Abstract
Lyotropic liquid crystals (LLCs) are liquids that have crystalline structures. LLCs as drug delivery systems that can deliver hydrophobic, hydrophilic, and amphiphilic agents. Due to their unique phases and structures, LLCs can protect both small molecules and biologics from the gastrointestinal tract’s harsh environment, thus making LLCs attractive as carriers for oral drug delivery. In this review, we discuss the advantages of LLCs and LLCs as oral formulations targeting intestinal lymphatic transport. In oral LLC formulations, the relationship between the micelle compositions and the resulting LLC structures as well as intestinal transport and absorption were determined. In addition, we further demonstrated approaches for the enhancement of intestinal lymphatic transport: (1) lipid-based LLCs promoting chylomicron secretion and (2) the design of LLC nanoparticles with M cell-triggered ligands for targeting the M cell pathway. In this review, we introduce LLC drug delivery systems and their characteristics. Our review focuses on recent approaches using oral LLC drug delivery strategies targeting the intestinal lymphatic system to enhance drug bioavailability.
1. Introduction
Peroral is the most common delivery route for drug administration [1,2,3]. Oral dosing is the most preferred by patients and convenient to handle for healthcare providers because taking medicines by mouth is non-invasive and requires no sterility constraints compared to injectables [2,3,4]. However, an orally administered drug formulation must survive the gastrointestinal tract’s harsh environment, gastric acidic conditions (pH 1.2), the presence of digestive enzymes, the mucus and mucosal barriers, the enteric epithelia whose tissue morphology inhibits drug absorption, and the hepatic metabolism for local and/or systemic therapy (Figure 1) [1,2,3,4,5]. Notably, hepatic first-pass metabolism, also known as drug pre-systemic exposure, heavily reduces the pharmacokinetic effects of drugs [2,4,5]. Thus, the oral bioavailability of many small molecule active pharmaceutical ingredients (API) (of biopharmaceutical classification system (BCS) III (high solubilitypoor permeability) and IV (poor solubilitypoor permeability) drug classes) is often very poor, and the oral delivery of biologics remains a great challenge [2,5].
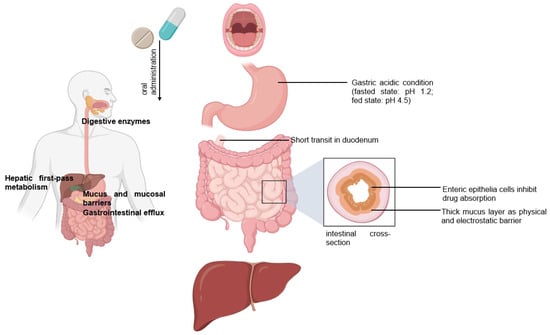
Figure 1.
Following the oral administration of a therapeutic, the drug travels from the mouth to its site of action. The drug enters the digestive tract where it faces challenges.
The intestinal lymphatic pathway can be considered as the ultimate solution for drug transport to avoid first-pass metabolism [1,2,5,6,7]. The intestine absorbs fat both through passive diffusion and protein-facilitated transfer [2,5,6,7,8]. The exogenous lipoprotein pathway utilizing chylomicronsthe fat carriers assembled in the intestinal epithelium, is responsible for transferring the food-derived lipids and fat-soluble vitamins to the cells [5,6,7,8]. Therefore, by targeting the chylomicrons in enterocytes, lipid-based drug formulations can be absorbed from the intestine via lymphatic transport through the chylomicron pathway, and they enter the bloodstream through the thoracic lymph duct (Figure 2a). Another promising lymphatic pathway for oral drug delivery is the microfold cells (M cells) channel [5,7,9]. The epithelium covering mucosa-associated lymphoid tissues is where M cells are typically found. In the ileum, M cells mediate the interface between the lumen and the lymphatic system by capturing and transporting pathogen-like particles. To initiate an immune response, M cells actively deliver luminal particles and antigens to intestinal dendritic cell subsets lying under lymphoid follicles (Figure 2b). The lymphatic vessels are closely associated with blood vessels, and they are well-distributed throughout the intestinal wall and lined with endothelial cells that are highly permeable to lipophilic molecules. Herein, we discuss several studies that have engineered drug delivery vehicles so that lymphatic vessels and immune cells in the intestines can absorb them more efficiently.
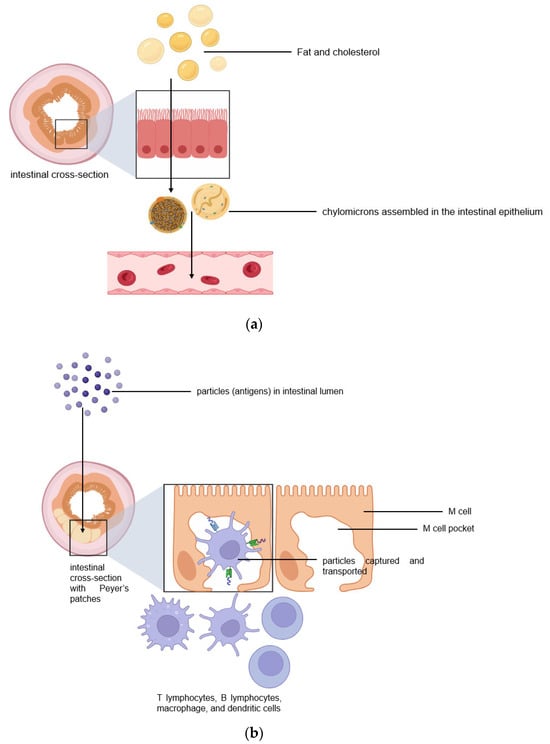
Figure 2.
Two pathways for efficient intestinal lymphatic transport: (a) the chylomicron pathway and (b) the M cell pathway.
Lyotropic liquid crystals (LLCs) result when amphiphiles (amphiphilic mesogens) dissolve into suitable solvent-forming solutions that flow like liquids but still have ordered crystalline structures of solid crystals [10,11]. The well-defined, thermodynamically stable structures of LLCs such as lamellar, hexagonal, and cubic (Figure 3 and Figure 4) are formed by the self-assembly of amphiphilic lipids due to hydrophobic forces. By dispersing these mesophase structures into water in the presence of stabilizers, they can be transformed into liposomes, hexosomes, and cubosomes, collectively known as LLC drug delivery systems [11]. In recent years, due to their unique structures offering matrices for prolonged drug release as well as their amphipathic nature for the encapsulation of hydrophobic, amphiphilic, and hydrophilic molecules of various molecular weights, LLC drug delivery systems have drawn significant attention as one of the advanced systems in the field of colloidal dispersions [11,12,13]. Figure 4 shows how a hydrophobic drug, a hydrophilic drug, and an amphiphilic drug are encapsulated in cubosomes. Many lipids that form LLCs are biocompatible and biodegradable and are applicable to the oral route of administration [12,14]. Intestinal lymphatic absorption is a pathway that an LLC drug delivery system may follow to gain access to the systemic circulation after its oral administration. A lipid-based LLC drug delivery system targeting the intestine can enhance intestinal lipid fluidity.
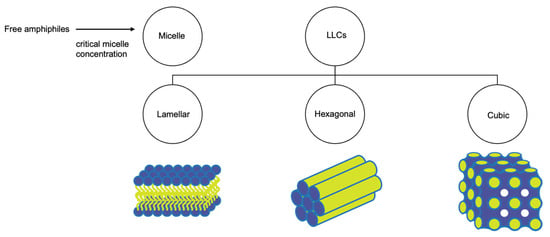
Figure 3.
Structural classification of LLCs.
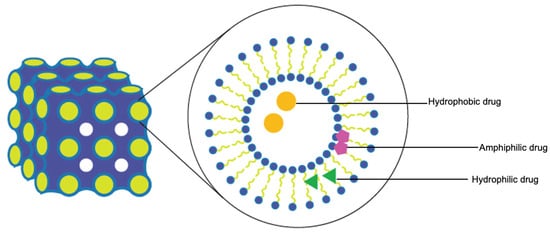
Figure 4.
Cubosomes encapsulate hydrophobic, hydrophilic, and amphiphilic model drugs.
In this review, we introduce LLC drug delivery systems, their characteristics, and their effects as oral formulations. Our review focuses on recent approaches using oral LLC drug delivery strategies targeting the intestinal lymphatic system to enhance drug bioavailability and reduce first-pass metabolism.
2. LLC Drug Delivery Systems for Efficient Intestinal Lymphatic Targeting
Preparation and Characterization of Oral LLC Drug Delivery Systems
Table 1 presents the compositions and phases of LLC systems. An LLC system includes water (aqueous phase), amphiphilic mesogen (lipid phase), and surfactant. The mesophase is often determined by the hydrophobicity and hydrophilicity of the system. When the concentration of micelles dispersed in water is above the critical aggregation concentration (CMC), micelles are forced to pack into a structure (micellar cubic phase—discontinuous cubic phase) [12,15]. The lamellar phase consists of lipid bilayers with hydrophilic heads (outer layer) and hydrophobic tails (inner layer). In water, these lipid bilayers are arranged linearly with water channels. Small changes in temperature and concentration can cause transitions between mesophases (Figure 4). Bicontinuous cubic and hexagonal phases are made of a 3D network of bicontinuous lipid bilayers arranged in a pattern of infinite periodic minimal surfaces (bicontinuous cubic phase) and in cylinders (hexagonal phase). These 3D networks have two distinct, continuous, non-intersecting, hydrophilic sections, and hydrophobic components are placed within. The cubic and hexagonal phase dispersions are especially advantageous for oral delivery because their structure could protect the drug in the gastrointestinal tract’s harsh environment [16]. Moreover, LLCs with a hydrophilic surface can easily traverse the water layer and contact the endothelial cell layer. Cubosomes were reported to have the ability to penetrate through the endothelial cell membrane [17,18].
Cubosomes and hexosomes are typically prepared for drug delivery by forming dispersions using energy input methods. Glyceryl monooleate and phytantriol are the two most used lipids to form cubic and hexagonal phases (Table 1). Surfactants and stabilizers are often incorporated into the preparation to maintain its colloidal stability. The “gold standard” steric stabilizer for the preparation of LLC is poloxamer 407 (Pluronic® F127 (BASF, Ludwigshafen, Germany), polyethylene-polypropylene glycol, hydrophilic–lipophilic balance (HLB) = 18) (Table 1) [19,20]. Poloxamer 407 kinetically stabilizes the formation of LLC by working on the interfacial tension, because it is composed of polyethylene oxide (PEO)-polypropylene oxide-PEO block copolymer that contains both hydrophilic and hydrophobic parts. The hydrophobic part interacts with the lipid bilayer, while the hydrophilic part faces the aqueous portion [21]. Bile salts, amphiphilic proteins, block polymers, other non-ionic surfactants, and other types of stabilizers (PEGylated lipids and customized lipid–copolymer (lipidic polymers)) including polysorbate 80, Cremophor®, and Labrasol® (BASF, Ludwigshafen, Germany), were investigated for their ability to stabilize LLCs [19,22]. The choice of surfactants for oral LLC systems was based on their required ability to protect APIs from premature metabolism by liver enzymes. Their high HLB value indicates their tendency to solubilize lipid (low HLB) in water, thereby forming the desired structure required to encapsulate a drug. For surfactants with HLB values > 6, cholesterol addition is recommended to form bilayered vesicles in the case of lamellar LLCs [23]. A chosen stabilizer is also required to have the bioactivity to inhibit the function of P-glycoprotein (P-gp) in the intestine, thereby increasing intestinal absorption [24]. Moreover, it was suggested that the PEO blocks of Poloxamer 407, covering the outer surfaces of formed cubosomes and/or hexosomes drain relatively faster into the lymphatic system, avoiding detection by lymph node macrophages, thus reaching the blood circulation and remaining there for a long period of time [25].


Table 1.
The compositions and phases of LLC systems.
Table 1.
The compositions and phases of LLC systems.
| Water Phase | Lipid Phase | Surfactant | Mesophase | References |
|---|---|---|---|---|
| low water content | glycerol monooleate, HLB = 4.2, forms micellar solution with water above CMC of 4 × 10−6 | Pluronic® F127 | bicontinuous cubic phase (V2) Ia3d or Im3m | [26,27,28,29,30] |
| high water content | bicontinuous cubic phase (V2) Pn3m | [17,26,27,28] | ||
| phosphate-buffered saline (PBS) | phytantriol, HLB = 3.8 | Pluronic® F127 | bicontinuous cubic phases (V2) with the Pn3m or Im3m space groups | [30,31] |
| water | phytantriol and oleic acid | Pluronic® F127 | reverse hexagonal (H2) | [25,29] |
| water | selachyl alcohol (glyceryl monooleyl ether) | Pluronic® F127 | reverse hexagonal phase (H2) | [29] |
| oleyl glycerate, HLB = 3.5 | reverse hexagonal phase (H2) |
LLC drug delivery systems are characterized by their mesophase identification, drug release patterns, stability, and drug entrapment efficiency (EE) that is calculated as follows:
For LLC formulations, EE and in vitro release are the most important characteristics. Both cubosomes and hexosomes can encapsulate the drug within the LLC system, and depending on the drug solubility, a water-insoluble drug can be found in the hydrophobic sections (surfactant hydrophobic parts and hydrophobic tails) and a water-soluble drug can be found in the hydrophilic sections (water channels) of the network. A general procedure to produce an LLC dispersion is to dissolve hydrophobic drugs in the oil phase with surfactants and then homogenize the oil phase mixture and water. Barauskas et al. compared different nanoparticle dispersions of self-assembled lipid mesophases and suggested that the release profile of hydrophobic drug-loaded cubosomes was significantly improved [26]. This can be explained by the fact that it is more difficult for hydrophobic drugs to escape from the LLC system compared to hydrophilic ones. The affinity of hydrophobic drugs with hydrophobic cores of reversed cubic phase is strong. Meanwhile, hydrophilic drugs trapped inside the LLC systems can flow out to the outer water through the water channels. Interestingly, amphiphilic drugs can be trapped in both hydrophilic and hydrophobic sections and along the interface of lipid and water (Figure 3 and Figure 4). The drug release from an LLC drug delivery system is based on the principle of drug diffusion following the Higuchi diffusion kinetic model [11,12,32,33]. Studies have shown that the LLC structure and the nature of lipids could be utilized to control the drug release rate, but an initial burst release before a sustained drug release seems unavoidable [11,12,34]. Initial burst release phenomenon and drug release kinetics are independent and are not related to EE. An LLC product with a high drug loading may cause local or systemic toxicity due to an initial burst release [34].
An LLC’s mesophase behavior can be affected by the choice of surfactant and mesogen and by several other factors. On the other hand, LLC mesophase behavior affects the physiochemical characteristics, EE (cubosomes were reported to be better than hexosomes in most cases), and drug release patterns (cubic phase often released drug faster than hexagonal phase) of the LLC system [35,36,37]. It is important to identify the LLC phase, especially when the phase determines whether the drug has been incorporated into it. Mesophases are detected and characterized by methods mentioned in Table 2. Structural details of an LLC can be observed using transmission electron microscopy (TEM), but small-angle X-ray scattering (SAXS) and small-angle neutron scattering (SANS) can visualize the structure as well as the phase behavior of LLCs using lattice parameters of the phase. The morphology of an LLC can be determined using TEM, in which samples are drop-casted on a mesh grid. Traditional TEM, in which materials are dried and stained on carbon grids before being observed using a microscope, is not recommended because of dehydration. Cryogenic electron microscopy (Cryo-EM) has been used to obtain the high-resolution images of LLCs, enabling the direct visualization of the internal phase structure and size assessment without fixating or staining [38]. To analyze LLC mesophases, experiments that are used are complex, and more than one technique can be used. Experiments are often run at various concentrations of mesogens. As an LLC phase transition occurs, fluctuations in heat capacities and enthalpy can be measured using differential scanning calorimetry (DSC). Particle size and zeta potential values measured using dynamic light scattering are often determined for the stability tests and quality control of LLCs.
The physical properties, stability, and physiological behavior of LLCs are highly critical characteristics. The nanostructured LLCs were considered as the template for nanostructured biodegradable hydrogel networks [39]. The choice of materials and the concentration of mesogens dramatically affected the network diffusivity and permeability, thus leading to changes in the degradation rate of the lamellar phase. For nanostructured LLCs, being nanosized may facilitate the release of drug from the carriers, and no significant relation between the mean particle size value and the release rate as well as the degradation rate was observed.


Table 2.
Advantages and limitations of techniques used to analyze LLC mesophases.
Table 2.
Advantages and limitations of techniques used to analyze LLC mesophases.
| Techniques | Advantages | Limitations | References | |
|---|---|---|---|---|
| Polarized optical microscopy | The most common tool to characterize liquid crystals. The original method for characterizing thermotropic mesophases. It detects the existence of liquid crystal phases in a solution. | Only allows the characterization of the pre-determined phase, because different phases are defined by their order, which must be observed. | [22,32,40,41,42] | |
| Thermal optical microscopy, DSC with polarized optical microscopy | Determine the thermal stability, phase transitions, transition enthalpies, phase sequences, temperature dependence of spontaneous polarization, and switching time. | |||
| Scanning electron microscopic (SEM) | Creates an image of the shape and surface of freeze-dried samples by detecting reflected electrons. SEM photographs detect possible morphological changes that occurred during sample treatment. | Dehydration Only sees the fractured surfaces of the treated samples. | [22,43,44] | |
| TEM | The structural details of the sample can be seen by transmitted electrons passing through the sample. Cryo-EM is where the TEM samples is studied at cryogenic temperatures. | Various TEM sample preparation techniques with multiple steps including cutting, fixation, filtration, staining, and dehydration. | [18,19,20,22,25,29,37] | |
| SAXS | Small-angle (2θ < 5°) (SA) X-ray scattering and/or neutron scattering are optimal tools to study LLC particles of 1–100 nm size. | Allow the measurements of size, shape, separation, and interactions between the scattering particles. The scattering of X-rays by electrons of the atoms in a crystal lattice is determined, thus leading to the identification of the space group related to the crystal structure of the sample. | The sequence of mesophases is evident (phase transition). Experiments are often run at various concentrations of mesogen. Mesophases must be in equilibrium and form colloidal dispersions stability. | [17,18,25,37,38,45] |
| SANS | Compared to SAXS, SANS is non-destructive and is a contrast variable. Neutron scattering can detect the exact location of movable monomers and crosslinkers within the sample. | |||
3. The Intestinal Lymphatic Pathway and LLC Approaches for the Enhancement of Intestinal Lymphatic Transport
The main advantage of LLCs is related to their ability to encapsulate drugs as cubosomes and hexosomes. Cubic phased LLCs can easily and instantly transform into lamellar phase and vice versa. The in situ phase transition makes LLCs uniquely applicable to different routes of administration and organ-specific target applications. This study focuses on LLCs as a delivery system targeting the intestinal lymphatic system.
3.1. Enhancement of Chylomicron Transport
The intestinal lymphatic system is responsible for the absorption and transportation of lipids [2,5,6,7,8,46]. Lymphatic delivery of small molecules is achievable by nanoparticles, polymers, liposomes, or via in situ association with proteins, lipoproteins, and lymphotropic leukocytes [5,6,7,9,30,47,48]. Fat-soluble particles are absorbed by intestinal epithelial cells, released as chylomicrons, and transferred by lymphatic vessels [8,49]. Similarly, upon enterocytes, drug–lipoprotein complexes are transported from the intestinal lamina propria via the lymphatics when the drug is administered with lipids (from food or a formulation). Inspired by the conventional oral formulations of lipophilic prodrugs and co-administered oil–drug formulations promoting chylomicron secretion, lipid-based drug delivery systems have emerged to become the most common and efficient solution when it comes to the enhancement of chylomicron transport. As discussed above, to avoid the water and mucosal layers found within the small intestine limiting the transport of drugs into endothelial cells, bile salts, phospholipids, cholesterol, and other types of lipids were used to help promote the formation of colloidal vesicles. These lipid droplets (multilayered LLCs) help increase the solubilization of drugs, especially poor-water soluble ones, by solubilizing them into micelles with the lamellar vesicles formed by the lipid layers of LLCs, thus increasing the oral bioavailability of drugs [5]. Therefore, LLCs consist of an oil phase and a solubilized micellar phase during lipid digestion, enhancing both the solubility and bioavailability of drugs in the lumen. Tran et al. ‘s quercetin-loaded-in situ self-emulsifying drug delivery system enhanced the oral bioavailability of quercetin by improving its solubility and lymphatic absorption. In male Sprague Dawley rat models, the Caco-2 permeability of quercetin oil/in/water nano-emulsion improved significantly due to the chylomicron uptake in the lymphatic system [50]. Quercetin, a hydrophobic drug, was confirmed to be transported by chylomicrons. Quercetin-loaded lipid liquid crystalline systems were developed, and they may increase quercetin’s lymphatic transport in the small intestine [50,51,52]. It was concluded that glyceryl monooleate cubosomes possibly did not show any ability to alter the integrity of the Caco-2 cellular tight junctions [53]; thus, the lymphatic transport pathway of lipid-based LLC nanoparticles mainly occurs via chylomicrons.
While the formation of chylomicrons does not relate directly to the ordered lipid mesophases in the gastrointestinal tract, the link between the influence of lipid digestion on the fate of orally administered cubosomes and the enhancement of oral bioavailability has been deciphered [52,53,54,55,56]. Moreover, the nanosized LLC delivery systems were stable in the gastrointestinal tract and were permeable across the gastrointestinal epithelium [17,54]. According to Leesajakul et al., the long in vivo circulation time of drugs in cubosomes was because of the sustained behavior of multilayered lipid-based LLCs [57]. Glyceryl monooleate and oleyl glycerate-based cubosomes containing cinnarizine showed a high drug loading with an improved sustained release of a drug compared to that of a drug suspension [31]. Glyceryl monooleate-based LLCs showed both a sustained-released mucoadhesiveness and an increased gastric residence time, thus enhancing their oral bioavailability [29,31]. Interestingly, lipid-based LLCs of piperine, a weakly hydrophobic drug (solubility in water of 4 × 104 ng/mL) [19], hydrophilic drugs (glucose, Allura Red, and FITC-dextrans) [58], and amphiphilic drugs (amphotericin B) were loaded in cubosomes as sustained-released oral formulations with improved bioavailability. Yang et al. explained that the enhanced solubility and gastrointestinal permeability of their amphotericin B-loaded cubosomes were because of their enhanced lymphatic drug transport and the fluidity of the enterocyte membrane [17]. Cubosomes containing glyceryl monooleate are widely accepted as candidates for lymphatic absorption via the chylomicron pathway after an oral administration.
3.2. Targeting the M Cell Pathway
Polysaccharide, an immunostimulant, was incorporated into cubosomes. The cubosomes were reported to have a higher immune activity compared to that of cubosomes or polysaccharides alone. The enhanced immune responses were explained by the promotion of antigen transport into draining lymph nodes and the efficient dendritic cell activation and memory T-helper cell differentiation in the draining lymph nodes [59]. M cells are mucosal epithelial cells specialized to transport antigens across the mucosa; therefore, particles can be decorated with ligands to mimic the transportation of antigens through the M cell pathway [5,7,9,60,61,62]. The intestinal M cell pathway is important for the uptake of proteins and peptides, which are nearly impossible to absorb through the intestinal epithelium.
Particle size influences the in vivo fate of particles and their ex vivo cellular interaction [63]. While smaller sized lipophilic LLCs can stay longer in the gastrointestinal tract due to their enhanced intestinal permeability, particles with size values of 550 nm and larger are preferred to be transported via the lymphatics [64], and microparticles (particle size of 1–5 μm) are often trapped in Peyer’s patches, thus activating the immune response [5]. Optimal particle surface hydrophobicity was also investigated. Hydrophobic particle surfaces that bind to proteins in the intestinal fluid can result in higher M cells transport efficiency for the particles [5]. Zeta potential values of the particles, indicating the particles’ stability, should be optimized in the development of micro/nanoparticles that target the M cell pathway. The ligand-conjugated nanoparticles (P2Ns–gambogic acid) targeting CD71s synthesized by Ganugula et al., polymeric-coated microparticles prepared by Shakweh et al., chitosan-modified solid lipid nanoparticles prepared by Shi et al., and chitosan-coated liposomes prepared by Channarong et al. successfully accumulated in Peyer’s patches due to their macromolecular size and lipophilicity [65,66,67,68]. These studies demonstrated the translational potential of lipid-based, polymeric, and hybrid polymer-lipid nanoparticles that can target lymphatic tissues through oral administration. Additionally, results showed that rod-like (hexagonal, cylindric) and disk-like particles had a longer retention time in the gastrointestinal tract with a higher penetration and achieved a higher lymphatic transport compared to spheres [63,69]. Overall, we can conclude that LLCs, the lipid-based, multilayered, hexagonal (rod-), and/or cubosomal (cubic) colloidal vesicles, can be conjugated and/or coated with other lipids, polymers, peptides, and proteins to form complex nanocomposites that target the lymphatic M cell pathway.
4. Facilitating Oral Drug and Vaccine Delivery using LLC Systems with Multiple Lymphatic Transport Mechanisms
Models mimicking the function of either chylomicrons or M cells have been developed for the evaluation of lymphatic transport. Caco-2 cell permeability assay has been established to measure the rate of flux of a drug across polarized human colon epithelial cell lines (monolayers) [50]. The Ussing chamber system has been used to measure intestinal permeability and the transport of drugs across the epithelial tissues [70]. Tissues from the ileum were isolated after the oral administration of bile-acid-conjugated solid nanoparticles and observed with confocal microscopy to visualize the enterocytes. Bile acid transporter was confirmed to mediate cellular uptake and chylomicron transport pathways [71,72]. Although chylomicron association predictive models were established [73] and permeability assays using Caco-2 cell lines with specific M cell-mimicking capacities were performed [74], lymphatic transport mechanisms could not be effectively clarified. When the uptake of particles through Peyer’s patches was higher, the enhanced absorption mechanisms of particles were related to the M cell pathway but were not limited to that. One of the goals of encapsulating therapeutics into LLCs is to target the lymphatic transport, but it was difficult to refer to the lymphatic transport with some LLC formulations, even though their intestinal permeability was enhanced. Usually, drugs transported to lymph should be highly lipophilic and should be associated with chylomicron. M cell pathway and other mechanisms of lymphatic transport can co-occur in the cases that hydrophilic drug-loaded LLCs cannot be taken up into epithelial cells as intact particles [17,19,75]. Multiple mechanisms of lymphatic transport including M cells, chylomicrons, and transcellular and paracellular pathways at the tight junction of epithelial cells have been reported [65,67,76]. We propose that lipid-based LLCs having chylomicron-binding capacity may take advantage of the chylomicrons to be delivered. Furthermore, the conjugation of LLCs with an antigen, a polymer, or a virus with proper chain length, molecular weight, and optimal proportions may help trigger the immune system. We recommend targeting different pathways and using different transport mechanisms to enhance lymphatic transport and oral bioavailability.
5. Conclusions
The oral route is one of the most common routes of drug administration, yet it remains a significant delivery challenge. The physiology of the gastrointestinal tract is complex. Overcoming the first-pass metabolism by targeting the intestinal lymphatic system with lipid nanoparticles, especially LLCs, can be a solution. Firstly, we characterized and described the unique structures of LLC phases, which influence the drug encapsulation, localization, and sustained-release behavior of LLCs. Secondly, LLCs can be engineered with bioactive lipids and polymers to increase their stability, optimize their particle shape, and size, and, ultimately, affect the intestinal lymphatic pathways. We recognize these LLC nanoplatforms as a promising generation of smart drug delivery systems for oral administration that can achieve a good lymphatic targeting efficacy while protecting the drug from the gastrointestinal environment.
Author Contributions
Conceptualization, L.D.; resources, B.Y.; writing—original draft preparation, L.D.; writing—review and editing, L.D. and B.Y.; visualization, L.D.; supervision, B.Y.; project administration, B.Y.; funding acquisition, B.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by National Institutes of Health (Grants: R01 AA030486 and R01 AI172959).
Data Availability Statement
The authors confirm that the data supporting this study are within the articles.
Acknowledgments
Figure 1 and Figure 2 were created with BioRender.com (accessed on 7 October 2023). We especially thank Sung-Joo Hwang from Yonsei University for his useful comments on an earlier draft of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hua, S. Advances in Oral Drug Delivery for Regional Targeting in the Gastrointestinal Tract—Influence of Physiological, Pathophysiological and Pharmaceutical Factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, P.; Muralidaran, Y.; Ragavan, G. Chapter 7—Challenges in oral drug delivery: A nano-based strategy to overcome. In Micro and Nano Technologies, Nanostructures for Oral Medicine; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 173–201. [Google Scholar] [CrossRef]
- Homayun, B.; Lin, X.; Choi, H.J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lu, Y.; Qi, J.; Wu, W. An update on oral drug delivery via intestinal lymphatic transport. Acta Pharm. Sin. B 2021, 11, 2449–2468. [Google Scholar] [CrossRef]
- Yáñez, J.A.; Wang, S.W.; Knemeyer, I.W.; Wirth, M.A.; Alton, K.B. Intestinal lymphatic transport for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 923–942. [Google Scholar] [CrossRef]
- Trevaskis, N.L.; Kaminskas, L.M.; Porter, C.J. From sewer to saviour—Targeting the lymphatic system to promote drug exposure and activity. Nat. Rev. Drug Discov. 2015, 14, 781–803. [Google Scholar] [CrossRef]
- Iqbal, J.; Hussain, M.M. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1183–E1194. [Google Scholar] [CrossRef]
- Fievez, V.; Plapied, L.; des Rieux, A.; Pourcelle, V.; Freichels, H.; Wascotte, V.; Vanderhaeghen, M.L.; Jerôme, C.; Vanderplasschen, A.; Marchand-Brynaert, J.; et al. Targeting nanoparticles to M cells with non-peptidic ligands for oral vaccination. Eur. J. Pharm. Biopharm. 2009, 73, 16–24. [Google Scholar] [CrossRef]
- Hansen, J.-P.; McDonald, I.R. Chapter 12—Applications to Soft Matter. In Theory of Simple Liquids, 4th ed.; Hansen, J.-P., McDonald, I.R., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 511–584. [Google Scholar]
- Huang, Y.; Gui, S. Factors affecting the structure of lyotropic liquid crystals and the correlation between structure and drug diffusion. RCS Adv. 2018, 8, 6978–6987. [Google Scholar] [CrossRef]
- Guo, C.; Wang, J.; Cao, F.; Lee, R.J.; Zhai, G. Lyotropic liquid crystal systems in drug delivery. Drug Discov. Today 2010, 15, 1032–1040. [Google Scholar] [CrossRef]
- Kim, D.H.; Jahn, A.; Cho, S.-J.; Kim, J.S.; Ki, M.-H.; Kim, D.-D. Lyotropic liquid crystal systems in drug delivery: A review. J. Pharm. Investig. 2015, 45, 1–11. [Google Scholar] [CrossRef]
- Ganem-Quintanar, A.; Quintanar-Guerrero, D.; Buri, P. Monoolein: A review of the pharmaceutical applications. Drug Dev. Ind. Pharm. 2000, 26, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Ruckenstein, E.; Nagarajan, R. Critical micelle concentration and the transition point for micellar size distribution. J. Phys. Chem. 1981, 85, 3010–3014. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, P.; Gui, S. Cubic and hexagonal liquid crystals as drug delivery systems. Biomed. Res. Int. 2014, 2014, 815981. [Google Scholar] [CrossRef]
- Yang, Z.; Tan, Y.; Chen, M.; Dian, L.; Shan, Z.; Peng, X.; Wu, C. Development of amphotericin B-loaded cubosomes through the SolEmuls technology for enhancing the oral bioavailability. AAPS PharmSciTech 2012, 13, 1483–1491. [Google Scholar] [CrossRef]
- Nazaruk, E.; Majkowska-Pilip, A.; Bilewicz, R. Lipidic Cubic-Phase Nanoparticles-Cubosomes for Efficient Drug Delivery to Cancer Cells. ChemPlusChem 2017, 82, 570–575. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Novel piperine-loaded Tween-integrated monoolein cubosomes as brain-targeted oral nanomedicine in Alzheimer’s disease: Pharmaceutical, biological, and toxicological studies. Int. J. Nanomed. 2015, 10, 5459–5473. [Google Scholar] [CrossRef]
- Nasr, M.; Ghorab, M.K.; Abdelazem, A. In vitro and in vivo evaluation of cubosomes containing 5-fluorouracil for liver targeting. Acta Pharm. Sin. B 2015, 5, 79–88. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, J.; Zheng, Y.; Gong, Y.; Fu, M.; Liu, C.; Xu, L.; Sun, C.C.; Gao, Y.; Qian, S. Cubosomes with surface cross-linked chitosan exhibit sustained release and bioavailability enhancement for vinpocetine. RSC Adv. 2019, 9, 6287–6298. [Google Scholar] [CrossRef]
- Paecharoenchai, O.; Teng, L.; Yung, B.C.; Teng, L.; Opanasopit, P.; Lee, R.J. Nonionic surfactant vesicles for delivery of RNAi therapeutics. Nanomedicine 2013, 8, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Duong, V.A.; Maeng, H.J. Pharmaceutical Formulations with P-Glycoprotein Inhibitory Effect as Promising Approaches for Enhancing Oral Drug Absorption and Bioavailability. Pharmaceutics 2021, 13, 1103. [Google Scholar] [CrossRef] [PubMed]
- Helvig, S.Y.; Andersen, H.; Antopolsky, M.; Airaksinen, A.J.; Urtti, A.; Yaghmur, A.; Moghimi, S.M. Hexosome engineering for targeting of regional lymph nodes. Materialia 2020, 11, 100705. [Google Scholar] [CrossRef]
- Barauskas, J.; Cervin, C.; Jankunec, M.; Spandyreva, M.; Ribokaite, K.; Tiberg, F.; Johnsson, M. Interactions of lipid-based liquid crystalline nanoparticles with model and cell membranes. Int. J. Pharm. 2010, 391, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Earnden, L.; Marangoni, A.G.; Laredo, T.; Stobbs, J.; Pensini, E. Self-Assembled glycerol monooleate demixes miscible liquids through selective hydrogen bonding to water. J. Mol. Liq. 2022, 367, 120551. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Y.; Zhong, Y.; Wang, Q.; Wu, W.; Gao, S. Ocular delivery of cyclosporine A based on glyceryl monooleate/poloxamer 407 liquid crystalline nanoparticles: Preparation, characterization, in vitro corneal penetration and ocular irritation. J. Drug Target 2012, 20, 856–863. [Google Scholar] [CrossRef]
- Boyd, B.J.; Khoo, S.M.; Whittaker, D.V.; Davey, G.; Porter, C.J. A lipid-based liquid crystalline matrix that provides sustained release and enhanced oral bioavailability for a model poorly water soluble drug in rats. Int. J. Pharm. 2007, 340, 52–60. [Google Scholar] [CrossRef]
- Cai, S.; Yang, Q.; Bagby, T.R.; Forrest, M.L. Lymphatic drug delivery using engineered liposomes and solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 901–908. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Hanley, T.; Porter, C.J.; Boyd, B.J. Nanostructured liquid crystalline particles provide long duration sustained-release effect for a poorly water soluble drug after oral administration. J. Control Release 2011, 153, 180–186. [Google Scholar] [CrossRef]
- Zewail, M.; Gaafar, P.M.E.; Ali, M.M.; Abbas, H. Lipidic cubic-phase leflunomide nanoparticles (cubosomes) as a potential tool for breast cancer management. Drug Deliv. 2022, 29, 1663–1674. [Google Scholar] [CrossRef]
- Paolino, D.; Tudose, A.; Celia, C.; Di Marzio, L.; Cilurzo, F.; Mircioiu, C. Mathematical Models as Tools to Predict the Release Kinetic of Fluorescein from Lyotropic Colloidal Liquid Crystals. Materials 2019, 12, 693. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control Release 2001, 73, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Nazaruk, E.; Miszta, P.; Filipek, S.; Górecka, E.; Landau, E.M.; Bilewicz, R. Lyotropic Cubic Phases for Drug Delivery: Diffusion and Sustained Release from the Mesophase Evaluated by Electrochemical Methods. Langmuir 2015, 31, 12753–12761. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, Y.; Prentice, R.N.; Boyd, B.J.; Rizwan, S.B. Comparison of cubosomes and hexosomes for the delivery of phenytoin to the brain. J. Colloid. Interface Sci. 2022, 605, 146–154. [Google Scholar] [CrossRef]
- Tran, N.; Mulet, X.; Hawley, A.M.; Hinton, T.M.; Mudie, S.T.; Muir, B.W.; Giakoumatos, E.C.; Waddington, L.J.; Kirbyb, N.M.; Drummond, C.J. Nanostructure and cytotoxicity of self-assembled monoolein–capric acid lyotropic liquid crystalline nanoparticles. R. Soc. Chem. Adv. 2015, 5, 26785–26795. [Google Scholar] [CrossRef]
- Gao, M.; Kim, Y.K.; Zhang, C.; Borshch, V.; Zhou, S.; Park, H.S.; Jákli, A.; Lavrentovich, O.D.; Tamba, M.G.; Kohlmeier, A.; et al. Direct observation of liquid crystals using cryo-TEM: Specimen preparation and low-dose imaging. Microsc. Res. Techn. 2014, 77, 754–772. [Google Scholar] [CrossRef]
- Clapper, J.D.; Iverson, S.L.; Guymon, C.A. Nanostructured Biodegradable Polymer Networks Using Lyotropic Liquid Crystalline Templates. Biomacromolecules 2007, 8, 2104–2111. [Google Scholar] [CrossRef]
- Miller, D.S.; Carlton, R.J.; Mushenheim, P.C.; Abbott, N.L. Introduction to optical methods for characterizing liquid crystals at interfaces. Langmuir 2013, 29, 3154–3169. [Google Scholar] [CrossRef]
- Tomczyk, W.; Marzec, M.; Juszyńska-Gałązka, E.; Węgłowska, D. Mesomorphic and physicochemical properties of liquid crystal mixture composed of chiral molecules with perfluorinated terminal chains. J. Mol. Struct. 2017, 1130, 503–510. [Google Scholar] [CrossRef]
- Juszyńska-Gałązka, E.; Gałązka, M.; Massalska-Arodź, M.; Bąk, A.; Chłędowska, K.; Tomczyk, W. Phase Behavior and Dynamics of the Liquid Crystal 4′-butyl-4-(2-methylbutoxy)azoxybenzene (4ABO5*). J. Phys. Chem. B 2014, 118, 14982–14989. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; He, W.; Yang, Z.; Cao, H.; Wang, D.; Li, Y. Research Progress of Cholesteric Liquid Crystals with Broadband Reflection. Molecules 2022, 27, 4427. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, I.; Palacio-Betancur, V.; Ayeb, H.; Demaille, D.; de Pablo, J.J.; Kamien, R.D.; Lacaze, E. Liquid Crystal Films as Active Substrates for Nanoparticle Control. ACS Appl. Nano Mater. 2021, 4, 6700–6708. [Google Scholar] [CrossRef]
- Alexandridis, P. Chapter 3—Small-Angle Scattering Characterization of Block Copolymer Micelles and Lyotropic Liquid Crystals. In Mesoscale Phenomena in Fluid Systems; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2003; Volume 861, pp. 60–80. [Google Scholar]
- Cifarelli, V.; Eichmann, A. The Intestinal Lymphatic System: Functions and Metabolic Implications. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Ali Khan, A.; Mudassir, J.; Mohtar, N.; Darwis, Y. Advanced drug delivery to the lymphatic system: Lipid-based nanoformulations. Int. J. Nanomed. 2013, 8, 2733–2744. [Google Scholar] [CrossRef]
- Attili-Qadri, S.; Karra, N.; Nemirovski, A.; Schwob, O.; Talmon, Y.; Nassar, T.; Benita, S. Oral delivery system prolongs blood circulation of docetaxel nanocapsules via lymphatic absorption. Proc. Natl. Acad. Sci. USA 2013, 110, 17498–17503. [Google Scholar] [CrossRef]
- Dixon, J.B. Mechanisms of chylomicron uptake into lacteals. Ann. N. Y. Acad. Sci. 2010, 1207 (Suppl. S1), E52–E57. [Google Scholar] [CrossRef]
- Tran, T.H.; Guo, Y.; Song, D.; Bruno, R.S.; Lu, X. Quercetin-containing self-nanoemulsifying drug delivery system for improving oral bioavailability. J. Pharm. Sci. 2014, 103, 840–852. [Google Scholar] [CrossRef]
- Linkevičiūtė, A.; Misiūnas, A.; Naujalis, E.; Barauskas, J. Preparation and characterization of quercetin-loaded lipid liquid crystalline systems. Colloids Surf. B Biointerfaces 2015, 128, 296–303. [Google Scholar] [CrossRef]
- Ali, M.A.; Kataoka, N.; Ranneh, A.-H.; Iwao, Y.; Noguchi, S.; Oka, T.; Itai, S. Enhancing the Solubility and Oral Bioavailability of Poorly Water-Soluble Drugs Using Monoolein Cubosomes. Chem. Pharm. Bull. 2017, 65, 42–48. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, M.; Yang, M.; Chen, J.; Fang, W.; Xu, P. Evaluating the potential of cubosomal nanoparticles for oral delivery of amphotericin B in treating fungal infection. Int. J. Nanomed. 2014, 9, 327–336. [Google Scholar] [CrossRef]
- Gabr, M.M.; Mortada, S.M.; Sallam, M.A. Hexagonal Liquid Crystalline Nanodispersions Proven Superiority for Enhanced Oral Delivery of Rosuvastatin: In Vitro Characterization and In Vivo Pharmacokinetic Study. J. Pharm. Sci. 2017, 106, 3103–3112. [Google Scholar] [CrossRef]
- Muheem, A.; Shakeel, F.; Warsi, M.H.; Jain, G.K.; Ahmad, F.J. A Combinatorial Statistical Design Approach to Optimize the Nanostructured Cubosomal Carrier System for Oral Delivery of Ubidecarenone for Management of Doxorubicin-Induced Cardiotoxicity: In Vitro-In Vivo Investigations. J. Pharm. Sci. 2017, 106, 3050–3065. [Google Scholar] [CrossRef] [PubMed]
- Yasser, M.; Teaima, M.; El-Nabarawi, M.; El-Monem, R.A. Cubosomal based oral tablet for controlled drug delivery of telmisartan: Formulation, in-vitro evaluation and in-vivo comparative pharmacokinetic study in rabbits. Drug Dev. Ind. Pharm. 2019, 45, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Leesajakul, W.; Nakano, M.; Taniguchi, A.; Handa, T. Interaction of cubosomes with plasma components resulting in the destabilization of cubosomes in plasma. Colloids Surf. B Biointerfaces 2004, 34, 253–258. [Google Scholar] [CrossRef]
- Lee, K.W.; Nguyen, T.H.; Hanley, T.; Boyd, B.J. Nanostructure of liquid crystalline matrix determines in vitro sustained release and in vivo oral absorption kinetics for hydrophilic model drugs. Int. J. Pharm. 2009, 365, 190–199. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, L.; Zheng, S.; Niu, Y.; Bo, R.; Huang, Y.; Xing, J.; Li, Z.; Wang, D. Cubosome nanoparticles potentiate immune properties of immunostimulants. Int. J. Nanomed. 2016, 11, 3571–3583. [Google Scholar] [CrossRef]
- Zhang, N.; Ping, Q.; Huang, G.; Xu, W.; Cheng, Y.; Han, X. Lectin-modified solid lipid nanoparticles as carriers for oral administration of insulin. Int. J. Pharm. 2006, 327, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Song, J.G.; Lee, S.H.; Han, H.K. Development of an M cell targeted nanocomposite system for effective oral protein delivery: Preparation, in vitro and in vivo characterization. J. Nanobiotechnol. 2021, 19, 15. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Han, S.; Dou, Y.; Liu, M.; Zhang, L.; Guo, J.; Shi, Q.; Gong, G.; Wang, R.; et al. Yeast Microcapsule-Mediated Targeted Delivery of Diverse Nanoparticles for Imaging and Therapy via the Oral Route. Nano Lett. 2017, 17, 1056–1064. [Google Scholar] [CrossRef]
- Banerjee, A.; Qi, J.; Gogoi, R.; Wong, J.; Mitragotri, S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J. Control Release 2016, 238, 176–185. [Google Scholar] [CrossRef]
- Shen, C.; Yang, Y.; Shen, B.; Xie, Y.; Qi, J.; Dong, X.; Zhao, W.; Zhu, W.; Wu, W.; Yuan, H.; et al. Self-discriminating fluorescent hybrid nanocrystals: Efficient and accurate tracking of translocation via oral delivery. Nanoscale 2017, 10, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Ganugula, R.; Arora, M.; Zou, D.; Agarwal, S.K.; Mohan, C.; Kumar, M.N.V.R. A highly potent lymphatic system–targeting nanoparticle cyclosporine prevents glomerulonephritis in mouse model of lupus. Sci. Adv. 2020, 6, eabb3900. [Google Scholar] [CrossRef] [PubMed]
- Shakweh, M.; Besnard, M.; Nicolas, V.; Fattal, E. Poly (lactide-co-glycolide) particles of different physicochemical properties and their uptake by peyer’s patches in mice. Eur. J. Pharm. Biopharm. 2005, 61, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.L.; Xie, H.; Lu, J.; Cao, Y.; Liu, J.Y.; Zhang, X.X.; Zhang, H.; Cui, J.H.; Cao, Q.R. Positively Charged Surface-Modified Solid Lipid Nanoparticles Promote the Intestinal Transport of Docetaxel through Multifunctional Mechanisms in Rats. Mol. Pharm. 2016, 13, 2667–2676. [Google Scholar] [CrossRef]
- Channarong, S.; Chaicumpa, W.; Sinchaipanid, N.; Mitrevej, A. Development and evaluation of chitosan-coated liposomes for oral DNA vaccine: The improvement of Peyer’s patch targeting using a polyplex-loaded liposomes. AAPS PharmSciTech 2011, 12, 192–200. [Google Scholar] [CrossRef]
- Li, D.; Zhuang, J.; He, H.; Jiang, S.; Banerjee, A.; Lu, Y.; Wu, W.; Mitragotri, S.; Gan, L.; Qi, J. Influence of Particle Geometry on Gastrointestinal Transit and Absorption following Oral Administration. ACS Appl. Mater. Interfaces 2017, 9, 42492–42502. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.J.; Lee, S.; Dinh, L.; Oh, H.; Abuzar, S.M.; Ahn, J.H.; Hwang, S.J. Preparation of Apixaban Solid Dispersion for the Enhancement of Apixaban Solubility and Permeability. Pharmaceutics 2023, 15, 907. [Google Scholar] [CrossRef]
- Kim, K.S.; Suzuki, K.; Cho, H.; Youn, Y.S.; Bae, Y.H. Oral Nanoparticles Exhibit Specific High-Efficiency Intestinal Uptake and Lymphatic Transport. ACS Nano 2018, 12, 8893–8900. [Google Scholar] [CrossRef]
- Dong, F.; Xie, Y.; Qi, J.; Hu, F.; Lu, Y.; Li, S.; Wu, W. Bile salt/phospholipid mixed micelle precursor pellets prepared by fluid-bed coating. Int. J. Nanomed. 2013, 8, 1653–1663. [Google Scholar] [CrossRef]
- Gershkovich, P.; Hoffman, A. Effect of a high-fat meal on absorption and disposition of lipophilic compounds: The importance of degree of association with triglyceride-rich lipoproteins. Eur. J. Pharm. Sci. 2007, 32, 24–32. [Google Scholar] [CrossRef]
- Fasciano, A.C.; Blutt, S.E.; Estes, M.K.; Mecsas, J. Induced Differentiation of M Cell-like Cells in Human Stem Cell-derived Ileal Enteroid Monolayers. J. Vis. Exp. 2019, 149, e59894. [Google Scholar] [CrossRef]
- Sultan, A.A.; El Nashar, N.F.; Ashmawy, S.M.; El Maghraby, G.M. Cubosomes for Enhancing Intestinal Absorption of Fexofenadine Hydrochloride: In situ and in vivo Investigation. Int. J. Nanomed. 2022, 17, 3543–3560. [Google Scholar] [CrossRef] [PubMed]
- Tang, V.W.; Goodenough, D.A. Paracellular ion channel at the tight junction. Biophys. J. 2003, 84, 1660–1673. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).