Abstract
Experimental solubilities were determined for 31 solid nonelectrolyte organic compounds dissolved in tert-butyl acetate at 298.15 K. Results of the experimental measurements were combined with published mole fraction solubility data for two lipid-lowering medicinal compounds (lovastatin and simvastatin) in order to derive Abraham model expressions for solute transfer into the tert-butyl acetate mono-solvent. The derived correlations provided an accurate mathematical description of the observed experimental data. As part of the current study, previously published Abraham model solvent correlations for both ethyl acetate and butyl acetate were updated using much larger datasets that contained an additional 64 and 35 experimental data points, respectively. The mathematical correlations presented in the current study describe the observed solubility ratios of solutes dissolved in tert-butyl acetate, ethyl acetate, and butyl acetate to within an overall standard deviation of 0.15 log units or less.
1. Introduction
Individuals employed by the chemical manufacturing sector handle and are exposed to organic solvents on a daily basis. Organic solvents serve as the solubilizing reaction media in the preparation of new chemical products, as cleansing and degreasing agents for chemical glassware and industrial machinery, as components of aqueous–organic biphasic extraction systems used in the removal of unwanted impurities from synthesized chemical materials, and as dispersing agents in paint and cosmetic products. Organic solvents have also been used to extract biochemical materials from plants and to preconcentrate and remove trace organic analytes from chemical samples prior to gas–liquid and high-performance chromatographic analyses. Several million tons of petroleum-based organic solvents are purchased and discarded on an annual basis. Governmental regulations pertaining to chemical waste disposal have encouraged the manufacturing sector to utilize more environmentally compatible organic solvents, to search for solvent-free synthetic processes, and to design effective solvent recovery methods in order to reduce the quantity of hazardous materials that are released into the natural environment.
Replacing hazardous organic solvents with safer chemical alternatives is not an easy task. Industrial processes are often designed around the specific solvent that is currently being used. Altering an existing process can be an expensive endeavor, even if one has identified a safer solvent which possesses suitable physical and chemical properties. Our contribution in the solvent selection and replacement process has been to develop mathematical Abraham model expressions [1,2,3,4] that enable process design engineers to predict molar solubilities of chemical reactants, synthesized chemical products, and reaction by-products in a wide range of organic solvents of varying polarity and hydrogen-bonding character. Unlike physical properties such as density, viscosity, and vapor pressure, one cannot easily locate needed solubility data in the published chemical and engineering literature. Solubility data is solute-solvent specific in nature, and it is not feasible to determine solubilities for every possible combination of chemical compounds. Currently there are more than 60 million known chemical compounds [5], and the number continually increases with each newly synthesized organic/inorganic molecule.
The Abraham model is among the simplest and most versatile predictive solubility expressions that have been developed in the past 30 years. The basic model [6,7,8,9] describes solute transfer, which, in the current study, is given by the logarithm of molar solubility ratios, log (CS,organic/CS,water), and log (CS,organic/CS,gas), in terms of:
the molecular solute–solvent interactions that govern the dissolution process. The subscripts “organic”, “water”, and “gas” on solubility ratios denote the phase to which the molar solute concentration pertains. Each molecular interaction is quantified as the product of a solute property multiplied by the complimentary solvent property. Solute properties (also called solute descriptors) are denoted by the capitalized alphabetical characters on the right-hand side of Equations (1) and (2) and are defined as follows: A and B refer to the respective overall hydrogen-bond donating and accepting capacities of the dissolved solute; E corresponds the molar refraction of the given solute (in units of (cm3 mol−1)/10) in excess of that of a linear alkane having a comparable molecular size; L is the logarithm of the solute’s gas-to-hexadecane partition coefficient determined at 298.15 K; S represents a combination of the electrostatic polarity and polarizability of the solute; V denotes the McGowan molecular volume of the solute (in units of (cm3 mol−1)/100) calculated from atomic sizes and chemical bond numbers. The complimentary solvent properties in Equations (1) and (2) are given by the lowercase alphabetical characters (ceq 1, eeq 1, seq 1, aeq 1, beq 1, veq 1, ceq 2, eeq 2, seq 2, aeq 2, beq 2, and leq 2). Numerical values of the solvent properties are determined by regressing measured molar solubility ratio data in accordance with Equations (1) and (2). Once determined, the lowercase alphabetical characters allow one to predict the molar solubilities of additional solutes in the given organic solvent, provided, of course, that the solute descriptors are known. Currently, equation coefficients are known for slightly more than 130 different organic solvents and binary aqueous-organic solvent mixtures [10]. This represents only a small fraction of the organic solvents currently used in industrial manufacturing processes and consumer product formulations. Less than half of the solvents for which equation coefficients have been obtained fall into the classification of “preferred” and/or “recommended” on the solvent selection guide developed by pharmaceutical companies [11,12,13].
log (CS,organic/CS,water) = eeq 1 × E + seq 1 × S + aeq 1 × A + beq 1 × B + veq 1 × V + ceq 1
log (CS,organic/CS,gas) = eeq 2 × E + seq 2 × S + aeq 2 × A + beq 2 × B + leq 2 × L + ceq 2
In the current study, we extend our earlier considerations to include the tert-butyl acetate mono-solvent, which is on the list of “recommended” organic solvents [14], along with several other alkyl acetates like ethyl acetate, propyl acetate, isopropyl acetate, and butyl acetate [11,15,16,17]. Alkyl acetates and other esters score well on published solvent selection guides because of their low toxicity and preparation from biomass materials [18]. Abraham predictive expressions are reported for tert-butyl acetate based on our measured solubility data for acenaphthene, acetylsalicylic acid, anthracene, benzil, benzoic acid, benzoin, 4-tert-butylbenzoic acid, 1-chloroanthraquinone, 3-chlorobenzoic acid, 4-chlorobenzoic acid, 2-chloro-5-nitrobenzoic acid, 4-chloro-3-nitrobenzoic acid, 3,4-dichlorobenzoic acid, 3,4-dimethoxybenzoic acid, 3,5-dinitrobenzoic acid, diphenyl sulfone, 2-ethylqnthraquinone, hippuric acid, 2-hydroxybenzoic acid, 2-methoxybenzoic acid, 4-methoxybenzoic acid, 2-methylbenzoic acid, 3-methylbenzoic acid, 2-methyl-3-nitrobenzoic acid, 3-methyl-4-nitrobenzoic acid, 4-methyl-3-nitrobenzoic acid, 3-nitrobenzoic acid, 4-nitrobenzoic acid, salicylamide, 3,4,5-trimethoxybenzoic acid, and xanthene. In total, mole fraction solubilities have been determined for 31 crystalline organic compounds dissolved in tert-butyl acetate at 298.15 K.
As part of the current study, we are also revising our existing Abraham model mathematical correlations for both ethyl acetate (dry, anhydrous) [19]:
and butyl acetate (dry, anhydrous) [19]:
as there has been sufficient new experimental data [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88] published since 2008, when the earlier correlations first appeared, to merit a redetermination of the equation coefficients. Equations (3)–(6) are based on 106 and 73 experimental molar solubility ratios; indirect water-to-alkyl acetate transfer coefficients, P; and gas-to-alkyl acetate partition coefficients, K, respectively. The updated correlations reported in the current study are based on much larger, more chemically diverse data sets, which include 170 (ethyl acetate) and 108 (butyl acetate) solutes. It is the chemical diversity, as reflected by the solute descriptor values, that defines the area of predictive chemical space over which a derived Abraham correlation can be used. One should not use a mathematical correlation to make predictions for solutes whose descriptor values fall too far outside of the range of values used in determining the equation coefficients.
log P and log (CS,organic/CS,water) = 0.328(0.034) + 0.369(0.057)E − 0.446(0.080)S − 0.700(0.069)A − 4.904(0.113)B + 4.150(0.033)V
(N = 106, SD = 0.165, R2 = 0.996, F = 4475.1)
(N = 106, SD = 0.165, R2 = 0.996, F = 4475.1)
log K and log (CS,organic/CS,gas) = 0.182(0.026) − 0.352(0.048)E + 1.316(0.050)S + 2.891(0.061)A + 0.916(0.008)L
(N = 106, SD = 0.148, R2 = 0.998, F = 15,635.1)
(N = 106, SD = 0.148, R2 = 0.998, F = 15,635.1)
log P and log (CS,organic/CS,water) = 0.248(0.047) + 0.356(0.065)E − 0.501(0.082)S − 0.867(0.096)A − 4.973(0.100)B + 4.281(0.027)V
(N = 73, SD = 0.160, R2 = 0.998, F = 7380)
(N = 73, SD = 0.160, R2 = 0.998, F = 7380)
log K and log (CS,organic/CS,gas) = 0.147(0.040) − 0.414(0.064)E + 1.212(0.077)S + 2.623(0.086)A + 0.954(0.007)L
(N = 73, SD = 0.157, R2 = 0.998, F = 6174.7)
(N = 73, SD = 0.157, R2 = 0.998, F = 6174.7)
The words ‘dry, anhydrous’ after the solvent name indicate that the organic solvent was not in direct contact with water, as would be the case for practical partitioning processes involving the removal of the solute from water with ethyl acetate or butyl acetate as the extracting organic solvent. Abraham model correlations have been published for “wet” ethyl acetate and “wet” butyl acetate in an earlier paper [19]; however, there has not been sufficient new experimental water-to-ethyl acetate and water-to-butyl acetate partition coefficient data to merit updating these existing “wet” Abraham model correlations.
The statistical information associated with Equations (3)–(6) appears immediately below the equation itself and includes the number of experimental data points used in the regression analysis, N; the standard deviation, SD; the squared correlation coefficient, R2; and the Fisher F-statistic, F. The numerical values contained within parenthesis that immediately follow each equation coefficient are the standard error in the respective calculated coefficient. As an informational item, the b × B term is missing in Equations (4) and (6), because both ethyl acetate and butyl acetate lack an acidic hydrogen, and thus, they cannot act as an H-bond donor. The term does appear in Equations (3) and (5), as here, the b-coefficients represent the difference in the H-bond acidity of the alkyl acetate solvent(s) and water. Water does possess an H-bond donor character.
2. Experimental Methodology
The crystalline organic solutes selected for the solubility study include 22 carboxylic acids as well as 9 noncarboxylic acid solutes possessing relatedly large E and S descriptor values. All chemicals used in the current study were purchased from commercial sources in the highest purity available. Several of the compounds were further purified by recrystallization from either acetone or anhydrous methanol prior to performing the solubility measurements. All solid compounds were dried for two days at 333 K. Purification details and chemical suppliers are given in Table 1, along with the final purities as determined by either a gas–liquid chromatographic analysis (noncarboxylic acid solutes, flame ionization detector) or the non-aqueous acid–base titrimetric method based on a modified procedure recommended by Fritz and Lisicki [89]. Our modified titration procedure replaced benzene with toluene as a component in the titration solvent for health reasons.

Table 1.
Chemical sources and final mass fraction purities of chemicals used in the solubility studies.
Solubilities of the organic compounds, except for 2-hydroxybenzoic acid and 2-methyl-3-nitrobenzoic acid, were determined using a well-established spectrophotometric method of chemical analysis. Solubilities of 2-hydroxybenzoic acid and 2-methyl-3-nitrobenzoic acid were measured by volumetric acid–base titrations to the phenolphthalein endpoint using a standardized aqueous-sodium hydroxide titrant. In both analytical methods, weighed aliquots of the saturated solutions were transferred into volumetric (for spectrophotometric method) or Erlenmeyer (for titrimetric method) flasks after an initial three-day equilibration in constant-temperature water at 298.15 ± 0.05 K. The samples were periodically shaken to facilitate mixing and dissolution of the solid solute. In the case of the spectrophotometric determinations, the transferred aliquot was quantitatively diluted with 2-propanol. Additional dilutions were performed if necessary in order for the samples’ absorbencies to fall on the Beer–Lambert Law calibration curve, established by graphing the measured absorbances versus the molar concentrations of the nine standard solutions having a known molar solute concentration. All absorbance measurements were recorded on a Milton Roy Spectronic 1000 Plus spectrophotometer. The analysis wavelengths and molar concentration ranges of the standard solutions are reported in Table 2 for each of the analytes, whose solubility was determined by the spectrophotometric method. Attainment of equilibrium was established by performing replicate measurements on the equilibrated samples after two (and in some cases three) additional days of equilibrium. In all instances, the replicate measurements confirmed that equilibrium had been obtained after the initial three-day equilibrium period.

Table 2.
Analysis wavelengths and concentration ranges of standard solutions used in the spectrophotometric determination of solubility.
To check for possible solid–solvate formation and/or possible solid-to-solid phase transition during the solution equilibration time, we did determine the melting point temperature of the solid material recovered from each saturated solution after the solubility measurements were performed. As shown in Table 3, the measured point temperature was within experimental error of the melting point temperature of the purchased commercial sample or the recrystallized compound prior to being placed in contact with the tert-butyl acetate mono-solvent. No indication of solid–solvate formation or polymorphism was observed.

Table 3.
Comparison of the melting point temperatures of the crystalline solutes prior to contact with tert-butyl acetate, Tmp,initial, and of the recovered crystalline solute in equilibrium with the saturated solution, Tmp,equilibrated.
3. Results and Discussion
The experimental mole fraction solubilities, XS,organic, of the 31 different crystalline organic solutes dissolved in tert-butyl acetate are tabulated in the second and fourth columns of Table 4. The numerical values represent the average of 4–7 independent experimental determinations, which were reproducible to within ±2.5% (relative error). We were not able to find, in the published chemical and engineering literature, solubility data for these organic solutes in tert-butyl acetate that we could compare our experimental values against. The only published experimental solubility data that we found was for lovastatin. Nti-Gyabaah and coworkers previously had measured the solubility of lovastatin [23] and simvastatin [88] in seven alkyl acetates between 285 K and 313 K using a high-performance liquid chromatographic method of chemical analysis. The solubility data for both lipid-lowering drug molecules will be used in our determination of the Abraham model equation coefficients for tert-butyl acetate. Both lovastatin (S = 2.730, B = 1.760, V = 3.2853, and L = 15.459) and simvastatin (S = 2.550, B = 1.860, V = 3.4628, and L = 15.551) possess large numerical values for several solute descriptors.

Table 4.
Mole fraction solubilities, XS,organic, of select crystalline nonelectrolyte organic compounds dissolved in tert-butyl acetate at a temperature of 298.15 K and ambient atmospheric pressure of 101 kPa a.
Development of a meaningful Abraham model correlation generally requires somewhere between 30 to 40 experimental values [90,91] that cover a sufficient range of solute descriptor values to enable one to make predictions for a large number of additional solutes. In the case of tert-butyl acetate, we have the 31 experimental mole fraction solubilities tabulated in Table 3, as well as the mole fraction solubility data for lovastatin [23] and simvastatin [88], which were retrieved from our search of the published literature. There were two additional experimental values that could be used in our regression analysis, and those were the gas-to-tert-butyl acetate partition coefficient and water-to-tert-butyl acetate transfer coefficient derived from the vapor pressure of tert-butyl acetate and the Raoult’s law infinite dilution activity coefficient of tert-butyl acetate dissolved in itself. By definition, the Raoult’s Law infinite dilution activity coefficient of a compound dissolved in itself is unity. The calculation of log K and log P from activity coefficients is described in greater detail in the published paper [19] that reported the existing Abraham model correlations for ethyl acetate and butyl acetate. In total, we have experimental solubilities and partition coefficients/transfer coefficients for 34 different solutes.
The Abraham model correlates the logarithms of molar solubility ratios, log (CS,organic/CS,water) and log (CS,organic/CS,gas), and not the mole fraction solubilities, as with our measured data given in Table 4. The tabulated mole fraction solubility data in Table 4 is converted into molar solubilities by dividing XS,organic by the ideal molar volume of the saturated solution (i.e., CS,organic ≈ XS,organic/[XS,organic VSolute + (1 − XS,organic) VSolvent]). A numerical value of Vsolvent = 0.13550 L mol−1 was used for the molar volume of tert-butyl acetate. The numerical values of the molar volumes of the hypothetical subcooled liquid solutes were given in our earlier publications [24,40,41,42,53,54,55,56,57,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107], along with the aqueous molar solubilities, CS,water, and solute molar gas concentrations, CS,gas, needed in obtaining the two molar solubility ratios. Published mole fraction solubilities of lovastatin and simvastatin were converted to molar solubility ratios in a similar fashion. The experimental log (CS,organic/CS,gas) and log (CS,organic/CS,water) values at 298.15 K for 33 solutes dissolved in tert-butyl acetate are listed in Table 5. Also included in Table 5 is the logarithm of the water-to-tert-butyl acetate transfer coefficient, log P, and gas-to-tert-butyl acetate partition coefficient, log K, for the solute tert-butyl acetate itself.

Table 5.
Experimental logarithms of molar solubility ratios; water-to-tert-butyl acetate transfer coefficients, log P; and gas-to-tert-butyl acetate partition coefficients, log K, at 298.15 K.
Once both sets of molar solubility ratios were calculated, we constructed a series of Abraham model log (CS,organic/CS,water) and log (CS,organic/CS,gas) equations by substituting the numerical solubility ratios and solute descriptors into Equations (1) and (2). Solute descriptors needed in constructing the Abraham model equations are given in Table 6. As an informational note, several of the compounds listed in Table 6 used the alternant hydrogen-bond basicity descriptor, B°, in “wet” water-organic solvents when the wet organic solvent contained appreciable quantities of water. For most solutes, B and B° were numerically equal but did differ mainly for alkylanilines, alkylpyridines, and sulfoxides.

Table 6.
Solute descriptors of the compounds used in the regression analysis for determining the Abraham model correlations for tert-butyl acetate, ethyl acetate, and butyl acetate.
Once the numerical values had been inserted into the equations, the only quantities left without numerical values were the two sets of equation coefficients (ceq 1, eeq 1, seq 1, aeq 1, beq 1, veq 1) and (ceq 2, eeq 2, seq 2, aeq 2, beq 2, leq 2) for the tert-butyl acetate mono-solvent. The 34 log (CS,organic/CS,water) equations and 34 log (CS,organic/CS,gas) equations were solved simultaneously to yield:
the values of the respective equation coefficients that best describe the logarithms of the observed molar solubility ratios. The b × B term is missing in Equation (8), because tert-butyl acetate lacks an acidic hydrogen, and thus, it cannot act as an H-bond donor. Both correlations were obtained using the IBM SPSS Statistical 22 commercial software.
Log (CS,organic/CS,water) = 0.456(0.110) + 0.324(0.090) E − 0.661(0.111) S − 1.068(0.084) A − 4.680(0.228) B + 4.101(0.115) V
(with N = 34, SD = 0.100, R2 = 0.994, F = 990.6)
(with N = 34, SD = 0.100, R2 = 0.994, F = 990.6)
Log (CS,organic/CS,gas) = 0.178(0.088) − 0.444(0.061) E + 1.045(0.090) S + 2.522(0.077) A + 0.964(0.017) L
(with N = 34, SD = 0.103, R2 = 0.999, F = 5319),
(with N = 34, SD = 0.103, R2 = 0.999, F = 5319),
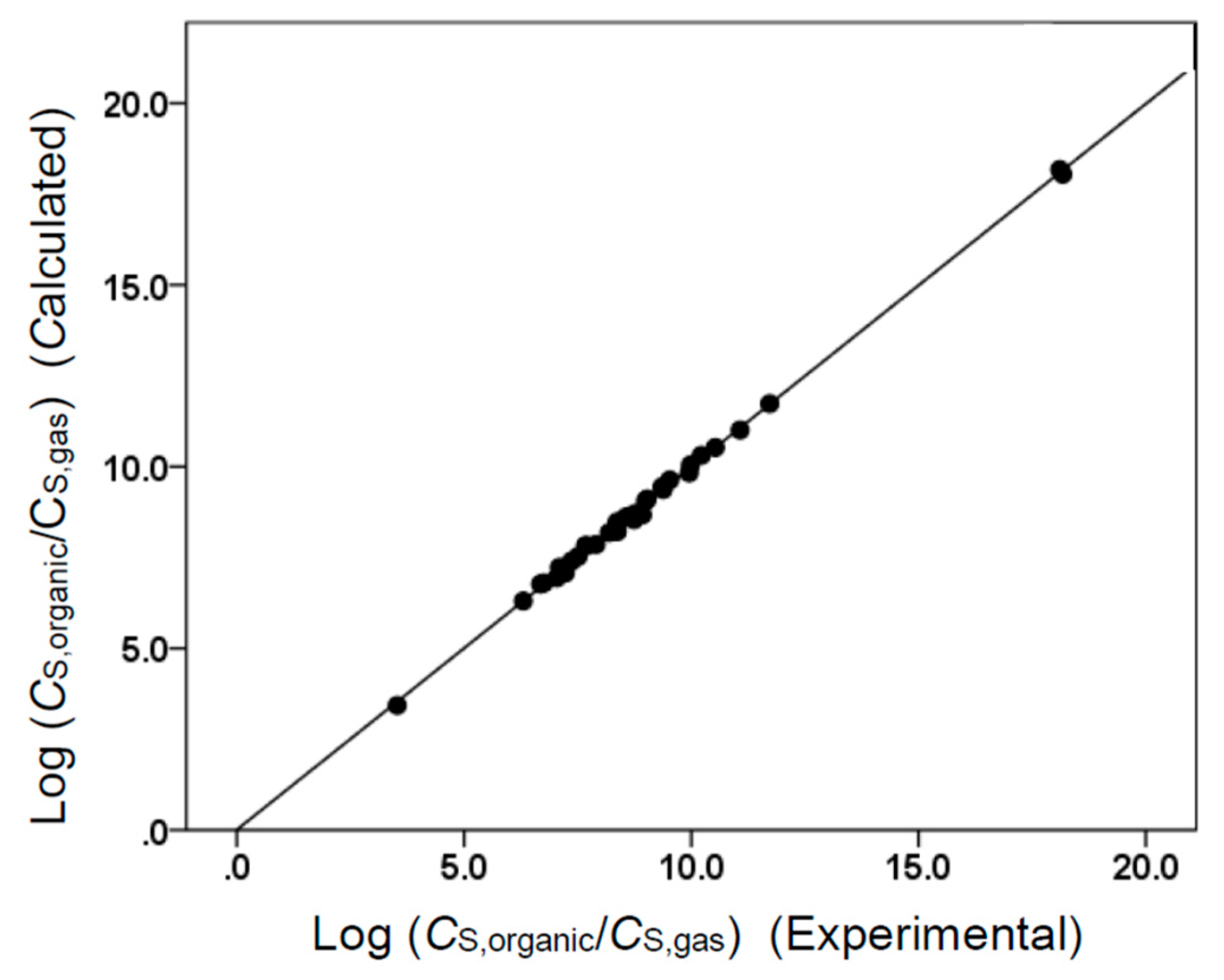
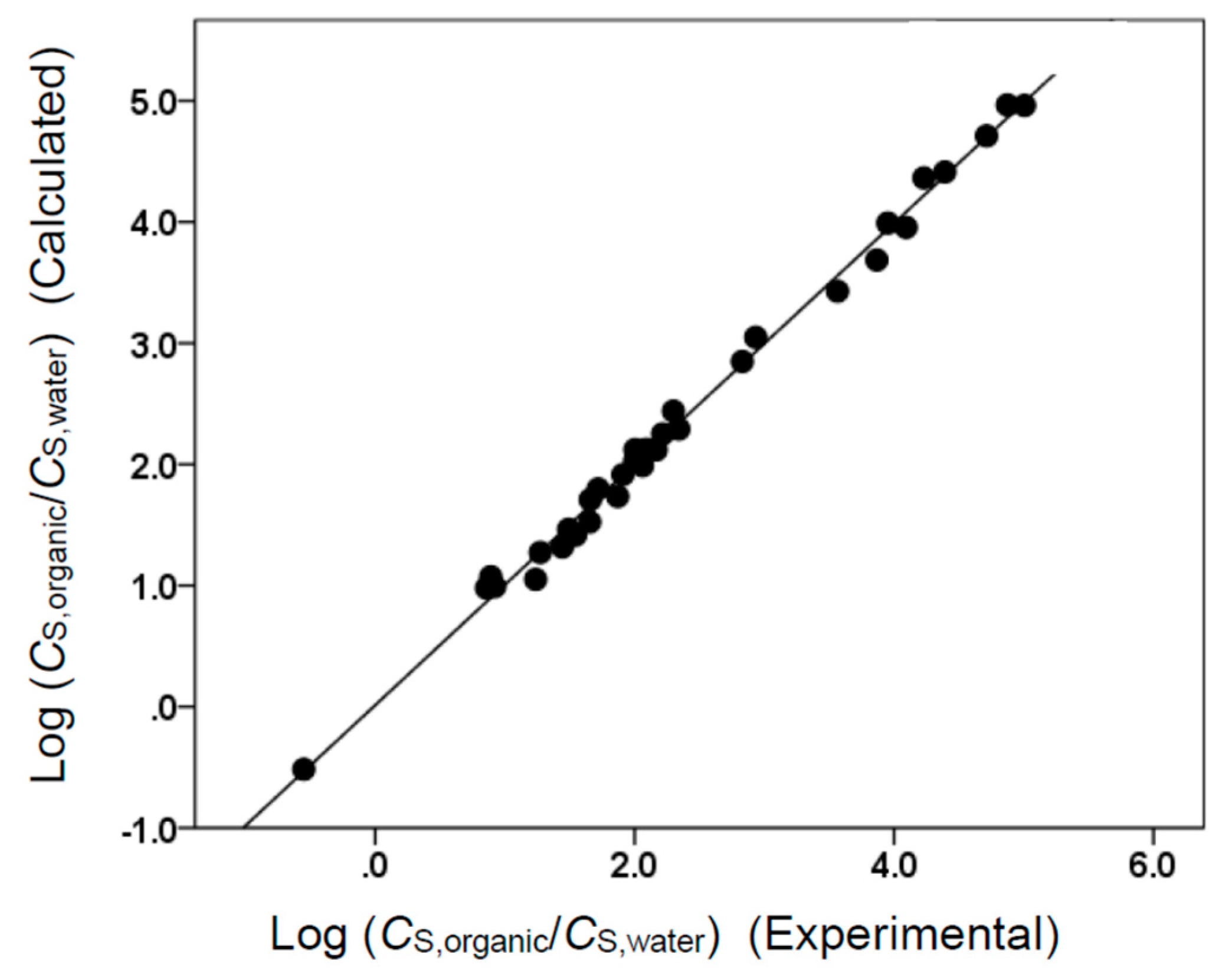
The two Abraham model correlations provided a very accurate mathematical description of the observed molar solubility ratios, as evidenced by the near-unity squared correlation coefficients (R2 = 0.994 for Equation (7) and R2 = 0.999 for Equation (8)) and low standard deviations (SD = 0.100 log units for Equation (7) and SD = 0.103 log units for Equation (8)). Figure 1 and Figure 2 provide a graphical comparison of the experimental data versus back-calculated values based on Equations (8) and (7), respectively. The experimental log (CS,organic/CS,gas) values spanned a range of approximately 14.6 log units. A slightly smaller range of approximately 5.6 log units was spanned by the log (CS,organic/CS,water) values. As an informational note, Equations (7) and (8) were built using a small dataset containing only 34 compounds. Several of the compounds in the dataset were structurally similar to each other, so there would have been some intercorrelation between their descriptor values. In the case of Equation (8), strong correlations were found between the B and S, B and L, and S and L descriptors. For Equation (7), strong correlations were noted between B and S, B and V, and S and V. Intercorrelations would diminish as more experimental values were added to the datasets.
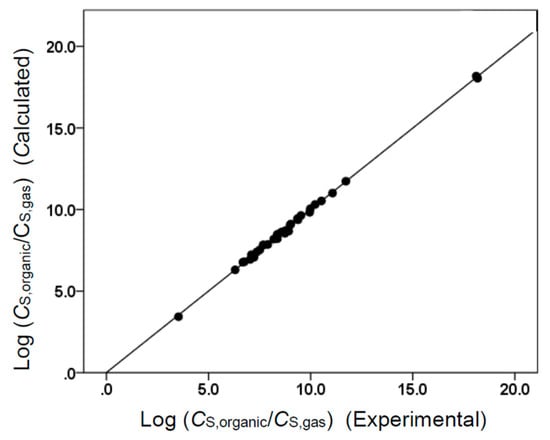
Figure 1.
Comparison of observed log (CS,organic/CS,gas) data versus back-calculated values based on Equation (8) for tert-butyl acetate. The straight line that is drawn corresponds to log (CS,organic/CS,gas) (Calculated) = log (CS,organic/CS,gas) (Experimental).
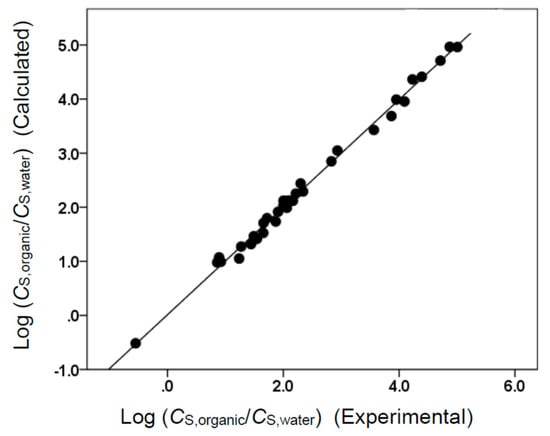
Figure 2.
Comparison of observed log (CS,organic/CS,water) data versus back-calculated values based on Equation (7) for tert-butyl acetate. The straight line that is drawn corresponds to log (CS,organic/CS,water) (Calculated) = log (CS,organic/CS,water) (Experimental).
The existing Abraham model correlations for both ethyl acetate and butyl acetate were published in 2008, based on the experimental solubility and infinite dilution activity coefficient data that were available at the time. During the last 10 years, there has been an enormous quantity of experimental solubility data reported for new pharmaceutical compounds, pesticides and herbicides, and important chemical reactants used in industrial manufacturing processes. A recent search of the published chemical literature managed to find experimental mole fraction solubility data for an additional 64 and 35 organic compounds dissolved in ethyl acetate and butyl acetate, respectively. The additional solubility data represent an approximate 50% increase in the number of experimental data points that are now available to update the earlier 2008 correlations. The additional compounds include not only important medicinal compounds (simvastatin, lovastatin, sorafenib, tinidazole, dapsone, chlorpropanamide, benorilate, probenecid), flavoring agents (vanillin, vanillic acid, vanillyl alcohol, ethyl vanillin), and substituted benzoic acid derivatives, but also a wide range of multi-functional organic compounds of varying shapes and sizes. The entire ethyl acetate and butyl acetate datasets are given in Table 6 and Table 7, respectively, along with the references from which the data were taken. In order to conserve journal space, the experimental values used in deriving the earlier correlations are referenced to the earlier paper [19] in which Equations (3)–(6) first appeared. Experimental-based solute descriptors of several of the additional compounds are reported for the first time in Table 6. As an additional note, the datasets associated with the Abraham model solvent equations have been used by several research groups [108,109,110,111,112,113,114] in developing group contribution approaches, machine learning models, quantitative structure-property relationships, and quantum-mechanical methods for predicting Gibbs energies of solvation and Gibbs energies of transfer for describing the equilibrium partitioning of solutes between two phases. Table 7 and Table 8 provide enlarged ethyl acetate and butyl acetate datasets to use in future modelling endeavors.

Table 7.
Experimental logarithms of molar solubility ratios; water-to-ethyl acetate transfer coefficients, log P; and gas-to-ethyl acetate partition coefficients, log K, at 298.15 K.

Table 8.
Experimental logarithms of molar solubility ratios; water-to-butyl acetate transfer coefficients, log P; and gas-to-butyl acetate partition coefficients, log K, at 298.15 K.
Analysis of the experimental values in Table 6 and Table 7 in accordance with the Abraham general solvation model yielded the following mathematical correlations:
For Ethyl Acetate:
Log (CS,organic/CS,water) = 0.328(0.025) + 0.314(0.033) E – 0.348(0.039) S − 0.847(0.043) A − 4.899(0.058) B + 4.142(0.025) V
(with N = 170, SD = 0.144, R2 = 0.996, F = 7548)
(with N = 170, SD = 0.144, R2 = 0.996, F = 7548)
Log (CS,organic/CS,gas) = 0.171(0.020) − 0.403(0.030) E + 1.428(0.028) S + 2.726(0.038) A + 0.914(0.006) L
(with N = 170, SD = 0.131, R2 = 0.999, F = 42942)
(with N = 170, SD = 0.131, R2 = 0.999, F = 42942)
For Butyl Acetate:
Log (CS,organic/CS,water) = 0.289(0.037) + 0.336(0.041) E − 0.501(0.050) S − 0.913(0.054) A − 4.964(0.063) B + 4.262(0.021) V
(with N = 108, SD = 0.140, R2 = 0.998, F = 11519)
(with N = 108, SD = 0.140, R2 = 0.998, F = 11519)
Log (CS,organic/CS,gas) = 0.154(0.034) − 0.439(0.041) E + 1.223(0.041) S + 2.586(0.056) A + 0.953(0.006) L
(with N = 108, SD = 0.148, R2 = 0.999, F = 17169)
(with N = 108, SD = 0.148, R2 = 0.999, F = 17169)
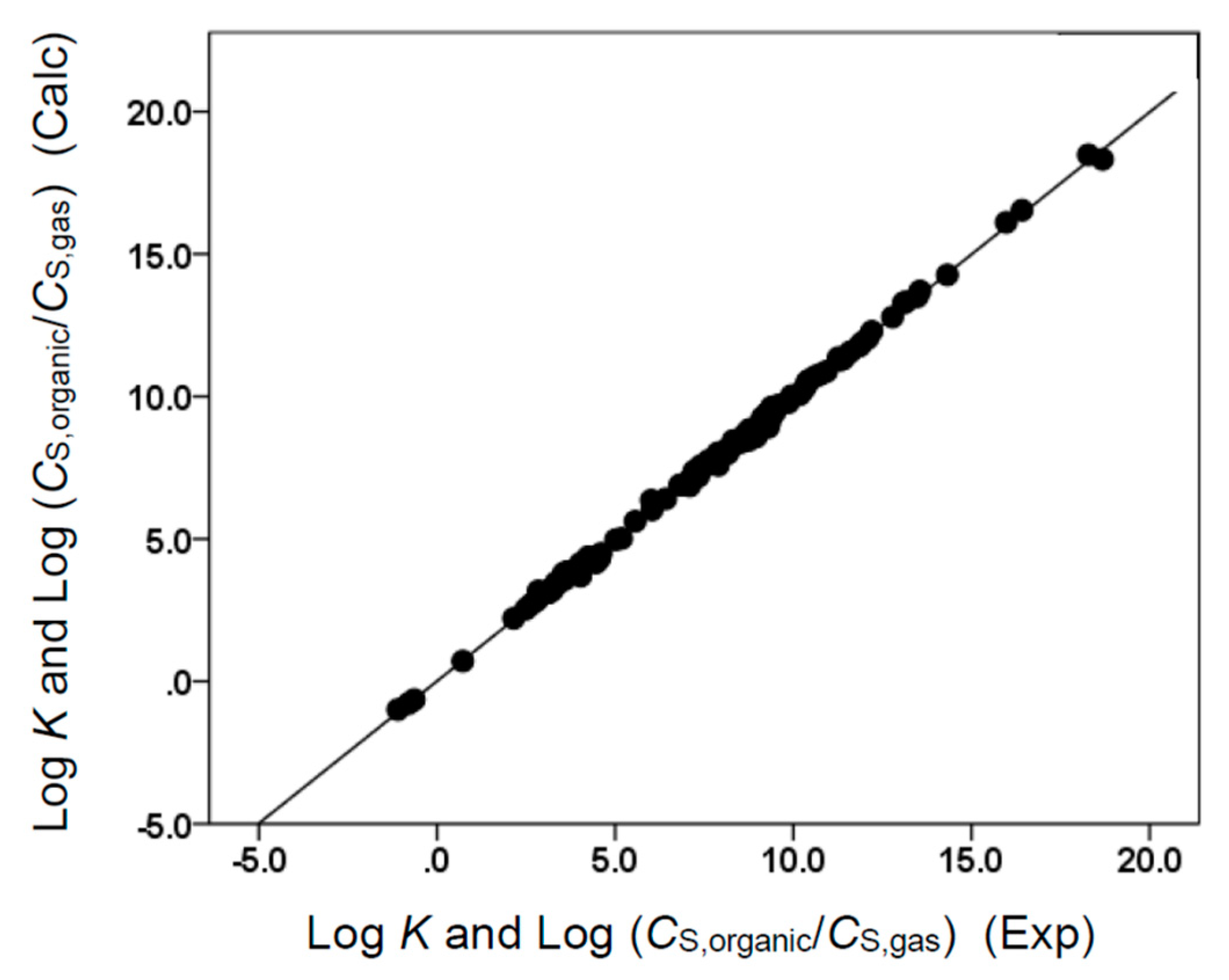
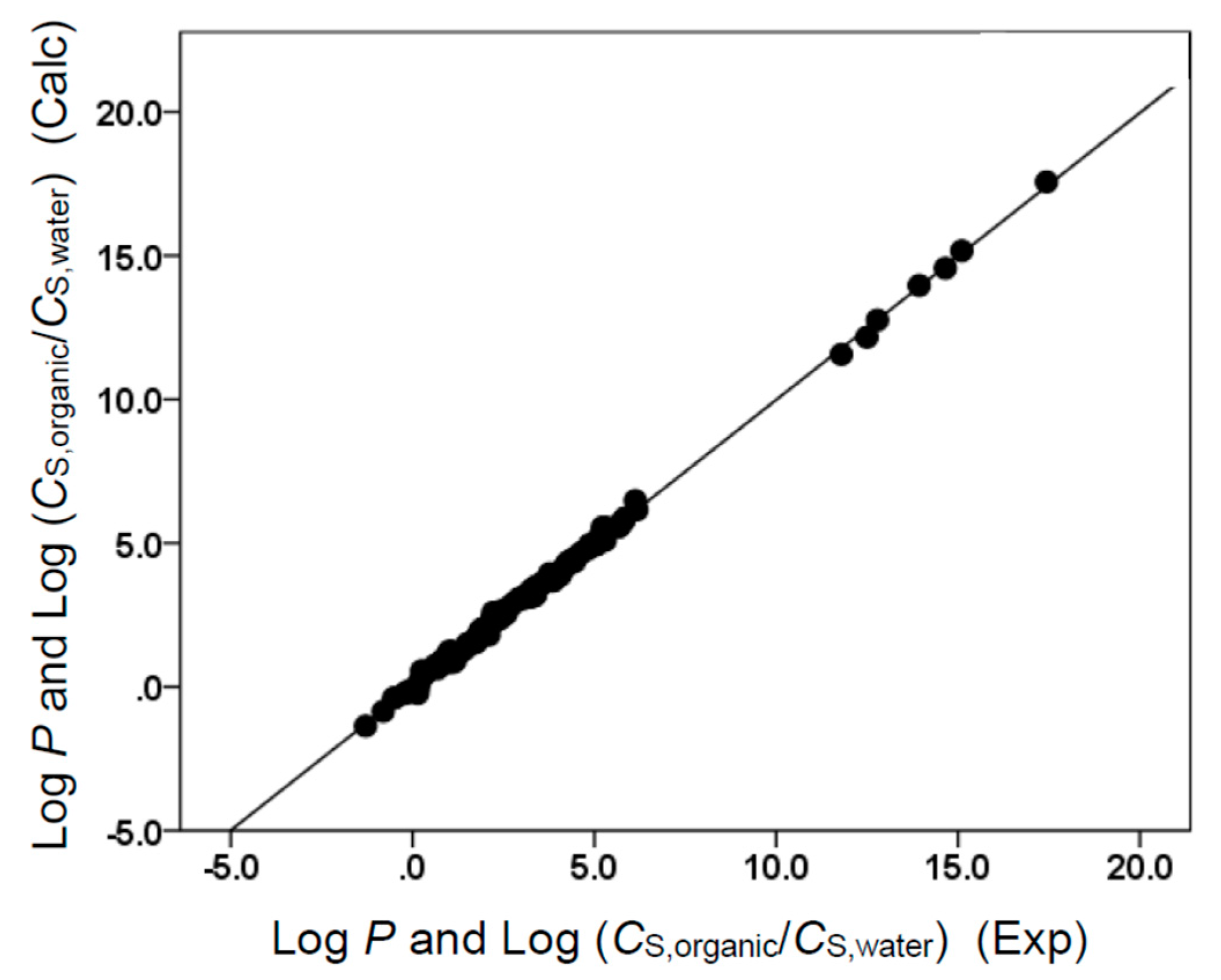
As, before, the b × B term was missing in Equations (10) and (12), neither ethyl acetate nor butyl acetate could act as an H-bond donor. Neither solvent molecule possesses an acidic hydrogen. All four derived correlations provided a reasonably accurate description of the observed solubility and partition coefficient data, as numerically reflected by the near unity squared correlation coefficient and the relatively small standard deviations. The descriptive ability is further illustrated in Figure 3, Figure 4, Figure 5 and Figure 6. For most of the solute molecules considered, the graphed points fell near the drawn straight line, indicating a near-perfect back-calculation.
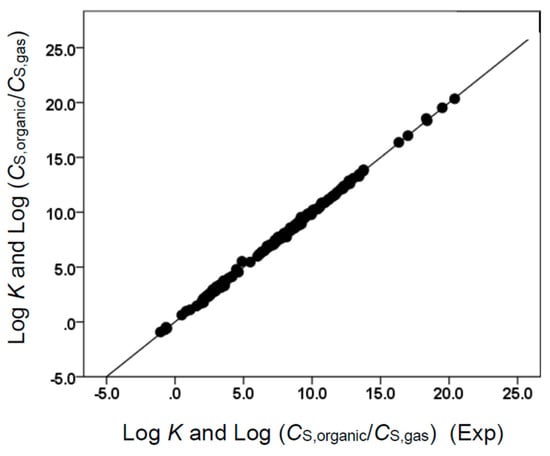
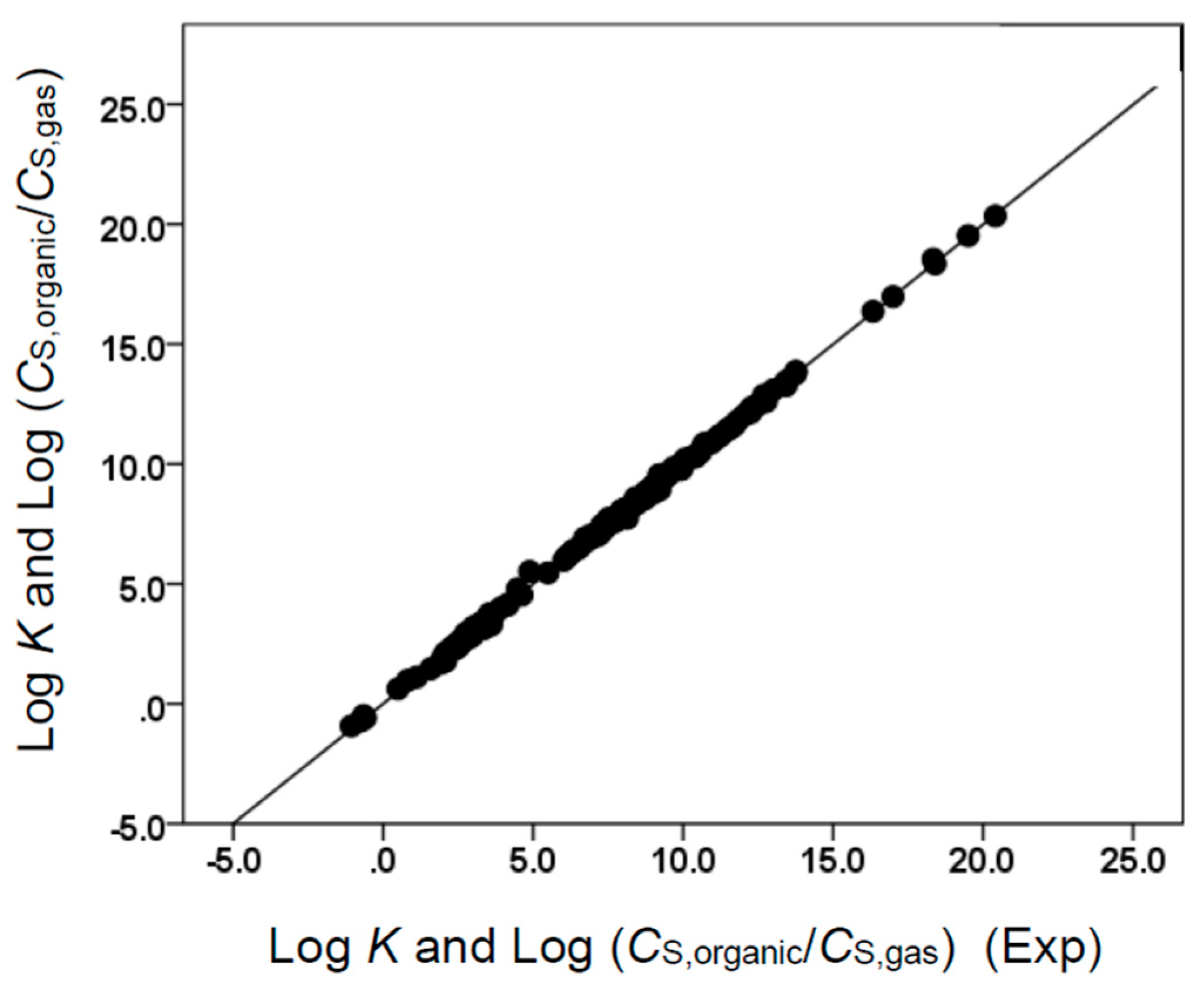
Figure 3.
Comparison of observed log K and log (CS,organic/CS,gas) data versus back-calculated values based on Equation (10) for ethyl acetate. The straight line that is drawn corresponds to log K and log (CS,organic/CS,gas) (Calc) = log K and log (CS,organic/CS,gas) (Exp).
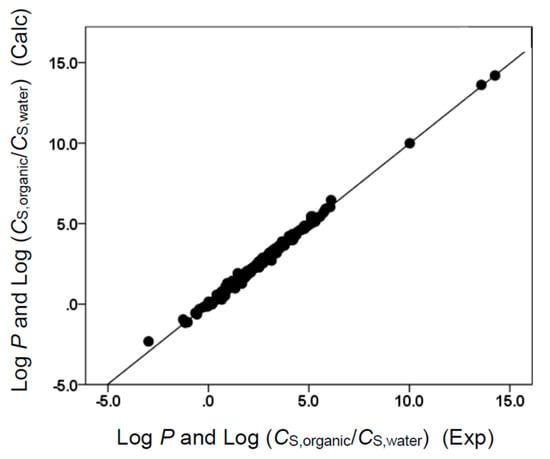
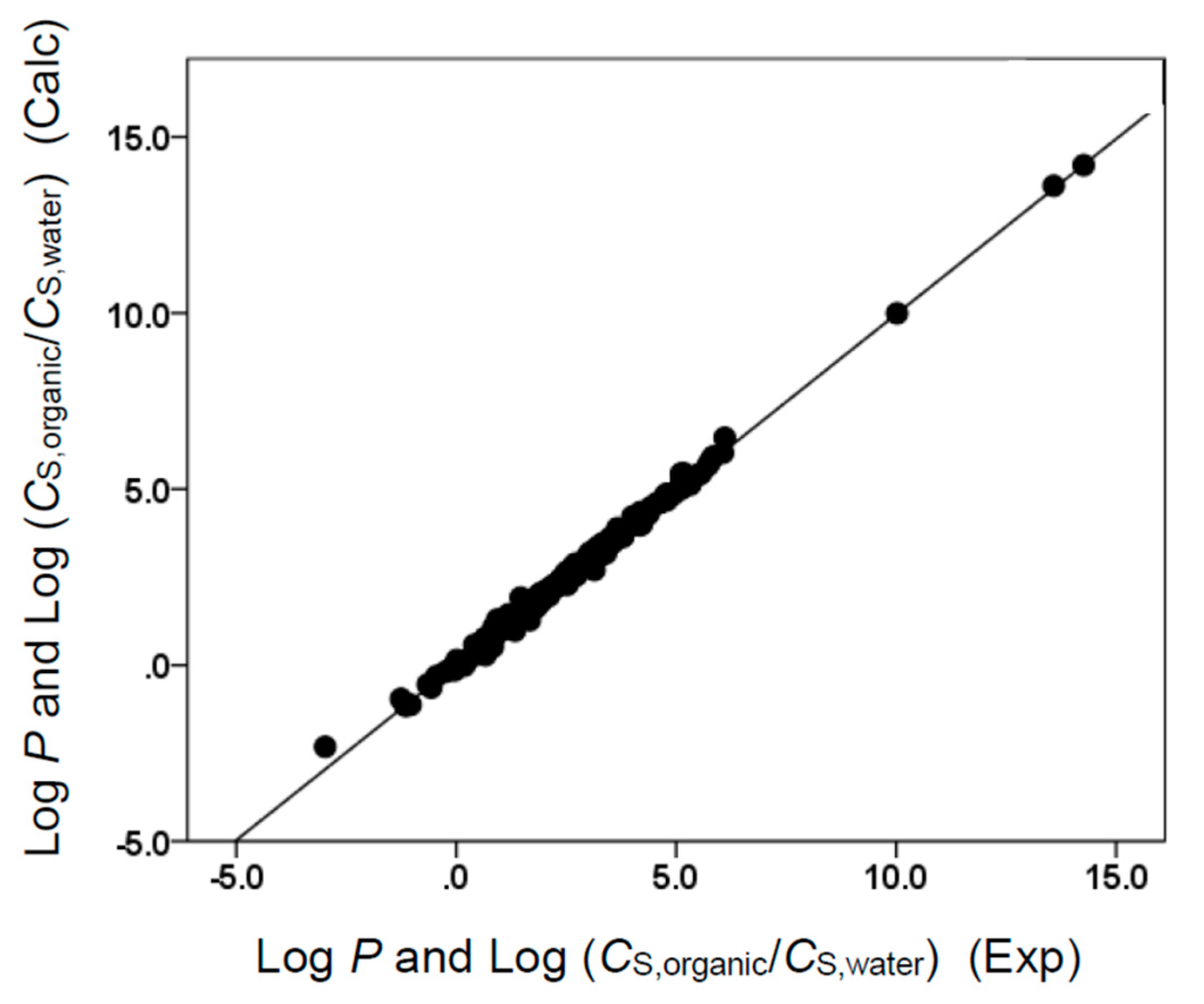
Figure 4.
Comparison of observed log P and log (CS,organic/CS,water) data versus back-calculated values based on Equation (9) for ethyl acetate. The straight line that is drawn corresponds to log P and log (CS,organic/CS,water) (Calc) = log P and log (CS,organic/CS,water) (Exp).
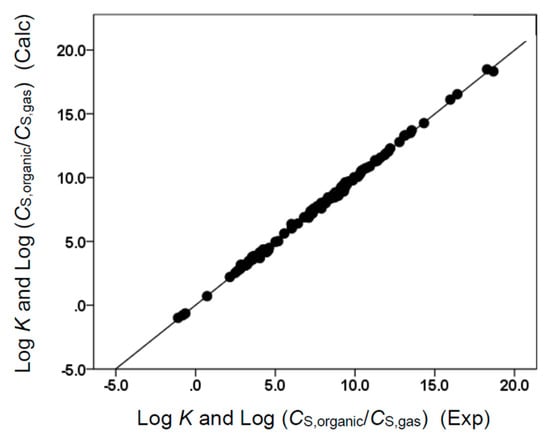
Figure 5.
Comparison of observed log K and log (CS,organic/CS,gas) data versus back-calculated values based on Equation (12) for butyl acetate. The straight line that is drawn corresponds to log K and log (CS,organic/CS,gas) (Calc) = log K and log (CS,organic/CS,gas) (Exp).
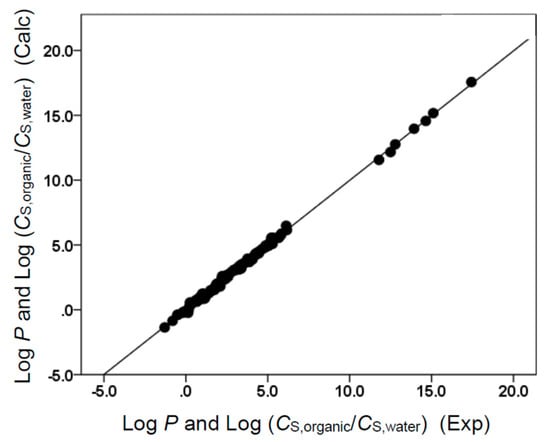
Figure 6.
Comparison of observed log P and log (CS,organic/CS,water) data versus back-calculated values based on Equation (11) for butyl acetate. The straight line that is drawn corresponds to log P and log (CS,organic/CS,water) (Calc) = log P and log (CS,organic/CS,water) (Exp).
We further note that the numerical values of the equation coefficients changed slightly from the values given in the earlier 2008 correlations (See Equations (3)–(6)). The change is likely reflected by the addition of several large, highly basic molecules (sorafenib, B = 1.494, V = 3.0195; lovastatin, B = 1.760, V = 3.2853; simvastatin, B = 1.860, V = 3.4268) to the datasets. Prior to the inclusion of an additional 64 compounds, betulin (B = 1.140) was the only compound in the ethyl acetate dataset having a B-solute descriptor that exceeded unity. It is important to periodically update existing correlations as new experimental data become available in order to expand the predictive area of chemical space. Ethyl acetate and, to a lesser extent, butyl acetate are solvents that researchers use in performing solubility studies on new drug molecules. These are also two organic solvents that we routinely use in calculating solute descriptor values. Datasets used in determining the Abraham model correlations need to contain solutes that possess the molecular size, polarity, and lipophilicity common to the newly approved medicinal compounds, if the correlations are to be used in calculating solute descriptors of these compounds. Newer drug molecules tend to be larger and more lipophilic and possess a greater H-bond acceptor capability than older drugs [115]. The predictive area of chemical space covered by the Abraham model correlations needs to keep pace with the molecular properties of today’s modern drug molecules.
Many of the compounds used in chemical manufacturing processes will have solute descriptors that fall within these ranges. Currently solute descriptors are readily available on the UFZ-LSER internet website [116] for more than 8500 different organic compounds. If not available, there are group contribution methods [117,118], as well as machine learning models [108,119], that can be used to estimate the desired descriptor values. The estimation requires simply inputting the canonical SMILES code of the desired solute into the software program found at either the UFZ-LSER website or at the RMG-MIT website link embedded in [89] in the published paper [108].
4. Conclusions
Mathematical expressions based upon the Abraham general solvation parameter model were obtained for predicting the solute transfer of molecular organic compounds and inorganic gases into three alkyl acetate mono-solvents (tert-butyl acetate, ethyl acetate, and butyl acetate). The predictive expressions for the three alkyl acetate solvents were determined using chemically diverse datasets, which contained 34, 170, and 108 solutes of various molecular sizes and shapes, polarities, and hydrogen-bonding characteristics. The mathematical correlations presented in the current study describe the observed solubility ratios of solutes dissolved in tert-butyl acetate, ethyl acetate, and butyl acetate to within an overall standard deviation of 0.15 log units or less. Based on our past experience using the Abraham model, we fully expect the derived mathematical expressions to provide comparable predictions for the solubility and partitioning behavior of additional organic solutes in the three fore-mentioned solvents, provided, of course, that the descriptor values of the additional solutes fall within the range of values used in deriving the respective predictive expression. Many of the compounds used in chemical manufacturing processes will have solute descriptors that fall within these ranges.
Author Contributions
Conceptualization and writing—original draft preparation, W.E.A.J.; formal data analysis, L.L., E.W., C.Y., M.Z., S.S. and A.V.; writing—review and editing, L.L., E.W., C.Y., M.Z., S.S. and A.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Emily Wu, Chelsea Yang, Miles Zhang, and Sneha Sinha thank the University of North Texas’s Texas Academy of Mathematics & Science (TAMS) program for providing a summer research scholarship to each student.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, A.; Varadharajan, A.; Shanmugam, N.; Kim, K.; Huang, E.; Cai, S.K.; Acree, W.E., Jr. Abraham model description of the solubilising properties of the isopropyl acetate organic mono-solvent. Phys. Chem. Liq. 2022, 60, 312–324. [Google Scholar] [CrossRef]
- Kim, K.; Shanmugam, N.; Xu, A.; Varadharajan, A.; Cai, S.K.; Huang, E.; Acree, W.E., Jr. Abraham model correlations for describing solute transfer into anisole based on measured activity coefficients and molar solubilities. Phys. Chem. Liq. 2022, 60, 452–462. [Google Scholar] [CrossRef]
- Cai, S.K.; Huang, E.; Kim, K.; Shanmugam, N.; Varadharajan, A.; Xu, A.; Acree, W.E., Jr. Development of Abraham model correlations for solute transfer into cyclopentanol from both water and the gas phase based on measured solubility ratios. Phys. Chem. Liq. 2022, 60, 287–296. [Google Scholar] [CrossRef]
- Strickland, S.; Ocon, L.; Zhang, A.; Wang, S.; Eddula, S.; Liu, G.; Tirumala, P.; Huang, J.; Dai, J.; Jiang, C.; et al. Abraham model correlations for describing dissolution of organic solutes and inorganic gases in dimethyl carbonate. Phys. Chem. Liq. 2021, 59, 181–195. [Google Scholar] [CrossRef]
- Ruddigkeit, L.; van Deursen, R.; Blum, L.C.; Reymond, J.-L. Enumeration of 166 billion organic small molecules in the Chemical Universe Database GDB-17. J. Chem. Inf. Model. 2012, 52, 2864–2875. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.H. Scales of solute hydrogen-bonding: Their construction and application to physicochemical and biochemical processes. Chem. Soc. Rev. 1993, 22, 73–83. [Google Scholar] [CrossRef]
- Abraham, M.H.; Ibrahim, A.; Zissimos, A.M. Determination of sets of solute descriptors from chromatographic measurements. J. Chromatogr. A 2004, 1037, 29–47. [Google Scholar] [CrossRef]
- Abraham, M.H.; Smith, R.E.; Luchtefeld, R.; Boorem, A.J.; Luo, R.; Acree, W.E., Jr. Prediction of solubility of drugs and other compounds in organic solvents. J. Pharm. Sci. 2010, 99, 1500–1515. [Google Scholar] [CrossRef]
- Abraham, M.H.; Acree, W.E., Jr. Descriptors for the prediction of partition coefficients of 8-hydroxyquinoline and its derivatives. Sep. Sci. Technol. 2014, 49, 2135–2141. [Google Scholar] [CrossRef]
- Sinha, S.; Yang, C.; Wu, E.; Acree, W.E., Jr. Abraham solvation parameter model: Examination of possible intramolecular hydrogen-bonding using calculated solute descriptors. Liquids. 2022, 2, 131–146. [Google Scholar] [CrossRef]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; McElroy, C.R.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 1–24. [Google Scholar] [CrossRef]
- Henderson, R.K.; Jimenez-Gonzalez, C.; Constable, D.J.C.; Alston, S.R.; Inglis, G.G.A.; Fisher, G.; Sherwood, J.; Binks, S.P.; Curzons, A.D. Expanding GSK’s solvent selection guide-embedding sustainability into solvent selection starting at medicinal chemistry. Green Chem. 2011, 13, 854–862. [Google Scholar] [CrossRef]
- Jimenez-Gonzalez, C.; Curzons, A.D.; Constable, D.J.C.; Cunningham, V.L. Expanding GSK’s solvent selection guide-application of life cycle assessment to enhance solvent selections. Clean Technol. Environ. Policy. 2005, 7, 42–50. [Google Scholar] [CrossRef]
- Welton, T. Solvents and sustainable chemistry. Proc. R. Soc. A. 2015, 471, 20150502. [Google Scholar] [CrossRef]
- Duereh, A.; Sato, Y.; Smith, R.L., Jr.; Inomata, H. Methodology for replacing dipolar aprotic solvents used in API processing with safe hydrogen-bond donor and acceptor solvent-pair mixtures. Org. Process Res. Dev. 2017, 21, 114–124. [Google Scholar] [CrossRef]
- Joshi, D.R.; Adhikari, N. An overview on common organic solvents and their toxicity. J. Pharm. Res. Int. 2019, 28, JPRI.49840. [Google Scholar] [CrossRef]
- Prat, D.; Hayler, J.; Wells, A. A survey of solvent selection guides. Green Chem. 2014, 16, 4546–4551. [Google Scholar] [CrossRef]
- Byrne, F.P.; Forier, B.; Bossaert, G.; Hoebers, C.; Farmer, T.J.; Hunt, A.J. A methodical selection process for the development of ketones and esters as bio-based replacements for traditional hydrocarbon solvents. Green Chem. 2018, 20, 4003–4011. [Google Scholar] [CrossRef]
- Sprunger, L.M.; Proctor, A.; Acree, W.E., Jr.; Abraham, M.H.; Benjelloun-Dakhama, N. Correlation and prediction of partition coefficient between the gas phase and water, and the solvents dry methyl acetate, dry and wet ethyl acetate, and dry and wet butyl acetate. Fluid Phase Equilib. 2008, 270, 30–44. [Google Scholar] [CrossRef]
- Xu, R.; Wang, J. Solubility measurement and thermodynamic model correlation and evaluation of 2-chloro-5-nitroaniline in 12 pure solvents. J. Chem. Eng. Data. 2019, 64, 1357–1365. [Google Scholar] [CrossRef]
- Ji, W.; Meng, Q.; Li, P.; Yang, B.; Wang, F.; Ding, L.; Wang, B. Measurement and correlation of the solubility of p-coumaric acid in nine pure and water + ethanol mixed solvents at temperatures from 293.15 to 333.15 K. J. Chem. Eng. Data. 2016, 61, 3457–3465. [Google Scholar] [CrossRef]
- Wu, H.; Dang, L.; Wei, H. Solid-liquid phase equilibrium of nicotinamide in different pure solvents: Measurements and thermodynamic modeling. Ind. Eng. Chem. Res. 2014, 53, 1707–1711. [Google Scholar] [CrossRef]
- Nti-Gyabaah, J.; Chiew, Y.C. Solubility of lovastatin in ethyl acetate, propyl acetate, isopropyl acetate, butyl acetate, sec-butyl acetate, isobutyl acetate, tert-butyl acetate, and 2-butanone, between (285 and 313) K. J. Chem. Eng. Data. 2008, 53, 2060–2065. [Google Scholar] [CrossRef]
- Holley, K.; Acree, W.E., Jr.; Abraham, M.H. Determination of Abraham model solute descriptors for 2-ethylanthraquinone based on measured solubility ratios. Phys. Chem. Liq. 2011, 49, 355–365. [Google Scholar] [CrossRef]
- Bao, Y.; Wu, J.; Zhao, X.; Zhao, H. 2-Methoxy-4-nitroaniline solubility in several monosolvents: Measurement, correlation, and solvent effect analysis. J. Chem. Eng. Data. 2020, 65, 757–765. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhang, Y.; Na, B.; Liu, Z.; Zhou, L.; Hao, H. Solubility determination of nicotinamide and its application for the cocrystallization with benzoic acid. J. Chem. Eng. Data. 2018, 63, 4157–4165. [Google Scholar] [CrossRef]
- Abraham, M.H.; Acree, W.E., Jr. Descriptors for ferrocene and some substituted ferrocenes. J. Mol. Liq. 2017, 232, 325–331. [Google Scholar] [CrossRef]
- Wu, K.; Li, Y. Solubility Measurement and thermodynamic modeling for o-toluenesulfonamide in 16 solvents from T = 273.15 to 323.85 K. J. Chem. Eng. Data. 2019, 64, 5238–5247. [Google Scholar] [CrossRef]
- Zhang, Z.; Qu, Y.; Li, M.; Wang, S.; Wang, J. Solubility and thermodynamic modeling of dimethyl terephthalate in pure solvents and the evaluation of the mixing properties of the solutions. J. Chem. Eng. Data. 2019, 64, 4565–4579. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Qu, C.; Li, M.; Qu, Y. Solid-liquid equilibrium of chlorpropamide in 14 pure solvents at temperature of 283.15 to 323.15 K. J. Chem. Eng. Data. 2020, 65, 2859–2871. [Google Scholar] [CrossRef]
- Peng, H.; Liu, Y.; Yan, H.; Yu, C.; Feng, S.; Yin, H.; Chen, M. Solubility measurement and data correlation of salicylanilide in 12 pure solvents at temperatures ranging from 283.15 to 323.15 K. J. Chem. Eng. Data. 2021, 66, 1501–1507. [Google Scholar] [CrossRef]
- Hu, W.; Shang, Z.; Wei, N.; Hou, B.; Gong, J.; Wang, Y. Solubility of benorilate in twelve monosolvents: Determination, correlation and COSMO-RS analysis. J. Chem. Thermodyn. 2021, 152, 106272/1–106272/10. [Google Scholar] [CrossRef]
- Wu, K.; Li, Y. Solid-liquid phase equilibrium and solution thermodynamics of 2-chlorobenzenesulfonamide in 16 mono solvents at temperature ranging from 273.15 K to 324.65 K. J. Mol. Liq. 2020, 304, 112795/1–112795/11. [Google Scholar] [CrossRef]
- Li, Y.; Lu, C.; Chen, R.; Wu, K. Determination, correlation, and thermodynamic analysis of the solid-liquid phase equilibrium of 1,4-dicyanobenzene in pure solvents at various temperatures. J. Chem. Eng. Data. 2020, 65, 4991–5002. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Zhang, Y.; Wang, W. Solubility measurement and correlation of 2-chlorothioxanthone (CTX) in twelve mono organic solvents. J. Mol. Liq. 2022, 360, 119503/1–119503/11. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, Q.; Bai, J.; Guo, C. Solubility determination, solute-solvent interactions, and model correlation of sorafenib in twelve pure organic solvents between T = 273.15 and 313.15 K. J. Chem. Eng. Data. 2021, 66, 4286–4292. [Google Scholar] [CrossRef]
- Gao, X.; Yu, S.; Wu, G.; Cheng, Y.; Xue, F. Solid-liquid phase equilibrium of 2-mercapto-1,3,4-thiadiazol in pure organic solvents. J. Chem. Eng. Data. 2021, 66, 4706–4713. [Google Scholar] [CrossRef]
- Li, T.; Zhu, L.; Li, J.; Cao, Z.; Sha, J.; Li, Y.; Ren, B. Solubility, thermodynamic properties and molecular simulation of tinidazole in fourteen mono-solvents at different temperatures. J. Chem. Thermodyn. 2022, 170, 106767/1–106767/14. [Google Scholar] [CrossRef]
- Wu, K.; Guan, Y.; Yang, Z.; Ji, H. Solid-liquid phase equilibrium of isophthalonitrile in 16 solvents from T = 273.15 to 324.75 K and mixing properties of solutions. J. Chem. Eng. Data. 2021, 66, 4442–4452. [Google Scholar] [CrossRef]
- Wilson, A.; Tian, A.; Chou, V.; Quay, A.N.; Acree, W.E., Jr.; Abraham, M.H. Experimental and predicted solubilities of 3,4-dichlorobenzoic acid in select organic solvents and in binary aqueous-ethanol mixtures. Phys. Chem. Liq. 2012, 50, 324–335. [Google Scholar] [CrossRef]
- Bowen, K.R.; Stephens, T.W.; Lu, H.; Satish, K.; Shan, D.; Acree, W.E., Jr.; Abraham, M.H. Experimental and predicted solubilities of 3,4-dimethoxybenzoic acid in select organic solvents of varying polarity and hydrogen-bonding character. Eur. Chem. Bull. 2013, 2, 577–583. [Google Scholar]
- Hart, E.; Klein, A.; Zha, O.; Wadawadigi, A.; Qian, E.; Dunn, S.; Herron, J.; Kankolongo, K.; Ryan, S.; Acree, W.E., Jr.; et al. Determination of Abraham model solute descriptors for monomeric 3,4,5-trimethoxybenzoic acid from experimental solubility data in organic solvents measured at 298.2 K. Phys. Chem. Liq. 2018, 56, 381–390. [Google Scholar] [CrossRef]
- Qian, E.; Lee, G.; Che, M.; Wang, L.; Yue, D.; Fischer, R.; Jodray, M.; Gupta, A.; Neal, R.; Liu, Y.; et al. Determination of Abraham model solute descriptors for xanthone based on experimental solubility measurements at 298.2 K. Phys. Chem. Liq. 2020, 58, 214–221. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Chen, H.; An, S.-F.; Li, T.-T. Solubility measurement and correlation of 2-phenyl-1H-indole in fourteen mono organic solvents from 289.05 K to 338.55 K. J. Mol. Liq. 2020, 302, 112491/1–112491/11. [Google Scholar] [CrossRef]
- Xu, R.; Wang, J.; Du, C.; Han, S.; Meng, L.; Zhao, H. Solubility determination and thermodynamic dissolution functions of 1,3-diphenylguanidine in nine organic solvents at evaluated temperatures. J. Chem. Thermodyn. 2016, 99, 86–95. [Google Scholar] [CrossRef]
- Xu, K.; Zhu, J.; Li, T. Measurement and correlation of the dissolution equilibria of o-iodoaniline and p-iodoaniline in pure solvents. J. Chem. Eng. Data. 2018, 63, 217–224. [Google Scholar] [CrossRef]
- Song, T.; Xiao, Y.; Shi, K.; Bai, Y.; Li, J.; Qi, L.; Xie, C. Solubility measurement and data correlation of pyrimethamine in 13 pure solvents at temperatures from 278.15 to 318.15 K. J. Chem. Eng. Data. 2022, 67, 28–36. [Google Scholar] [CrossRef]
- Li, J.; Yao, M.; Kang, X.; Yu, C.; Liu, Y.; Yan, H.; Yin, H.; Chen, M. Equilibrium solubility determination and dissolution property analysis of 5,6-dimethoxy-1-indanone in 15 pure solvents from 283.15 to 323.15 K. J. Chem. Eng. Data. 2021, 66, 1813–1820. [Google Scholar] [CrossRef]
- Sinha, S.; Varadharajan, A.; Xu, A.; Wu, E.; Acree, W.E., Jr. Determination of Abraham model solute descriptors for hippuric acid from measured molar solubilities in several organic mono-solvents of varying polarity and hydrogen-bonding ability. Phys. Chem. Liq. 2022, 60, 563–571. [Google Scholar] [CrossRef]
- Xu, S.; Cao, T.; Ma, M.; Wang, Y. Uncover the effect of solvent and temperature on solid-liquid equilibrium behavior of 2-bromodibenzofuran. J. Chem. Thermodyn. 2022, 171, 106813. [Google Scholar] [CrossRef]
- Xu, R.; Xu, A.; Du, C.; Cong, Y.; Wang, J. Solubility determination and thermodynamic modeling of 2,4-dinitroaniline in nine organic solvents from T = (278.15 to 318.15) K and mixing properties of solutions. J. Chem. Thermodyn. 2016, 102, 178–187. [Google Scholar]
- Wang, J.; Xu, A.; Xu, R. Determination and correlation of terephthaldialdehyde solubility in (ethanol, isopropanol, ethyl acetate, isopentanol) + N,N-dimethylformamide mixed solvents at temperatures from 273.15 K to 318.15 K. J. Chem. Thermodyn. 2017, 105, 327–336. [Google Scholar] [CrossRef]
- Hart, E.; Lee, G.; Qian, E.; Jodray, M.; Barrera, M.; Fischer, R.; Che, M.; Liu, Y.; Zha, O.; Woods, D.; et al. Determination of Abraham model solute descriptors for 4-tert-butylbenzoic acid from experimental solubility data in organic mono-solvents. Phys. Chem. Liq. 2019, 57, 445–452. [Google Scholar] [CrossRef]
- Hart, E.; Ramirez, A.M.; Cheeran, S.; Barrera, M.; Horton, M.Y.; Wadawadigi, A.; Acree, W.E., Jr.; Abraham, M.H. Determination of Abraham model solute descriptors for 2-methyl-3-nitrobenzoic acid from measured solubility data in alcohol, alkyl ether, alkyl acetate and 2-alkoxyalcohol mono-solvents. Phys. Chem. Liq. 2017, 55, 796–804. [Google Scholar] [CrossRef]
- Acree, W.E., Jr.; Bowen, K.R.; Horton, M.Y.; Abraham, M.H. Computation of Abraham model solute descriptors for 3-methyl-4-nitrobenzoic acid from measured solubility data. Phys. Chem. Liq. 2017, 55, 482–491. [Google Scholar] [CrossRef]
- Wang, S.; Liu, K.; Zhang, A.; Dai, J.; Gupta, A.; Zhu, S.; Eddula, S.; Jiang, C.; Acree, W.E., Jr.; Abraham, M.H. Solubility of 4-methyl-3-nitrobenzoic acid in organic mono-solvents: Calculation of Abraham model solute descriptors. Phys. Chem. Liq. 2020, 58, 782–791. [Google Scholar] [CrossRef]
- Stephens, T.W.; Loera, M.; Calderas, M.; Diaz, R.; Montney, N.; Acree, W.E., Jr.; Abraham, M.H. Determination of Abraham model solute descriptors for benzoin based on measured solubility ratios. Phys. Chem. Liq. 2012, 50, 254–265. [Google Scholar] [CrossRef]
- Fletcher, K.A.; Coym, K.S.; Roy, L.E.; Hernandez, C.E.; McHale, M.E.R.; Acree, W.E., Jr. Solubility of thioxanthen-9-one in organic nonelectrolyte solvents. Comparison of observed versus predicted values based upon mobile order theory (MOT). Phys. Chem. Liq. 1998, 35, 243–252. [Google Scholar] [CrossRef]
- Ye, S.; Saifullah, M.; Grubbs, L.M.; McMillan-Wiggins, M.C.; Acosta, P.; Mejorado, D.; Flores, I.; Acree, W.E., Jr.; Abraham, M.H. Determination of the Abraham model solute descriptors for 3,5-dinitro-2-methylbenzoic acid from measured solubility data in organic solvents. Phys. Chem. Liq. 2011, 49, 821–829. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, M.; Zhu, P.; Ouyanga, J.; Gong, J.; Chen, W.; Xu, F. Solubility and solution thermodynamics of sorbic acid in eight pure organic solvents. J. Chem. Thermodyn. 2015, 85, 202–209. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, B.; Yang, Z.; Yao, G.; Zhao, H. Solubility of dichloronitrobenzene in eight organic solvents from T = (278.15 to 303.15) K: Measurement and thermodynamic modeling. J Chem. Eng, Data. 2014, 59, 1281–1287. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, H.; Yang, Z.; Li, R. Solubility of 3,4-dichloronitrobenzene in methanol, ethanol, and liquid mixtures (methanol + water, ethanol + water): Experimental measurement and thermodynamic modeling. J. Chem. Eng. Data. 2013, 58, 3061–3068. [Google Scholar] [CrossRef]
- Lee, G.; Che, M.; Qian, E.; Wang, L.; Gupta, A.; Neal, R.; Yue, D.; Downs, S.; Mayes, T.; Rose, O.; et al. Determination of Abraham model solute descriptors for o-acetoacetanisidide based on experimental solubility data in organic mono-solvents. Phys. Chem. Liq. 2019, 57, 528–535. [Google Scholar] [CrossRef]
- Sun, R.; He, H.; Wan, Y.; Li, L.; Sha, J.; Jiang, G.; Li, Y.; Li, T.; Ren, B. Kojic acid in fourteen mono-solvents: Solubility data, Hansen solubility parameter and thermodynamic properties. J. Chem. Thermodyn. 2021, 152, 106280/1–116280/13. [Google Scholar] [CrossRef]
- Zhang, K.; Shen, H.; Xu, S.; Zhang, H.; Zhu, M.; Shi, P.; Fu, X.; Yang, Y.; Gong, J. Thermodynamic study of solubility for pyrazinamide in ten solvents from T = (283.15 to 323.15) K. J. Chem. Thermodyn. 2017, 112, 204–212. [Google Scholar] [CrossRef]
- Jia, S.; Zhang, K.; Wan, X.; Gao, Z.; Gong, J.; Rohani, S. Effects of temperature and solvent properties on the liquid-solid phase equilibrium of γ-pyrazinamide. J. Chem. Eng. Data. 2020, 65, 3667–3678. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Z.; Jiao, W.; Cheng, X.; Yang, J.; Hu, Y.; Mi, L. Determination and analysis of solubility of 2-bromo-9-fluorenone in 10 different organic solvents and three binary solvent mixtures at different temperatures (T = 278.15-323.15 K). J. Chem. Eng. Data 2022, in press. [Google Scholar] [CrossRef]
- Li, W.; Ma, Y.; Yang, Y.; Xu, S.; Shi, P.; Wu, S. Solubility measurement, correlation and mixing thermodynamics properties of dapsone in twelve mono solvents. J. Mol. Liq. 2019, 280, 175–181. [Google Scholar] [CrossRef]
- Long, B.; Yang, Z. Measurements of the solubilities of m-phthalic acid in acetone, ethanol and acetic ether. Fluid Phase Equilib. 2008, 266, 38–41. [Google Scholar] [CrossRef]
- Noubigh, A.; Abderrabba, M. Solid-liquid phase equilibrium and thermodynamic properties of vanillic acid in different pure solvents. J Mol Liq. 2016, 223, 261–266. [Google Scholar] [CrossRef]
- Shakeel, F.; Haq, N.; Siddiqui, N.A. Solubility and thermodynamic function of vanillin in ten different environmentally benign solvents. Food Chem. 2015, 180, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, J.; Zhou, Y.; Guo, N.; Liu, Q.; Zong, S.; Bao, Y.; Hao, H. Solid-liquid phase equilibrium and dissolution properties of ethyl vanillin in pure solvents. J. Chem. Thermodyn. 2017, 105, 345–351. [Google Scholar] [CrossRef]
- Guo, Y.; Hao, Y.; Zhou, Y.; Han, Z.; Xie, C.; Su, W.; Hao, H. Solubility and thermodynamic properties of vanillyl alcohol in some pure solvents. J. Chem. Thermodyn. 2017, 106, 276–284. [Google Scholar] [CrossRef]
- Wu, K.; Li, Y. Solubility and solution thermodynamics of p-toluenesulfonamide in 16 solvents from T = 273.15 to 324.75 K. J. Mol. Liq. 2019, 293, 111577. [Google Scholar] [CrossRef]
- Li, J.; Hao, H.; Guo, N.; Wang, N.; Hao, Y.; Luan, Y.; Chen, K.; Huang, X. Solubility and thermodynamic properties of maltol in different pure solvents. J. Mol. Liq. 2017, 243, 313–323. [Google Scholar] [CrossRef]
- Shi, J.; Liu, L.; Wang, L.; Ding, Z.; Wu, S. Solubility measurement and correlation of probenecid in 12 pure organic solvents and thermodynamic properties of mixing of solutions. J. Chem. Eng. Data. 2019, 64, 624–631. [Google Scholar] [CrossRef]
- Wu, K.; Han, R.; Xu, L.; Li, X.; Li, Y. Thermodynamic modelling for solubility of methyl 2-sulfamoylbenzoate in sixteen organic solvents from T (272.15-324.15 K) and dissolution properties. J. Mol. Liq. 2021, 337, 116618. [Google Scholar] [CrossRef]
- Li, Y.; Wu, K.; Liang, L. Solubility determination, modeling, and thermodynamic dissolution properties of benzenesulfonamide in 16 neat solvents from 273.15 to 324.45 K. J. Chem. Eng. Data. 2019, 64, 3606–3616. [Google Scholar] [CrossRef]
- Xu, J.; Han, S.; Cong, Y.; Du, C.; Cheng, C.; Wang, J.; Zhao, H. Thermodynamic functions of 1-methyl-4-(methylsulfonyl)benzene solubility in nine organic solvents from T = (278.15 to 318.15) K. J. Chem. Thermodyn. 2016, 103, 234–243. [Google Scholar] [CrossRef]
- Vilas-Boas, S.M.; Vieira, V.; Brandao, P.; Alves, R.S.; Coutinho, J.A.P.; Pinho, S.P.; Ferreira, O. Solvent and temperature effects on the solubility of syringic, vanillic or veratric acids: Experimental, modeling and solid phase studies. J. Mol. Liq. 2019, 289, 111089. [Google Scholar] [CrossRef]
- Buchowski, H.; Jodzewicz, W.; Milek, R.; Ufnalski, W.; Maczynski, A. Solubility and hydrogen bonding. I. Solubility of 4-nitro-5-methylphenol in one-component solvents. Roczniki Chemii. 1975, 49, 1879–1887. [Google Scholar] [CrossRef]
- Tong, Y.; Wang, Z.; Yang, E.; Pan, B.; Jiang, J.; Dang, L.; Wei, H. Determination and correlation of solubility and solution thermodynamics of ethenzamide in different pure solvents. Fluid Phase Equilib. 2016, 427, 549–556. [Google Scholar] [CrossRef]
- Wang, K.; Shang, Z.; Zhang, J.; Liu, Y.; Han, J.; Tang, W. Solubility determination and thermodynamic correlation of 2-ethoxybenzamide in 12 pure solvents from 288.15 to 328.15 K. J. Chem. Eng. Data. 2021, 66, 1508–1514. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, Y.; Cheng, X.; Yang, J.; Zhou, B.; Mi, L.; Hu, Y. Investigation on Hansen solubility parameter, solvent effect and thermodynamic properties of 3-methylflavone-8-carboxylic acid dissolved in different solvents. J. Mol. Liq. 2022, 356, 119017. [Google Scholar] [CrossRef]
- Abraham, M.H.; Acree, W.E., Jr. Gas-solvent and water-solvent partition of trans-stilbene at 298 K. J. Mol. Liq. 2017, 238, 58–61. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Dai, J.; Niu, Y.; Yin, Q.; Zhou, L. Solubility determination, model evaluation and solution thermodynamics of isovanillin in 15 pure solvents and 4 binary solvents. J. Mol. Liq. 2021, 340, 116847. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, C.; Zhao, C.; Qi, L.; Bao, Y.; Zhou, L.; Yin, Q. Analysis of solid-liquid equilibrium behavior of highly water-soluble beet herbicide metamitron in thirteen pure solvents using experiments and molecular simulations. J. Mol. Liq. 2022; in press. [Google Scholar] [CrossRef]
- Nti-Gyabaah, J.; Chan, V.; Chiew, Y.C. Solubility and limiting activity coefficient of simvastatin in different organic solvents. Fluid Phase Equilib. 2009, 280, 35–41. [Google Scholar] [CrossRef]
- Fritz, J.S.; Lisicki, N.M. Titration of acids in nonaqueous solvents. Anal Chem. 1951, 23, 589–591. [Google Scholar] [CrossRef]
- Knofczynski, G.T.; Daniel Mundfrom, D. Sample sizes when using multiple linear regression for prediction. Educ. Psychol. Meas. 2008, 68, 431–442. [Google Scholar] [CrossRef]
- Austin, P.C.; Steyerberg, E.W. The number of subjects per variable required in linear regression analyses. J. Clin. Epidemiol. 2015, 68, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Charlton, A.K.; Daniels, C.R.; Acree, W.E., Jr.; Abraham, M.H. solubility of crystalline nonelectrolyte solutes in organic solvents: Mathematical correlation of acetylsalicylic acid solubilities with the Abraham general solvation model. J. Solution Chem. 2003, 32, 1087–1102. [Google Scholar] [CrossRef]
- Abraham, M.H.; Zissimos, A.M.; Acree, W.E., Jr. Partition of solutes into wet and dry ethers; an LFER analysis. New J. Chem. 2003, 27, 1041–1044. [Google Scholar] [CrossRef]
- Blake-Taylor, B.H.; Deleon, V.H.; Acree, W.E., Jr.; Abraham, M.H. Mathematical correlation of salicylamide solubilities in organic solvents with the Abraham solvation parameter model. Phys. Chem. Liq. 2007, 45, 389–398. [Google Scholar] [CrossRef]
- Flanagan, K.B.; Hoover, K.R.; Garza, O.; Hizon, A.; Soto, T.; Villegas, N.; Acree, W.E., Jr.; Abraham, M.H. Mathematical correlation of 1-chloroanthraquinone solubilities in organic solvents with the Abraham solvation parameter model. Phys. Chem. Liq. 2006, 44, 377–386. [Google Scholar] [CrossRef]
- Stovall, D.M.; Givens, C.; Keown, S.; Hoover, K.R.; Barnes, R.; Harris, C.; Lozano, J.; Nguyen, M.; Rodriguez, E.; Acree, W.E., Jr.; et al. Solubility of crystalline nonelectrolyte solutes in organic solvents: Mathematical correlation of 4-chloro-3-nitrobenzoic acid and 2-chloro-5-nitrobenzoic acid solubilities with the Abraham solvation parameter model. Phys. Chem. Liq. 2005, 43, 351–360. [Google Scholar] [CrossRef]
- Stovall, D.M.; Acree, W.E., Jr.; Abraham, M.H. Solubility of 9-fluorenone, thianthrene and xanthene in organic solvents. Fluid Phase Equilib. 2005, 232, 113–121. [Google Scholar] [CrossRef]
- Hoover, K.R.; Pop, K.; Acree, W.E., Jr.; Abraham, M.H. Solubility of crystalline nonelectrolyte solutes in organic solvents: Mathematical correlation of 3-chlorobenzoic acid solubilities with the Abraham solvation parameter model. S. Afr. J. Chem. 2005, 58, 25–29. [Google Scholar]
- Hoover, K.R.; Stovall, D.M.; Pustejovsky, E.; Coaxum, R.; Pop, K.; Acree, W.E., Jr.; Abraham, M.H. Solubility of crystalline nonelectrolyte solutes in organic solvents—Mathematical correlation of 2-methoxybenzoic acid and 4-methoxybenzoic acid solubilities with the Abraham solvation parameter model. Can. J. Chem. 2004, 82, 1353–1360. [Google Scholar] [CrossRef]
- Charlton, A.K.; Daniels, C.R.; Wold, R.M.; Pustejovsky, E.; Acree, W.E., Jr.; Abraham, M.H. Solubility of crystalline nonelectrolyte solutes in organic solvents: Mathematical correlation of 3-nitrobenzoic acid solubilities with the Abraham general solvation model. J. Mol. Liq. 2004, 116, 19–28. [Google Scholar] [CrossRef]
- Hoover, K.R.; Coaxum, R.; Pustejovsky, E.; Acree, W.E., Jr.; Abraham, M.H. Thermochemical behavior of dissolved carboxylic acid solutes: Part 5-mathematical correlation of 3,5-dinitrobenzoic acid solubilities with the Abraham solvation parameter model. Phys. Chem. Liq. 2004, 42, 457–466. [Google Scholar] [CrossRef]
- Hoover, K.R.; Coaxum, R.; Pustejovsky, E.; Stovall, D.M.; Acree, W.E., Jr.; Abraham, M.H. Thermochemical behavior of dissolved carboxylic acid solutes: Part 4—Mathematical correlation of 4-nitrobenzoic acid solubilities with the Abraham solvation parameter model. Phys. Chem. Liq. 2004, 42, 339–347. [Google Scholar] [CrossRef]
- Coaxum, R.; Hoover, K.R.; Pustejovsky, E.; Stovall, D.M.; Acree, W.E., Jr.; Abraham, M.H. Thermochemical behavior of dissolved carboxylic acid solutes: Part 3—MATHEMATICAL correlation of 2-methylbenzoic acid solubilities with the Abraham solvation parameter model. Phys. Chem. Liq. 2004, 42, 313–322. [Google Scholar] [CrossRef]
- Daniels, C.R.; Charlton, A.K.; Wold, R.M.; Acree, W.E., Jr.; Abraham, M.H. Thermochemical behavior of dissolved carboxylic acid solutes: Solubilities of 3-methylbenzoic acid and 4-chlorobenzoic acid in organic solvents. Can. J. Chem. 2003, 81, 1492–1501. [Google Scholar] [CrossRef]
- Acree, W.E., Jr.; Abraham, M.H. Solubility of crystalline nonelectrolyte solutes in organic solvents: Mathematical correlation of benzil solubilities with the Abraham general solvation model. J. Solution Chem. 2002, 31, 293–303. [Google Scholar] [CrossRef]
- Acree, W.E., Jr.; Abraham, M.H. Solubility predictions for crystalline nonelectrolyte solutes dissolved in organic solvents based upon the Abraham general solvation model. Can. J. Chem. 2001, 79, 1466–1476. [Google Scholar] [CrossRef]
- Acree, W.E., Jr.; Abraham, M.H. Solubility predictions for crystalline polycyclic aromatic hydrocarbons (PAHs) dissolved in organic solvents based upon the Abraham general solvation model. Fluid Phase Equilib. 2002, 201, 245–258. [Google Scholar] [CrossRef]
- Chung, Y.; Vermeire, F.H.; Wu, H.; Walker, P.J.; Abraham, M.H.; Green, W.H. Group contribution and machine learning approaches to predict Abraham solute parameters, solvation free energy, and solvation enthalpy. J. Chem. Inf. Model. 2022, 62, 433–446. [Google Scholar] [CrossRef]
- Brown, T.N. Empirical regressions between system parameters and solute descriptors of polyparameter linear free energy relationships (PPLFERs) for predicting solvent-air partitioning. Fluid Phase Equilib. 2021, 540, 113035. [Google Scholar] [CrossRef]
- Hille, C.; Ringe, S.; Deimel, M.; Kunkel, C.; Acree, W.E.; Reuter, K.; Oberhofer, H. Generalized molecular solvation in non-aqueous solutions by a single parameter implicit solvation scheme. J. Chem. Phys. 2019, 150, 041710. [Google Scholar] [CrossRef]
- Kashefolgheta, S.; Oliveira, M.P.; Rieder, S.R.; Horta, B.A.C.; Acree, W.E., Jr.; Hunenberger, P.H. Evaluating classical force fields against experimental cross-solvation free energies. J. Chem. Theory Comput. 2020, 16, 7556–7580. [Google Scholar] [CrossRef]
- Kashefolgheta, S.; Wang, S.; Acree, W.E.; Hunenberger, P.H. Evaluation of nine condensed-phase force fields of the GROMOS, CHARMM, OPLS, AMBER, and OpenFF families against experimental cross-solvation free energies. Phys. Chem. Chem. Phys. 2021, 23, 13055–13074. [Google Scholar] [CrossRef]
- Lee, S.; Cho, K.-H.; Acree, W.E., Jr.; No, K.T. Development of surface-SFED models for polar solvents. J. Chem. Inf. Model. 2012, 52, 440–448. [Google Scholar] [CrossRef]
- Ma, S.; Hwang, S.; Lee, S.; Acree, W.E.; No, K.T. Incorporation of hydrogen bond angle dependency into the generalized solvation free energy density model. J. Chem. Inf. Model. 2018, 58, 761–772. [Google Scholar] [CrossRef]
- Avdeef, A.; Kansy, M. Predicting solubility of newly-approved drugs (2016–2020) with a simple ABSOLV and GSE(flexible-acceptor) consensus model outperforming Random Forest regression. J. Solut. Chem. 2022, 51, 1020–1055. [Google Scholar] [CrossRef]
- Ulrich, N.; Endo, S.; Brown, T.N.; Watanabe, N.; Bronner, G.; Abraham, M.H.; Goss, K.-U. UFZ-LSER Database v 3.2.1 [Internet], Leipzig, Germany, Helmholtz Centre for Environmental Research-UFZ. 2017. Available online: http://www.ufz.de/lserd (accessed on 1 September 2022).
- Platts, J.A.; Butina, D.; Abraham, M.H.; Hersey, A. Estimation of molecular linear free energy relation descriptors using a group contribution approach. J. Chem. Inf. Comput. Sci. 1999, 39, 835–845. [Google Scholar] [CrossRef]
- Platts, J.A.; Abraham, M.H.; Butina, D.; Hersey, A. Estimation of molecular linear free energy relationship descriptors by a group contribution approach. 2. Prediction of partition coefficients. J. Chem. Inf. Comput. Sci. 2000, 40, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, N.; Ebert, A. Can deep learning algorithms enhance the prediction of solute descriptors for linear solvation energy relationship approaches? Fluid Phase Equilib. 2022, 555, 113349. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).