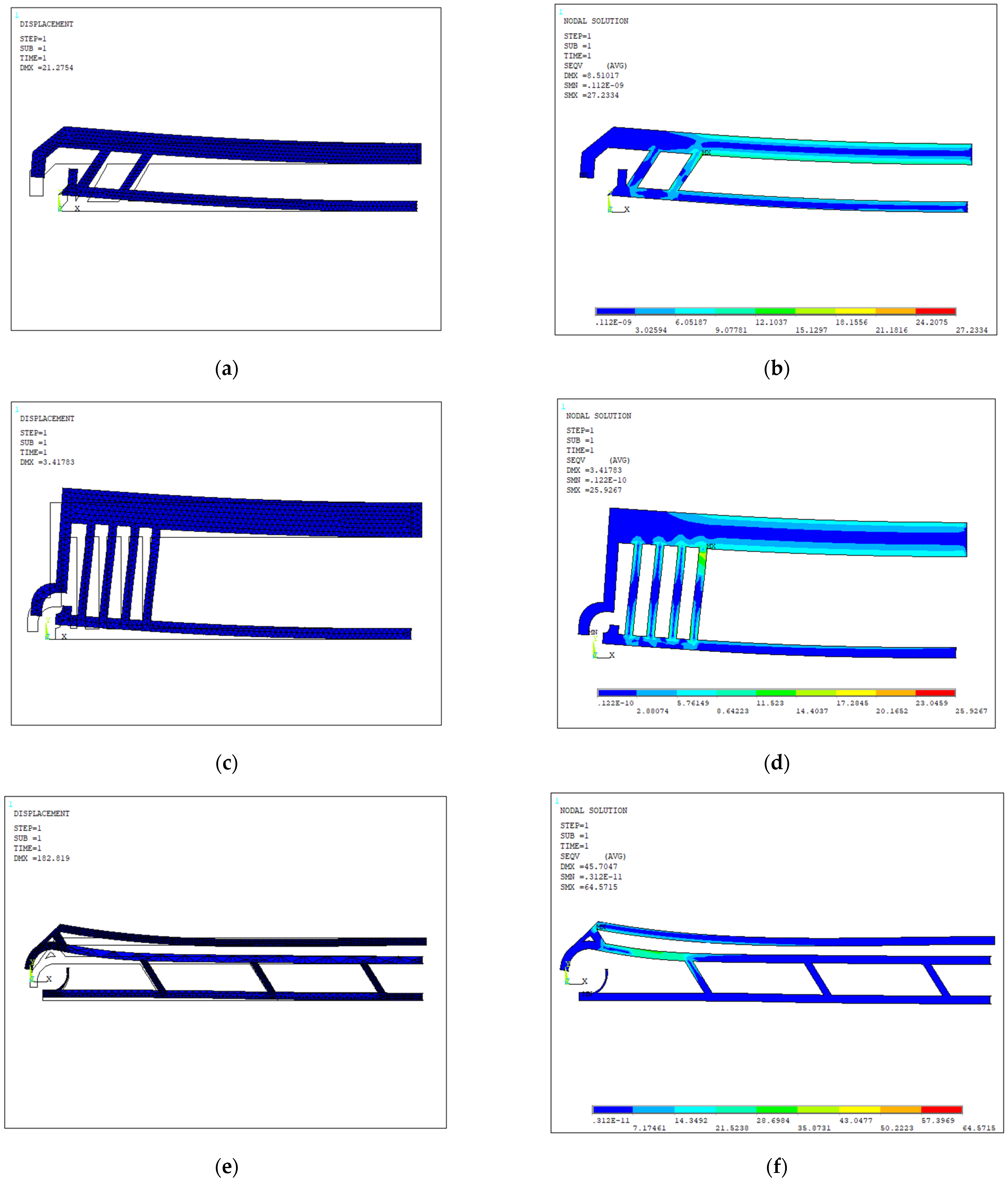
4.1. Design Optimization
4.2. Operative Sequence
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zubir, M.N.M.; Shirinzadeh, B.; Tian, Y. A New Design of Piezoelectric Driven Compliant-Based Microgripper for Micromanipulation. Mech. Mach. Theory 2009, 44, 2248–2264. [Google Scholar] [CrossRef]
- Nah, S.K.; Zhong, Z.W. A Microgripper Using Piezoelectric Actuation for Micro-Object Manipulation. Sens. Actuators A Phys. 2007, 133, 218–224. [Google Scholar] [CrossRef]
- Verotti, M.; Di Giamberardino, P.; Belfiore, N.P.; Giannini, O. A Genetic Algorithm-Based Method for the Mechanical Characterization of Biosamples Using a MEMS Microgripper: Numerical Simulations. J. Mech. Behav. Biomed. Mater. 2019, 96, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Beyeler, F.; Neild, A.; Oberti, S.; Bell, D.J.; Sun, Y.; Dual, J.; Nelson, B.J. Monolithically Fabricated Microgripper with Integrated Force Sensor for Manipulating Microobjects and Biological Cells Aligned in an Ultrasonic Field. J. Microelectromech. Syst. 2007, 16, 7–15. [Google Scholar] [CrossRef]
- Chen, T.; Sun, L.; Chen, L.; Rong, W.; Li, X. A Hybrid-Type Electrostatically Driven Microgripper with an Integrated Vacuum Tool. Sens. Actuators A Phys. 2010, 158, 320–327. [Google Scholar] [CrossRef]
- Volland, B.E.; Ivanova, K.; Ivanov, T.; Sarov, Y.; Guliyev, E.; Persaud, A.; Zöllner, J.P.; Klett, S.; Kostic, I.; Rangelow, I.W. Duo-Action Electro Thermal Micro Gripper. Microelectron. Eng. 2007, 84, 1329–1332. [Google Scholar] [CrossRef]
- Vurchio, F.; Fiori, G.; Scorza, A.; Sciuto, S.A. Comparative Evaluation of Three Image Analysis Methods for Angular Displacement Measurement in a MEMS Microgripper Prototype: A Preliminary Study. Acta IMEKO 2021, 10, 119–125. [Google Scholar] [CrossRef]
- Belfiore, N.P.; Bagolini, A.; Rossi, A.; Bocchetta, G.; Vurchio, F.; Crescenzi, R.; Scorza, A.; Bellutti, P.; Sciuto, S.A. Design, Fabrication, Testing and Simulation of a Rotary Double Comb Drives Actuated Microgripper. Micromachines 2021, 12, 1263. [Google Scholar] [CrossRef]
- Andersen, K.N.; Carlson, K.; Petersen, D.H.; Mølhave, K.; Eichhorn, V.; Fatikow, S.; Bøggild, P. Electrothermal Microgrippers for Pick-and-Place Operations. Microelectron. Eng. 2008, 85, 1128–1130. [Google Scholar] [CrossRef]
- Ivanova, K.; Ivanov, T.; Badar, A.; Volland, B.E.; Rangelow, I.W.; Andrijasevic, D.; Sümecz, F.; Fischer, S.; Spitzbart, M.; Brenner, W.; et al. Thermally Driven Microgripper as a Tool for Micro Assembly. Microelectron. Eng. 2006, 83, 1393–1395. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Ho, S.S.; Low, C.L.N. A Polymeric Microgripper with Integrated Thermal Actuators. J. Micromech. Microeng. 2004, 14, 969–974. [Google Scholar] [CrossRef]
- Roy, A.; Nabi, M. Modeling of MEMS Electrothermal Microgripper Employing POD-DEIM and POD Method. Microelectron. Reliab. 2021, 125, 114338. [Google Scholar] [CrossRef]
- Roy, A.; Sarkar, R.; Banerjee, A.; Nabi, M. An Efficient Design Procedure for MEMS Electrothermal Microgripper. ASME Lett. Dyn. Syst. Control 2021, 2, 1–6. [Google Scholar] [CrossRef]
- Huang, W. On the Selection of Shape Memory Alloys for Actuators. Mater. Des. 2002, 23, 11–19. [Google Scholar] [CrossRef]
- Kohl, M.; Skrobanek, K.D. Linear Microactuators Based on the Shape Memory Effect. Sens. Actuators A Phys. 1998, 70, 104–111. [Google Scholar] [CrossRef]
- Kohl, M.; Krevet, B.; Just, E. SMA Microgripper System. Sens. Actuators A Phys. 2002, 97–98, 646–652. [Google Scholar] [CrossRef]
- Giouroudi, I.; Hötzendorfer, H.; Kosel, J.; Andrijasevic, D.; Brenner, W. Development of a Microgripping System for Handling of Microcomponents. Precis. Eng. 2008, 32, 148–152. [Google Scholar] [CrossRef]
- Suriyage, M.P.; Prabath, T.A.B.; Wickramathilaka, Y.L.G.C.L.; Silva, S.K.M.M.; Gunawardane, M.A.S.V.; Jayawardana, J.A.D.N.; Bandara, H.M.N.W.; Withanapathirana, W.P.V.V.; Amarasinghe, Y.W.R. Design of a Novel Mems-Based Microgripper with Hybrid Actuation to Determine Circulating Tumor Cell (CTC) Progression. In Innovation in Medicine and Healthcare; Chen, Y.W., Tanaka, S., Howlett, R., Jain, L., Eds.; Smart Innovation, Systems and Technologies; Springer: Singapore, 2020; Volume 192. [Google Scholar]
- Majidi Fard-Vatan, H.; Hamedi, M. Design, Analysis and Fabrication of a Novel Hybrid Electrothermal Microgripper in Microassembly Cell. Microelectron. Eng. 2020, 231, 111374. [Google Scholar] [CrossRef]
- Wei, D.; Hall, M.B.; Sherehiy, A.; Popa, D.O. Design and Evaluation of Human-Machine Interface for NEXUS: A Custom Microassembly System. J. Micro Nano-Manuf. 2020, 8, 041011. [Google Scholar] [CrossRef]
- Kim, J.W.; Yoshida, K.; Ide, T.; Yokota, S. Fabrication, Experiment, and Simulation of a Flexible Microvalve-Integrated Microarm for Microgrippers Using Electrorheological Fluid. J. Robot. Mechatron. 2020, 32, 333–343. [Google Scholar] [CrossRef]
- Chronis, N.; Lee, L.P. Electrothermally Activated SU-8 Microgripper for Single Cell Manipulation in Solution. J. Microelectromech. Syst. 2005, 14, 857–863. [Google Scholar] [CrossRef]
- Han, K.; Lee, S.H.; Moon, W.; Park, J.S. Fabrication of the Micro-Gripper with a Force Sensor for Manipulating a Cell. In Proceedings of the 2006 SICE-ICASE International Joint Conference, Busan, Korea, 18–21 October 2006. [Google Scholar]
- Neagu, C.; Jansen, H.; Gardeniers, H.; Elwenspoek, M. Electrolysis of Water: An Actuation Principle for MEMS with a Big Opportunity. Mechatronics 2000, 10, 571–581. [Google Scholar] [CrossRef]
- Vurchio, F.; Fiori, G.; Scorza, A.; Sciuto, S.A. A Comparison among Three Different Image Analysis Methods for the Displacement Measurement in a Novel MEMS Device. In Proceedings of the 24th IMEKO TC4 International Symposium and 22nd International Workshop on ADC and DAC Modelling and Testing, Palermo, Italy, 14–16 September 2020. [Google Scholar]
- Vurchio, F.; Orsini, F.; Scorza, A.; Fuiano, F.; Sciuto, S.A. A Preliminary Study on a Novel Automatic Method for Angular Displacement Measurements in Microgripper for Biomedical Applications. In Proceedings of the 2020 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Bari, Italy, 1 June–1 July 2020. [Google Scholar]
- Schindler, C.B.; Gomez, H.C.; Acker-James, D.; Teal, D.; Li, W.; Pister, K.S.J. 15 Millinewton Force, 1 Millimeter Displacement, Low-Power MEMS Gripper. In Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Vancouver, BC, Canada, 18–22 January 2020. [Google Scholar]
- Ferrara-Bello, A.; Vargas-Chable, P.; Vera-Dimas, G.; Vargas-Bernal, R.; Tecpoyotl-Torres, M. XYZ Micropositioning System Based on Compliance Mechanisms Fabricated by Additive Manufacturing. Actuators 2021, 10, 68. [Google Scholar] [CrossRef]
- Li, W.J.; Xi, N. Novel Micro Gripping, Probing, and Sensing Devices for Single-Cell Surgery. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004; Volume 26 IV. [Google Scholar]
- Solano, B.; Wood, D. Design and Testing of a Polymeric Microgripper for Cell Manipulation. Microelectron. Eng. 2007, 84, 1219–1222. [Google Scholar] [CrossRef]
- MacKay, R.E.; Le, H.R.; Donnelly, K.; Keatch, R.P. Micro-Gripping of Small Scale Tissues. In Proceedings of the 4th European Conference of the International Federation for Medical and Biological Engineering. IFMBE Proceedings; Vander Sloten, J., Verdonck, P., Nyssen, M., Haueisen, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 22. [Google Scholar]
- Sochol, R.D.; Sweet, E.; Glick, C.C.; Wu, S.Y.; Yang, C.; Restaino, M.; Lin, L. 3D Printed Microfluidics and Microelectronics. Microelectron. Eng. 2018, 189, 52–68. [Google Scholar] [CrossRef]
- Rupal, B.S.; Garcia, E.A.; Ayranci, C.; Qureshi, A.J. 3D Printed 3D-Microfluidics: Recent Developments and Design Challenges. J. Integr. Des. Process Sci. 2019, 22, 5–20. [Google Scholar] [CrossRef]
- Lifton, V.A.; Lifton, G.; Simon, S. Options for Additive Rapid Prototyping Methods (3D Printing) in MEMS Technology. Rapid Prototyp. J. 2014, 20, 403–412. [Google Scholar] [CrossRef]
- Vaezi, M.; Seitz, H.; Yang, S. A Review on 3D Micro-Additive Manufacturing Technologies. Int. J. Adv. Manuf. Technol. 2013, 67, 1721–1754. [Google Scholar] [CrossRef]
- ISO/ASTM 52900:2015(E); Standard Terminology for Additive Manufacturing—General Principles—Terminology; International Organization for Standardization: Geneva, Switzerland, 2015; pp. 1–9.
- Kamat, A.M.; Pei, Y.; Jayawardhana, B.; Kottapalli, A.G.P. Biomimetic Soft Polymer Microstructures and Piezoresistive Graphene MEMS Sensors Using Sacrificial Metal 3D Printing. ACS Appl. Mater. Interfaces 2021, 13, 1094–1104. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [Green Version]
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired Structural Materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Ovsianikov, A.; Khademhosseini, A.; Mironov, V. The Synergy of Scaffold-Based and Scaffold-Free Tissue Engineering Strategies. Trends Biotechnol. 2018, 36, 348–357. [Google Scholar] [CrossRef] [PubMed]
- De Pasquale, G.; Ruggeri, V. Sensing Strategies in Wearable Bio-Mechanical Systems for Medicine and Sport: A Review. J. Micromech. Microeng. 2019, 29, 103001. [Google Scholar] [CrossRef]
- Duan, B. State-of-the-Art Review of 3D Bioprinting for Cardiovascular Tissue Engineering. Ann. Biomed. Eng. 2017, 45, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Yue, K.; Aleman, J.; Mollazadeh-Moghaddam, K.; Bakht, S.M.; Yang, J.; Jia, W.; Dell’Erba, V.; Assawes, P.; Shin, S.R.; et al. 3D Bioprinting for Tissue and Organ Fabrication. Ann. Biomed. Eng. 2017, 45, 148–163. [Google Scholar] [CrossRef] [Green Version]
- Joshi, S.; Cook, E.; Mannoor, M.S. Bacterial Nanobionics via 3D Printing. Nano Lett. 2018, 18, 7448–7456. [Google Scholar] [CrossRef]
- Park, M.; Do, K.; Kim, J.; Son, D.; Koo, J.H.; Park, J.; Song, J.-K.; Kim, J.H.; Lee, M.; Hyeon, T.; et al. Skin Electronics: Oxide Nanomembrane Hybrids with Enhanced Mechano- and Thermo-Sensitivity for Semitransparent Epidermal Electronics (Adv. Healthcare Mater. 7/2015). Adv. Healthc. Mater. 2015, 4, 991. [Google Scholar] [CrossRef] [Green Version]
- Kanao, K.; Harada, S.; Yamamoto, Y.; Honda, W.; Arie, T.; Akita, S.; Takei, K. Printable Flexible Tactile Pressure and Temperature Sensors with High Selectivity against Bending. In Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Estoril, Portugal, 18–22 January 2015. [Google Scholar]
- Kong, Y.L.; Gupta, M.K.; Johnson, B.N.; McAlpine, M.C. 3D Printed Bionic Nanodevices. Nano Today 2016, 11, 330–350. [Google Scholar] [CrossRef] [Green Version]
- Yazdi, A.A.; Popma, A.; Wong, W.; Nguyen, T.; Pan, Y.; Xu, J. 3D Printing: An Emerging Tool for Novel Microfluidics and Lab-on-a-Chip Applications. Microfluid. Nanofluid. 2016, 20, 1–18. [Google Scholar] [CrossRef]
- Pérez, R.; Chaillet, N.; Domanski, K.; Janus, P.; Grabiec, P. Fabrication, Modeling and Integration of a Silicon Technology Force Sensor in a Piezoelectric Micro-Manipulator. Sens. Actuators A Phys. 2006, 128, 367–375. [Google Scholar] [CrossRef] [Green Version]
- De Pasquale, G. Experimental Analysis of Viscous and Material Damping in Microstructures through the Interferometric Microscopy Technique with Climatic Chamber. J. Sound Vib. 2013, 332, 4103–4121. [Google Scholar] [CrossRef] [Green Version]
- De Pasquale, G.; Veijola, T. Comparative Numerical Study of FEM Methods Solving Gas Damping in Perforated MEMS Devices. Microfluid. Nanofluid. 2008, 5, 517–528. [Google Scholar] [CrossRef]
- Seidemann, V.; Bütefisch, S.; Büttgenbach, S. Fabrication and Investigation of In-Plane Compliant SU8 Structures for MEMS and Their Application to Micro Valves and Micro Grippers. Sens. Actuators A Phys. 2002, 97–98, 457–461. [Google Scholar] [CrossRef]
- Ouyang, P.R.; Tjiptoprodjo, R.C.; Zhang, W.J.; Yang, G.S. Micro-Motion Devices Technology: The State of Arts Review. Int. J. Adv. Manuf. Technol. 2008, 38, 463–478. [Google Scholar] [CrossRef]
- Potekhina, A.; Voicu, R.C.; Muller, R.; Al-Zandi, M.H.M.; Wang, C. Design and Characterization of a Polymer Electrothermal Microgripper with a Polynomial Flexure for Efficient Operation and Studies of Moisture Effect on Negative Deflection. Microsyst. Technol. 2021, 27, 2723–2731. [Google Scholar] [CrossRef]
- Pedrazzoli, P.; Rinaldi, R.; Boër, C.R. A Rule Based Approach to the Gripper Selection Issue for the Assembly Process. In Proceedings of the IEEE International Symposium on Assembly and Task Planning, Fukuoka, Japan, 29 May 2001. [Google Scholar]
- Fahlbusch, S.; Fatikow, S. Implementation of Self-Sensing SPM Cantilevers for Nano-Force Measurement in Microrobotics. Ultramicroscopy 2001, 86, 181–190. [Google Scholar] [CrossRef]
- Liu, X.; Kim, K.; Zhang, Y.; Sun, Y. Nanonewton Force Sensing and Control in Microrobotic Cell Manipulation. Int. J. Rob. Res. 2009, 28, 1065–1076. [Google Scholar] [CrossRef]
- Carrozza, M.C.; Eisinberg, A.; Menciassi, A.; Campolo, D.; Micera, S.; Dario, P. Towards a Force-Controlled Microgripper for Assembling Biomedical Microdevices. J. Micromech. Microeng. 2000, 10, 271–276. [Google Scholar] [CrossRef]
- Fantoni, G.; Porta, M. A Critical Review of Releasing Strategies in Microparts Handling. In Micro-Assembly Technologies and Applications. IPAS 2008. IFIP—International Federation for Information Processing; Ratchev, S., Koelemeijer, S., Eds.; Springer: Boston, MA, USA, 2008; Volume 260. [Google Scholar]
- Hoxhold, B.; Büttgenbach, S. Easily Manageable, Electrothermally Actuated Silicon Micro Gripper. Microsyst. Technol. 2010, 16, 1609–1617. [Google Scholar] [CrossRef]
- Sun, C.; Fang, N.; Wu, D.M.; Zhang, X. Projection Micro-Stereolithography Using Digital Micro-Mirror Dynamic Mask. Sens. Actuators A Phys. 2005, 121, 113–120. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, X.N.; Sun, C. Micro-Stereolithography for MEMS. In Proceedings of the ASME 1998 International Mechanical Engineering Congress and Exposition. Micro-Electro-Mechanical Systems (MEMS), Anaheim, CA, USA, 15–20 November 1998; pp. 3–9. [Google Scholar] [CrossRef]
- De Pasquale, G. Additive Manufacturing of Micro-electro-mechanical Systems (MEMS). Micromachines 2021, 12, 1374. [Google Scholar] [CrossRef] [PubMed]
- Hull, C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent No 4,575,300, 8 August 1984. [Google Scholar]
- Fritzler, K.B.; Prinz, V.Y. 3D Printing Methods for Micro- and Nanostructures. Uspekhi Fiz. Nauk 2019, 189, 55–71. [Google Scholar] [CrossRef]
- Bártolo, P.J. Stereolithography—Materials, Processes and Applications; Springer Science & Business Media: Boston, MA, USA, 2011. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B. Additive Manufacturing Technologies: 3D Printing and Direct Digital Manufacturing; Springer: New York, NY, USA, 2015; Volume 59, pp. 94–97. [Google Scholar]
- De Pasquale, G.; Bertana, V.; Scaltrito, L. Experimental Evaluation of Mechanical Properties Repeatability of SLA Polymers for Labs-on-Chip and Bio-MEMS. Microsyst. Technol. 2018, 24, 3487–3497. [Google Scholar] [CrossRef]
- De Pasquale, G.; Zappulla, L.; Scaltrito, L.; Bertana, V. Numerical and Experimental Evaluation of SLA Polymers Adhesion for Innovative Bio-MEMS. Mater. Today Proc. 2019, 7, 572–577. [Google Scholar] [CrossRef]
- Bertana, V.; De Pasquale, G.; Ferrero, S.; Scaltrito, L.; Catania, F.; Nicosia, C.; Marasso, S.L.; Cocuzza, M.; Perrucci, F. 3D Printing with the Commercial UV-Curable Standard Blend Resin: Optimized Process Parameters towards the Fabrication of Tiny Functional Parts. Polymers 2019, 11, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Y.; Jiang, L.; Sun, Y.; Yang, Y.; Quan, Y.; Wei, S.; Lu, G.; Li, R.; Rong, J.; Chen, Y.; et al. 3D-Printing Piezoelectric Composite with Honeycomb Structure for Ultrasonic Devices. Micromachines 2020, 11, 713. [Google Scholar] [CrossRef]
- Janusziewicz, R.; Tumbleston, J.R.; Quintanilla, A.L.; Mecham, S.J.; DeSimone, J.M. Layerless Fabrication with Continuous Liquid Interface Production. Proc. Natl. Acad. Sci. USA 2016, 113, 11703–11708. [Google Scholar] [CrossRef] [Green Version]
- Tumbleston, J.R.; Shirvanyants, D.; Ermoshkin, N.; Janusziewicz, R.; Johnson, A.R.; Kelly, D.; Chen, K.; Pinschmidt, R.; Rolland, J.P.; Ermoshkin, A.; et al. Continuous Liquid Interface Production of 3D Objects. Science 2015, 347, 1349–1352. [Google Scholar] [CrossRef]
- Wang, Z.; Abdulla, R.; Parker, B.; Samanipour, R.; Ghosh, S.; Kim, K. A Simple and High-Resolution Stereolithography-Based 3D Bioprinting System Using Visible Light Crosslinkable Bioinks. Biofabrication 2015, 7, 045009. [Google Scholar] [CrossRef]
- Knowlton, S.; Yu, C.H.; Ersoy, F.; Emadi, S.; Khademhosseini, A.; Tasoglu, S. 3D-Printed Microfluidic Chips with Patterned, Cell-Laden Hydrogel Constructs. Biofabrication 2016, 8, 025019. [Google Scholar] [CrossRef] [Green Version]
- Soltanzadeh, R.; Afsharipour, E.; Anssari, N.; Mansouri, B.; Shafai, C. Structural and Performance Comparison between SU-8 Microfabricated and 3D-Printed Microneedle Electrodes. J. 3D Print. Med. 2020, 4, 29–44. [Google Scholar] [CrossRef]
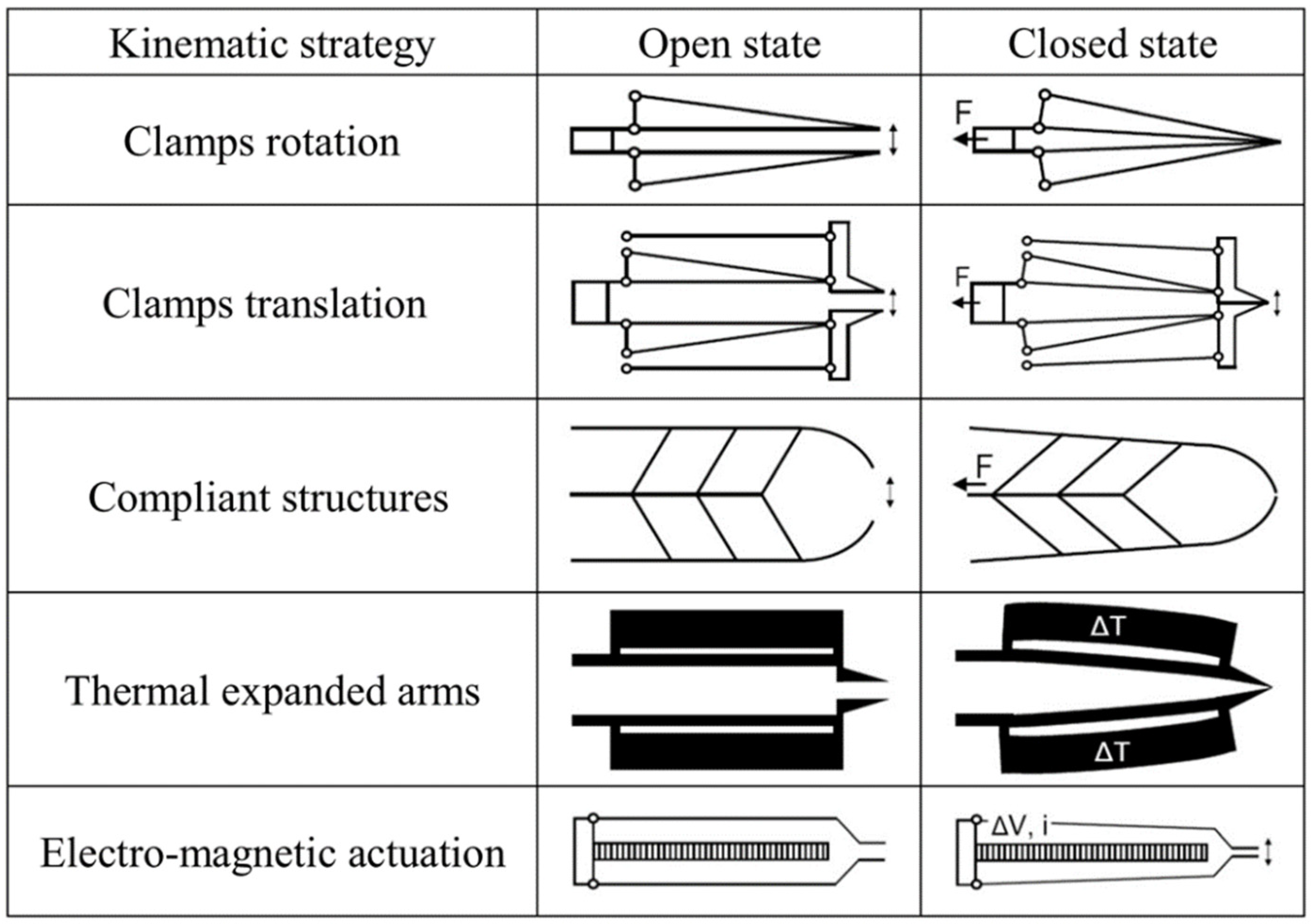
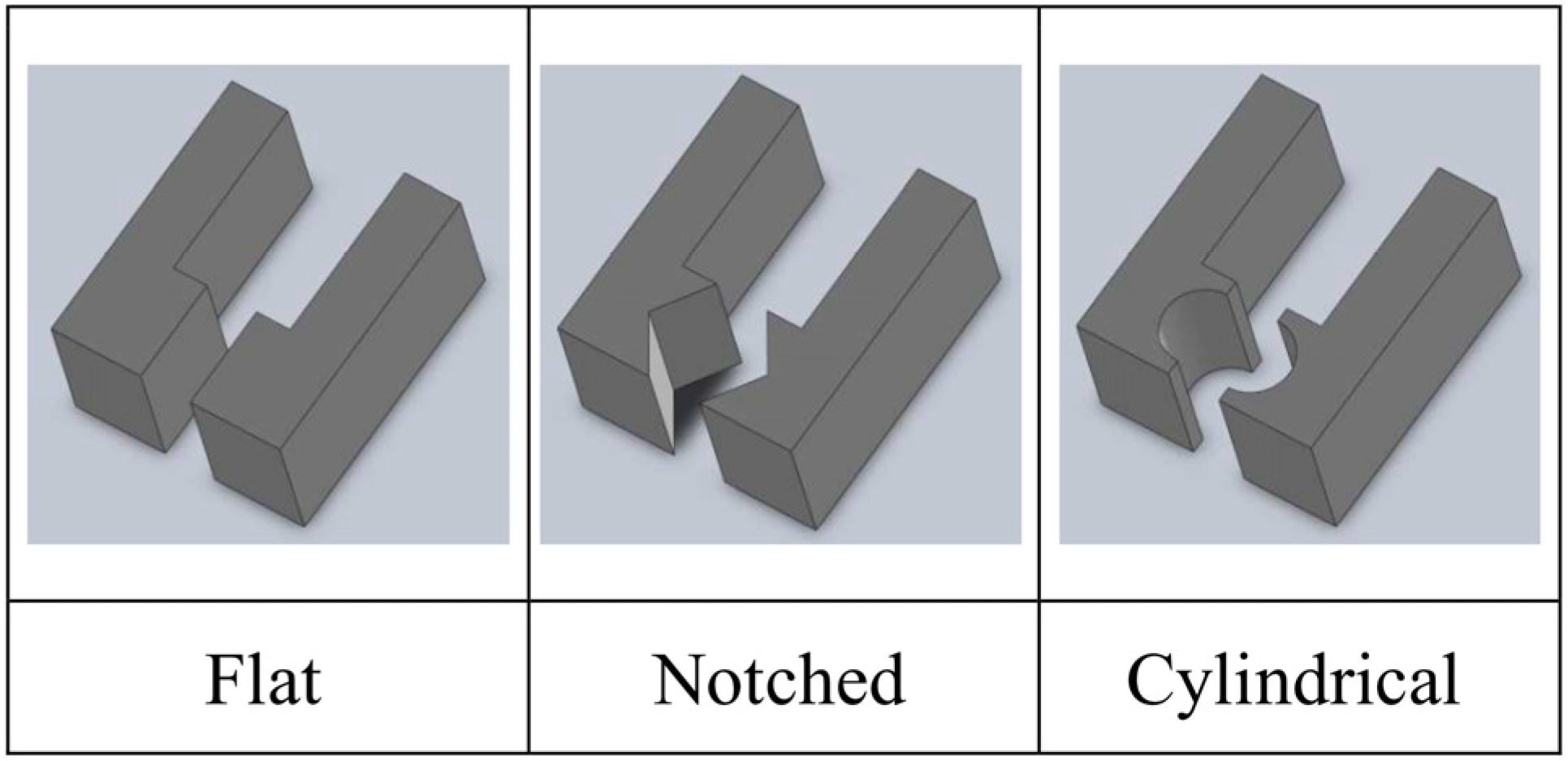
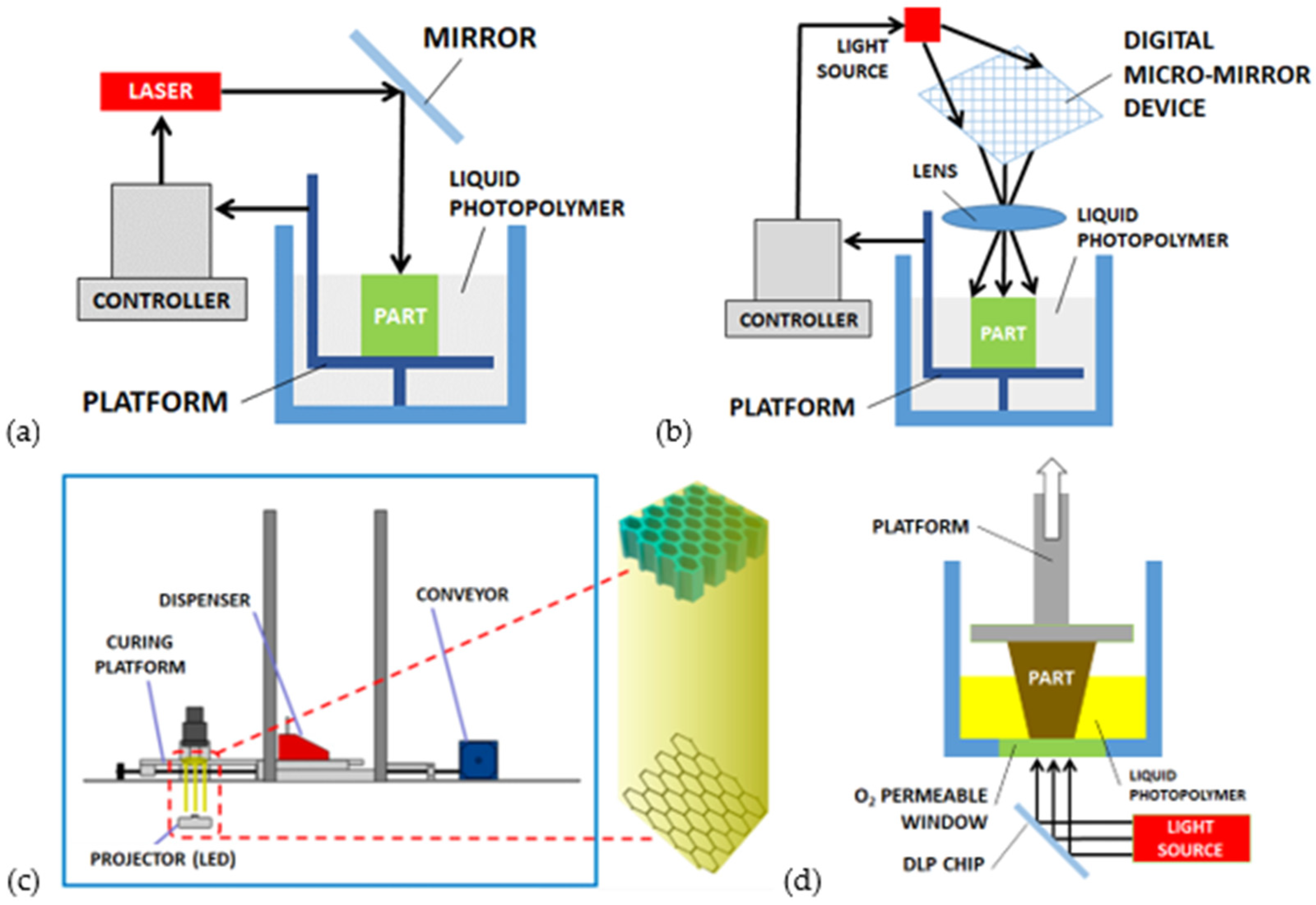
| Actuation Strategy | Advantages | Limitations | |
|---|---|---|---|
| Internal actuation | Piezoelectric | Thermal stability, high accuracy, high responsiveness. | Nonlinearity, high supply voltage, small motion range, creep, fatigue, hysteresis, low biocompatibility. |
| Electrostatic capacitive | Consolidated micromachining manufacturing process, direct motion feedback. | Complicated geometry, small motion range, electrolysis, and bubble formation. | |
| Thermal | Consolidated micromachining manufacturing process. | High temperature, slow response. | |
| SMA actuators | Faster response then thermal actuation, large motion range. | Fatigue, small motion range, nonlinearity, hysteresis, hard manufacturing process, high cost. | |
| Electromagnetic | Preservation of cell integrity. | Coil heating, magnetic field weakness, field leakage. | |
| Hydraulic and pneumatic | Reliability, preservation of cell integrity. | Limited applicability. | |
| External actuation | DC motors | Thermal insulation, high speed, high accuracy. | Heat generation, dimensions, hysteresis, interface connection, feedback control needed. |
| Step motors | Thermal insulation, very large motion range. | Heat generation, low precision, dimensions, unsmooth motion, interface connection, noise. | |
| Piezoelectric motors | Large force, high accuracy, high responsiveness, thermal insulation, small size, no wear and tear, low power consumption. | Interface connection. | |
| Releasing Strategy | Description | |
|---|---|---|
| Passive release | Rough surfaces | The contact area is reduced by roughness, and the electrostatic adhesion force also reduces. |
| Hydrophobic coating | The coating reduces the superficial tension. | |
| Conductive coating | Conductive coatings/materials reduce the electrostatic forces through the small potential difference with the gripped object. | |
| Vacuum environment | The vacuum reduces the superficial tension. | |
| Fluid environment | The fluid eliminates the superficial tension and reduces the electrostatic forces. | |
| Ionized air | The ionized air reduces the electrostatic forces. | |
| Active release | Vibrations | The acceleration imposed produces the object release due to inertial force. |
| Air pressure | A pressurized airflow is used to overcome the adhesion force. | |
| Heating | The temperature reduces the capillary forces. | |
| Electrostatic control | The electrostatic force is controlled by shorting the gripper electrodes or by inverting the polarity. | |
| Adhesion to the substrate | The object adheres to an external substrate by means of higher adhesion forces, by gluing it, or by engaging it on the substrate. | |
| Additional tools | Additional tools are used to detach the object. |
| Laser-Based Process for MEMS Microgripper Fabrication | Min. Feature (μm) | Materials | References |
|---|---|---|---|
| Micro-stereolithography (μ-SLA) | 30–70 | Photosensitive polymers, Formlabs clear resin | [74,75] |
| Mask–image–projection stereolithography (MIP-SLA) | 450 | Photosensitive polymers | [71] |
| Continuous liquid interphase printing (CLIP) | 100 | Photosensitive polymers | [72,73] |
| Layout | Dimensions (µm) | Width Open-Close States (µm) | Actuated Arm | Actuation Displ. Applied a (µm) | Force (µN) | Vertical Tips Displ. (Fixed Arm, Moving Arm) | Opening Distance (Vert.) b (µm) | Opening Distance (Horiz.) b (µm) | Overall Stiffness (µN/µm) | Max Stress (Mpa) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1000 × 127 × 20 | 40–18 | Lower | −20 | 28 | (106, 102) | +4 | −10 | 3.1 | 33.93 |
| 2 | 1000 × 390 × 20 | 100–20 | Lower | −20 | 25 | (20, 19) | +1 | −10 | 2.6 | 32.94 |
| 3 | 1000 × 70 × 20 | 30–5 | Upper | 10 | 5.5 | (4, 42) | +38 | +0.5 | 0.14 | 30.95 |
| 4 | 1000 × 140 × 20 | 30–5 | Upper | 10 | 4.2 | (5, 41) | +35 | +6 | 0.11 | 32.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Pasquale, G. Design and Modeling of MEMS Microgrippers for Laser-Based Additive Manufacturing. Micro 2022, 2, 225-239. https://doi.org/10.3390/micro2020015
De Pasquale G. Design and Modeling of MEMS Microgrippers for Laser-Based Additive Manufacturing. Micro. 2022; 2(2):225-239. https://doi.org/10.3390/micro2020015
Chicago/Turabian StyleDe Pasquale, Giorgio. 2022. "Design and Modeling of MEMS Microgrippers for Laser-Based Additive Manufacturing" Micro 2, no. 2: 225-239. https://doi.org/10.3390/micro2020015
APA StyleDe Pasquale, G. (2022). Design and Modeling of MEMS Microgrippers for Laser-Based Additive Manufacturing. Micro, 2(2), 225-239. https://doi.org/10.3390/micro2020015






