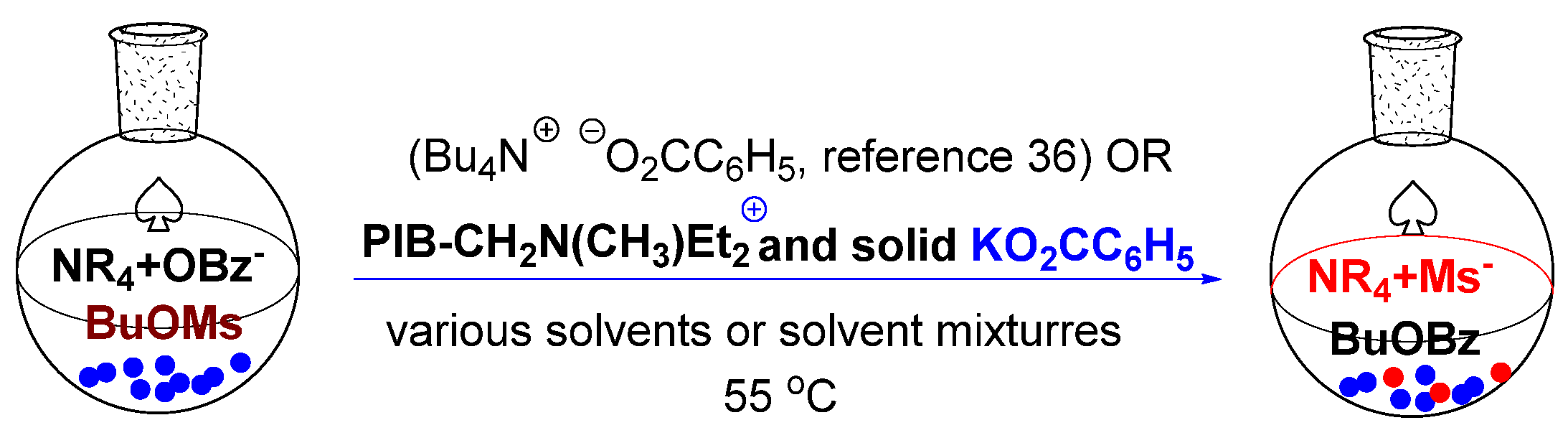1. Introduction
Solvents are an essential part of chemistry and serve important roles. Solvents allow reagents to react more readily with substrates. They influence reaction rates, stereoselectivity, and regioselectivity, and improve heat transfer in exothermic processes. Solvents are also important at the end of a reaction since most separation processes depend on solubility differences in substates, products, by-products, and impurities. Nonetheless, solvents pose sustainability and Green Chemistry issues [
1,
2,
3,
4,
5]. Solvents are the major mass component in a reaction or a separation process. Solvent sustainability, toxicity, volatility, flammability, and recycling, thus, are all important Green Chemistry issues.
A common approach to address these sustainability and Green Chemistry issues has been to design new solvents. This has included invention of new low-molecular-weight solvents derived from renewable resources [
6,
7,
8]. It has also included development of less conventional systems like polymeric solvents [
9,
10,
11,
12,
13], switchable solvents [
14,
15], deep eutectic solvents (DESs) [
16], ionic liquids (Ils) [
17,
18,
19,
20], scCO
2 [
21,
22], and aqueous solutions of surfactant-based amphiphiles [
23,
24].
Solvent recycling is also an important Green Chemistry issue [
25]. It is often assumed that volatile solvents will be recycled by distillation. This is practiced on a large scale, but it requires a significant energy input. Distillation, though, is not always simple, efficient, or safe. Ethers and acetals (e.g., THF, MeTHF, or Cyrene) and many other solvents contain CH bonds that can autoxidize to form unsafe peroxides that would be concentrated in any distillation [
26]. Solvent recycling involving other low-energy, safe, simple, and efficient processes is, thus, of interest. For example, physically recycling non-volatile ILs or polymeric solvents via biphasic separations without concentration makes these solvents greener and more sustainable.
Our past work has focused on making homogeneous catalysis greener and more sustainable [
27]. That involved using solvent mixtures to facilitate catalyst recycling by physical biphasic separations. Those studies used heptane or miscible heptane/polar solvent mixtures, separating heptane-soluble catalysts from products after the reaction. Later studies replaced heptane with greener non-volatile poly-α-olefin (PAO) solvents. However, alkane solvents are generally poor solvents for many reactions. Thus, we often used 1/1 (
v/
v) solvent blends. Later, we found that modest amounts of the polar solvent were enough to facilitate a reaction [
28,
29,
30]. This empirical addition of cosolvents allowed us to expand our repertoire of reactions where we could use recyclable catalysts bound to terminally functionalized polyisobutylene (PIB) oligomers. This paper explores the nature of these solvent mixtures to understand why as little as 0.1 M of an added cosolvent in what is visually a monophasic heptane or PAO solvent mixture is effective at facilitating a reaction. It also shows that polymeric analogs of polar solvents are similarly effective.
Our hypothesis is that addition of a small amount of a polar cosolvent to heptane or PAO produces a microheterogeneous solvent mixture. In this scenario, even small amounts of a polar cosolvent in a hydrocarbon would lead to a local environment in the solvent system where a substrate, reagent, or catalyst has a solvent environment like that in a bulk polar cosolvent. Given that solvation significantly affects reaction yields and rates, such microheterogeneity could explain why small amounts of a cosolvent can have a large effect on PIB-bound catalysts in heptane or in a recyclable polymeric hydrocarbon solvent like PAO. This work explores polymeric analogs of low-molecular-weight solvents too. We show that recyclable polymeric cosolvents have the same effects as low-molecular-weight solvents, producing similar microheterogeneous environments. Finally, we used two reactions—a transesterification and a phase transfer catalyzed SN2 reaction—to show that the microheterogeneity we observed affects reaction rates. In the latter case, we show that the combination of a polymeric solvent, a polymeric cosolvent, and a polymeric catalyst affords a fully recyclable catalyst/solvent system.
2. Materials and Methods
Tetrahydrofuran (THF) was distilled from sodium benzophenone ketyl. Dichloromethane (DCM) was purified using a SPBT-1 Bench Top Solvent Purification System before use. While neither THF nor DCM is considered a ‘green’ solvent, they have been used in some of the reactions below because they were historically used in our group for similar chemistry. Alkene-terminated PIB polymers with molecular weights (
Mn) of 450, 1000, and 2300 Da (
1a,
1b, and
1c, respectively) are commercially available and were obtained from Texas Polymer Corp [
31]. The poly(α-olefin) (PAO) solvents used had various molecular weights reflecting the fact that they were obtained from hydrogenated dimers, trimers, and tetramers of 1-decene or 1-dodecene. They are commercially available and were obtained from ExxonMobil (decene oligomers) or Chevron Phillips (dodecene oligomers [
32,
33].
1H,
13C, and
31P NMR spectra were recorded on a Varian 500 MHz Bruker or Avance Neo 400 spectrometer operating at 499.39 and 400.09 MHz interfaced to a Linux computer, and were worked up using the VNMR-J software (version 4.2). Chemical shifts are reported in ppm and referenced to the residual proton in CDCl
3. Spin multiplicities are indicated by the following symbols: s (singlet), d (doublet), t (triplet), dd (doublet of doublet), and m (multiplet). UV–visible spectra were recorded using a Shimadzu UV-2600 UV–visible spectrophotometer. Gas chromatography analyses were performed using a 15 m SPB-5 fused-silica capillary column. Fluorescence spectra were measured on a Horiba FluorEssenceTM fluorescence spectrometer. PIB polymers with end groups (
2, (PIB-CH
2CH(CH
3)CH
2OH),
3 (PIB-CH
2COCH
3),
4 (PIB-CH
2CO
2H),
5 (PIB-CH
2CO
2CH
2CH
3),
6 (PIB-CH
2CH(CH
3)CH
2I),
7 (PIB-CH
2(CH
3)
2C
6H
4OH),
8 (PIB-HMPA (PIB-CH
2CH(CH
3)CH
2N(C
3H
7)PO(N(CH
3)
2)), and PIB-bound ammonium salts (e.g.,
9 PIB-CH
2CH(CH
3)CH
2N
+(C
3H
7)(CH
2CH
3)
2 C
6H
5CO
2-) are compounds that have been prepared and described in the literature [
34,
35,
36,
37].
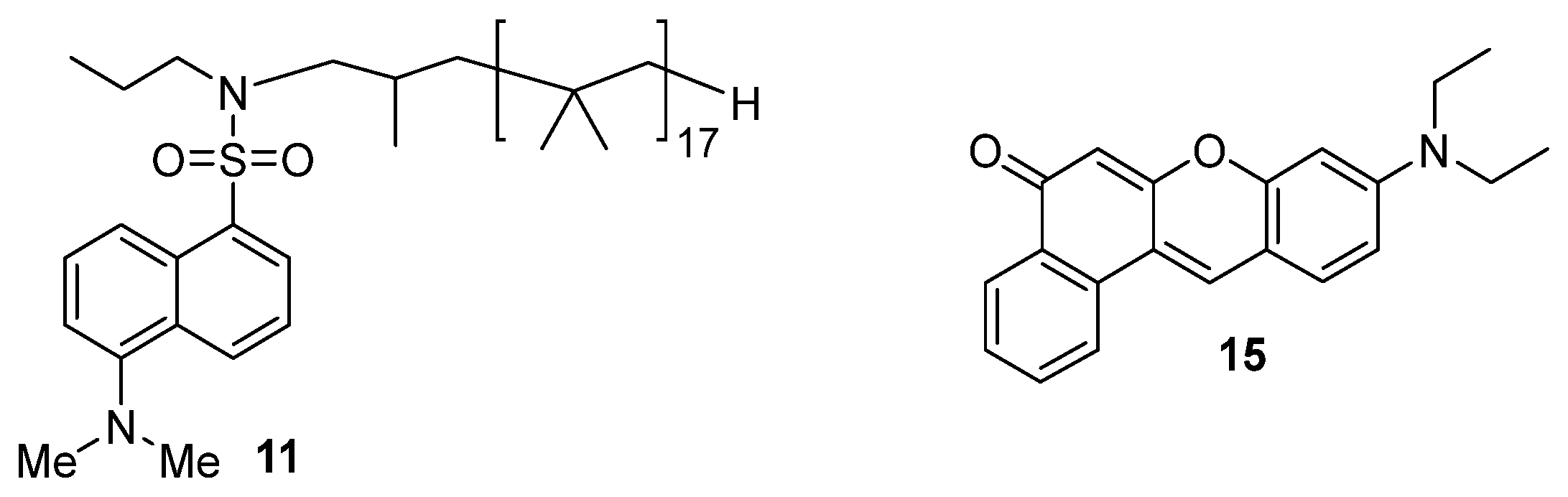
Synthesis of N-Propyl Dansyl Sulfonamide (10): 1-Aminopropane (2.63 g, 44.5 mmol) was added to a round-bottomed flask equipped with a magnetic stir bar. Then 50 mL of MeCN containing dansyl chloride (1.00 g, 0.370 mmol) was added at 0 °C. The reaction mixture was allowed to stir at room temperature for 3 h, and then the solvent was removed under reduced pressure. The residue was dissolved in 100 mL of DCM and washed with water (3 × 30 mL). The organic phase was filtered through silica, and the solvent was removed under reduced pressure. The product N-propylsulfonamide was isolated in 60% yield. 1H NMR (300 MHz, CDCl3): δ 1H (300 MHz, CDCl3) δ: 8.58 (d, J = 8.5 Hz, 1H), 8.28 (t, J = 8.5 Hz, 2H), 7.48 (m, 2H), 7.22 (d, J = 6.0 Hz, 1H), 4.53, (t, J = 6.0 Hz, 2H), 2.92 (s, 6H), 1.38, (m, 2H), 0.78 (t, J = 9.0 Hz, 3).
Synthesis of Dansyl-Terminated PIB (11b): The procedure used to prepare this PIB-bound fluorophore was based on earlier work where we functionalized polymers with
N-butyl dansylsulfonamide [
38,
39]. Dansyl-
N-propyl sulfonamide (1.10 g, 3.76 mmol) was added to a round-bottomed flask equipped with a magnetic stir bar and dissolved in 50 mL of heptane. Then 50 mL of DMF containing 0.72 g (3.8 mmol) of potassium carbonate was added. Heating to 90 °C produced a single phase that was allowed to stir for 1 h. Then 1 mmol of PIB-CH
2CH(CH
3)CH
2I
6b in 5 mL of heptane was added, and the solution was stirred for 96 h. The solution was cooled, and the heptane phase was isolated by a gravity separation. The heptane was then removed at reduced pressure using a rotary evaporator. The product was isolated as a viscous oil in 75% yield.
1H NMR (300 MHz, CDCl
3)
δ:
1H (300 MHz, CDCl
3) δ: 8.53 (d, J = 9.0 Hz, 1H), 8.26 (t, J = 8.0 Hz, 2H), 7.54 (m, 2H), 7.19 (d, J = 6.0 Hz, 1H), 3.30 (m, 2H), 3.06 (m, 2H), 2.90 (s, 6H), 1.6–0.8 (m, 180H).
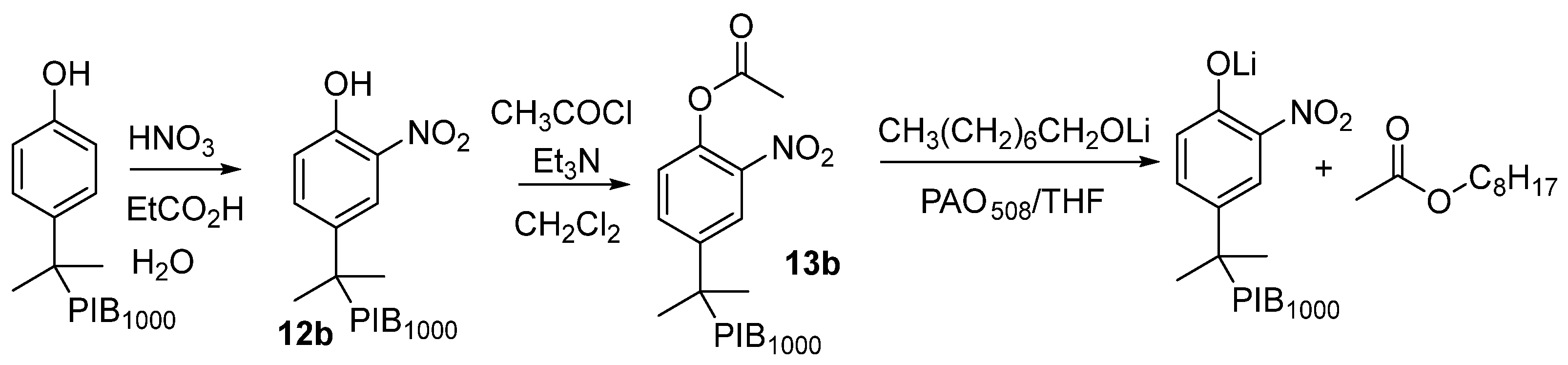
Synthesis of PIB-Bound Nitrophenol (12b): PIB-phenol (7b, PIB1000-CH2(CH3)2C6H4OH) (10 g or 10 mmol) was dissolved in 20 mL of propionic acid in a round-bottomed flask with a stir bar. Then, 2.5 mL of water and 2.5 mL of HNO3 were added at 0 °C, and the reaction was allowed to proceed for 1 h. The product 12b was obtained by adding 30 mL of water and 20 mL of hexane and extracting the product into hexane. The hexane layer was dried with Na2SO4, filtered, and the solvent was removed at reduced pressure using a rotary evaporator to obtain 9.3 g (93% yield) of a viscous pale orange oil: 1H NMR (400 MHz, CDCl3) δ: 7.98 (s, 1 H), 7.57 (d, J = 8.5 Hz, 1 H), 7.08 (d, J = 8.5 Hz, 1 H), 1.81 (s, 2 H), 1.4–0.8 (m, 80H).
Synthesis of PIB-bound Nitrophenyl Acetate (13b): PIB-nitrophenol (12b) (10 g, 10 mmol) was dissolved in 50 mL of DCM in a round-bottomed flask with a stir bar. Then, 3 g (30 mmol) of triethylamine was added to form a dark orange solution. This solution was cooled to 0 °C, and 2 g (25 mmol) of acetyl chloride was added dropwise. The reaction mixture was allowed to stir overnight and then refluxed for 2 h. After cooling, the solvent was removed at reduced pressure. The residue was taken up in 10 mL of hexane and then washed successively with acetonitrile (3 × 10 mL), 90% ethanol/water (3 × 10 mL), and brine (1 × 10 mL). The hexane phase was dried with Na2SO4, filtered, and the solvent was removed at reduced pressure to yield 6.9 g (69%) of a viscous pale oil: 1H NMR (400 MHz, CDCl3) δ: 8.08 (s, 1 H), 7.65 (d, J = 9 Hz, 1 H), 7.14 (d, J = 9 Hz, 1 H), 2.37 (s, 3 H), 1.87 (s, 2 H), 1.4–0.8 (m, 80 H). End group analysis by 1H NMR spectroscopy using dichloroethane as an internal standard showed that 13b had a loading of ester of 1.2 mmol/g.
Lithium Octanoxide (prepared in situ): 1-Octanol (1.05 mmol, 0.14 g) was added to a 20 mL scintillation vial with a septum cap along with 9.6 mL of dried and distilled THF. The vial was then placed in an ice bath, and 2.5 M n-BuLi (1 mmol, 0.4 mL) was added to the vial by syringe. The product solution was titrated with 0.01 M HCl to determine the concentration of the lithium alkoxide was 0.07 M.
Synthesis of the Diisopropylamide-Terminated PIB Oligomer (14c): A carboxylic acid-terminated PIB oligomer (4c) (58.6 g, 25 mmol) was dissolved in 150 mL of dry DCM, and 5 drops of DMF were added to the mixture as a catalyst. Then, oxalyl chloride (3.8 mL, 44.2 mmol) was added dropwise to the reaction mixture. The reaction mixture was allowed to stir for 45 min. At that point, excess diisopropylamine (18.3 mL, 130 mmol) was slowly added to the reaction solution, and the reaction mixture was allowed to react overnight at ambient temperature. The DCM was then removed under reduced pressure. Hexane (200 mL) was then used to dissolve the crude reaction products. The hexane layer was washed with 2 M NaOH (200 mL × 2), MeCN (200 mL × 3), 80% EtOH:H2O (200 mL × 2), and brine (200 mL × 2). The hexane solvent was subsequently removed under reduced pressure using a rotary evaporator to yield 22.2 g of product (80% yield over two steps). 1H NMR (400 MHz, CDCl3) δ: 4.14–4.02 (m, 1H), 3.55–3.37 (m, 1H), 2.30 (s, 2H), 1.48–1.40 (m, 80H), 1.18–1.08 (m, 240H).). 13C NMR (100 MHz, CDCl3) δ: 170.9, multiple peaks between 59.6 and 58.3, 56.9, 47.0, 45.7, 42.1; multiple peaks between 38.4 and 37.8, 36.1, 34.7, 34.6; multiple peaks between 32.6 and 30.9, 30.3, 27.0, 25.4, 22.7, 20.9, 14.3, and 11.6.
Solvatochromic Studies in Solvent Mixtures: Solutions of the PIB-bound dansyl dye 11 (1 × 10−6 M) or Nile red 15 (1 × 10−8 M) were prepared in 2 mL of heptane or PAO, and the fluorescence was measured. Then known amounts of cosolvent were added, and the fluorescence emission spectrum was measured. In these studies, the solutions of the dansyl dye were excited at 354 nm, and solutions of Nile red were excited at 420 nm. The max emission wavelength (the λEM) of the fluorophores in pure alkane solvent or polar cosolvent was used to determine the ∆λEM of the fluorophore in the solvent mixtures. The λEM of 15 was 525 nm in heptane and 529 to 531 nm in the PAOs. In more polar solvents, the λEM of each of the dyes increased. Dye 11 has a working range (a ∆λEM) of 65 nm corresponding to the difference between the λEM of 11 in heptane and ethanol. Nile red has a working range (a ∆λEM) of 98 nm corresponding to the difference between the λEM of 15 in heptane and ethanol (525 nm and 623 nm, respectively.
In calculating the volume percent of added polar solvent to alkanes, we assume that the solvent volumes are additive. Thus, volume % of a polar solvent/alkane solvent mixture refers to the added volume of a solvent divided by the volume of the original alkane solvent and volume of the added solvent. While we recognize that these are not ideal solvents, we expected the volume of the solvent mixture to be roughly equal to the volume of the components. This assumption was experimentally tested qualitatively for several mixtures—a 10/90, 50/50, and 90/10 vol/vol mixture of THF and heptane, ethyl acetate and heptane, ethanol and heptane, and dichloromethane and heptane using a 10 mL graduated cylinder to measure volumes of each component and of the product solvent mixture. Visually, the total volume of these 10 mL mixtures in these experiments was > 95% and < 105% of the sum of the volume of each solvent.
General Procedure for Transesterification Reactions: The general procedure to study transesterification involved preparing a solution of the PIB-bound nitrophenylacetate 13b in a solvent or solvent mixture (PAO508, PAO508/2.4 M THF, PAO508/6.0 M THF, or THF) and measuring its concentration by UV spectroscopy (λmax of 268 nm). Then a stoichiometric amount of 0.07 M lithium octanoxide solution was added. The reaction was followed by UV visible spectroscopy for the first 20% of the reaction by following the appearance of the anion of 12b (λmax of 450 nm).
General Procedure of Nucleophilic Substitution Reactions: The kinetic studies followed the procedure we described previously [
36]. A 0.1 M solution of equivalent amounts of the substrates, the nucleophile source, and solvent was prepared. The reaction was stirred at 55 °C. An aliquot (100 μL) of the reaction mixture was taken at different time intervals. In cases where polar solvents were used, the aliquot was diluted with 1 mL of diethyl ether and washed with 1 mL of water. The two phases were separated, and the organic layer was dried with anhydrous sodium sulfate. The solution was then submitted for GC analysis. When alkane solvents were used, an aliquot (100 μL) of the reaction mixture was passed through a pipette silica gel column using 1 mL of hexane. The eluent was submitted to GC for analysis. The rate constant, k, was calculated by using Solver in Excel for nonlinear curve fitting.
The recycling experiment using a PIB-bound HMPA cosolvent was carried out using a 0.1 M solution of 1-butylmesylate and 10 mol % of the PIB-bound ammonium salt in PAO (10 mL). The solid alkali metal acetate salt (3 equiv) was added to the reaction mixture, and the reaction was stirred for 24–36 h at 55 °C. At that point, the product ester was isolated by vacuum distillation (0.004 Torr). The PAO solution containing the PIB-bound ammonium salt catalyst was then separated from the solids using centrifugation for 15 min at 25 °C with a spinning speed of 3000 rpm. After decanting the solution from the solid, fresh 1-butylmesylate and potassium benzoate were added to the recovered PAO solution, and the reaction was repeated. The product butyl benzoate was isolated after each cycle, and the average yield per cycle was 88%. While the isolated yield varied from cycle to cycle slightly (89%, 83%, 85%, and 91% in cycles 1, 2, 3, and 4), GC analysis indicated complete conversion of the butyl mesylate to the butyl benzoate.
3. Results and Discussion
Miscible solvent mixtures have properties like refractive index, density, viscosity, and bulk polarity that vary in a roughly linear manner with the mole fraction of each component. As noted above, our prior work with heptane or PAOs as recyclable hydrocarbon solvents often showed that adding as little as 0.1–2 M of a polar cosolvent could often significantly facilitate a reaction. While these mixtures of heptane or PAO with a polar solvent were visibly homogeneous, it is known that otherwise macrohomogeneous solvent mixtures can be inhomogeneous on the nano scale. For example, water and ethanol, water and acetonitrile, and water and DMSO are miscible at any mixing ratio. However, these visually homogeneous solutions on a nano scale deviate from ideal mixing. Such solutions’ microscopic phase separation is described as microheterogeneity [
40].
Microheterogeneity effects are well established for many solvent mixtures, but have been most studied for mixtures of H-bonding solvent mixtures. Theoretical studies [
41], mass spectroscopy [
42], NMR spectroscopy [
43,
44], absorption spectroscopy [
45], and solvatochromic dyes have all been used to study microheterogeneity [
46,
47,
48]. While microheterogeneity has been less studied in alkane or polymeric solvents, we found that solvatochromatic probes like those that have been used previously to study microheterogeneity in other solvent mixtures worked well in these solvents too. [
46,
47,
48,
49]
The most common solvatochromic dye used for assaying solvent polarity is Reichart’s dye [
49]. However, this dye was not a good choice for our purposes as it has little alkane solubility. While this could be remedied by synthesis of an alkane-soluble analog, that was deemed less feasible than using alternative dyes. Two such alternative fluorescent dyes were used—dansyl bound to PIB (
11) and Nile red (
15) (
Scheme 1).
We previously prepared polymer-bound dansyl sulfonamides as probes of poly mer-solubility [
38,
39]. Similar chemistry afforded an alkane-soluble PIB-bound dansyl sulfonamide
11 (
Scheme 2). Nile red (
15) is commercially available and has been used by others to probe polarity and microviscosity effects in mixtures of polar and nonpolar solvents [
50]. To study microheterogeneity effects of polar solvents in alkanes or PAOs, we measured the bathochromic shifts for the maximum emission wavelength (λ
EM) of the fluorescence of
11 or
15. We first measured the emission of the fluorophores in the bulk solvents.
Table 1 lists the λ
EM of the dyes in different solvents. The λ
EM of the
11 was 447 nm in heptane and 448 to 449 nm in the PAOs. The λ
EM of
15 was 525 nm in heptane and 529 to 531 nm in the PAOs. In more polar solvents, the λ
EM of each of the dyes increased. Dye
11 has a working range (∆λ
EM) of 65 nm corresponding to the difference between the λ
EM of
11 in heptane and ethanol. Nile red has a working range (a ∆λ
EM) of 98 nm corresponding to the difference between the λ
EM of
15 in heptane and ethanol (525 nm and 623 nm, respectively).
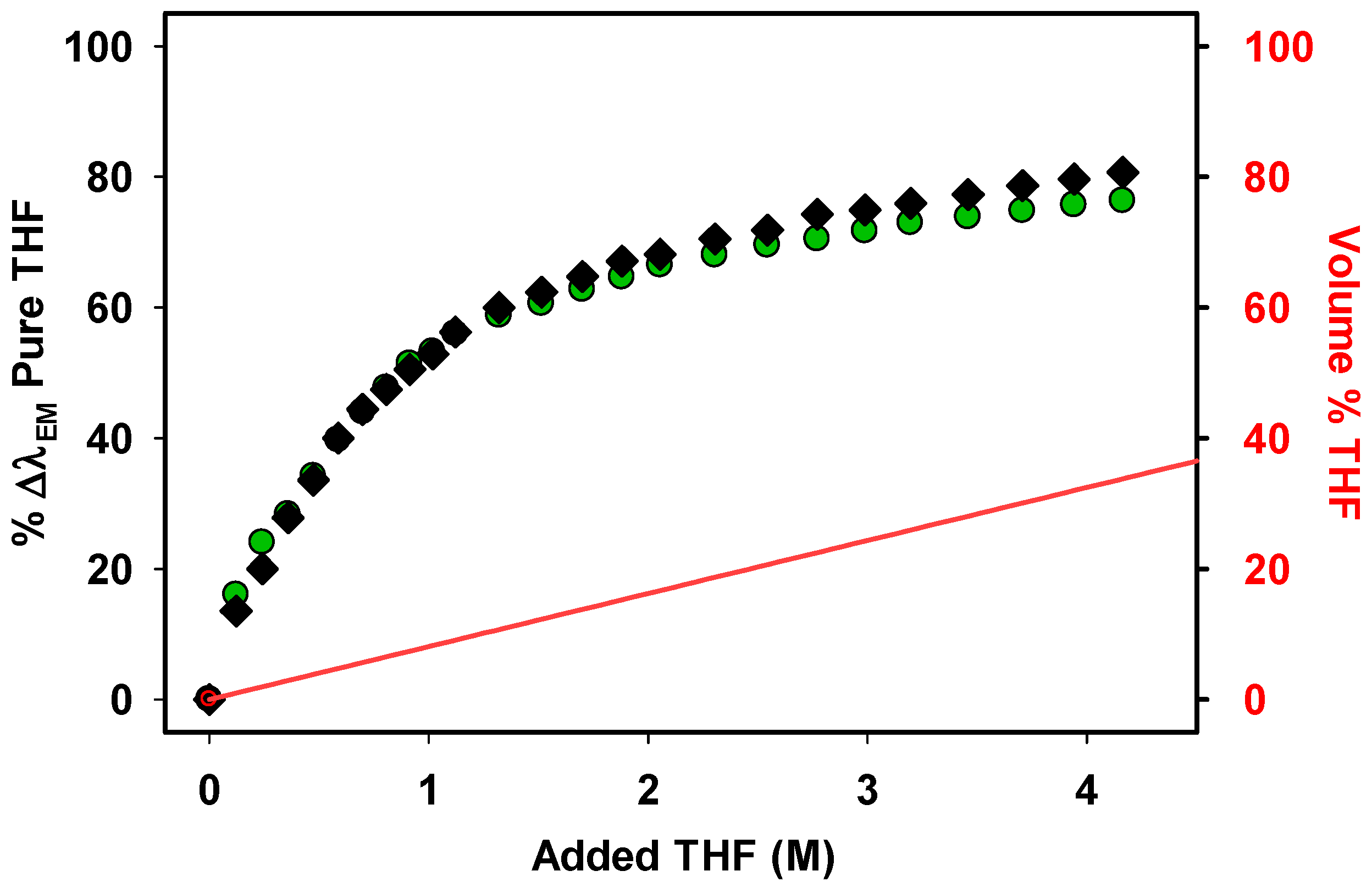
While microheterogeneity in solvent mixtures of low-molecular-weight solvents has been studied and while solvatochromic dyes have been used in such studies, we are unaware of studies using polymer-bound dyes to study microheterogeneity in polymeric solvent systems. We first used
11 to study the effect of adding various amounts of THF to a solution of
11 in heptane, PAO
283, or PAO
687 (
Figure 1). We prepared a solution of
11 in heptane or PAO and then added a known volume of THF and recorded the change in λ
EM. This process continued about 15 times for each alkane solvent. We calculated the % change in the emission λ
EM—the %∆λ
EM—of
11 and then plotted the %∆λ
EM as a function of the molarity of the polar solvent in the solvent mixture. For example, for
11, the λ
EM in heptane is 447 nm and 493 nm in pure THF, and the maximum ∆λ
EM is 46 nm. When 2 M THF is present, the λ
EM is ca 470 nm or 43% of the ∆λ
EM for
11 in heptane versus THF.
The nonlinear behavior in
Figure 2 for the %∆λ
EM as a function of solvent composition relative to the linear change in concentration of THF is evidence that the mixtures of THF in heptane, PAO
283, or PAO
687 are microheterogeneous. We hypothesize that this microheterogeneity occurs because THF at low concentrations solvates the dye by dipole–dipole interactions better than an alkane solvent that only interacts with the dye by dispersion forces. Thus, small amounts of THF lead to a significant solvatochromatic shift in the λ
EM. In contrast, when adding alkane to pure THF, the alkane has little effect on the dye’s λ
EM since alkane does not compete with THF in solvating the polar dye solute.
We also studied the effects of THF addition on solutions of Nile red in heptane or PAO
432 (
Figure 2). The plot of ∆λ
EM that resulted from assays of the λ
EM of
15 in ca. 24 different mixtures of THF and these two alkanes had the same sort of nonlinear behavior seen in
Figure 1. In this case, the presence of 2 M THF led to a %∆λ
EM of 64%. Similar studies of solvatochromatic shifts with other polar solvents further confirmed that macrohomogeneous solutions of low-molecular-weight polar solvents in heptane or PAO generally exhibit microheterogeneity, with the polar solvent preferentially solvating the polar dye, whether it is bound to PIB (
11) or is a non-polymeric dye
15. Graphs of these studies using ethyl acetate (EtOAc), dichloromethane (DCM), or 1-octyl-2-pyrrolidone are provided in the
supporting information (Figures S1, S2, S3, and S4, respectively). These cosolvents, like THF, stabilize the excited state of
11 and
15 by dipole–dipole interactions, leading to bathochromic shifts in dye fluorescence. We postulated that cosolvents that have stronger H-bonding interactions with the solvatochromatic dyes
11 and
15 using H-bonding cosolvents would have greater effects.
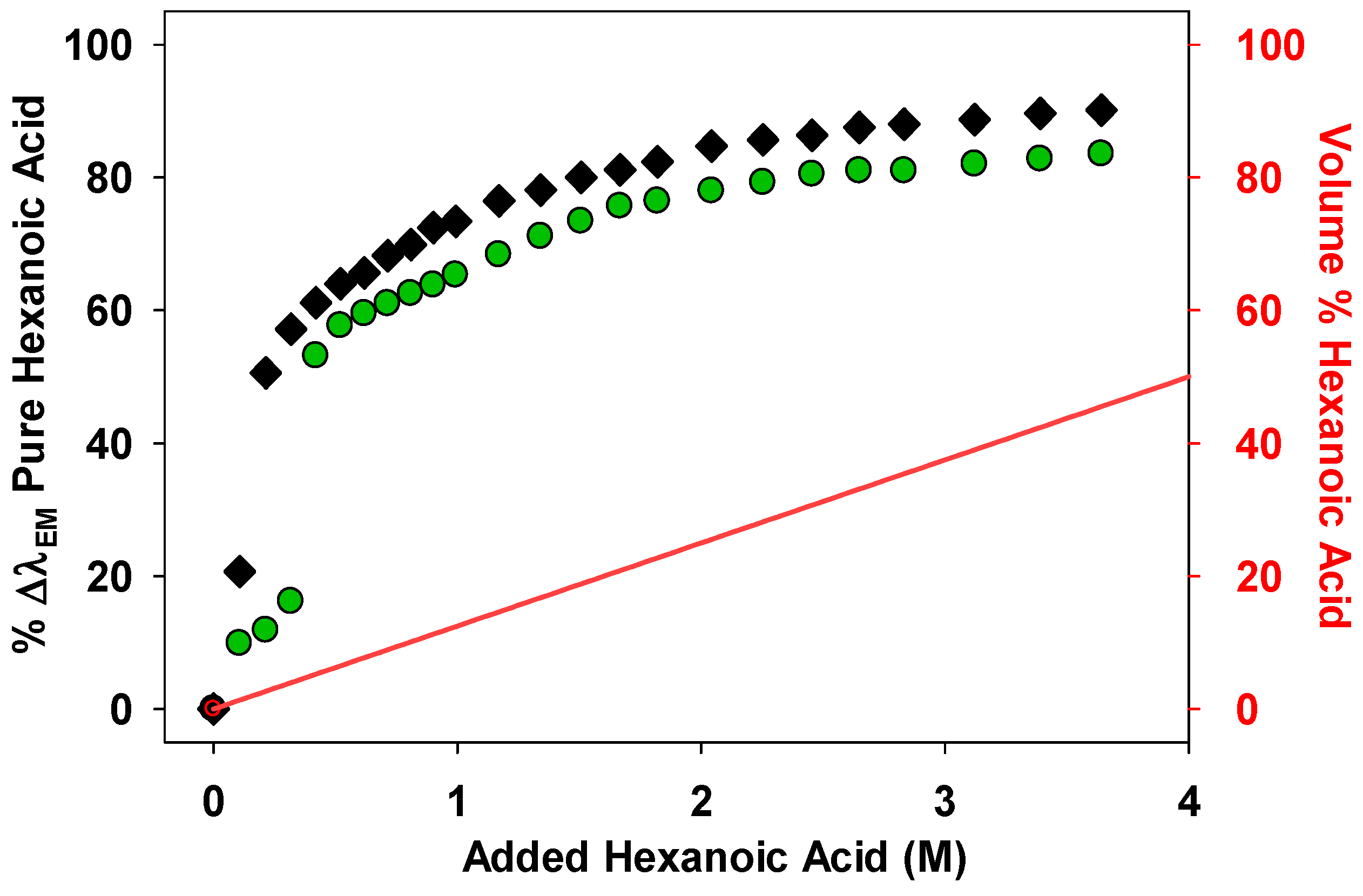
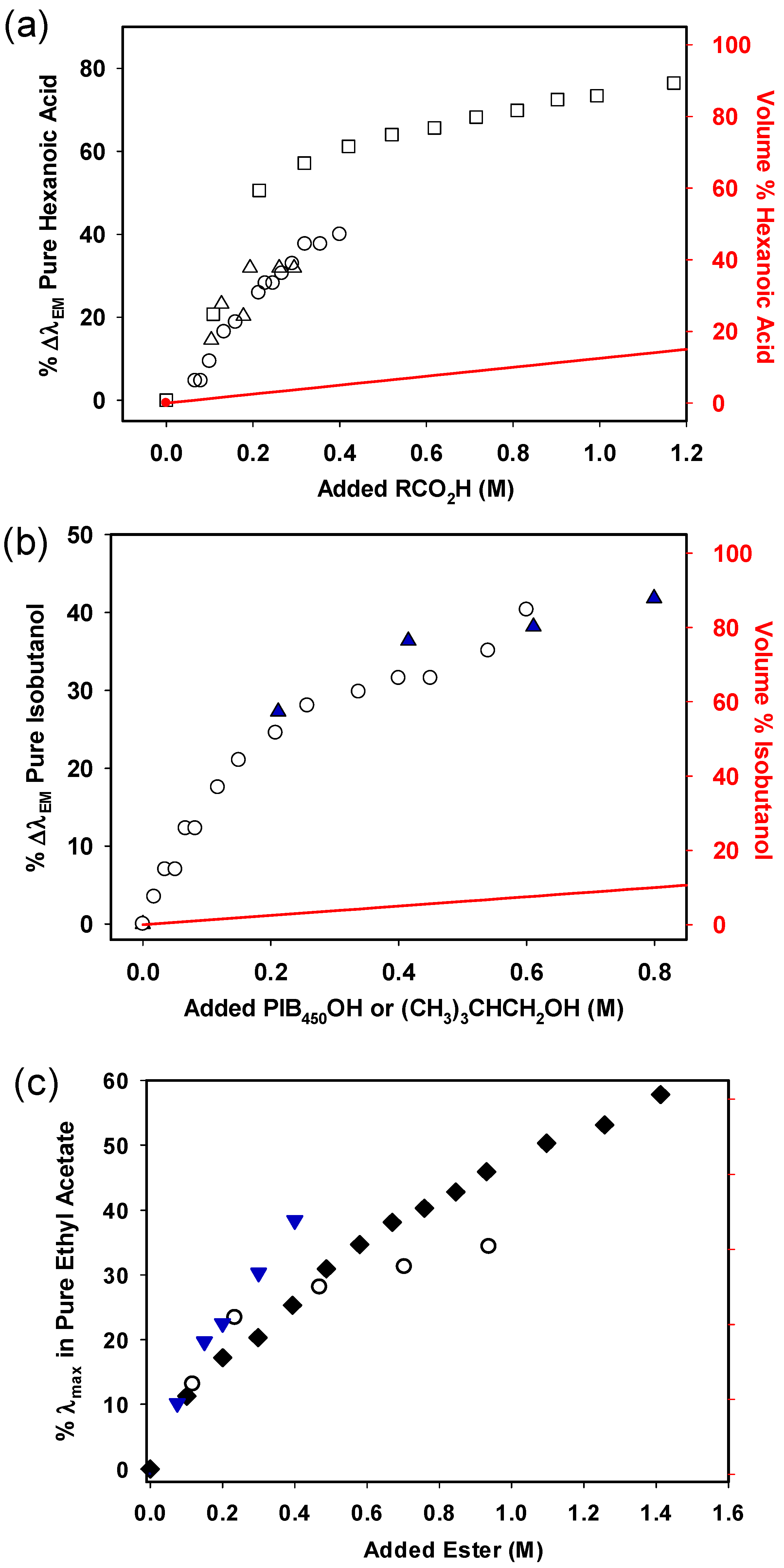
These studies began by studying how the %∆λ
EM changes when hexanoic acid was used as a cosolvent in solutions of
15 in heptane or PAO
432 (
Figure 3). The effect of the hexanoic acid on the λ
EM of the Nile red fluorophore is much more pronounced at low concentrations compared to solvents like THF, which cannot hydrogen bond with the fluorophore. For example, while 1 M THF in an alkane solvent was necessary to produce a 50%∆λ
EM for the Nile red fluorophore
15, ca. 0.2 M hexanoic acid achieved this same 50% ∆λ
EM. We ascribe the larger impact of the cosolvent to the fact that unlike THF, hexanoic acid has stronger H-bond interactions with the Nile red fluorophore, making it more effective at lower concentrations.
Figure 4 shows that alcohol cosolvents in heptane solutions of
11 also exhibit microheterogeneity behavior. In this case too, alcohols can interact with the dye by H-bonding. As shown, over half of the change in ∆λ
EM is seen with <0.4 M of
n-butanol cosolvent. The more hindered
tert-butanol is somewhat less effective than other alcohols, but at ca. 1.0 M, all the alcohol cosolvents other than
tert-butanol have at least a 50% ∆λ
EM.
These results have Green Chemistry implications for using PAOs as recyclable and tunable alternative solvent systems. They suggest that it would be possible to use PAO as an alternative recyclable bulk solvent and fine-tune the polarity experienced by polar solute molecules by adding a small volume of polar cosolvents. While the added cosolvent might not be fully recyclable, we could significantly reduce the amount of solvent needed for a reaction by taking advantage of microheterogeneity effects. What would be more interesting would be to design a fully recyclable system using PAO and a recyclable polymeric cosolvent.
Our prior work has shown that soluble homogenous polymer-bound ligands or catalysts at the termini of polymers behave identically to their low-molecular-weight analogs under similar conditions [
51,
52,
53]. We hypothesized that this effect could also be seen with a polymer-bound cosolvent. We also expected a PIB-bound solvent that behaved like its low-molecular-weight analog would be as recyclable as the PIB-bound catalysts we have already explored. In such a case, such a cosolvent in a PAO solution would be a fully recyclable polarized solvent system.
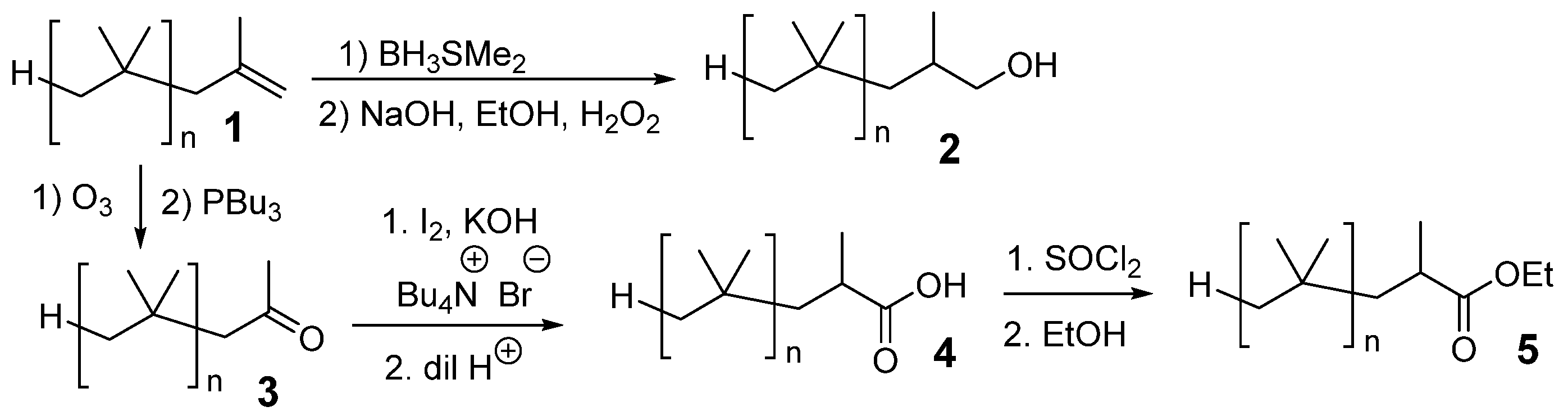
To explore whether a PIB-bound cosolvent could behave like a low-molecular-weight solvent in creating microheterogeneous solutions, we prepared PIB-bound alcohols, PIB-bound acids, and PIB-bound esters as analogs of butanol, hexanoic acid, and EtOAc (
Scheme 3) [
34]. However, while the products of these syntheses could act like EtOH, hexanoic acid, or EtOAc, they also have limitations. Most of the mass of these PIB-bound cosolvents is the PIB group. Thus, neat PIB-bound cosolvents with ca. 9, 17, or 40 repeating isobutylene groups (i.e., PIB350, PIB1000, or PIB2300) have a molarity of ca. 2 M, 1 M, and 0.5 M, respectively. As a result, a PIB cosolvent diluted with heptane or PAO has a maximum usable molarity of polar groups that is generally less than 1 M.
To test how a PIB-bound alcohol
2, the PIB-bound carboxylic acid
4, and the PIB-bound ester
5 compare with low-molecular-weight cosolvents, we measured the λ
EM shift of
15 using 0–1 M solutions of these three PIB-bound cosolvents in either heptane or PAO.
Figure 5a–c compare the % ∆λ
max for
15 in the presence of
2,
4, and
5, respectively, to data from earlier experiments with hexanoic acid, butanol, and ethyl acetate. The results show that these PIB-bound cosolvents affect the solute dye in much the same way as their low-molecular-weight analogs. However, unlike their low-molecular-weight analogs, these PIB-bound species are recyclable.
These dye studies suggest microheterogeneity effects could be useful in facilitating a stoichiometric or catalytic reaction in a more sustainable and recyclable solvent mixture. We used two reactions to this possibility. In the first case we studied the kinetics of a transesterification in a microheterogeneous THF/PAO mixture. This chemistry used a PAO soluble PIB-bound ester substrate we prepared and lithium octanoxide to effect a transesterification (
Scheme 4). This transesterification was chosen as a model reaction because the kinetics for formation of the nitrophenolate anion can be measured using UV–visible spectroscopy since the nitrophenoxy group in the ester has a λ
max of 268 nm and the anion has a λ
max of 450 nm. In this case, the kinetics for the first 20% of this transesterification were studied in pure PAO
508, 2.4 M THF in PAO
508, 6.0 M THF in PAO
508, or pure THF as a solvent. The solvent mixtures chosen resemble mixtures studied in
Figure 2 and
Figure 3 that exhibited microheterogeneity.
As expected, transesterification was faster in more polar solvents and faster in PAO with THF as a cosolvent. As shown by the data in
Table 2, transesterification was ca. 200 times faster in pure THF than in PAO
506. However, in 2.4 M THF, the transesterification rate was 80 times faster than in PAO
506 and roughly 40% the rate in pure THF. The studies with dyes in the THF/PAO mixtures in
Figure 2 and
Figure 3 suggested that a dye had a solvent environment in 2 M THF/heptane or PAO that was 43% (for dye
11) or 64% (for dye
15) the same polarity as pure THF, suggesting that microheterogeneity had roughly the same effect on this transesterification reaction as it did on dye fluorescence.
The second reaction we examined involved studying S
N2 chemistry we previously showed occurred in heptane or PAOs [
36]. That work showed that tetrabutylammonium benzoate or PIB
2300-tetraalkylammonium benzoate (
9c) effected an S
N2 reaction in heptane or PAO (
Scheme 5). In this chemistry, the PIB
2300-bound ammonium salt acts as a phase transfer catalyst to synthesize butyl benzoate from butyl mesylate using solid potassium benzoate.
In order to examine how added polar PIB-bound cosolvents might affect this reaction in an alkane, we looked at three different versions of this experiment that used PAO
432 as a solvent. We used the same phase transfer catalyst but with 0.5 M PIB
1000-CH
2CH(CH
3)CH
2OH (
2b), 0.2 M PIB
2300-CON(iPr)
2, (
14c), or 0.5 M PIB
1000-bound HMPA (
8b) as a cosolvent. The results shown in
Table 3 show that a PIB
2300-bound carboxamide (
8b) cosolvent had a modest effect, accelerating the reaction by a factor of ca. 2.4 over the reaction in PAO alone. The effect of the PIB
1000-CH
2CH(CH
3)CH
2OH (
2b) cosolvent was more striking. In this case, the presence of PIB
1000-bound alcohol lowered the rate by 10-fold, a change consistent with the known effects of H-bonding solvents on S
N2 chemistry. Finally, we examined a PIB-HMPA analog
8 that we had previously studied as an organocatalyst [
29] but now used as a cosolvent. In this case, the reaction rate increased by a factor of ca. 3.3. In this latter case, after we had obtained kinetic data, we left the reaction to continue until the butyl mesylate substrate had been consumed. At that point, we isolated the butyl benzoate product at reduced pressure. The volume of the solution of the PAO
432, the PIB-bound HMPA, and the PIB-bound phase transfer catalyst did not measurably decrease in this process. We then separated the PAO solution of the catalyst from the solid and added more butyl mesylate and solid potassium benzoate. The resulting suspension of potassium benzoate in PAO
432 containing the PIB-bound HMPA, and the PIB-bound phase transfer catalyst containing reaction mixture was reheated to 55 °C for 24 h. We repeated this process three more times. The volatile butyl benzoate product that was isolated in a liquid nitrogen trap was then measured gravimetrically. The yield of the ester product averaged 88% over those four cycles, suggesting the catalyst, solvent, and polymeric cosolvent can be successfully recycled as we expected.
The studies in
Table 3 not only show the effects of microheterogeneity of a polymer-bound cosolvent on a reaction, but they also highlight another advantage of these systems. While in our prior work we were able to show that an added dipolar solvent like DMF accelerated the chemistry in
Scheme 5, HMPA was not studied because it had no appreciable solubility in the alkane solvents we used. While the DMF result showed an effect, neither DMF nor HMPA is a green solvent. Both the PIB-bound carboxamide and the PIB-bound HMPA would be fully recyclable in this chemistry and could be greener versions of these solvents. Given that these cosolvents contain a polyisobutylene group, we believe both would have little or no bioavailability, making them potentially environmentally more friendly versions of these dipolar aprotic solvents.