Revisiting the Total Hildebrand and Partial Hansen Solubility Parameters of Analgesic Drug Meloxicam
Abstract
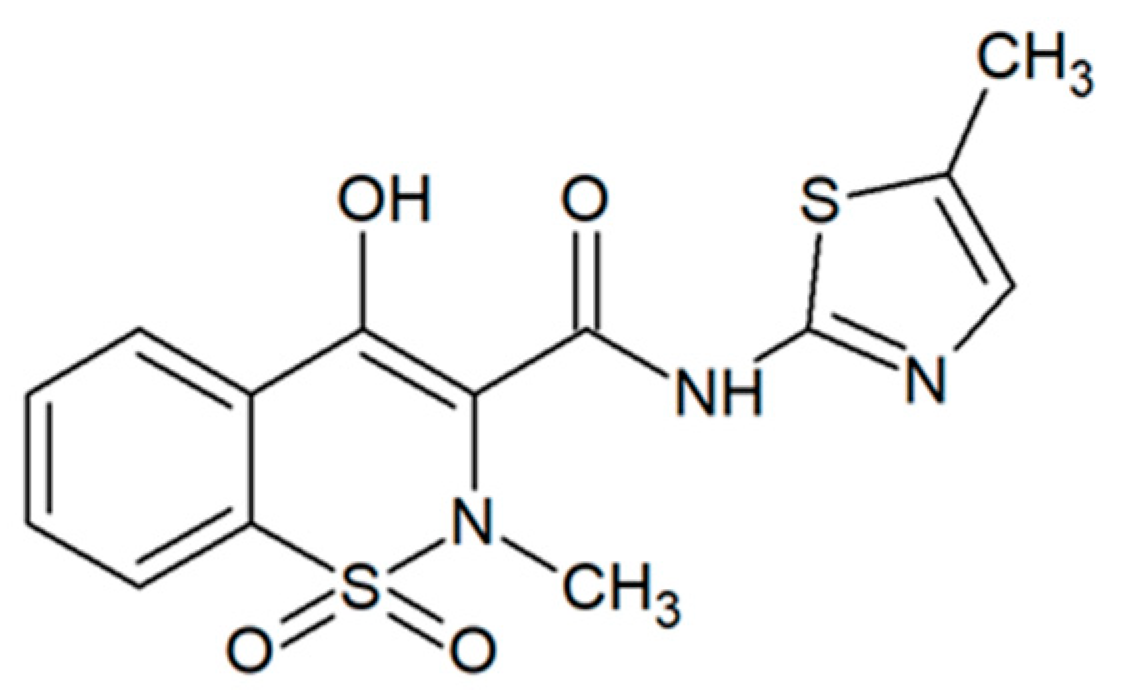
:1. Introduction
2. Methodology
2.1. Meloxicam Solubility Values
2.2. Physicochemical Properties of Solvents
| Solvent a | ln x2 | Solvatochromic Parameters b | V° c (cm3·mol−1) | Hansen Solubility Parameters c | δT (MPa1/2) c | ||||
|---|---|---|---|---|---|---|---|---|---|
| α | β | π | δd (MPa1/2) | δp (MPa1/2) | δh (MPa1/2) | ||||
| Hexane | −11.774 | 0.00 | 0.00 | −0.11 | 131.6 | 14.9 | 0.0 | 0.0 | 14.9 |
| Cyclohexane | −12.317 | 0.00 | 0.00 | 0.00 | 108.7 | 16.8 | 0.0 | 0.0 | 16.8 |
| Butyl acetate | −7.834 | 0.00 | 0.45 | 0.46 | 132.5 | 15.8 | 3.7 | 6.3 | 17.4 |
| CCl4 | −9.694 | 0.00 | 0.10 | 0.21 | 97.1 | 17.8 | 0.0 | 0.6 | 17.8 |
| Ethyl acetate | −7.701 | 0.00 | 0.45 | 0.45 | 98.5 | 15.8 | 5.3 | 7.2 | 18.1 |
| Toluene | −8.681 | 0.00 | 0.11 | 0.49 | 106.8 | 18.0 | 1.4 | 2.0 | 18.2 |
| Benzene | −8.733 | 0.00 | 0.10 | 0.55 | 89.4 | 18.4 | 0.0 | 2.0 | 18.6 |
| Chloroform | −6.312 | 0.20 | 0.10 | 0.58 | 80.7 | 17.8 | 3.1 | 5.7 | 19.0 |
| Acetone | −7.409 | 0.08 | 0.48 | 0.62 | 74.0 | 15.5 | 10.4 | 7.0 | 20.0 |
| 1,4-Dioxane | −6.576 | 0.00 | 0.37 | 0.49 | 85.7 | 19.0 | 1.8 | 7.4 | 20.5 |
| 1-Octanol | −8.082 | 0.77 | 0.81 | 0.40 | 157.7 | 17.0 | 3.3 | 11.9 | 20.9 |
| 1-Heptanol | −8.395 | 141.8 | 16.0 | 5.3 | 11.7 | 21.0 | |||
| 1-Hexanol | −8.888 | 0.80 | 0.84 | 0.40 | 135.8 | 15.9 | 5.8 | 12.5 | 21.3 |
| PEG400 | −4.676 | 354.5 d | 16.6 d | 3.7 d | 13.3 d | 21.6 d | |||
| 1-Pentanol | −9.124 | 0.84 | 0.86 | 0.40 | 109.0 | 16.0 | 4.5 | 13.9 | 21.7 |
| Acetophenone | −6.077 | 0.04 | 0.49 | 0.81 | 117.4 | 19.6 | 8.6 | 3.7 | 21.8 |
| Carbitol | −6.645 | 130.9 | 16.2 | 9.2 | 12.3 | 22.3 | |||
| NMP | −3.313 | 0.00 | 0.77 | 0.92 | 96.5 | 18.0 | 12.3 | 7.2 | 22.9 |
| 1-Butanol | −9.338 | 0.84 | 0.84 | 0.47 | 91.5 | 16.0 | 5.7 | 15.8 | 23.1 |
| Benzyl alcohol | −6.299 | 0.60 | 0.52 | 0.98 | 103.6 | 18.4 | 6.3 | 13.7 | 23.8 |
| ACN | −9.010 | 0.19 | 0.40 | 0.66 | 52.6 | 15.3 | 18.0 | 6.1 | 24.4 |
| 1-Propanol | −9.797 | 0.84 | 0.90 | 0.52 | 75.2 | 16.0 | 6.8 | 17.4 | 24.5 |
| DMF | −4.361 | 0.00 | 0.69 | 0.88 | 77.0 | 17.4 | 13.7 | 11.3 | 24.8 |
| Ethanol | −9.778 | 0.86 | 0.75 | 0.54 | 58.5 | 15.8 | 8.8 | 19.4 | 26.5 |
| DMSO | −4.840 | 0.00 | 0.76 | 1.00 | 71.3 | 18.4 | 16.4 | 10.2 | 26.7 |
| Methanol | −9.930 | 0.98 | 0.66 | 0.60 | 40.7 | 15.1 | 12.3 | 22.3 | 29.6 |
| NMF | −6.444 | 0.62 | 0.80 | 0.90 | 59.1 | 17.4 | 18.8 | 15.9 | 30.1 |
| PG | −10.078 | 0.83 | 0.78 | 0.76 | 73.6 | 16.8 | 9.4 | 23.3 | 30.2 |
| Glycerol | −12.610 | 1.21 | 0.51 | 0.62 | 73.3 | 17.4 | 12.1 | 29.3 | 36.1 |
| Formamide | −7.196 | 0.71 | 0.48 | 0.97 | 39.8 | 17.2 | 26.2 | 19.0 | 36.6 |
| Water | −13.687 | 1.17 | 0.47 | 1.09 | 18.0 | 15.6 | 16.0 | 42.3 | 47.8 |
2.3. Meloxicam Parameter Calculations
3. Results and Discussion
3.1. Meloxicam Mole Fraction Solubility in Pure Solvents at 298.15 K
3.2. Hildebrand Solubility Parameter of Meloxicam
3.3. Hansen Solubility Parameters of Meloxicam
3.4. Solvent Effects: KAT-LSER Model
3.5. Comparison between Hansen Solubility Parameters and KAT-LSER Model for Intermolecular Interactions Analyses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Budavari, S.; O’Neil, M.J.; Smith, A.; Heckelman, P.E.; Obenchain, J.R., Jr.; Gallipeau, J.A.R. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 13th ed.; Merck & Co., Inc.: Whitehouse Station, NJ, USA, 2001. [Google Scholar]
- Brooks, P.M.; Day, R.O. Non steroidal anti-inflammatory drugs—Differences and similarities. N. Engl. J. Med. 1991, 324, 1716–1725. [Google Scholar] [CrossRef]
- Engelhardt, G.; Homma, D.; Schlegel, K.; Utzmann, R.; Schnitzler, C. Anti-inflammatory, analgesic, antipyretic and related properties of meloxicam, a new non-steroidal anti-inflammatory agent with favourable gastrointestinal tolerance. Inflamm. Res. 1995, 44, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Türck, D.; Roth, W.; Busch, U. A review of the clinical pharmacokinetics of meloxicam. Br. J. Rheumatol. 1996, 35, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, S.C. Martindale: The Complete Drug Reference, 36th ed.; Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Jouyban, A. Handbook of Solubility Data for Pharmaceutical; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Washington, N.; Washington, C.; Wilson, C.G. Physiological Pharmaceutics: Barriers to Drug Absorption, 2nd ed.; Taylor & Francis: London, UK, 2001. [Google Scholar]
- Avdeef, A. Absorption and Drug Development: Solubility, Permeability, and Charge State; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Martin, A.; Bustamante, P.; Chun, A.H.C. Physical Pharmacy: Physical Chemical Principles in the Pharmaceutical Sciences, 4th ed.; Lea & Febiger: Philadelphia, PA, USA, 1993. [Google Scholar]
- Florence, A.T.; Attwood, D. Physicochemical Principles of Pharmacy, 5th ed.; Pharmaceutical Press: London, UK, 2011. [Google Scholar]
- Allen, L.V., Jr.; Ansel, A.C. Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems, 10th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2014. [Google Scholar]
- Taylor, K.M.G.; Aulton, M.E. Aulton’s Pharmaceutics: The Design and Manufacture of Medicines, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Seedher, N.; Bhatia, S. Solubility enhancement of Cox-2 inhibitors using various solvent systems. AAPS PharmSciTech 2003, 4, 33. [Google Scholar] [CrossRef]
- Babu, P.R.; Subrahmanyam, C.V.; Thimmasetty, J.; Manavalan, R.; Valliappan, K. Solubility of meloxicam in mixed solvent systems. Ethiop. Pharm. J. 2007, 25, 23–28. [Google Scholar] [CrossRef]
- Sathesh-Babu, P.R.; Subrahmanyam, C.V.S.; Thimmasetty, J.; Manavalan, R.; Valliappan, K.; Kedarnath, S.S. Solubility enhancement of Cox-II inhibitors by cosolvency approach. Dhaka Univ. J. Pharm. Sci. 2008, 7, 119–126. [Google Scholar] [CrossRef]
- Jain, D.; Pathak, K. Design, characterization, and evaluation of meloxicam gel prepared by suspension and solution polymerization using solubility parameter as the basis for development. AAPS PharmSciTech 2010, 11, 133–142. [Google Scholar] [CrossRef]
- Delgado, D.R.; Holguin, A.R.; Almanza, O.A.; Martinez, F.; Marcus, Y. Solubility and preferential solvation of meloxicam in ethanol + water mixtures. Fluid Phase Equilib. 2011, 305, 88–95. [Google Scholar] [CrossRef]
- Holguín, A.R.; Delgado, D.R.; Martínez, F.; Marcus, Y. Solution thermodynamics and preferential solvation of meloxicam in propylene glycol + water mixtures. J. Solut. Chem. 2011, 40, 1987–1999. [Google Scholar] [CrossRef]
- Delgado, D.R.; Jouyban, A.; Martínez, F. Solubility and preferential solvation of meloxicam in methanol + water mixtures at 298.15 K. J. Mol. Liq. 2014, 197, 368–373. [Google Scholar] [CrossRef]
- Jiménez, D.M.; Cárdenas, Z.J.; Delgado, D.R.; Jouyban, A.; Martínez, F. Solubility and solution thermodynamics of meloxicam in 1,4-dioxane and water mixtures. Ind. Eng. Chem. Res. 2014, 53, 16550–16558. [Google Scholar] [CrossRef]
- Cárdenas, Z.J.; Jiménez, D.M.; Martínez, F. Solubility and solution thermodynamics of meloxicam in polyethylene glycol 400 + water mixtures. J. Mol. Liq. 2015, 211, 233–238. [Google Scholar] [CrossRef]
- Tinjacá, D.A.; Martínez, F.; Almanza, O.A.; Jouyban, A.; Acree, W.E., Jr. Solubility of meloxicam in aqueous binary mixtures of formamide, N-methylformamide and N,N-dimethylformamide: Determination, correlation, thermodynamics and preferential solvation. J. Chem. Thermodyn. 2021, 154, 106332. [Google Scholar] [CrossRef]
- Tinjacá, D.A.; Martínez, F.; Almanza, O.A.; Jouyban, A.; Acree, W.E., Jr. Dissolution thermodynamics and preferential solvation of meloxicam in (acetonitrile + water) mixtures. Phys. Chem. Liq. 2021, 59, 733–752. [Google Scholar] [CrossRef]
- Tinjacá, D.A.; Martínez, F.; Almanza, O.A.; Jouyban, A.; Acree, W.E., Jr. Solubility, dissolution thermodynamics and preferential solvation of meloxicam in (methanol + water) mixtures. J. Solut. Chem. 2021, 50, 667–689. [Google Scholar] [CrossRef]
- Tinjacá, D.A.; Martínez, F.; Almanza, O.A.; Jouyban, A.; Acree, W.E., Jr. Solubility of meloxicam in (Carbitol® + water) mixtures: Determination, correlation, dissolution thermodynamics and preferential solvation. J. Mol. Liq. 2021, 324, 114671. [Google Scholar] [CrossRef]
- Golgoun, S.; Mokhtarpour, M.; Shekaari, H. Solubility enhancement of betamethasone, meloxicam and piroxicam by use of choline-based deep eutectic solvents. Pharm. Sci. 2021, 27, 86–101. [Google Scholar] [CrossRef]
- Tinjacá, D.A.; Martínez, F.; Almanza, O.A.; Jouyban, A.; Acree, W.E., Jr. Solubility, correlation, dissolution thermodynamics and preferential solvation of meloxicam in aqueous mixtures of 2-propanol. Pharm. Sci. 2022, 28, 130–144. [Google Scholar] [CrossRef]
- Tinjacá, D.A.; Martínez, F.; Almanza, O.A.; Peña, M.A.; Jouyban, A.; Acree, W.E., Jr. Increasing the equilibrium solubility of meloxicam in aqueous media by using dimethyl sulfoxide as a cosolvent: Correlation, dissolution thermodynamics and preferential solvation. Liquids 2022, 2, 161–182. [Google Scholar] [CrossRef]
- Tinjacá, D.A.; Martínez, F.; Almanza, O.A.; Jouyban, A.; Acree, W.E., Jr. Effect of N-methyl-pyrrolidone (NMP) on the equilibrium solubility of meloxicam in aqueous media: Correlation, dissolution thermodynamics, and preferential solvation. ACS Omega 2022, 7, 37988–38002. [Google Scholar] [CrossRef]
- Sathesh-Babu, P.R.; Subrahmanyam, C.V.S.; Thimmasetty, J.; Manavalan, R.; Valliappan, K. Extended Hansen’s solubility approach: Meloxicam in individual solvents. Pak. J. Pharm. Sci. 2007, 20, 311–316. [Google Scholar]
- Cristancho, D.M.; Martínez, F. Solubility and preferential solvation of meloxicam in ethyl acetate + ethanol mixtures at several temperatures. J. Mol. Liq. 2014, 200, 122–128. [Google Scholar] [CrossRef]
- Castro, G.T.; Filippa, M.A.; Sancho, M.I.; Gasull, E.I.; Almandoz, M.C. Solvent effect on the solubility and absorption spectra of meloxicam: Experimental and theoretical calculations. Phys. Chem. Liq. 2020, 58, 337–348. [Google Scholar] [CrossRef]
- Hansen, C.M. The three-dimensional solubility parameters. Key to paint component affinities. J. Paint Technol. 1967, 39, 505–511. [Google Scholar]
- Petříková, E.; Patera, J.; Gorlová, O. Influence of active pharmaceutical ingredient structures on Hansen solubility parameters. Eur. J. Pharm. Sci. 2021, 167, 106016. [Google Scholar] [CrossRef]
- Lara, J.; Zimmermann, F.; Drolet, D.; Hansen, C.; Chollot, A.; Monta, N. The use of the Hansen solubility parameters in the selection of protective polymeric materials resistant to chemicals. Int. J. Current Res. 2017, 9, 47860–47867. [Google Scholar]
- Peña, M.A.; Martinez, F. Hansen Solubility Parameters: A tool for solvent selection in drugs. Pharm. Sci. 2023, 29, 133–134. [Google Scholar] [CrossRef]
- Peña, M.A.; Spaò, G.; Torres-Pabón, N.S.; Martínez, F. Solubility data and solubility parameters of barnidipine in different pure solvents. Ars Pharm. 2023, 64, 329–341. [Google Scholar] [CrossRef]
- Li, W.; Qi, S.; Wang, N.; Fei, Z.; Farajtabar, A.; Zhao, H. Solute-solvent and solvent-solvent interactions and preferential solvation of limonin in aqueous co-solvent mixtures of methanol and acetone. J. Mol. Liq. 2018, 263, 357–365. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Cao, Y.; Cong, Y.; Farajtabar, A.; Zhao, H. Solubility modeling, solvent effect, and preferential solvation of thiamphenicol in cosolvent mixtures of methanol, ethanol, N,N-dimethylformamide, and 1,4-dioxane with water. J. Chem. Eng. Data 2018, 63, 2219–2227. [Google Scholar] [CrossRef]
- Li, W.X.; Farajtabar, A.; Wang, N.; Liu, Z.T.; Fei, Z.H.; Zhao, H.K. Solubility of chloroxine in aqueous co-solvent mixtures of N, N-dimethylformamide, dimethyl sulfoxide, N-methyl-2-pyrrolidone and 1,4-dioxane: Determination, solvent effect and preferential solvation analysis. J. Chem. Thermodyn. 2019, 138, 288–296. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Gao, X.; Lv, H. Equilibrium solubility, preferential solvation and solvent effect study of clotrimazole in several aqueous co-solvent solutions. J. Chem. Thermodyn. 2020, 151, 106255. [Google Scholar] [CrossRef]
- Cong, Y.; Du, C.; Xing, K.; Bian, Y.; Li, X.; Wang, M. Investigation on co-solvency, solvent effect, Hansen solubility parameter and preferential solvation of fenbufen dissolution and models correlation. J. Mol. Liq. 2022, 348, 118415. [Google Scholar] [CrossRef]
- Choi, E.; Heynderickx, P.M. Solubility measurement and correlation of 2-aminoterephthalic acid in eight alcoholic solvents at different temperatures. J. Chem. Thermodyn. 2023, 177, 106948. [Google Scholar] [CrossRef]
- Rubino, J.T. Cosolvents and cosolvency. In Encyclopedia of Pharmaceutical Technology; Swarbrick, J., Boylan, J.C., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1988; Volume 3, pp. 375–398. [Google Scholar]
- Marcus, Y. The Properties of Solvents; John Wiley & Sons: Chichester, UK, 1998. [Google Scholar]
- Barton, A.F.M. Handbook of Solubility Parameters and Other Cohesion Parameters, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Liu, B.; Du, Q.; Yang, Y. The phase diagrams of mixtures of EVAL and PEG in relation to membrane formation. J. Membr. Sci. 2000, 180, 81–82. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K.A. Phase solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Luger, P.; Daneck, K.; Engel, W.; Trummlitz, G.; Wagner, K. Structure and physicochemical properties of meloxicam, a new NSAID. Eur. J. Pharm. Sci. 1996, 4, 175–187. [Google Scholar] [CrossRef]
- Banerjee, R.; Sarkar, M. Spectroscopic studies of microenvironment dictated structural forms of piroxicam and meloxicam. J. Luminescence 2002, 99, 255–263. [Google Scholar] [CrossRef]
- Noolkar, S.B.; Jadhav, N.R.; Bhende, S.A.; Killedar, S.G. Solid-state characterization and dissolution properties of meloxicam–moringa coagulant–PVP ternary solid dispersions. AAPS PharmSciTech 2013, 14, 569–577. [Google Scholar] [CrossRef]
- Wu, X.Q.; Tang, P.X.; Li, S.S.; Zhang, L.L.; Li, H. X-ray powder diffraction data for meloxicam, C14H13N3O4S2. Powder Diffr. 2014, 29, 196–198. [Google Scholar] [CrossRef]
- Sirisolla, J. Solubility enhancement of meloxicam by liquisolid technique and its characterization. Int. J. Pharm. Sci. Res. 2015, 6, 835–840. [Google Scholar] [CrossRef]
- Freitas, J.T.J.; Santos-Viana, O.M.M.; Bonfilio, R.; Doriguetto, A.C.; Benjamim de Araújo, M. Analysis of polymorphic contamination in meloxicam raw materials and its effects on the physicochemical quality of drug product. Eur. J. Pharm. Sci. 2017, 109, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Alnaief, M.; Obaidat, R.; Mashaqbeh, H. Loading and evaluation of meloxicam and atorvastatin in carrageenan microspherical aerogels particles. J. Appl. Pharm. Sci. 2019, 9, 83–88. [Google Scholar] [CrossRef]
- Yalkowsky, S.H. Solubility and Solubilization in Aqueous Media; American Chemical Society and Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Fedors, R.F. A method for estimating both the solubility parameters and molar volumes of liquids. Polym. Eng. Sci. 1974, 14, 147–154. [Google Scholar] [CrossRef]
- Mohammad, M.A.; Alhalaweh, A.; Velaga, S.P. Hansen solubility parameter as a tool to predict cocrystal formation. Int. J. Pharm. 2011, 407, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, P.; Martin, A.; González-Guisandez, M.A. Partial-solubility parameters and solvatochromic parameters for predicting the solubility of single and multiple drugs in individual solvents. J. Pharm. Sci. 1993, 82, 635–640. [Google Scholar] [CrossRef]
- Bustamante, P.; Peña, M.A.; Barra, J. Partial solubility parameters of naproxen and sodium diclofenac. J. Pharm. Pharmacol. 1998, 50, 975–982. [Google Scholar] [CrossRef]
- Bevington, P.R. Data Reduction and Error Analysis for the Physical Sciences; McGraw-Hill Book, Co.: New York, NY, USA, 1969; pp. 56–65. [Google Scholar]
- Carstensen, J.T. Modeling and Data Treatment in the Pharmaceutical Sciences; Technomic Publishing Co., Inc.: Lancaster, PA, USA, 1996; pp. 127–159. [Google Scholar]
- Barrante, J.R. Applied Mathematics for Physical Chemistry, 2nd ed.; Prentice Hall, Inc.: Upper Saddle River, NJ, USA, 1998; 227p. [Google Scholar]
- Hoftyzer, P.J.; Van Krevelen, D.W. Properties of Polymers, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1976; pp. 152–155. [Google Scholar]
- Bustamante, P.; Peña, M.A.; Barra, J. The modified Hansen method to determine partial solubility parameters of drugs containing single hydrogen bonding group and their sodium derivatives: Benzoic acid/Na and ibuprofen/Na. Int. J. Pharm. 2000, 194, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Just, S.; Sievert, F.; Thommes, M.; Breitkreutz, J. Improved group contribution parameter set for the application of solubility parameters to melt extrusion. Eur. J. Pharm. Biopharm. 2013, 85, 1191–1199. [Google Scholar] [CrossRef]
- Rahimpour, E.; Jouyban, A. Utilizing Abraham and Hansen solvation parameters for solubility prediction of meloxicam in cosolvency systems. J. Mol. Liq. 2021, 328, 115400. [Google Scholar] [CrossRef]
| δT (MPa1/2) | Solvent Mixture | Mixture Composition (w1) of Maximum Meloxicam Solubility |
|---|---|---|
| 29.1 a | Ethanol (1) + water (2) | 0.85 |
| 21.2 b | 1,4-Dioxane (1) + water (2) | 0.975 |
| 26.0 c | Acetonitrile (1) + water (2) | 0.80 |
| 25.9 d | 2-Propanol (1) + water (2) | 0.70 |
| 19.8 e | Ethyl acetate (1) + Ethanol | 0.70 |
| Group | Number | Fd (J·cm3/2·mol−1) a | (J·cm3·mol−2) a | Uh (J·mol−1) a |
|---|---|---|---|---|
| –CH3 | 2 | 673.2 | 0 | 0 |
| =CH– | 1 | 255.0 | 1444 | 0 |
| =C< | 4 | −226.8 | 1600 | 0 |
| Phenylene | 1 | 1173.0 | 4057.69 | 40.4 |
| –OH | 1 | 76.5 | 1,500,625 | 6060 |
| –CO-NH– | 1 | 225.0 | 160,000 | 11,000 |
| –N< | 1 | 30.0 | 22,500 | 750 |
| –N= | 1 | 380.0 | 10,000 | 250 |
| –S– | 1 | 815.9 | 38,416 | 297.5 |
| –SO2– | 1 | 295.8 | 19,018,321 | 200 |
| Ring ≥ 5 | 2 | 285.6 | 0 | 0 |
| Double bond | 3 | 45.0 | 613.47 | 250.5 |
| Σ | 4028.2 | 20,757,577 | 18,848.4 | |
| δd | 17.9 MPa1/2 | |||
| δp | 20.3 MPa1/2 | |||
| δh | 9.2 MPa1/2 | |||
| δT | 28.6 MPa1/2 |
| Interaction | HSP (Bustamante et al.) a | HSP (Hoftyzer–Van Krevelen) b | KAT-LSER c |
|---|---|---|---|
| Dispersion | 46.7% | 37.9% | 26.9% |
| Dipolar | 39.8% | 42.8% | 45.1% |
| Hydrogen bonding | 13.5% | 19.3% | 28.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tinjacá, D.A.; Martinez, F.; Peña, M.A.; Jouyban, A.; Acree, W.E., Jr. Revisiting the Total Hildebrand and Partial Hansen Solubility Parameters of Analgesic Drug Meloxicam. Liquids 2023, 3, 469-480. https://doi.org/10.3390/liquids3040030
Tinjacá DA, Martinez F, Peña MA, Jouyban A, Acree WE Jr. Revisiting the Total Hildebrand and Partial Hansen Solubility Parameters of Analgesic Drug Meloxicam. Liquids. 2023; 3(4):469-480. https://doi.org/10.3390/liquids3040030
Chicago/Turabian StyleTinjacá, Darío A., Fleming Martinez, María Angeles Peña, Abolghasem Jouyban, and William E. Acree, Jr. 2023. "Revisiting the Total Hildebrand and Partial Hansen Solubility Parameters of Analgesic Drug Meloxicam" Liquids 3, no. 4: 469-480. https://doi.org/10.3390/liquids3040030
APA StyleTinjacá, D. A., Martinez, F., Peña, M. A., Jouyban, A., & Acree, W. E., Jr. (2023). Revisiting the Total Hildebrand and Partial Hansen Solubility Parameters of Analgesic Drug Meloxicam. Liquids, 3(4), 469-480. https://doi.org/10.3390/liquids3040030










