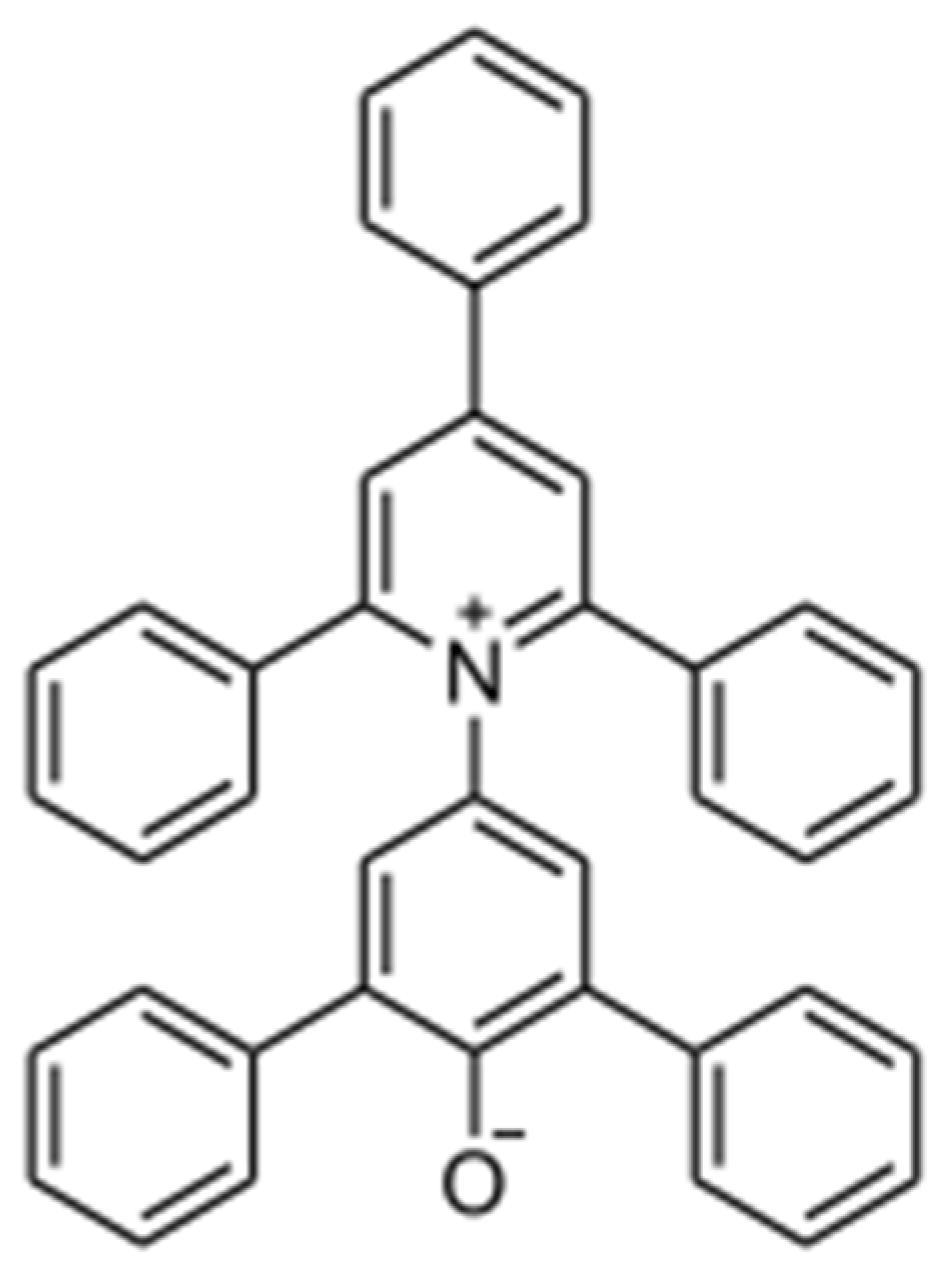
Polarity of Organic Solvent/Water Mixtures Measured with Reichardt’s B30 and Related Solvatochromic Probes—A Critical Review
Abstract
1. Introduction
- i.
- The solvent mixture (true micelles are a different situation) is inherently inhomogeneous and the solute B30 is therefore preferentially entrapped by a specific microdomain.
- ii.
- The solute probe such as B30 preferably forms a specific complex with one of the two solvent components.
νmax (cm−1)) = N (mol/cm3) εmax (cm2/mol).
2. Methods
3. Results
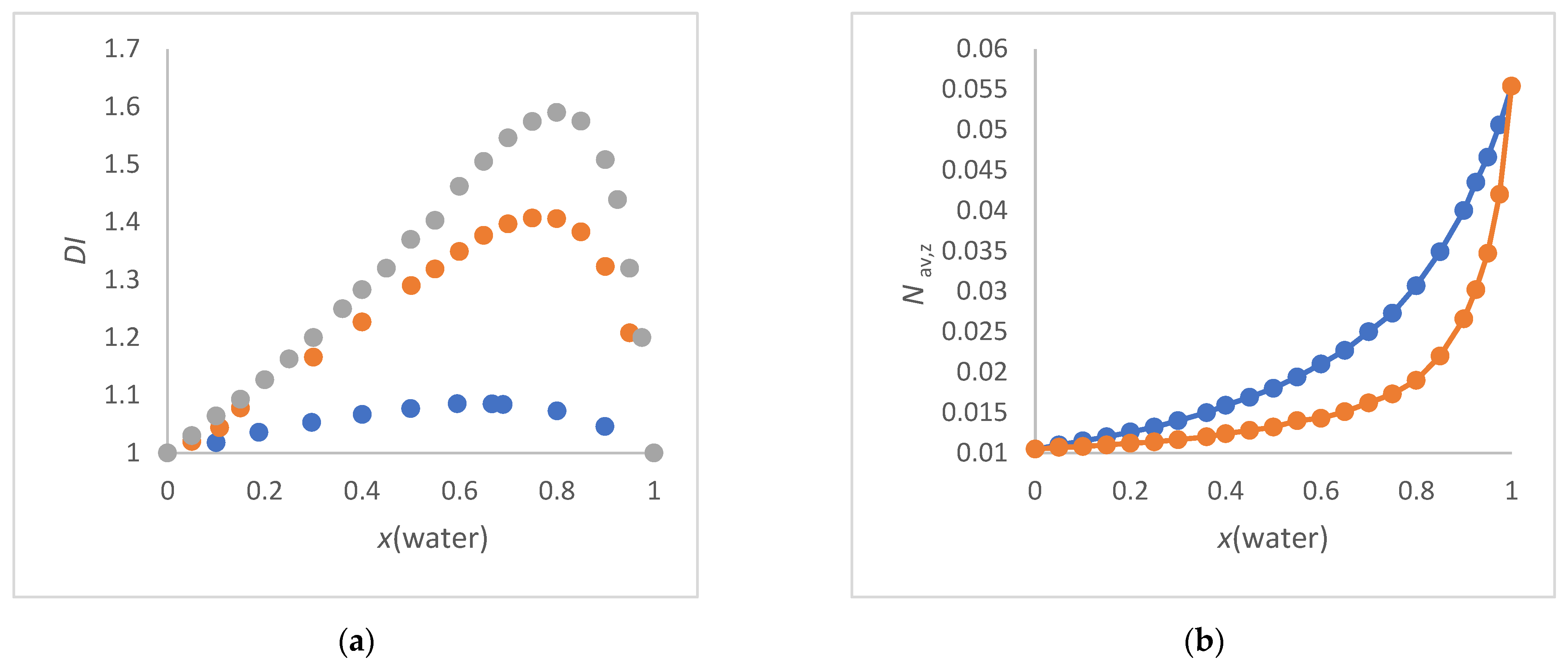
3.1. Selection of the Solvent Mixtures
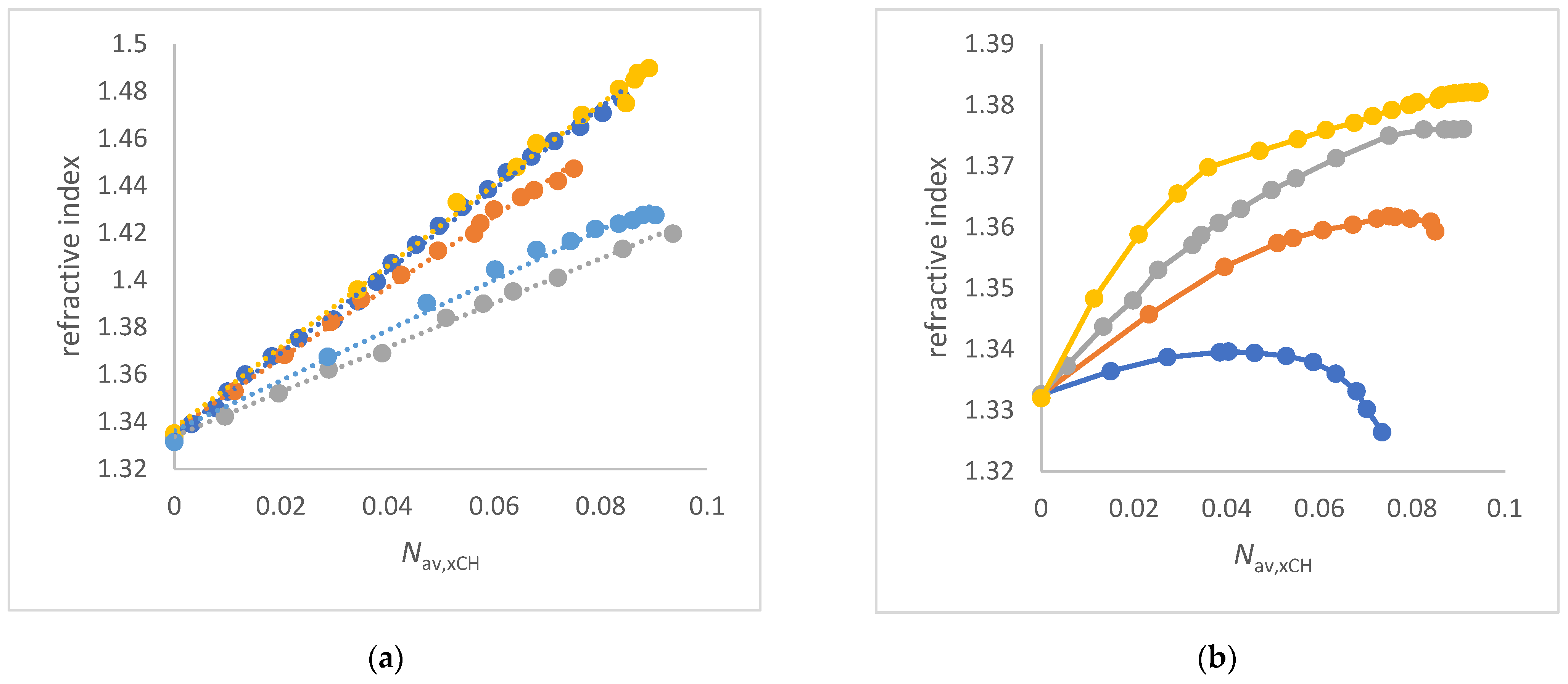
3.2. Refractive Index of Aqueous Solvent Mixtures
3.3. Temperature Influence on ET(30) in Terms of Density Impact
n = 8 (Reichardt), r = 0.9969.
n = 7 (Linert), r = 0.9978
3.4. Solvatochromism of B30 in Aqueous Solvent Mixtures
3.4.1. 1,2-Ethanediol/Water, Methanol/Water and Ethanol/Water Mixtures
n = 12 (1,2-ethanediol/water); r = 0.999.
n = 12 (1,2-ethanediol/water); r = 0.999.
n = 7 (methanol/water; Nav,x >0.04); r = 0.9957.
n = 6 (methanol/water; Nav,x < 0.04; r = 0.9985.
n = 8 (ethanol/water; Nav,x > 0.04; r = 0.995.
n = 10 (ethanol/water; Nav,x < 0.04); r = 0.998.
n = 42 (methanol/water, ethanol/water and primary alcohol); r = 0.994.
n = 42 (methanol/water, ethanol/water and primary n-alcohol); r = 0.994.
n = 10 (B1 in ethanol/water and water), r = 0.988.
n = 11 (BM in methanol/water), r = 0.997.
n = 11 (Fe in methanol/water), r = 0.992.
3.4.2. Formamide/Water and other Amide/Water Mixtures
n = 16 (FA/water); r = 0.999.
n = 17 (NMF/water); r = 0.993.
3.4.3. DMSO/Water Mixture
n = 22 (DMSO/water) r = 0.993.
n = 9 (DMSO/water-rich; Nav,x > 0.02); r = 0.998.
n = 5 (DMSO/water low; Nav,x < 0.02); r = 0.999.
n = 12 (DMSO/water), r = 0.996.
3.4.4. 1,4-Dioxane/Water Mixtures
r = 0.944, n= 7 (Nav,x < 0.02 mol/cm3, 1,4-dioxane rich section)
r = 0.997, n= 12 (Nav,x > 0.02 mol/cm3, water-rich section)
3.4.5. 2-Propanol/Water and 2-methyl-2-propanol/Water Mixtures
3.4.6. 2-Butoxyethanol/Water Mixtures
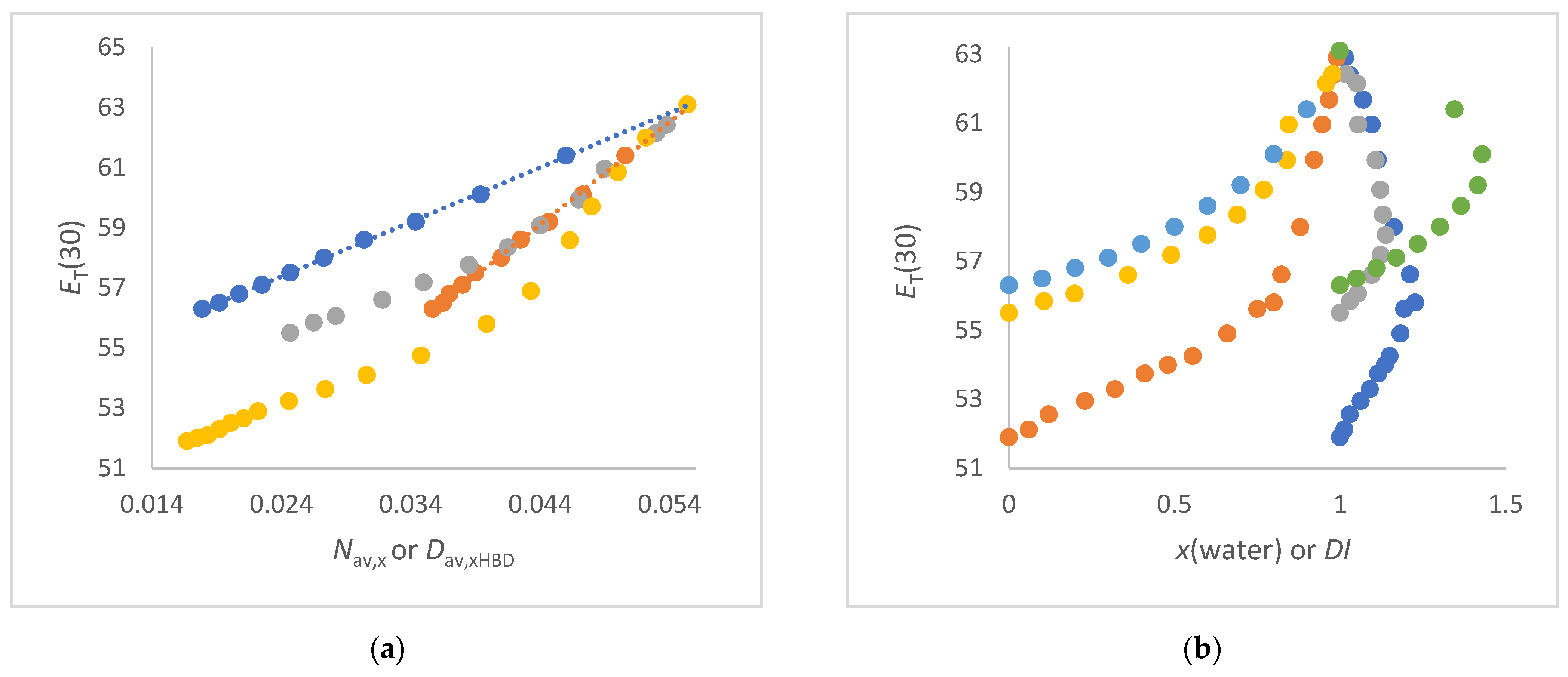
4. Discussion
- A.
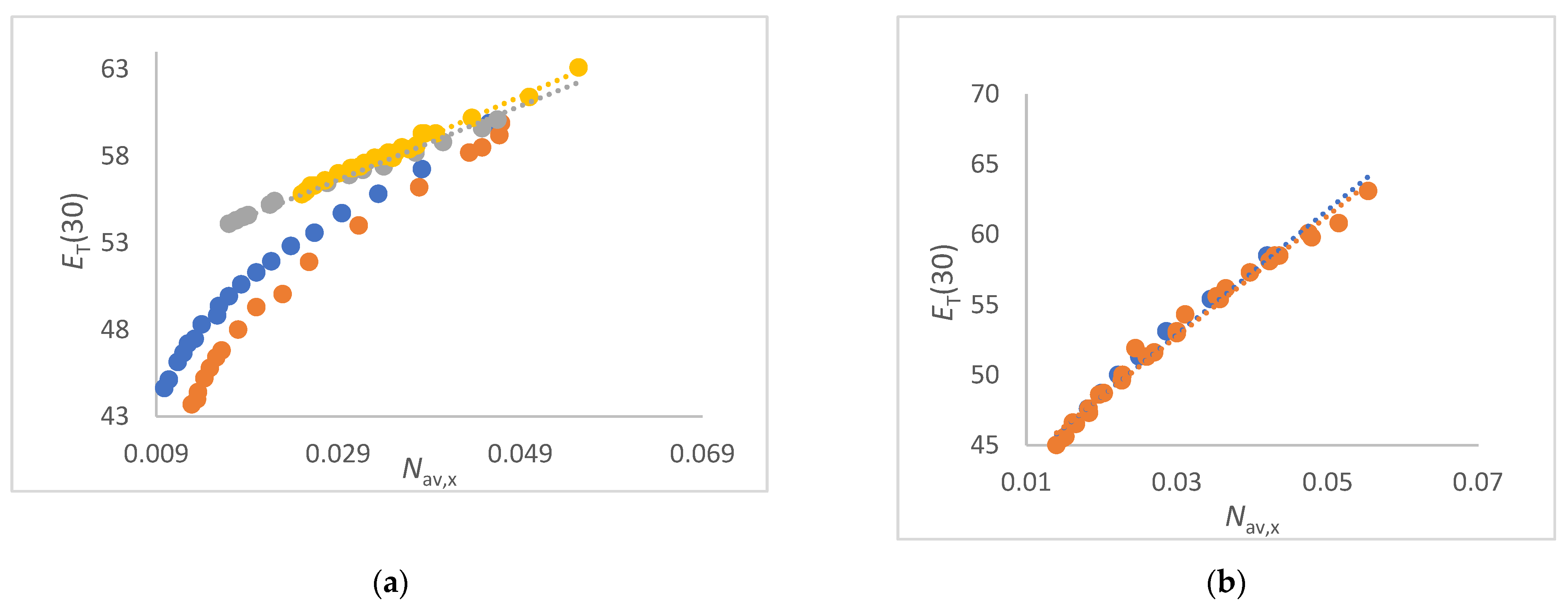
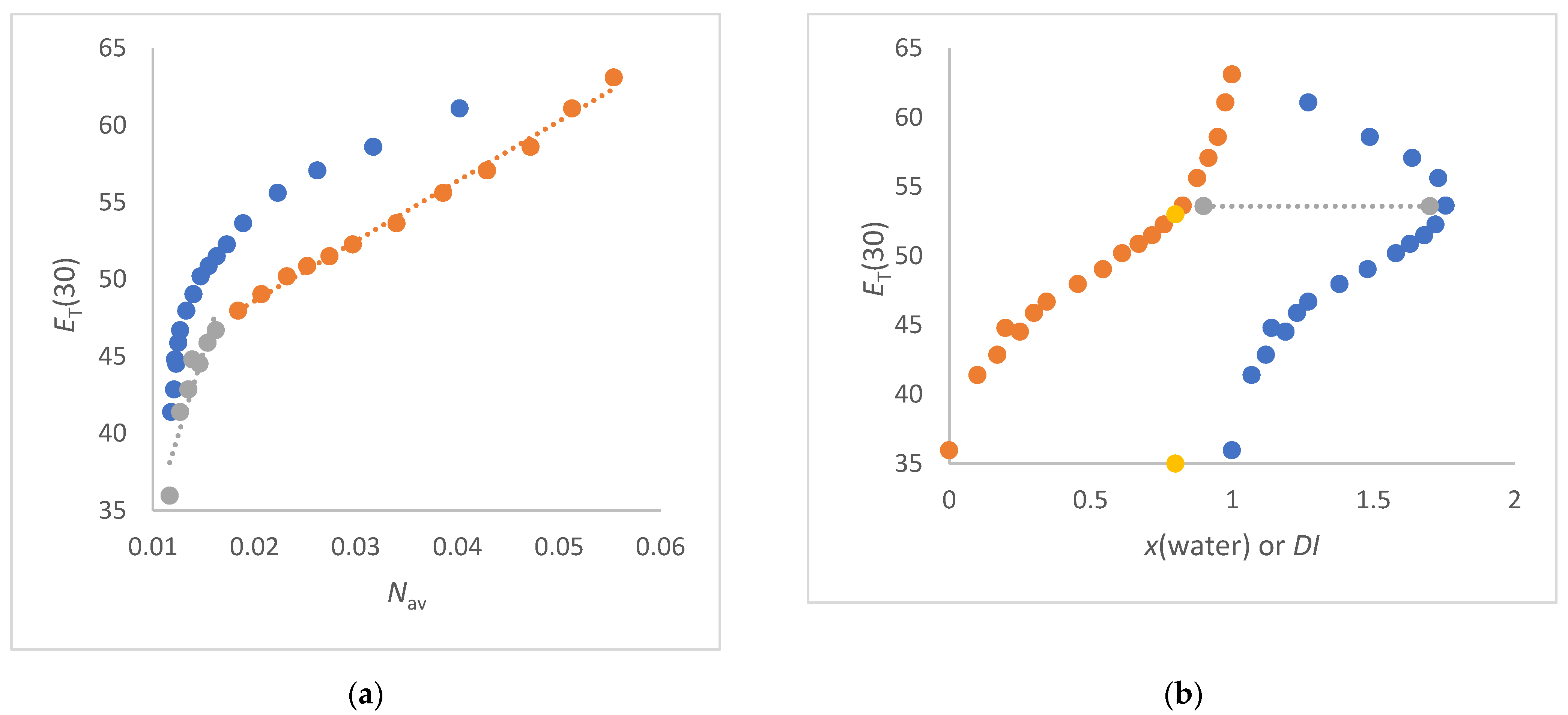
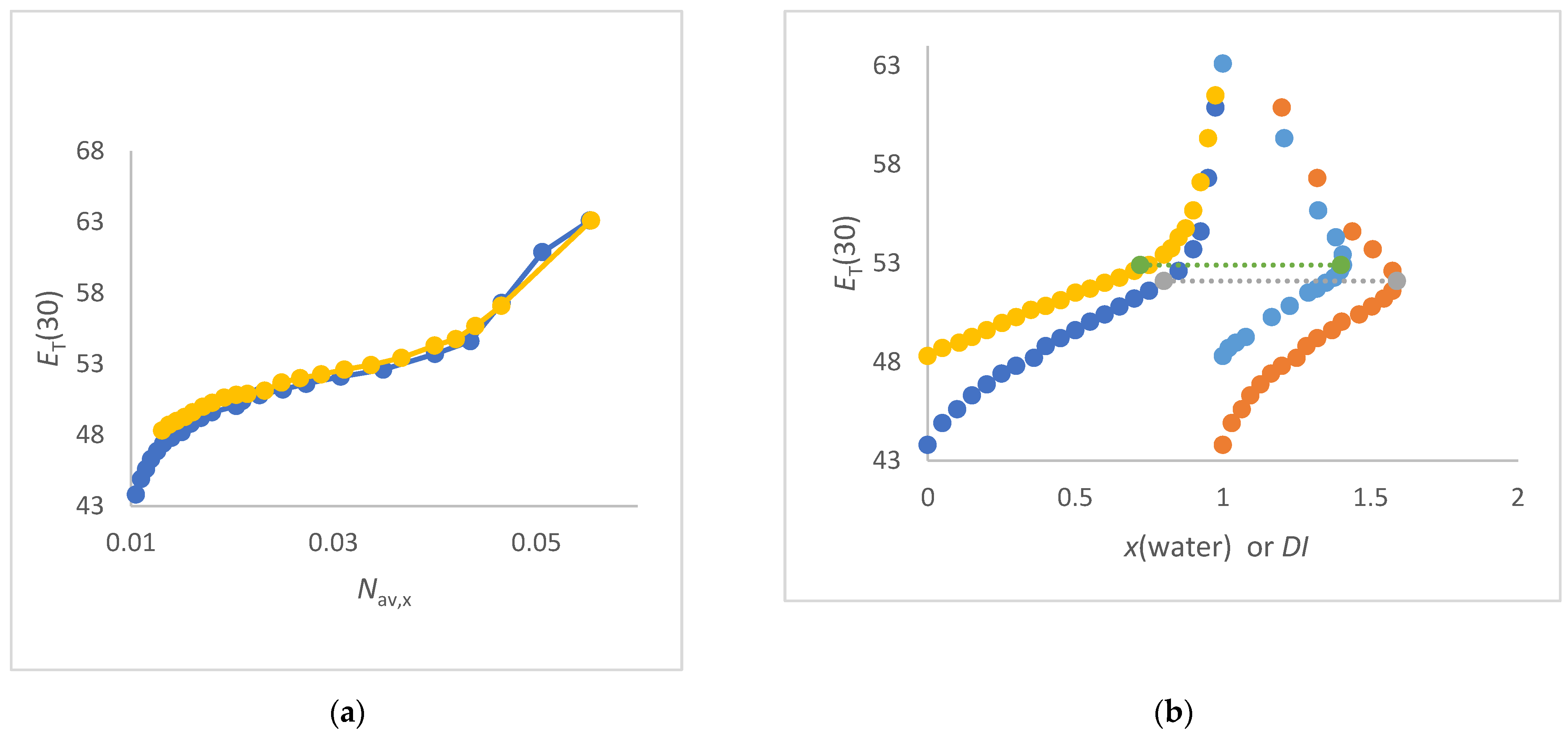
- The ET(30) increases significantly and linearly with Nav,x (1,2-ethanediol/water, FA/water, urea/water, NMF/ water and DMSO/water mixtures) (see Figure 3a and Figure 4a,b). These co-solvents belong to the group of solvents that do not enhance the water structure at all and form strong hydrogen bonds with water. In these cases, the ET(30) of the pure co-solvent is fitted to the linear plot.
- B.
- The ET(30) increases asymptotically with increasing Nav,x where the ET(30) value is always higher than with a linear dependence (1,4-dioxane/water, DMF/water and NFM/water mixtures) (see Figure 4a and Figure 5a). In these cases, the co-solvent-rich fraction shows the non-linearity ET(30) as function of Nav,x. These co-solvents do not enhance the water structure but form weaker hydrogen bonds with water than those belonging to scenario (A).
- C.
- The ET(30) increases as Nav,x increases, with the ET(30) value always being lower than expected for a linear dependence (see Figure 3a). This scenario applies to methanol/water and ethanol/water mixtures. These co-solvents enhance the water structure.
- D.
- ET(30) shows an S-shaped curve as a function of Nav,x (see Figure 6a). With increasing Nav,x the ET(30) value is always higher than expected with a linear dependence in the co-solvent-rich part. In the water-rich part, the ET(30) is lower than with a linear dependence according to scenario (C). The mixtures 2-propanol/water, 2-methyl-2-propanol/water and 2-butoxyethanol/water belong to this group. This scenario applies to binary solvent mixtures that interact either on the structure of the water or on the structure of the co-solvent.
5. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Dimroth, K.; Reichardt, C.; Siepmann, T.; Bohlmann, F. On pyridinium-N-phenol-betaines and their use in characterising the polarity of solvents (original in german). Liebigs Ann. Chem. 1963, 661, 1–37. [Google Scholar] [CrossRef]
- Dimroth, K.; Reichardt, C. Die colorimetrische Analyse binärer organischer Lösungsmittelgemische mit Hilfe der Solvatochromie von Pyridinium-N-phenolbetainen, the colourimetric analysis of binary organic solvent mixtures using the solvatochromism of pyridinium-N-phenolbetaines. Fresenius Z. Anal. Chem. 1966, 215, 344–350. [Google Scholar] [CrossRef]
- Maksimovic, Z.B.; Reichardt, C.; Spiric, A. Determination of empirical parameters of solvent polarity ET in binary mixtures by solvatochromic pyridinium-N-phenol betaine dyes. Z. Anal. Chem. 1974, 270, 100–104. [Google Scholar] [CrossRef]
- Krygowski, T.M.; Wrona, P.K.; Zielkowska, U.; Reichardt, C. Empirical parameters of Lewis acidity and basicity for aqueous binary solvent mixtures. Tetrahedron 1985, 41, 4519–4527. [Google Scholar] [CrossRef]
- Langhals, H. Die quantitative Beschreibung der Lösungsmittelpolarität binärer Gemische unter Berücksichtigung verschiedener Polaritätsskalen. Chem. Ber. 1981, 114, 2907–2913. [Google Scholar] [CrossRef][Green Version]
- Langhals, H. Polarity of binary liquid mixtures. Angew. Chem. Int. Ed. Engl. 1982, 21, 724–733. [Google Scholar] [CrossRef]
- Langhals, H. The description of the polarity of alcohols as a function of their molar content of OH groups. Nouv. J. Chim. 1982, 6, 265–267. Available online: https://epub.ub.uni-muenchen.de/93533/ (accessed on 2 November 2022).
- Haak, J.R.; Engberts, J.B.F.N. Solvent polarity and solvation effects in highly aqueous mixed solvents. Application of the Dimroth-Reichardt ET(30) parameter. Recl. Trav. Chim. Pays-Bas 1986, 105, 307–311. [Google Scholar] [CrossRef]
- Michels, J.J.; Dorsey, J.G. Retention in reversed-phase liquid chromatography: Solvatochromic investigation of homologous alcohol-water binary mobile phases. J. Chromatogr. 1988, 21, 85–98. [Google Scholar] [CrossRef]
- Chatterjee, P.; Bagchi, S. Preferential solvation of a dipolar solute in mixed binary solvent. A study by UV–visible spectroscopy. J. Phys. Chem. 1991, 95, 3311–3314. [Google Scholar] [CrossRef]
- Bosch, E.E.; Rosés, M. Relationships between ET Polarity and Composition in Binary Solvent Mixtures. J. Chem. Soc. Faraday Trans. 1992, 88, 3541–3546. [Google Scholar] [CrossRef]
- Marcus, Y. The use of chemical probes for the characterization of solvent mixtures. Part 2. Aqueous mixtures. J. Chem. Soc. Perkin Trans. 1994, 2, 1751–1758. [Google Scholar] [CrossRef]
- Kipkemboi, P.K.; Easteal, A.J. Solvent Polarity Studies of the Water+t-Butyl Alcohol and Water+t-Butylamine Binary Systems with the Solvatochromic Dyes Nile Red and Pyridinium-N-phenoxide Betaine, Refractometry and Permittivity Measurements. Austr. J. Chem. 1994, 47, 1771–1781. [Google Scholar] [CrossRef]
- Banerjee, D.; Laha, A.K.; Bagchi, S. Preferential solvation in mixed binary solvent. J. Chem. Soc. Faraday Trans. 1995, 91, 631–636. [Google Scholar] [CrossRef]
- Skwierczynski, R.D.; Connors, K.A. Solvent effects on chemical processes. Part 7. Quantitative description of the composition dependence of the solvent polarity measure ET(30) in binary aqueous–organic solvent mixtures. J. Chem. Soc. Perkin Trans. 1994, 2, 467–472. [Google Scholar] [CrossRef]
- Marcus, Y. Use of chemical probes for the characterization of solvent mixtures. Part 1. Completely non-aqueous mixtures. J. Chem. Soc. Perkin Trans. 2 1994, 5, 1015–1021. [Google Scholar] [CrossRef]
- Rosés, M.; Ràfols, C.; José Ortega, J.; Bosch, E. Solute–solvent and solvent–solvent interactions in binary solvent mixtures. Part 1. A comparison of several preferential solvation models for describing ET(30) polarity of bipolar hydrogen bond acceptor-cosolvent mixtures. J. Chem. Soc. Perkin Trans. 1995, 2, 1607–1615. [Google Scholar] [CrossRef]
- Bosch, E.E.; Rosés, M.; Herodes, K.; Koppel, I.; Leito, I. Solute-solvent and solvent-solvent interactions in binary solvent mixtures. Part 3. The ET(30) polarity of binary mixtures of hydroxylic solvents. J. Chem. Soc. Perkin Trans. 1996, 2, 1497–1503. [Google Scholar] [CrossRef]
- Rosés, M.; Buhvestov, U.; Ràfols, C.; Rived, F.; Bosch, E. Solute–solvent and solvent–solvent interactions in binary solvent mixtures. Part 6. A quantitative measurement of the enhancement of the water structure in 2-methylpropan-2-ol–water and propan-2-ol–water mixtures by solvatochromic indicators. J. Chem. Soc. Perkin Trans. 1997, 2, 1341–1348. [Google Scholar] [CrossRef]
- Buhvestov, U.; Rived, F.; Ràfols, C.; Bosch, E.; Rosés, M. Solute–solvent and solvent–solvent interactions in binary solvent mixtures. Part 7. Comparison of the enhancement of the water structure in alcohol–water mixtures measured by solvatochromic indicators. J. Phys. Org. Chem. 1998, 11, 185–196. [Google Scholar] [CrossRef]
- Herodes, K.; Leito, I.; Koppel, I.; Rosés, M. ET(30) Polarity of Binary Mixtures of Formamides with Hydroxylic Solvent. J. Phys. Org. Chem. 1999, 12, 109–115. [Google Scholar] [CrossRef]
- Tada, E.B.; Novaki, L.P.; El Seoud, O.A. Solvatochromism in pure and binary solvent mixtures: Effects of the molecular structure of the zwitterionic probe. J. Phys. Org. Chem. 2000, 13, 679–687. [Google Scholar] [CrossRef]
- El Seoud, O.A. Solvation in pure and mixed solvents: Some recent developments. Pure Appl. Chem. 2007, 79, 1135–1151. [Google Scholar] [CrossRef]
- Ghoneim, N. Study of the preferential solvation of some betaine dyes in binary solvent mixtures. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2001, 57, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.G.; Tabata, M.; Takamuku, T. Preferential Solvation in Aqueous–Organic Mixed Solvents Using Solvatochromic Indicators. J. Sol. Chem. 2002, 31, 381–395. [Google Scholar] [CrossRef]
- Bevilaqua, T.; da Silva, D.C.; Machado, V.G. Preferential solvation of Brooker’s merocyanine in binary solvent mixtures composed of formamides and hydroxylic solvents. Spectrochim. Acta Part A 2004, 60, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Bentley, T.W.; Koo, I.S. Role of hydroxyl concentrations in solvatochromic measures of solvent polarity of alcohols and alcohol–water mixtures—Evidence that preferential solvation effects may be overestimated. Org. Biomol. Chem. 2004, 21, 2376–2380. [Google Scholar] [CrossRef]
- El Seoud, O.A. Understanding solvation. Pure Appl. Chem. 2009, 8, 697–707. [Google Scholar] [CrossRef]
- Morisue, M.; Ueno, I. Preferential Solvation Unveiled by Anomalous Conformational Equilibration of Porphyrin Dimers: Nucleation Growth of Solvent–Solvent Segregation. J. Phys. Chem. B 2018, 122, 5251–5259. [Google Scholar] [CrossRef]
- Duereh, A.; Anantpinijwatna, A.; Latcharote, P. Prediction of Solvatochromic Polarity Parameters for Aqueous Mixed-Solvent Systems. Appl. Sci. 2020, 10, 8480. [Google Scholar] [CrossRef]
- Pasham, F.; Jabbari, M.; Farajtabar, A. Solvatochromic Measurement of KAT Parameters and Modeling Preferential Solvation in Green Potential Binary Mixtures of N-Formylmorpholine with Water, Alcohols, and Ethyl Acetate. J. Chem. Eng. Data 2020, 65, 5458–5466. [Google Scholar] [CrossRef]
- Sandri, J.C.; de Melo, C.E.A.; Giusti, L.A.; Rezende, M.C.; Machado, V.G. Preferential solvation index as a tool in the analysis of the behavior of solvatochromic probes in binary solvent mixtures. J. Mol. Liq. 2021, 328, 1155450. [Google Scholar] [CrossRef]
- Pavel, C.M.; Ambrosi, E.; Dimitriu, D.G.; Dorohoi, D.O. Complex formation and microheterogeneity in water–alcohol binary mixtures investigated by solvatochromic study. Eur. Phys. J. Spec. Top. 2023, 232, 415–425. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry, 4th ed.; Wiley-VCH: Weinheim, Germany, 2011; ISBN 978-3-527-32473-6. [Google Scholar]
- Reichardt, C. Solvatochromic Dyes as Solvent Polarity Indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Kamlet, M.-J.; Abboud, J.L.M.; Abraham, M.H.; Taft, R.W. Linear Solvation Energy Relationships. 23. A Comprehensive Collection of the Solvatochromic Parameters, π*, α, and ß, and Some Methods for Simplifying the Generalized Solvatochromic Equation. J. Org. Chem. 1983, 48, 2877–2887. [Google Scholar] [CrossRef]
- Catalán, J.; Diaz, C. A generalized solvent acidity scale: The solvatochromism of o-tert-Butylstilbazolium Betaine dye and ist homomorph o,o’-di-tert-butylstilbazolium betaine dye. Liebigs Ann. 1997, 1997, 1941–1949. [Google Scholar] [CrossRef]
- Catalán, J.; Díaz, C. Extending the Solvent Acidiy Scale to Highly Acidic Organic Solvents: The Unique Photophysical Behaviour of 3,6-Diethyltetrazine. Eur. J. Org. Chem. 1999, 4, 885–891. [Google Scholar] [CrossRef]
- Catalán, J. Toward a Generalized Treatment of the Solvent Effect Based on Four Empirical Scales: Dipolarity (SdP, a New Scale), Polarizability (SP), Acidity (SA), and Basicity (SB) of the Medium. J. Phys. Chem. B 2009, 113, 5951–5960. [Google Scholar] [CrossRef] [PubMed]
- Cerón-Carrasco, J.P.; Jacquemin, D.; Laurence, C.; Planchat, A.; Reichardt, C.; Sraïdi, K. Determination of a Solvent Hydrogen-Bond Acidity Scale by Means of the Solvatochromism of Pyridinium-N-phenolate Betaine Dye 30 and PCM-TD-DFT Calculations. J. Phys. Chem. B 2014, 118, 4605–4614. [Google Scholar] [CrossRef]
- Laurence, C.; Mansour, S.; Vuluga, D.; Sraidi, K.; Legros, J. Theoretical, Semiempirical, and Experimental Solvatochromic Comparison Methods for the Construction of the α1 Scale of Hydrogen-Bond Donation of Solvents. J. Org. Chem. 2022, 87, 6273–6287. [Google Scholar] [CrossRef]
- Laurence, C.; Mansour, S.; Vuluga, D.; Planchat, D.; Legros, J. Hydrogen-Bond Acceptance of Solvents: A 19F Solvatomagnetic β1 Database to Replace Solvatochromic and Solvatovibrational Scales. J. Org. Chem. 2021, 86, 4143–4158. [Google Scholar] [CrossRef]
- Spange, S.; Weiß, N. Empirical Hydrogen Bonding Donor (HBD) Parameters of Organic Solvents Using Solvatochromic Probes–A Critical Evaluation. ChemPhysChem 2023, 24, e202200780. [Google Scholar] [CrossRef]
- Pike, S.J.; Lavagnini, E.; Varley, L.M.; Cook, J.L.; Hunter, C.A. H-Bond donor parameters for cations. Chem. Sci. 2019, 10, 5943–5951. [Google Scholar] [CrossRef] [PubMed]
- Spange, S.; Lienert, C.; Friebe, N.; Schreiter, K. Complementary interpretation of ET(30) polarity parameters of ionic liquids. Phys. Chem. Chem. Phys. 2020, 160, 9954–9966. [Google Scholar] [CrossRef]
- Mero, A.; Guglielmero, L.; Guazzelli, L.; D’Andrea, F.; Mezzetta, A.; Pomelli, C.S. A specific interaction between ionic liquids’ cations and Reichardt’s Dye. Molecules 2022, 27, 7205. [Google Scholar] [CrossRef]
- Böttcher, C.J.F. Theory of Electric Polarization, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1973; ISBN 978-0-444-41019-1. [Google Scholar] [CrossRef]
- Marcus, Y. Preferential solvation of ions in mixed solvents. Part 2—The solvent composition near the ion. J. Chem. Soc. Faraday Trans. I 1988, 84, 1465–1473. [Google Scholar] [CrossRef]
- Marcus, Y. Preferential solvation in mixed solvents. Part 5—Binary mixtures of water and organic solvents. J. Chem. Soc. Faraday Trans. 1990, 86, 2215–2224. [Google Scholar] [CrossRef]
- Marcus, Y. Solvent Mixtures: Properties and Selective Solvation; CRC Press: Boca Raton, FL, USA, 2014; pp. 9–15. ISBN 9780429175664. [Google Scholar]
- Marcus, Y. Preferential Solvation in Mixed Solvents X. Completely Miscible Aqueous Co-Solvent Binary Mixtures at 298.15 K. Chem. Mon. 2001, 132, 1387–1411. [Google Scholar] [CrossRef]
- Spange, S.; Lauterbach, M.; Gyra, A.K.; Reichardt, C. Über Pyridinium-N-phenolat-Betaine und ihre Verwendung zur Charakterisierung der Polarität von Lösungsmitteln, XVI. Bestimmung der empirischen Lösungsmittelpolaritäts-Parameter ET(30) und AN für 55 substituierte Phenole. Liebigs Ann. Chem. 1991, 323–329. [Google Scholar] [CrossRef]
- Coleman, C.A.; Murray, C.J. Hydrogen bonding between N-pyridinium phenolate and O-H donors in acetonitrile. J.Org. Chem. 1992, 57, 3578–3582. [Google Scholar] [CrossRef]
- Laurence, C.; Berthelot, M.; Graton, J. Hydrogen-Bonded Complexes of Phenols. In The Chemistry of Phenols; Rappoport, Z., Ed.; Wiley: New York, NY, USA, 2004; ISBN 978-0-470-86945-1. [Google Scholar]
- Cook, J.L.; Hunter, C.A.; Low, C.M.R.; Perez-Velasco, A.; Vinter, J.G. Solvent Effects on Hydrogen Bonding. Angew. Chem. Int. Ed. 2007, 46, 3706–3709. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.L.; Hunter, C.A.; Low, C.M.R.; Perez-Velasco, A.; Vinter, J.G. Preferential Solvation and Hydrogen Bonding in Mixed Solvents. Angew. Chem. Int. Ed. 2008, 47, 6275–6277. [Google Scholar] [CrossRef]
- Reichardt, C.; Eschner, M. On pyridinium N-phenolate betaines and their use in characterising the polarity of solvents, XVIII. Synthesis and UV/Vis spectroscopic properties of a negatively solvatochromic pyridinium N-thiophenolate betaine dye (in german). Liebigs Ann. Chem. 1991, 1991, 1003–1012. [Google Scholar] [CrossRef]
- Bolz, I.; Schaarschmidt, D.; Rüffer, T.; Lang, H.; Spange, S. A pyridinium barbiturate betaine dye with pronounced negative solvatochromism: A new approach to molecular recognition. Angew. Chem. Int. Ed. 2009, 48, 7440–7443. [Google Scholar] [CrossRef] [PubMed]
- Plenert, C.; Mendez-Vega, E.; Sander, W. Micro- vs. Macrosolvation in Reichardt’s Dyes. J. Am. Chem. Soc. 2021, 143, 13156–13166. [Google Scholar] [CrossRef]
- Henkel, S.; Misuraca, M.C.; Troselj, P.; Davidson, J.; Hunter, C.A. Polarisation effects on the solvation properties of alcohols. Chem. Sci. 2018, 9, 88–89. [Google Scholar] [CrossRef]
- Spange, S.; Weiß, N.; Schmidt, C.; Schreiter, K. Reappraisal of Empirical Solvent Polarity Scales for Organic Solvents. Chem. Methods 2021, 1, 42–60. [Google Scholar] [CrossRef]
- Spange, S.; Weiß, N.; Mayerhöfer, T.G. The Global Polarity of Alcoholic Solvents and Water–Importance of the Collectively Acting Factors Density, Refractive Index and Hydrogen Bonding Forces. Chem. Open 2022, 11, e202200140. [Google Scholar] [CrossRef]
- Lerf, C.; Suppan, P. Hydrogen Bonding and Dielectric Effects in Solvatochromic Shifts. J. Chem. Soc. Faraday Trans. 1992, 88, 963–969. [Google Scholar] [CrossRef]
- Rezende, M.C.; Machado, V.G.; Morales, S.; Pastenes, C.; Vidal, M. Use of Nonideality Parameters for the Analysis of the Thermodynamic Properties of Binary Mixtures. ACS Omega 2021, 6, 16553–16564. [Google Scholar] [CrossRef]
- Morales, S.; Pastenes, C.; Machado, V.G.; Rezende, M.C. Applications of a preferential–solvation index (PSI) for the comparison of binary mixtures with ionic liquids. J. Mol. Liq. 2021, 343, 117644. [Google Scholar] [CrossRef]
- Lüdecke, C.; Lüdecke, D. Thermodynamic properties of homogeneous mixtures. In Thermodynamik: Physikalisch-Chemische Grundlagen der Thermischen Verfahrenstechnik, Thermodynamics: Physical-Chemical Fundamentals of Thermal Process Engineering; Springer: Berlin/Heidelberg, Germany, 2000; 424p. [Google Scholar] [CrossRef]
- Mayerhöfer, T.G.; Dabrowska, A.; Schwaighofer, A.; Lendl, B.; Popp, J. Beyond Beer’s Law: Why the Index of Refraction Depends (Almost) Linearly on Concentration. ChemPhysChem. 2020, 21, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Mayerhöfer, T.G.; Popp, J. Beyond Beer’s Law: Revisiting the Lorentz-Lorenz Equation. ChemPhysChem 2020, 21, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Mayerhöfer, T.G.; Spange, S. Understanding refractive index changes in homologous series of hydrocarbons based on Beer’s law. ChemPhysChem 2023, 24, e202300430. [Google Scholar] [CrossRef] [PubMed]
- Spange, S.; Mayerhöfer, T.G. The Negative Solvatochromism of Reichardt’s Dye B30–A Complementary Study. Chem. Phys. Chem. 2022, 23, e202200100. [Google Scholar] [CrossRef] [PubMed]
- Spange, S.; Kaßner, L.; Mayerhöfer, T.G. New insights into the negative solvatochromism of various merocyanines. PhysChemPhys 2023. submitted. [Google Scholar]
- Deye, J.F.; Berger, T.A.; Anderson, A.G. Nile Red as a Solvatochromic Dye for Measuring Solvent Strength in Normal Liquids and Mixtures of Normal Liquids with Supercritical and Near Critical Fluids. Anal. Chem. 1990, 62, 615–622. [Google Scholar] [CrossRef]
- Kasap, S.; Capper, P. Springer Handbook of Electronic and Photonic Materials; Springer: Berlin/Heidelberg, Germany, 2006; p. 49. ISBN 978-0-387-26059-4. [Google Scholar]
- Suppan, P. Polarizability of excited molecules from spectroscopic studies. Spectrochim. Acta 1967, 24A, 1161–1165. [Google Scholar] [CrossRef]
- Suppan, P. Solvent effects on the energy of electronic transitions: Experimental observations and applications to structural problems of excited molecules. J. Chem. Soc. A 1968, 3125–3133. [Google Scholar] [CrossRef]
- Dei, L.; Grassi, S. Peculiar Properties of Water as Solute. J. Phys. Chem. B 2006, 110, 12191–12197. [Google Scholar] [CrossRef]
- Lee, H.; Hong, W.-H.; Kim, H. Excess Volumes of Binary and Ternary Mixtures of Water, Methanol and Ethylene Glycol. J. Chem. Eng. Data 1990, 35, 371–374. [Google Scholar] [CrossRef]
- Franks, F.; Ives, D.J.G. The structural properties of alcohol–water mixtures. Q. Rev. Chem. Soc. 1966, 20, 1–44. [Google Scholar] [CrossRef]
- Armitage, D.A.; Blandamer, M.J.; Morcom, K.W.; Treloar, N.C. Partial Molar Volumes and Maximum Density Effects in Alcohol–Water Mixtures. Nature 1968, 219, 718–720. [Google Scholar] [CrossRef]
- Lama, F.R.; Lu, C.Y. Excess Thermodynamic Properties of Aqueous Alcohol Solutions. J. Chem. Eng. Data 1965, 10, 216–219. [Google Scholar] [CrossRef]
- Douheret, G.; Khadir, A.; Pal, A. Thermodynamic Characterization of the Water + Methanol System, at 298.15 K. Thermochim. Acta 1989, 142, 219–243. [Google Scholar] [CrossRef]
- Peeters, D.; Huyskens, P. Endothermicity or exothermicity of water/alcohol mixtures. J. Mol. Struct. 1993, 300, 539–550. [Google Scholar] [CrossRef]
- Xiao, C.; Bianchi, H.; Tremaine, P.R. Excess molar volumes and densities of (methanol+water) at temperatures between 323 K and 573 K and pressures of 7.0 MPa and 13.5 MPa. J. Chem. Thermodyn. 1997, 29, 261–286. [Google Scholar] [CrossRef]
- Petong, P.; Pottel, R.; Kaatze, U. Water-Ethanol Mixtures at Different Compositions and Temperatures. A Dieletric Relaxation Stud. J. Phys. Chem. A 2000, 104, 7420–7428. [Google Scholar] [CrossRef]
- Sato, T.; Chiba, A.; Nozaki, R. Hydrophobic hydration and molecular association in methanol–water mixtures studied by microwave dielectric analysis. J. Chem. Phys. 2000, 112, 2924–2932. [Google Scholar] [CrossRef]
- Sato, T.; Buchner, R. Dielectric Relaxation Processes in Ethanol/Water Mixtures. J. Phys. Chem. A 2004, 108, 5007–5015. [Google Scholar] [CrossRef]
- Moldoveanu, S.; David, V. Chapter 6—Solvent Extraction. In Modern Sample Preparation for Chromatography; Elsevier: Amsterdam, The Netherlands, 2015; pp. 131–139. [Google Scholar] [CrossRef]
- Kaatze, U. The Dielectric Properties of Water in Its Different States of Interaction. J. Solution Chem. 1997, 26, 1049–1112. [Google Scholar] [CrossRef]
- Behrends, R.; Fuchs, K.; Kaatze, U. Dielectric properties of glycerol/water mixtures at temperatures between 10 and 50 °C. J. Chem. Phys. 2006, 124, 144512. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Crain, J.; Poon, W.C.K.; Finney, J.L.; Soper, A.K. Molecular segregation observed in a concentrated alcohol–water solution. Nature 2002, 416, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Pascal, T.A.; Goddard, W.A. Hydrophobic Segregation, Phase Transitions and the Anomalous Thermodynamics of Water/Methanol Mixtures. J. Phys. Chem. B 2012, 116, 13905–13912. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.-L.; Miller, B.T.; Te, J.; Cendagorta, J.R.; Brooks, B.R.; Ichiye, T. Hydrophobic hydration and the anomalous partial molar volumes in ethanol-water mixtures. J. Chem. Phys. 2015, 142, 064501. [Google Scholar] [CrossRef] [PubMed]
- Moučka, C.F.; Nezbeda, I. Partial molar volume of methanol in water: Effect of polarizability. Coll. Czech. Chem. Commun. 2009, 74, 559–563. [Google Scholar] [CrossRef]
- Besford, Q.A.; Van den Heuvel, W.; Christofferson, A.J. Dipolar Dispersion Forces in Water–Methanol Mixtures: Enhancement of Water Interactions upon Dilution Drives Self-Association. J. Phys. Chem. B 2022, 126, 6231–6239. [Google Scholar] [CrossRef]
- Guevara-Carrion, G.; Fingerhut, R.; Vrabec, J. Density and partial molar volumes of the liquid mixture water + methanol + ethanol + 2-propanol at 298.15 K and 0.1 MPa. J. Chem. Eng. Data 2021, 66, 2425–2435. [Google Scholar] [CrossRef]
- Han, C.; Gao, J.; Sun, W.; Han, C.; Li, F.; Li, B. Structure study of water in alcohol-water binary system based on Raman spectroscopy. J. Phys. Conf. Ser. 2022, 2282, 012021. [Google Scholar] [CrossRef]
- Nunes, N.; Ventura, C.; Martins, F.; Elvas-Leitão, R. Modeling Preferential Solvation in Ternary Solvent Systems. J. Phys. Chem. B 2009, 113, 3071–3079. [Google Scholar] [CrossRef]
- Nunes, N.; Elvas-Leitão, R.; Martins, F. Using solvatochromic probes to investigate intermolecular interactions in 1,4-dioxane/methanol/acetonitrile solvent mixtures. J. Mol. Liq. 2018, 266, 259–268. [Google Scholar] [CrossRef]
- Atkins, P.W.; de Paula, J. Physical Chemistry, 4th ed.; Wiley-VCH: Weinheim, Germany, 2006; p. 724. ISBN 3-527-31546-2. [Google Scholar]
- Lechner, M.D.; Gehrke, K.; Nordmeier, E.H. Macromolecular Chemistry: A Textbook for Chemists, Physicists, Materials Scientists and Chemical Engineers, 5th ed.; Springer: Berlin/Heidelberg, Germany, 2014; 15p, ISBN 978-3-642-41768-9. [Google Scholar] [CrossRef]
- Stepto, T.F.T. Dispersity in Polymer Science. Pure Appl. Chem. 2009, 8, 351–353. [Google Scholar] [CrossRef]
- Heller, W. Remarks on Refractive Index Mixture Rules. J. Phys. Chem. 1965, 69, 1123–1129. [Google Scholar] [CrossRef]
- Tasic, A.Z.; Djordjevic, B.D.; Grozdanic, D.K.; Radojkovic, N. Use of mixing rules in predicting refractive indexes and specific refractivities for some binary liquid mixtures. J. Chem. Eng. Data 1992, 37, 310–313. [Google Scholar] [CrossRef]
- Pretorius, F.; Focke, W.W.; Androsch, R.; du Toi, E. Estimating binary liquid composition from density and refractive index measurements: A comprehensive review of mixing rules. J. Mol. Liq. 2021, 332, 115893. [Google Scholar] [CrossRef]
- IUPAC. Compendium of Chemical Terminology, 2nd ed.; McNaught, A.D., Wilkinson, A., Eds.; Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar] [CrossRef]
- Markgraf, H.G.; Nikuradse, A. Über den Volumeneffekt in binären Gemischen einiger organischer Flüssigkeiten. On the volume effect in binary mixtures of some organic liquids. Z. Naturforsch. A 1954, 9, 27–34. [Google Scholar] [CrossRef]
- Redlich, O.; Kister, A.T. Algebraic Representation of Thermodynamic Properties and the Classification of Solutions. Ind. Eng. Chem. 1948, 40, 341–348. [Google Scholar] [CrossRef]
- Speight, J.G. Atomic and Group Refractions. In Lange’s Handbook of Chemistry, 16th ed.; McGraw-Hill Education LLC: New York, NY, USA; ISBN 10:0070163847. Available online: https://www.labxing.com/files/lab_data/1340-1625805401-Qqoazhfj.pdf (accessed on 2 November 2022).
- Marcus, Y. Water structure enhancement in water-rich binary solvent mixtures. J. Mol. Liq. 2011, 158, 23–26. [Google Scholar] [CrossRef]
- Marcus, Y. Water structure enhancement in water-rich binary solvent mixtures. Part II. The excess partial molar heat capacity of the water. J. Mol. Liq. 2012, 166, 62–66. [Google Scholar] [CrossRef]
- Hsu, W.-H.; Yen, T.-C.; Chen, C.-C.; Yang, C.-W.; Fang, C.-K.; Hwang, I.-S. Observation of mesoscopic clathrate structures in ethanol-water mixtures. J. Mol. Liq. 2022, 366, 120299. [Google Scholar] [CrossRef]
- Langhals, H. The relationship between the refractive index and the composition of binary liquid mixtures. Ζ. Phys. Chem. 1985, 266, 775–780. [Google Scholar] [CrossRef][Green Version]
- Herraez, J.V.; Beld, R. Refractive Indices, Densities and Excess Molar Volumes of Monoalcohols + Water. J. Solution Chem. 2006, 35, 1315–1328. [Google Scholar] [CrossRef]
- El-Dossoki, F.I. Refractive index and density measurements for selected binary protic-protic, aprotic-aprotic, and aprotic-protic systems at temperatures from 298.15 K to 308.15 K. J. Chin. Chem. Soc. 2007, 54, 1129–1137. [Google Scholar] [CrossRef]
- Riobóo, R.J.; Philipp, M.; Ramos, M.A.; Krüger, J.K. Concentration and temperature dependence of the refractive index of ethanol-water mixtures: Influence of intermolecular interactions. Eur. Phys. J. E 2009, 30, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Linert, W.; Jameson, R. Acceptor properties of solvents: The use of isokinetic relationships to elucidate the relationship between the acceptor number and the solvatochromism of N-phenolate betaine dyes. J. Chem. Soc. Perkin Trans. 1993, 2, 1415–1421. [Google Scholar] [CrossRef]
- Zhao, X.; Knorr Jeanne, F.J.; Mc Hal, L. Temperature-dependent absorption spectrum of betaine-30 in methanol. Chem. Phys. Lett. 2002, 356, 214–220. [Google Scholar] [CrossRef]
- Tada, E.B.; Silva, P.L.; El Seoud, O.A. Thermosolvatochromism of betaine dyes in aqueous alcohols: Explicit consideration of the water–alcohol complex. J. Phys. Org. Chem. 2003, 16, 691–699. [Google Scholar] [CrossRef]
- Bosch, E.; Rosés, M.; Herodes, K.; Koppel, I.; Leito, I.; Koppel, I.; Taal, V. Solute-solvent and solvent-solvent interactions in binary solvent mixtures. 2. Effect of temperature on the ET(30) polarity parameter of dipolar hydrogen bond acceptor-hydrogen bond donor mixtures. J. Phys. Org. Chem. 1996, 9, 403–410. [Google Scholar] [CrossRef]
- Papadakis, R. Preferential Solvation of a Highly Medium Responsive Pentacyanoferrate(II) Complex in Binary Solvent Mixtures: Understanding the Role of Dielectric Enrichment and the Specificity of Solute–Solvent Interactions. J. Phys. Chem. B 2016, 120, 9422–9433. [Google Scholar] [CrossRef]
- Hernandez-Perni, G.; Leuenberger, H. The characterization of aprotic polar liquids and percolation phenomena in DMSO/water mixtures. Eur J. Pharm. Biopharm. 2005, 61, 201–213. [Google Scholar] [CrossRef]
- Yalcin, A.J.D.; Christofferson, C.J.; Drummond, T.L. Greaves, Solvation properties of protic ionic liquid–molecular solvent mixtures. Phys. Chem. Chem. Phys. 2020, 22, 10995–11011. [Google Scholar] [CrossRef]
- Kosower, E.M. The Effect of Solvent on Spectra. I. A New Empirical Measure of Solvent Polarity: Z-Values. J. Am. Chem. Soc. 1958, 80, 3253–3260. [Google Scholar] [CrossRef]
- Kosower, E.M. The Effect of Solvent on Spectra. II. Correlation of Spectral Absorption Data with Z-Values. J. Am. Chem. Soc. 1958, 80, 3261–3267. [Google Scholar] [CrossRef]
- Kosower, E.M.; Dodiuk, H.; Tanizawa, K.; Ottolenghi, M.; Orbach, N. Intramolecular Donor-Acceptor Systems Radiative and Nonradiative Processes for the Excited States of 2-iV-Arylamino-6-naphthalenesulfonates. J. Am. Chem. Soc. 1975, 97, 2167–2178. [Google Scholar] [CrossRef]
- Brownstein, S. The effect of solvents upon equilibria, spectra, and reaction rates. Can. J. Chem. 1960, 38, 1590–1596. [Google Scholar] [CrossRef]
- Gowland, J.H.; Schmid, J.H. Two linear correlations of pKa vs. solvent composition. Can. J. Chem. 1969, 47, 2953–2958. [Google Scholar] [CrossRef]
- Brooker, L.G.S.; Arnold, C.; Craig, C.; Heseltine, D.W.; Jenkins, P.W.; Lincoln, L.L. Color and Constitution. XIII. Merocyanines as Solvent Property Indicators. J. Am. Chem. Soc. 1965, 87, 2443–2450. [Google Scholar] [CrossRef]
- Taha, A.; Ramadan, A.A.T.; El-Behairy, M.A.; Ismaila, A.I.; Mahmoud, M.M. Preferential solvation studies using the solvatochromic dicyanobis (1, 10-phenanthroline) iron (II) complex. New J. Chem. 2001, 25, 1306–1312. [Google Scholar] [CrossRef]
- Huot, J.Y.; Battistel, E.; Lumry, R.; Villeneuve, G.; Lavallee, J.-F.; Anusiem, A.; Jolicoeur, C. A comprehensive thermodynamic investigation of water-ethylene glycol mixtures at 5, 25, and 45° C. J. Solution Chem 1988, 17, 601–636. [Google Scholar] [CrossRef]
- Langhals, H. Polarity of liquid mixtures with components of limited miscibility. Tetrahedron Lett. 1986, 27, 339–342. [Google Scholar] [CrossRef]
- Langhals, H. Polarität von Flüssigkeitsgemischen mit begrenzt mischbaren Komponenten. Z. Phys. Chem. 1987, 268, 91–96. [Google Scholar] [CrossRef]
- Jyoti, N.; Meena, A.S.; Beniwal, V. Evaluation of the Polarity in Binary Liquid Mixtures as a Function of Volume Fraction. Asian J. Chem. 2023, 35, 721–726. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kawashima, Y.; Yoshida, N.; Nakano, H. Solvatochromism and preferential solvation of Brooker’s merocyanine in water–methanol mixtures. J. Comp. Chem. 2017, 38, 2411–2419. [Google Scholar] [CrossRef] [PubMed]
- Kiss, B.; Fábián, B.; Idrissi, A.; Szőri, M.; Jedlovszky, P. Miscibility and Thermodynamics of Mixing of Different Models of Formamide and Water in Computer Simulation. J. Phys. Chem. B 2017, 121, 7147–7155. [Google Scholar] [CrossRef] [PubMed]
- Egan, E.P., Jr.; Luff, B.B. Heat of Solution, Heat Capacity, and Density of Aqueous Formamide Solutions at 25° C. J. Chem. Eng. Data 1966, 11, 194–196. [Google Scholar] [CrossRef]
- Campos, V.; Marigliano, A.C.G.; Sólimo, H.N. Density, Viscosity, Refractive Index, Excess Molar Volume, Viscosity, and Refractive Index Deviations and Their Correlations for the (Formamide + Water) System. Isobaric (Vapor + Liquid) Equilibrium at 2.5 kPa. J. Chem. Eng. Data 2008, 53, 211–216. [Google Scholar] [CrossRef]
- Egorov, G.I.; Makarov, D.M. Densities and Molar Isobaric Thermal Expansions of the Water + Formamide Mixture over the Temperature Range from 274.15 to 333.15 K at Atmospheric Pressure. J. Chem. Eng. Data 2017, 62, 1247–1256. [Google Scholar] [CrossRef]
- Perticaroli, S.; Comez, L.; Sassi, P.; Morresi, A.; Fioretto, D.; Paolanton, M. Water-like Behavior of Formamide: Jump Reorientation Probed by Extended Depolarized Light Scattering. J. Phys. Chem. Lett. 2018, 9, 120–125. [Google Scholar] [CrossRef]
- de Visser, C.; Pel, P.; Somsen, G. Volumes and Heat Capacities of Water and N-Methylformamide in Mixtures of These Solvents. J. Solution Chem. 1977, 6, 571–580. [Google Scholar] [CrossRef]
- Cilense, M.; Benedetti, A.V.; Vollet, D.R. Thermodynamic properties of liquid mixtures. II. Dimethylformamide-water. Thermochim. Acta 1983, 63, 151–156. [Google Scholar] [CrossRef]
- Kota Venkata Sivakumar, K.; Murthy Neriyanuri, K.; Krishnadevaraya, S.; Surahmanyam, S.V. Excess thermodynamic functions of the systems water+ N-methyl formamide and water+ N, N-dimethyl formamide. Acustica 1981, 48, 341–345. [Google Scholar]
- Spange, S.; Keutel, D. Untersuchungen zur Polarität wäßriger Harnstoff-und Zucker-Lösungen mit der Methode der vergleichenden Solvatochromie. Liebigs Ann. 1993, 1993, 981–985. [Google Scholar] [CrossRef]
- Stroka, J.; Herfort, I.; Schneider, H. Dimethylpropyleneurea—Water Mixtures: 1. Physical Properties. J. Solution Chem. 1990, 19, 743–753. [Google Scholar] [CrossRef]
- Civera, C.; del Valle, J.C.; Elorza, M.A.; Elorza, B.; Arias, C.; Díaz-Oliva, C.; Catalán, J.; Blanco, F.G. Solvatochromism in urea/water and urea-derivative/water solutions. Phys. Chem. Chem. Phys. 2020, 22, 25165. [Google Scholar] [CrossRef]
- LeBel, R.G.; Goring, D.A.I. Density, Viscosity, Refractive Index, and Hygroscopicity of Mixtures of Water and Dimethyl Sulfoxide. Chem. Eng. Data 1962, 7, 100–101. [Google Scholar] [CrossRef]
- Clever, H.L.; Pigott, S.P. Enthalpies of mixing of dimethylsulfoxide with water and with several ketones at 298.15 K. J. Chem. Thermodyn. 1971, 3, 221–225. [Google Scholar] [CrossRef]
- Egorov, G.I.; Makarov, D.M. The bulk properties of the water-dimethylsulfoxide system at 278–323.15 K and atmospheric pressure. Rus. J. Phys. Chem. A 2009, 83, 693–698. [Google Scholar] [CrossRef]
- Wong, D.B.; Sokolowsky, K.P.; El-Barghouthi, M.I.; Fenn, E.E.; Giammanco, C.H.; Sturlaugson, A.L.; Fayer, M.D. Water Dynamics in Water/DMSO Binary Mixtures. J. Phys. Chem. B 2012, 116, 5479–5490. [Google Scholar] [CrossRef] [PubMed]
- Kaatze, U.; Pottel, R.; Schaefer, M. Dielectric spectrum of dimethyl sulfoxide/water mixtures as a function of composition. J. Phys. Chem. 1989, 93, 5623–5627. [Google Scholar] [CrossRef]
- Płowaś, I.; Świergiel, J.; Jadżyn, J. Relative Static Permittivity of Dimethyl Sulfoxide + Water Mixtures. J. Chem. Eng. Data 2013, 58, 1741–1746. [Google Scholar] [CrossRef]
- Kirchner, B.; Reiher, M. The Secret of Dimethyl Sulfoxide−Water Mixtures. A Quantum Chemical Study of 1DMSO−nWater Clusters. J. Am. Chem. Soc. 2002, 124, 6206–6215. [Google Scholar] [CrossRef]
- Özal, T.A.; van der Vegt, N.F.A. Confusing cause and effect: Energy–entropy compensation in the preferential solvation of a nonpolar solute in dimethyl sulfoxide/water mixtures. J. Phys. Chem. B 2006, 110, 12104–12112. [Google Scholar] [CrossRef]
- Idrissi, A.; Marekha, B.; Barj, M.; Jedlovszky, P. Thermodynamics of mixing water with dimethyl sulfoxide, as seen from computer simulations. J. Phys. Chem. B 2014, 118, 8724–8733. [Google Scholar] [CrossRef]
- Catalán, J.; Dıaz, C.; Garcıa-Blanco, F. Characterization of binary solvent mixtures of DMSO with water and other cosolvents. J. Org. Chem. 2001, 66, 5846–5852. [Google Scholar] [CrossRef]
- Inamdar, S.R.; Gayathri, B.R.; Mannekutla, J.R. Rotational diffusion of coumarins in aqueous DMSO. J. Fluoresc. 2009, 19, 693–703. [Google Scholar] [CrossRef]
- Jabbari, M.; Khosravi, N.; Feizabadi, M.; Ajloo, D. Solubility temperature and solvent dependence and preferential solvation of citrus flavonoid naringin in aqueous DMSO mixtures: An experimental and molecular dynamics simulation study. RSC Adv. 2017, 7, 14776–14789. [Google Scholar] [CrossRef]
- Goates, J.R.; Sullivan, R.J. Thermodynamic Properties of the System Water-p-Dioxane. J. Phys. Chem. 1958, 62, 188–190. [Google Scholar] [CrossRef]
- Sakurai, M. Partial Molar Volumes for 1,4-Dioxane + Water. J. Chem. Eng. Data 1992, 37, 492–496. [Google Scholar] [CrossRef]
- Schott, H. Densities, Refractive Indices, and Molar Refractions of the System Water-Dioxane at 25 °C. J. Chem. Eng. Data 1961, 6, 19–20. [Google Scholar] [CrossRef]
- Ahn-Ercan, G.; Krienke, H.; Schmeer, G. Structural and dielectric properties of 1,4-dioxane–water mixtures. J. Mol. Liq. 2006, 129, 75–79. [Google Scholar] [CrossRef]
- Schrödle, S.; Fischer, B.; Helm, H.; Buchner, R. Picosecond dynamics and microheterogenity of water+ dioxane mixtures. J. Phys. Chem. A 2007, 111, 2043–2046. [Google Scholar] [CrossRef]
- Schrödle, S.; Hefter, G.; Buchner, R. Dielectric Spectroscopy of Hydrogen Bond Dynamics and Microheterogenity of Water + Dioxane Mixtures. J. Phys. Chem. B 2007, 111, 5946–5955. [Google Scholar] [CrossRef]
- Kumbharkhane, A.C.; Joshi, Y.S.; Mehrotra, S.C.; Yagihara, S.; Sudo, S. Study of hydrogen bonding and thermodynamic behavior in water–1,4-dioxane mixture using time domain reflectometry. Phys. B Conden. Matter 2013, 421, 1–7. [Google Scholar] [CrossRef]
- Casassas, E.; Fonrodona, G.; de Juan, A. Solvatochromic parameters for binary mixtures and a correlation with equilibrium constants. Part I. Dioxane-water mixtures. J. Solution Chem. 1992, 21, 147–162. [Google Scholar] [CrossRef]
- Hüttenhain, S.H.; Balzer, W. Solvatochromic Fluorescence of 8-(Phenylamino)-l-naphthalene-ammonium-sulfonate (8,1 ANS) in 1,4-Dioxane/Water Mixtures, revisited. Z. Naturforsch. A 1993, 48, 709–712. [Google Scholar] [CrossRef]
- Raju, B.B.; Costa, S.M.B. Photophysical properties of 7-diethylaminocoumarin dyes in dioxane–water mixtures: Hydrogen bonding, dielectric enrichment and polarity effects. Phys. Chem. Chem. Phys. 1999, 1, 3539–3547. [Google Scholar] [CrossRef]
- Sánchez, F.; Díaz, A.N.; Algarra, M.J.; Lovillo, J.; Aguilar, A. Time resolved spectroscopy of 2-(dimethylamine)fluorene. Solvent effects and photophysical behavior. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 83, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, R.; Jana, D. Seth, Photophysical study of an alkaloid harmaline in 1,4-dioxane-water mixtures. Chem. Phys. Lett. 2018, 706, 158–163. [Google Scholar] [CrossRef]
- de Visser, C.; Perron, G.; Desnoyers, J.E. The heat capacities, volumes, and expansibilities of tert-butyl alcohol–water mixtures from 6 to 65 °C. Can. J. Chem. 1977, 55, 856–862. [Google Scholar] [CrossRef]
- Sakurai, M. Partial molar volumes in aqueous mixtures of nonelectrolytes, I. tert. butyl alcohol. Bull. Chem. Soc. Jpn. 1987, 60, 1–7. [Google Scholar] [CrossRef]
- Bowron, D.T.; Sober, A.K.; Finney, J.L. Temperature dependence of the structure of a 0.06 mole fraction tertiary butanol-water solution. J. Chem. Phys. 2001, 114, 6203–6219. [Google Scholar] [CrossRef]
- Egorov, G.I.; Makarov, D.M. Densities and volume properties of (water + tert-butanol) over the temperature range of (274.15 to 348.15) K at pressure of 0.1 MPa. J. Chem. Thermodyn. 2011, 43, 430–441. [Google Scholar] [CrossRef]
- Subramanian, D.; Klauda, J.B.; Leys, J.; Anisimov, M.A. Thermodynamic anomalies and structural fluctuations in aqueous solutions of tertiary butyl alcohol. arXiv 2013, arXiv:1308.3676. [Google Scholar] [CrossRef]
- Aman-Pommier, F.; Jallut, C. Excess specific volume of water + tert-butyl alcohol solvent mixtures: Experimental data, modeling and derived excess partial specific thermodynamic quantities. Fluid Phase Equilibria 2017, 439, 43–66. [Google Scholar] [CrossRef]
- Gavrylyak, M.S. Investigation of dynamic fluctuations of refraction index of water tertiary butanol solutions. In Proceedings of the Eighth International Conference on Correlation Optics, Chernivsti, Ukraine, 11–14 September 2007. [Google Scholar]
- Gazi, A.H.R.; Biswas, R. Heterogeneity in Binary Mixtures of (Water + Tertiary Butanol): Temperature Dependence Across Mixture Composition. J. Phys. Chem. A 2011, 115, 2447–2455. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, D.; Anisimov, M.A. Resolving the Mystery of Aqueous Solutions of Tertiary Butyl Alcohol. J. Phys. Chem. B 2011, 115, 9179–9183. [Google Scholar] [CrossRef] [PubMed]
- Banik, D.; Bhattacharya, S.; Kumar Datta, P.; Sarkar, N. Anomalous Dynamics in tert-Butyl Alcohol–Water and Trimethylamine N-Oxide–Water Binary Mixtures: A Femtosecond Transient Absorption Study. ACS Omega 2018, 3, 383–392. [Google Scholar] [CrossRef]
- Arnett, E.M.; Hufford, D.; McKelvey, D.R. The Ground-State Solvation Contribution to an Electronic Spectral Shift. J. Am. Chem. Soc. 1966, 88, 3142–3148. [Google Scholar] [CrossRef]
- Duereh, A.; Sato, Y.; Smith, R.L.; Inomata, H.; Pichierri, F. Does Synergism in Microscopic Polarity Correlate with Extrema in Macroscopic Properties for Aqueous Mixtures of Dipolar Aprotic Solvents? J. Phys. Chem. B 2017, 121, 6033–6041. [Google Scholar] [CrossRef]
- Akay, S.; Kayan, B.; Martínez, F. Solubility, dissolution thermodynamics and preferential solvation of 4-nitroaniline in (ethanol + water) mixtures. Phys. Chem. Liq. 2021, 59, 956–968. [Google Scholar] [CrossRef]
- Ellis, C.-M. The 2-butoxyethanol-water system: Critical solution temperatures and salting-out effects. J. Chem. Educ. 1967, 44, 405–407. [Google Scholar] [CrossRef]
- Quirion, F.; Magid, L.J.; Drifford, M. Aggregation and Critical Behavior of 2-Butoxyethanol in Water. Langmuir 1990, 6, 244–249. [Google Scholar] [CrossRef]
- Kaatze, U.; Menzel, K.; Pottel, R.; Schwerdtfeger, S. Microheterogeneity of 2-Butoxyethanol/Water Mixtures at Room Temperature. An Ultrasonic Relaxation Study. Z. Phys. Chem. 1994, 186, 141–170. [Google Scholar] [CrossRef]
- Kaatze, U.; Pottel, R.; Schumacher, A. Dielectric spectroscopy of 2-butoxyethanol/water mixtures in the complete composition range. J. Phys. Chem. 1992, 96, 6017–6020. [Google Scholar] [CrossRef]
- Siu, W.; Koga, Y. Excess partial molar enthalpies of 2-butoxyethanol and water in 2-butoxyethanol-water mixtures. Can. J. Chem. 1989, 67, 671–676. [Google Scholar] [CrossRef]
- Andersson, B.; Olofsson, G. Partial molar enthalpies of solution as indicators of interactions in mixtures of 2-butoxyethanol and 2-butanol with water. J. Solution Chem. 1988, 17, 1169–1182. [Google Scholar] [CrossRef]
- Yoshida, K.; Yamaguchi, T.; Nagao, M.Y.; Kawabata, Y.; Seto, H.; Takeda, T. Slow dynamics of n-butoxyethanol–water mixture by neutron spin echo technique. Appl. Phys. A 2002, 74, 386–388. [Google Scholar] [CrossRef]
- Joshi, Y.S.; Kumbharkhan, A.C. Study of dielectric relaxation and hydrogen bonding in water + 2-butoxyethanol mixtures using TDR technique. Fluid Phase Equilib. 2012, 317, 96–101. [Google Scholar] [CrossRef]
- Chiou, D.-R.; Chen, S.-Y.; Chen, L.J. Density, Viscosity, and Refractive Index for Water + 2-Butoxyethanol and + 2-(2-Butoxyethoxy) ethanol at Various Temperatures. J. Chem. Eng. Data 2010, 55, 1012–1016. [Google Scholar] [CrossRef]
- Kajimoto, S.; Moria, A.; Fukumura, H. Photo-controlled phase separation and mixing of a mixture of water and 2-butoxyethanol caused by photochromic isomerisation of spiropyran. Photochem. Photobiol. Sci. 2010, 9, 208–212. [Google Scholar] [CrossRef]
- Indra, S.; Biswas, R. Heterogeneity in (2-butoxyethanol + water) mixtures: Hydrophobicity-induced aggregation or criticality-driven concentration fluctuations? J. Chem. Phys. 2015, 142, 204501. [Google Scholar] [CrossRef] [PubMed]
- Catalán, J.; Díaz-Oliva, C.; García-Blanco, F. On the hydrophobic effect in water–alcohol mixtures. Chem. Phys. 2019, 527, 110467. [Google Scholar] [CrossRef]
- Li, R.; Agostino, C.; McGregor, J.; Mantle, M.D.; Zeitler, A.; Gladden, L.F. Mesoscopic Structuring and Dynamics of Alcohol/Water Solutions Probed by Terahertz Time-Domain Spectroscopy and Pulsed Field Gradient Nuclear Magnetic Resonance. J. Phys. Chem. B 2014, 118, 10156–10166. [Google Scholar] [CrossRef] [PubMed]
- Baksi, A.; Biswas, R. Dynamical Anomaly of Aqueous Amphiphilic Solutions: Connection to Solution H-Bond Fluctuation Dynamics? ACS Omega 2022, 7, 10970–10984. [Google Scholar] [CrossRef] [PubMed]
- Koohyar, F.; Kiani, F.; Sharifi, S.; Sharifirad, M.; Rahmanpour, S.H. Study on the Change of Refractive Index on Mixing, Excess Molar Volume and Viscosity Deviation for Aqueous Solution of Methanol, Ethanol, Ethylene Glycol, 1-Propanol and 1, 2, 3-Propantriol at T = 292.15 K and Atmospheric Pressure. Res. J. Appl. Sci. Eng. Technol. 2012, 4, 3095–3101. [Google Scholar]
- Zhang, Z.; Wang, W.; Korpacz, A.N.; Dufour, C.R.; Weiland, Z.J.; Lambert, C.R.; Timko, M.T. Binary Liquid Mixture Contact-Angle Measurements for Precise Estimation of Surface Free Energy. Langmuir 2019, 35, 12317–12325. [Google Scholar] [CrossRef]
- Davis, M.I. Determination and analysis of the excess molar volumes of some amide-water systems. Thermochim. Acta 1987, 120, 299–314. [Google Scholar] [CrossRef]
- Marcus, Y. Preferential solvation in mixed solvents. 15. Mixtures of acetonitrile with organic solvents. J. Chem. Thermodyn. 2019, 135, 55–59. [Google Scholar] [CrossRef]
- Marcus, Y. The structure of mixtures of water and acetone derived from their cohesive energy densities and internal pressures. J. Mol. Liq. 2020, 320, 112801. [Google Scholar] [CrossRef]
- Reichardt, C. Pyridinium-N-phenolate betaine dyes as empirical indicators of solvent polarity: Some new findings. Pure Appl. Chem. 2008, 80, 1415–1432. [Google Scholar] [CrossRef]
- Kiselev, V.D.; Kashaeva, E.A.; Luzanova, N.A.; Konovalov, A.I. Enthalpies of solution of lithium perchlorate and Reichardt’ dye in some organic solvents. Thermochim. Acta 1997, 303, 225–228. [Google Scholar] [CrossRef]
- Machado, C.; da Graça Nascimento, M.; Rezende, M.C.; Beezer, A.E. Calorimetric evidence of aggregation of the ET(30) dye in alcoholic solutions. Thermochim. Acta 1999, 328, 150–159. [Google Scholar] [CrossRef]
- Catalán, J.; Gomez, J.; Saiz, J.L.; Couto, A.; Ferraris, M.; Laynez, J. Calorimetric quantification of the hydrogen-bond acidity of solvents and its relationship with solvent polarity. J. Chem. Soc. Perkin Trans. 2 1995, 1, 2301–2305. [Google Scholar] [CrossRef]
- Bose, K.; Kundu, K.K. Thermodynamics of transfer of p-nitroaniline from water to alcohol + water mixtures at 25 °C and the structure of water in these media. Can. J. Chem. 1977, 55, 3961–3966. [Google Scholar] [CrossRef]
- Cowie, J.M.G.; Toporowski, P.M. Association in the binary liquid system dimethylsulphoxide-water. Can. J. Chem. 1961, 39, 2240–2243. [Google Scholar] [CrossRef]
- Schrader, A.M.; Donaldson, S.H., Jr.; Song, J.; Israelachvili, J.N. Correlating steric hydration forces with water dynamics through surface force and diffusion NMR measurements in a lipid–DMSO–H2O system. Proc. Natl. Acad. Sci. USA 2015, 112, 10708–10713. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.N.; Singh, K.K.; Goswam, D. Sensing non-ideal microheterogeneity in binary mixtures of dimethyl sulfoxide and water. J. Opt. 2022, 24, 054001. [Google Scholar] [CrossRef]
- Cerar, J.; Jamnik, A.; Pethes, I.; Temleitner, L.; Pusztai, L.; Tomšič, M. Structural, rheological and dynamic aspects of hydrogen-bonding molecular liquids: Aqueous solutions of hydrotropic tert-butyl alcohol. J. Coll. Interf. Sci. 2020, 560, 730–742. [Google Scholar] [CrossRef]
- Chakraborty, S.; Pyne, P.; Mitra, R.K.; Das Mahanta, D. A subtle interplay between hydrophilic and hydrophobic hydration governs butanol (de)mixing in water. Chem. Phys. Lett. 2022, 807, 140080. [Google Scholar] [CrossRef]
- Langhals, H. Ein neues, unkompliziertes Verfahren zur Bestimmung der Zusammensetzung binärer Fliissigkeitsgemische, A new, uncomplicated method for the determination of the composition of binary liquid mixtures. Fresenius Z. Anal. Chem. 1981, 308, 441–444. [Google Scholar] [CrossRef][Green Version]
- Królicki, R.; Jarzȩba, W.; Mostafavi, M.; Lampre, I. Preferential Solvation of Coumarin 153 The Role of Hydrogen Bonding. J. Phys. Chem. A 2002, 106, 1708–1713. [Google Scholar] [CrossRef]
- Marcus, Y. The structure of and interactions in binary acetonitrile + water mixtures. J. Phys. Org. Chem. 2012, 35, 1072–1085. [Google Scholar] [CrossRef]
- Shalmashi, A.; Amani, F. Densities and excess molar volumes for binary solution of water + ethanol, + methanol and + propanol from (283.15 to 313.15) K). Lat. Am. Appl. Res. 2014, 44, 163–166. [Google Scholar] [CrossRef]
- Galicia-Andrés, E.; Dominguez, H.; Pusztai, L.; Pizio, O. On the composition dependence of thermodynamic, dynamic and dielectric properties of water-methanol model mixtures. Molecular dynamics simulation results. Condens. Mater. Phys. 2015, 18, 43602. [Google Scholar] [CrossRef]
- Martens, M.; Hadrich, M.J.; Nestler, F.; Ouda, M.; Schaad, A. Combination of Refractometry and Densimetry–A Promising Option for Fast Raw Methanol Analysis. Chem. Ing. Tech. 2020, 92, 1474–1481. [Google Scholar] [CrossRef]
- Ortega, J. Densities and Refractive Indices of Pure Alcohols as a Function of Temperature. J. Chem. Eng. Data 1982, 27, 312–317. [Google Scholar] [CrossRef]
- Zaichikov, A.M.; Krestyaninov, M.A. Thermodynamic characteristics of and intermolecular interactions in aqueous solutions of N-methylformamide. Russ. J. Phys. Chem. 2006, 80, 1249–1254. [Google Scholar] [CrossRef]
- de Visser, C.; Perron, G.; Desnoyers, J.E.; Heuvelsland, W.J.M.; Somsen, G. Volumes and Heat Capacities of Mixtures of N, N- Dimethylformamide and Water at 298.15 K. J. Chem. Eng. Data 1977, 22, 74–79. [Google Scholar] [CrossRef]
- Al-Azzawl, S.F.; Allo, E.I. Density, Viscosity, and Refractivity Data off Solutions of Potassium Iodide in /V-Formylmorpholine-Water at 25, 35, and 45 °C. J. Chem. Eng. Data 1992, 37, 158–162. [Google Scholar] [CrossRef]
- Chen, G.; Hou, Y.; Knapp, H. Diffusion Coefficients, Kinematic Viscosities, and Refractive Indices for Heptane + Ethylbenzene, Sulfolane + 1-Methylnaphthalene, Water + N,N-Dimethylformamide, Water + Methanol, Water + N-Formylmorpholine, and Water + N-Methylpyrrolidone. J. Chem. Eng. Data 1995, 40, 1005–1010. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spange, S. Polarity of Organic Solvent/Water Mixtures Measured with Reichardt’s B30 and Related Solvatochromic Probes—A Critical Review. Liquids 2024, 4, 191-230. https://doi.org/10.3390/liquids4010010
Spange S. Polarity of Organic Solvent/Water Mixtures Measured with Reichardt’s B30 and Related Solvatochromic Probes—A Critical Review. Liquids. 2024; 4(1):191-230. https://doi.org/10.3390/liquids4010010
Chicago/Turabian StyleSpange, Stefan. 2024. "Polarity of Organic Solvent/Water Mixtures Measured with Reichardt’s B30 and Related Solvatochromic Probes—A Critical Review" Liquids 4, no. 1: 191-230. https://doi.org/10.3390/liquids4010010
APA StyleSpange, S. (2024). Polarity of Organic Solvent/Water Mixtures Measured with Reichardt’s B30 and Related Solvatochromic Probes—A Critical Review. Liquids, 4(1), 191-230. https://doi.org/10.3390/liquids4010010







