Abstract
Peng–Robinson equation of state (PR EoS) has good prediction accuracy for phase diagrams of pure substances or mixtures, but liquid density, especially for high polar substances, is known to be ~20% lower value compared with experimental data at standard atmospheric temperature and pressure (SATP) conditions. To overcome this issue, translation via entropy-based solubility parameter (eSP) Peng–Robinson EoS (eSPT-PR EoS) is proposed in this work. The technique uses eSP for the liquid phase at SATP conditions and correlates the ideal value and a constant C for each substance as a correction. As a result, the C value can be linearly correlated with critical compressibility factor (ZC). Finally, the liquid density was improved and gave an average relative deviation (ARD) value of 4.2% for the generally used 27 chemicals selected at SATP condition. Furthermore, critical density was also improved and gave ARD values of 3.9% compared with the original PR EoS of 21.8%. Thus, a universal calculation method based on PR EoS was developed for improving liquid density representation with the eSPT-PR EoS.
1. Introduction
Peng–Robinson equation of state (PR EoS) [1] is widely used by scientists and engineers to predict high-pressure phase diagrams for manufacturing products. Not only pure substances, but also multiple mixtures are studied in the prediction of phase diagrams. The utility of PR EoS for petroleum industries [2,3] is very wide and is commonly applied to simulation of both super- and sub-critical processes in the manufacture of chemical products [4,5,6,7,8].
However, the PR EoS has low prediction ability for liquid density, especially for high polar substances like ethanol, methanol and water. Therefore, volume-translated PR EoS (VT-PR EoS) has been previously proposed by researchers [9,10,11,12] to improve volumetric representation. The VT-PR EoS method has become well established, but always needs experimental density data and a fixed reference condition, which is disadvantageous at times, because experimental density data may be limited or may require estimation when conditions of the simulation are very different from those where density data are available. Therefore, a different type of translation technique is needed to predict phase diagrams reliably, keeping the future development of chemical products in mind.
Hildebrand Solubility Parameter (SP) is widely used for processing extraction and separation systems that involve chemical-dominated phenomena. Entropy-based solubility parameter (eSP) [13,14], which is linearly correlated to the Hildebrand SP [15] at the standard temperature and pressure (SATP) condition, is used not only for vapor–liquid multiple phases but also for a single phase like supercritical fluids for both pure substances and mixtures. From this point of view, eSP has been used for extraction and separation processing of high-pressure systems like subcritical fluid separation (SFS) or supercritical fluid extractions (SFE). In preliminary research, calculated eSP values with the original PR EoS for liquid density at SATP condition deviated from the theoretical values. Therefore, in this study, it was decided to consider a correction to PR EoS as a translation. For adding this eSP correction to PR EoS, a database of chemicals was used as a basis, as described in detail later. These solvents are usually seen in chemistry, for example, in the processing of organic–inorganic hybrid nano particles [16] for dispersion, coating and catalyst preparation.
The added eSP translation correction to the PR EoS is denoted as eSPT-PR EoS. In the eSPT-PR EoS, a newly added constant C is proposed, and the resulting C value is generalized with fundamental properties of chemical species.
2. Materials and Methods
Table 1 shows the fundamental properties for the 27 selected substances that are generally used in chemistry. Critical properties and acentric factors are listed. The Hildebrand solubility parameter (SP) is generally evaluated at ambient conditions [15] and is defined for organic compounds by Equation (1):

Table 1.
Fundamental properties of 27 selected chemicals.
In Equation (1), is the cohesive energy and is the molar volume of the liquid. Usually, the Hildebrand SP is calculated at a SATP condition (298.2 K and 101.3 kPa), where is the enthalpy of vaporization and is the molar volume of gas.
The eSP [13,14] defined in Equation (2) is more widely used not only for multiple phases such as vapor and liquid phases, but also for homogeneous supercritical fluid phases. The eSP is defined as
In Equation (2), s is the molar entropy, and a Maxwell relationship is used to determine its volumetric dependence. The relationship between SP and eSP is estimated with the following thermodynamic relationships:
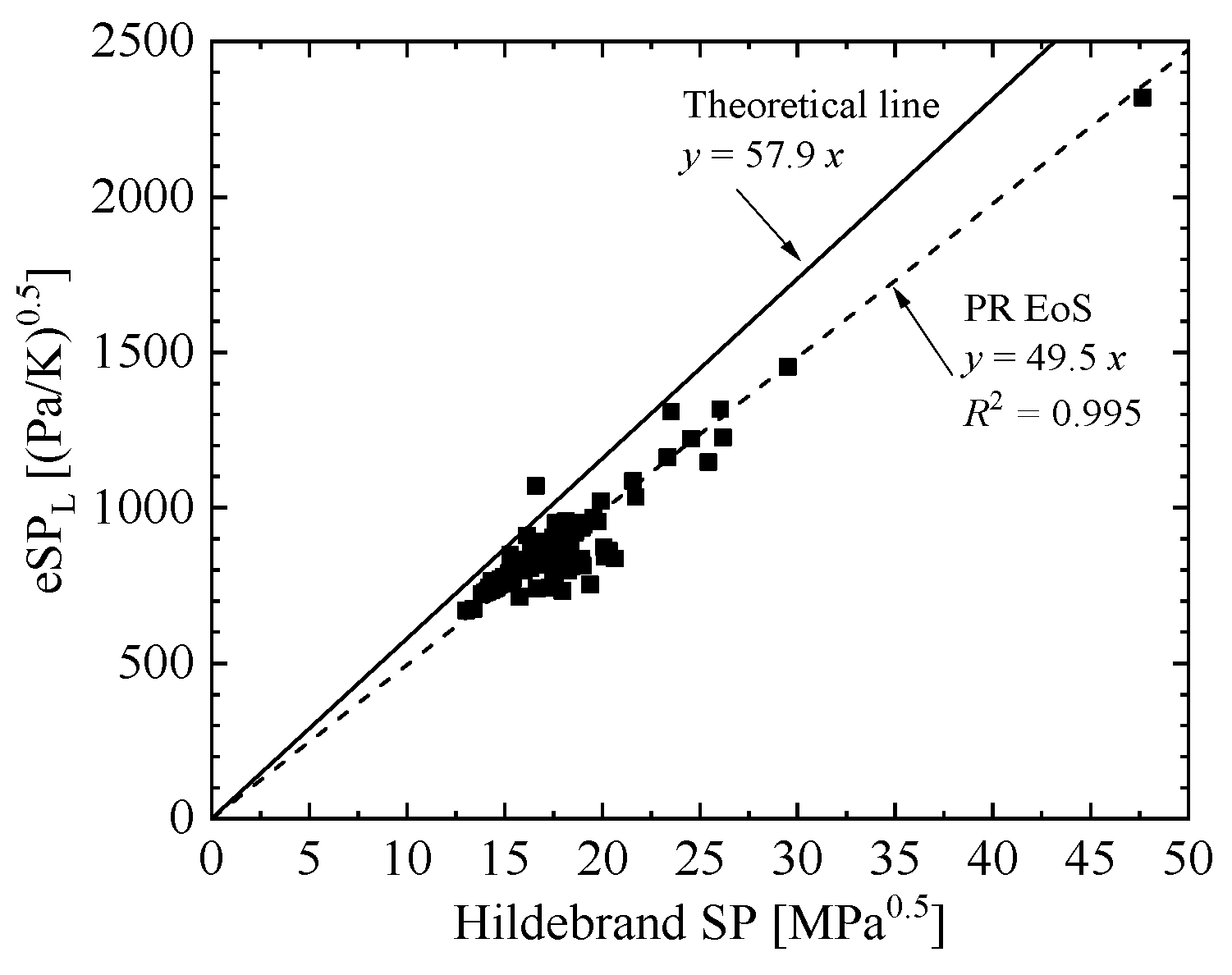
where eSPIdeal is the theoretical eSP, which was plotted as a function of Hildebrand SP, as shown in Figure 1 for 124 substances from ref. [17] at SATP conditions (298.2 K and 101.3 kPa). In Figure 1, the results calculated with the original PR EoS were added, which shows a large deviation from the theoretical line. The slope of theoretical line was 1.17 (= 57.9/49.5) times larger than the slope of the original PR EoS. Therefore, it is clear that a correction in form of eSP can be added to improve liquid density representation compared with the original PR EoS.
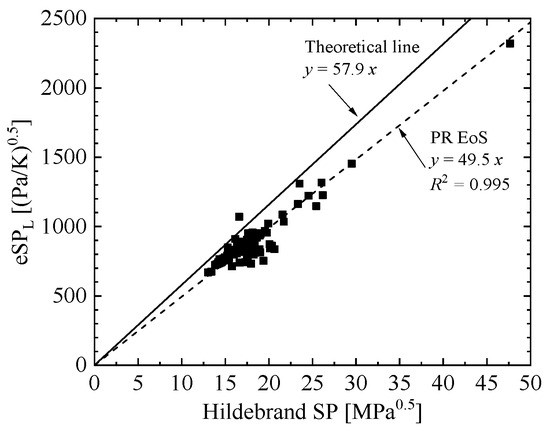
Figure 1.
Theoretical eSP (eSPIdeal) at SATP conditions plotted as a function of Hildebrand SP at 298.2 K and 101.3 kPa.
For the liquid density corrections with eSPT-PR EoS, the original PR EoS in Equation (4):
was modified as given by Equation (5):
A constant C was newly introduced into the eSPT-PR EoS, even though the original PR EoS has only two constants. In the eSPT-PR EoS model, Equation (5), f (v) = v − b and β = 1 were adopted.
For EoS parameters ac and b in Equation (5), the constant C was also introduced as follows:
The function of original PR EoS was adopted and is described by Equation (8):
In Equation (8), Tr is a reduced temperature (= T/TC). For eSPT-PR EoS, pure parameters in Equation (6), in Equation (7) and , , in Equation (8) were redetermined by fitting to experimental data for the compounds in the database.
The objective function for data fitting was chosen to be dimensionless as follows:
In Equation (9), arbitrary weights were used to adjust each term to have the same order of magnitude. Average Relative Deviation (ARD) was defined as follows:
3. Results
The five pure-component parameters ( in Equation (6), in Equation (7) and , , in Equation (8)) and each constant C in Equation (5), which is substance-dependent due to the 27 selected chemicals, were determined using the objective function in Equation (9) by a least-squares method. Namely, a total of 32 (=5 + 27) parameters were simultaneously determined by data fitting. The resulting parameters are listed in Table 2 and detailed results are given in Table 3.

Table 2.
Pure-component parameters in original PR EoS given by Equation (4) and eSPT-PR EoS given by Equation (5).

Table 3.
Obtained constant C of 27 selected chemicals with eSPT-PR EoS and deviations from experimental data values.
4. Discussion
At first, the resulting constant C was plotted against many fundamental properties including compressibility factor ZC. As a result, it was found that the constant C was proportional to the value of ZC as follows:
In Equation (12), D = −28.537 and E = 8.2377 (R2 = 0.9285). Then, the calculated data were refit to the experimental data to redetermine constant D and E in Equation (12), as well as five pure parameters ( in Equation (6), in Equation (7) and , , in Equation (8)). According to this treatment, the number of fitting parameters was reduced from a total of 32 parameters to a total of 7 parameters, and universal constants were determined as D = −27.6704 and E = 8.73306 (R2 = 1.000).
In Table 4, the eSPT-PR EoS was compared with the original PR EoS and the VTPR-EoS. For calculation with the eSPT-PR EoS, the objective function was changed to Equation (13) to account for all terms. In Equation (13), the saturated pressure was added to the standard atmospheric temperature () as follows:

Table 4.
Comparison of generalized eSPT-PR EoS given by Equations (5) and (12) with other equations of state.
The arbitrarily weighted calculation method was also applied to Equation (13) because of the different magnitudes of the terms. The eSPT-PR EoS compared favorably with the original and volume-translated PR-EoS (Table 4). In Table 5, four pure substances (n-hexane, CO2, methanol and water) were predicted with the eSPT-PR EoS given by Equations (5) and (12) at several (T, P) conditions and compared with the pc-SAFT EoS [18]. For water, the pc-SAFT EoS was fit to an intermediate isotherm of NIST data (523.15 K) and then was applied to calculate densities at the other stated conditions. In the calculation of ARD [%], values from the NIST chemistry webbook [19] were used for reference as follows:

Table 5.
Prediction of properties for four pure chemicals with eSPT-PR EoS given by Equation (5) according to Equation (12) at several (T, P) conditions. For water, the pc-SAFT EoS [18] was fit to an intermediate isotherm (523.15 K) for parameter determination.
ARDs of liquid densities of the eSPT-PR EoS (Table 5) were generally comparable with those of the pc-SAFT EoS for two non-polar substances (n-hexane, CO2) but were higher than those of the pc-SAFT EoS for two polar substances (methanol, water). Furthermore, ARDs of gas densities were much lower for the pc-SAFT EoS than the eSPT-PR EoS (Table 5). The pc-SAFT EoS is neither a cubic equation nor any fixed-degree polynomial, but includes statistical mechanic integral terms that capture the trend of liquid and vapor densities in the critical region better than cubic EoS. All cubic EoSs have a cubic critical isotherm and a quadratic coexistence curve as conditions [20], unlike that of the pc-SAFT EoS. Nevertheless, generalized form of the eSPT-PR EoS improves liquid density representation compared with the PR-EoS (Table 4) and provides comparable representation of liquid densities calculated with the pc-SAFT EoS.
5. Conclusions
A translation type of correction (eSP) was added to the PR EoS. The resulting eSPT-PR EoS showed good prediction ability for both liquid molar volumes and critical molar volumes. In the eSPT-PR EoS, the newly added constant C could be well correlated with compressibility factor ZC and thus, a predictive form was produced based on the original PR EoS.
For the use of predictive eSPT-PR EoS, if experimental density data are available, the data can be used for data-fitting to redetermine the more accurate C value. If data are unavailable, then the generalized values of D and E given in this work can be used to estimate C.
Author Contributions
Conceptualization, M.O. and R.L.S.J.; methodology, H.I.; software, M.O.; validation, N.Y. and H.K.; formal analysis, M.O.; investigation, N.Y.; resources, M.O.; data curation, R.L.S.J.; writing—original draft preparation, M.O.; writing—review and editing, R.L.S.J.; visualization, M.O. and R.L.S.J.; supervision, H.I.; project administration, H.I.; funding acquisition, M.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Process Science Project of Ministry of Education, Culture, Sports, Science and Technology, Grant Number JPMXP0219192801. This result was also obtained as a result of a grant project (JPNP20004) from the New Energy and Industrial Technology Development Organization (NEDO). This research was financially supported by Grant-in-Aid for Scientific Research (B) 21H01685 and Grant-in-Aid for Scientific Research (C) 17K06884.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
This research was supported by Amano Institute of Technology, KOSÉ Cosmetology Research Foundation, Lotte Foundation, Tobe Maki Foundation and Urakami Foundation for Food and Food Culture Pro-motion.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
References
- Peng, D.-Y.; Robinson, D.B. A new two-constant equation of state. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Mushrif, S.H.; Phoenix, A.V. Effect of Peng−Robinson Binary Interaction Parameters on the Predicted Multiphase Behavior of Selected Binary Systems. Ind. Eng. Chem. Res. 2008, 47, 6280–6288. Available online: https://pubs.acs.org/doi/10.1021/ie800599t (accessed on 23 August 2025). [CrossRef]
- Jaubert, J.-N.; Mutelet, F. VLE predictions with the Peng–Robinson equation of state and temperature dependent kij calculated through a group contribution method. Fluid Phase Equilib. 2004, 224, 285–304. [Google Scholar] [CrossRef]
- Maeta, Y.; Ota, M.; Sato, Y.; Smith, R.L.; Inomata, H. Measurements of vapor–liquid equilibrium in both binary carbon dioxide–ethanol and ternary carbon dioxide–ethanol–water systems with a newly developed flow-type apparatus. Fluid Phase Equilib. 2015, 405, 96–100. [Google Scholar] [CrossRef]
- Soma, S.; Ota, M.; Sato, Y.; Smith, R.L.; Inomata, H. Measurement and correlation of vapor-liquid distribution coefficients of flavonoids in high pressure carbon dioxide—Ethanol—Water systems. Fluid Phase Equilib. 2019, 489, 90–98. [Google Scholar] [CrossRef]
- Urabe, M.; Ota, M.; Smith, R.L.; Watanabe, M. High-pressure phase equilibria of liquid CO2 with ethanol—Water mixtures. Fluid Phase Equilib. 2025, 597, 114450. [Google Scholar] [CrossRef]
- Ueno, Y.; Hoshino, Y.; Ota, M.; Sato, Y.; Inomata, H. Experiments and simulation of counter-current extraction (subcritical fluid separation) by supercritical CO2 with hops-extract ethanol solution. Kagaku Kogaku Rombunshu 2021, 47, 23–27. [Google Scholar] [CrossRef]
- Hoshino, Y.; Ueno, Y.; Ota, M.; Sato, Y.; Inomata, H. Measurement and correlation of vapor–liquid distribution coefficients of compounds contained in hops-extract ethanol solution with supercritical CO2. Kagaku Kogaku Rombunshu 2021, 47, 17–22. [Google Scholar] [CrossRef]
- Péneloux, A.; Rauzy, E.; Fréze, R. A consistent correction for Redlich-Kwong-Soave volumes. Fluid Phase Equilib. 1982, 8, 7–23. [Google Scholar] [CrossRef]
- Ahlers, J.; Gmehling, J. Development of an universal group contribution equation of state. I. Prediction of liquid densities for pure compounds with a volume translated Peng-Robinson equation of state. Fluid Phase Equilib. 2001, 191, 177–188. [Google Scholar] [CrossRef]
- Tsai, J.-C.; Chen, Y.-P. Application of a volume-translated Peng-Robinson equation of state on vapor-liquid equilibrium calculations. Fluid Phase Equilib. 1998, 145, 193–215. [Google Scholar] [CrossRef]
- Schmid, B.; Gmehling, J. Present status of the group contribution equation of state VTPR and typical applications for process development. Fluid Phase Equilib. 2016, 425, 443–450. [Google Scholar] [CrossRef]
- Ota, M.; Hashimoto, Y.; Sato, M.; Sato, Y.; Smith, R.L.; Inomata, H. Solubility of flavone, 6-methoxyflavone and anthracene in supercritical CO2 with/without a co-solvent of ethanol correlated by using a newly proposed entropy-based solubility parameter. Fluid Phase Equilib. 2016, 425, 65–71. [Google Scholar] [CrossRef]
- Ota, M.; Sugahara, S.; Sato, Y.; Smith, R.L.; Inomata, H. Vapor-liquid distribution coefficients of hops extract in high pressure CO2 and ethanol mixtures and data correlation with entropy-based solubility parameters. Fluid Phase Equilib. 2017, 434, 44–48. [Google Scholar] [CrossRef]
- Hildebrand, J.H.; Scott, R.L. The Solubility of Nonelectrolytes, 3rd ed.; Reinhold Pub. Corporation: New York, NY, USA, 1950. [Google Scholar]
- Tomai, T.; Tajima, N.; Kimura, M.; Yoko, A.; Seong, G.; Adschiri, T. Solvent accommodation effect on dispersibility of metal oxide nanoparticle with chemisorbed organic shell. J. Colloid Interface Sci. 2021, 587, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.R.; Diky, V.; Knotts, T.A., IV; Wilding, W.V. The Properties of Gases and Liquids, 6th ed.; Mc Graw Hill: New York, NY, USA, 2023; pp. 687–747. [Google Scholar]
- Gross, J.; Sadowski, G. Perturbed-Chain SAFT: An Equation of State Based on a Perturbation Theory for Chain Molecules. Ind. Eng. Chem. Res. 2001, 40, 1244–1260. [Google Scholar] [CrossRef]
- NIST Chemistry Webbook. Available online: https://webbook.nist.gov/chemistry/fluid (accessed on 23 August 2025).
- Levelt Sengers, J.M.H. From Van der Waals’ equation to the scaling laws. Physica 1974, 73, 73–106. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).