Acid Adaptation Leads to Sensitization of Salmonella Challenge Cultures During Processing of Air-Dried Beef (Biltong, Droëwors)
Abstract
1. Introduction
- Test every lot of edible ingredients for Salmonella prior to use (must test negative) and use a process that is validated to provide ≥ 2-log reduction of a pathogen of concern (i.e., Salmonella), or
- Use a process that is validated to give ≥ 5 log reduction of a pathogen of concern (Salmonella).
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Evaluation of Salmonella-Selective Agar Media for Enumeration of Salmonella from Droëwors Processing
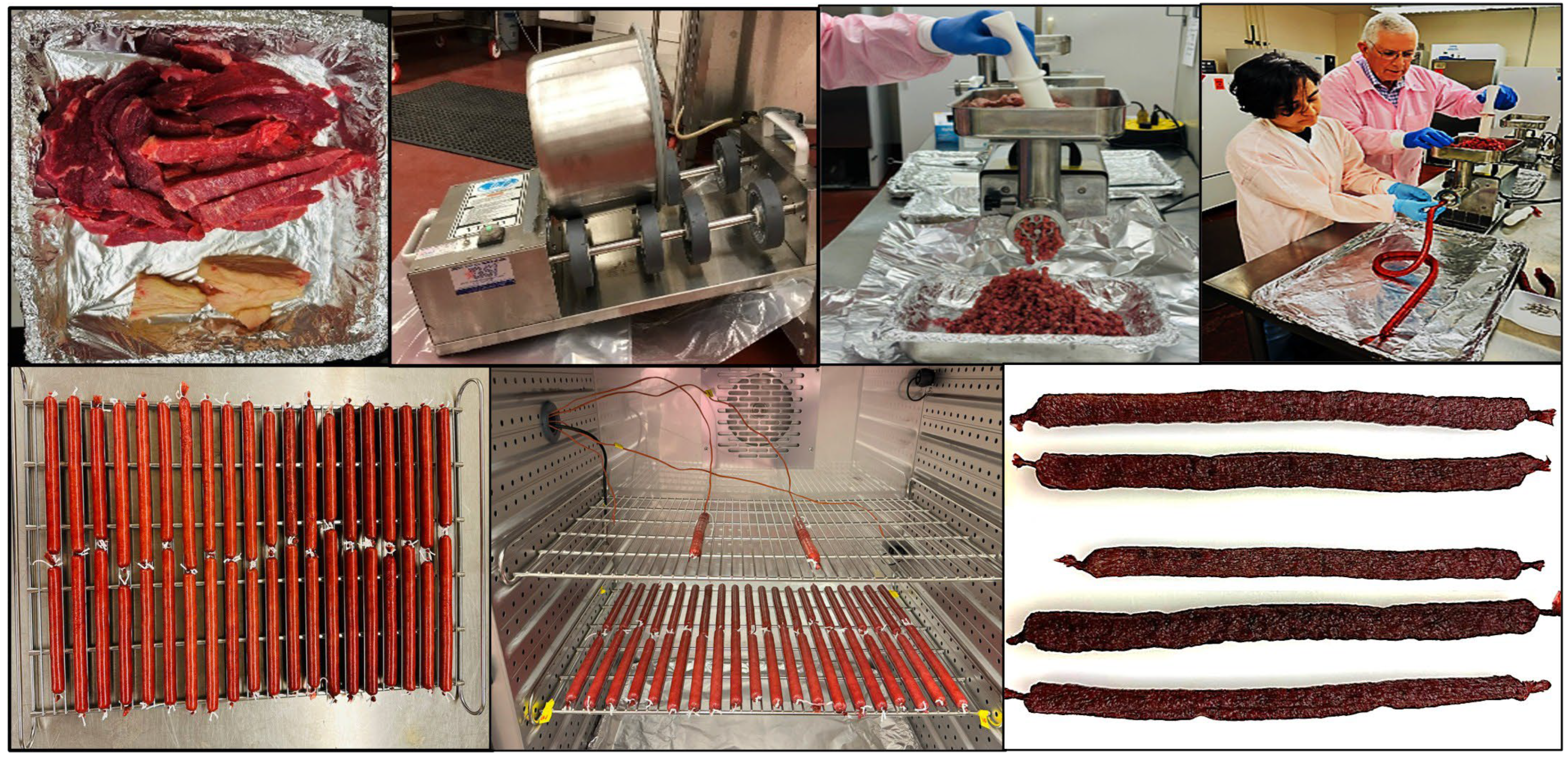
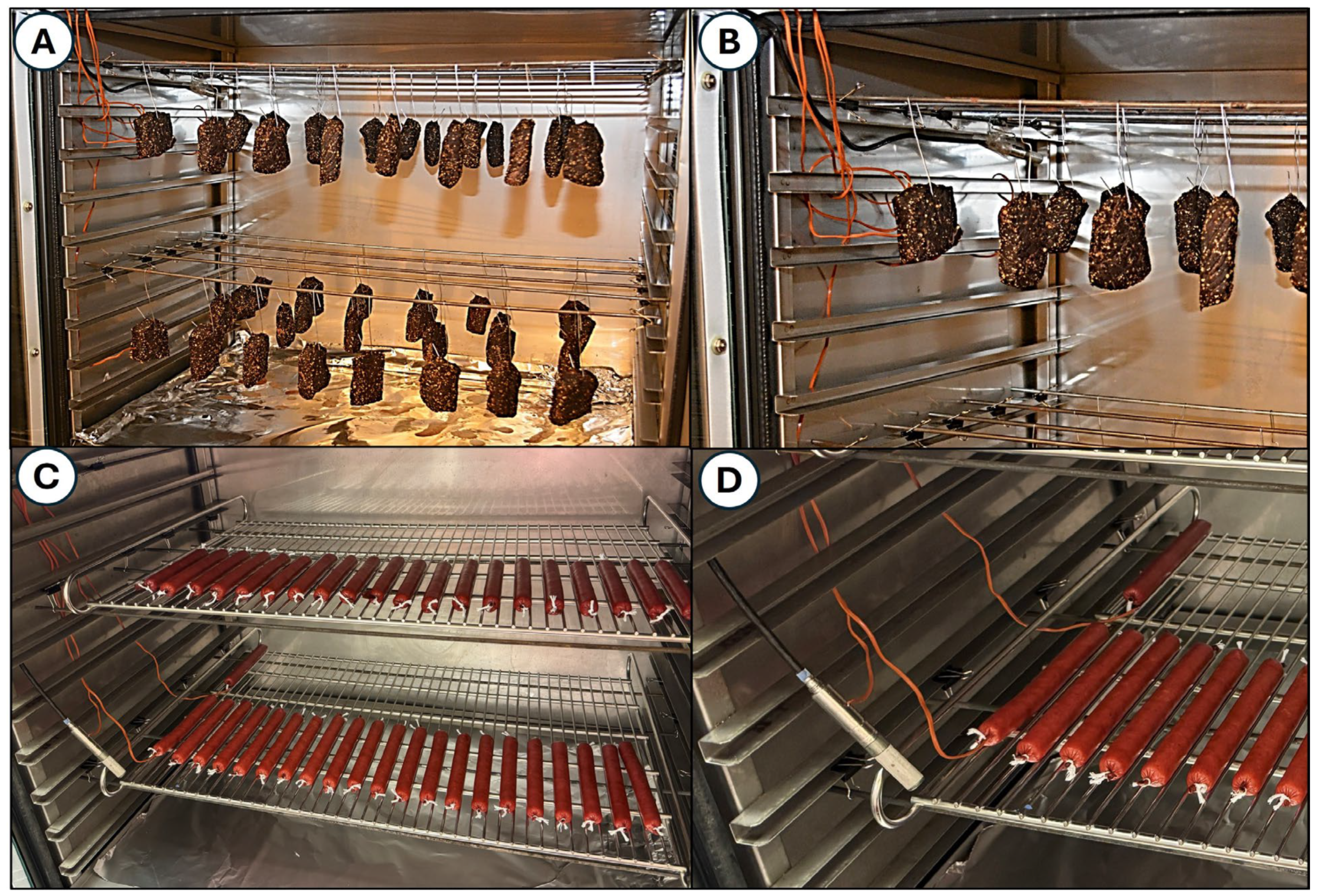
2.3. Air-Dried Biltong Beef Process
2.4. Air-Dried Droëwors Beef Process
2.5. Processing Parameters for Biltong and Droëwors (Temperature, Relative Humidity, and pH)
2.6. Statistical Analysis
3. Results and Discussion
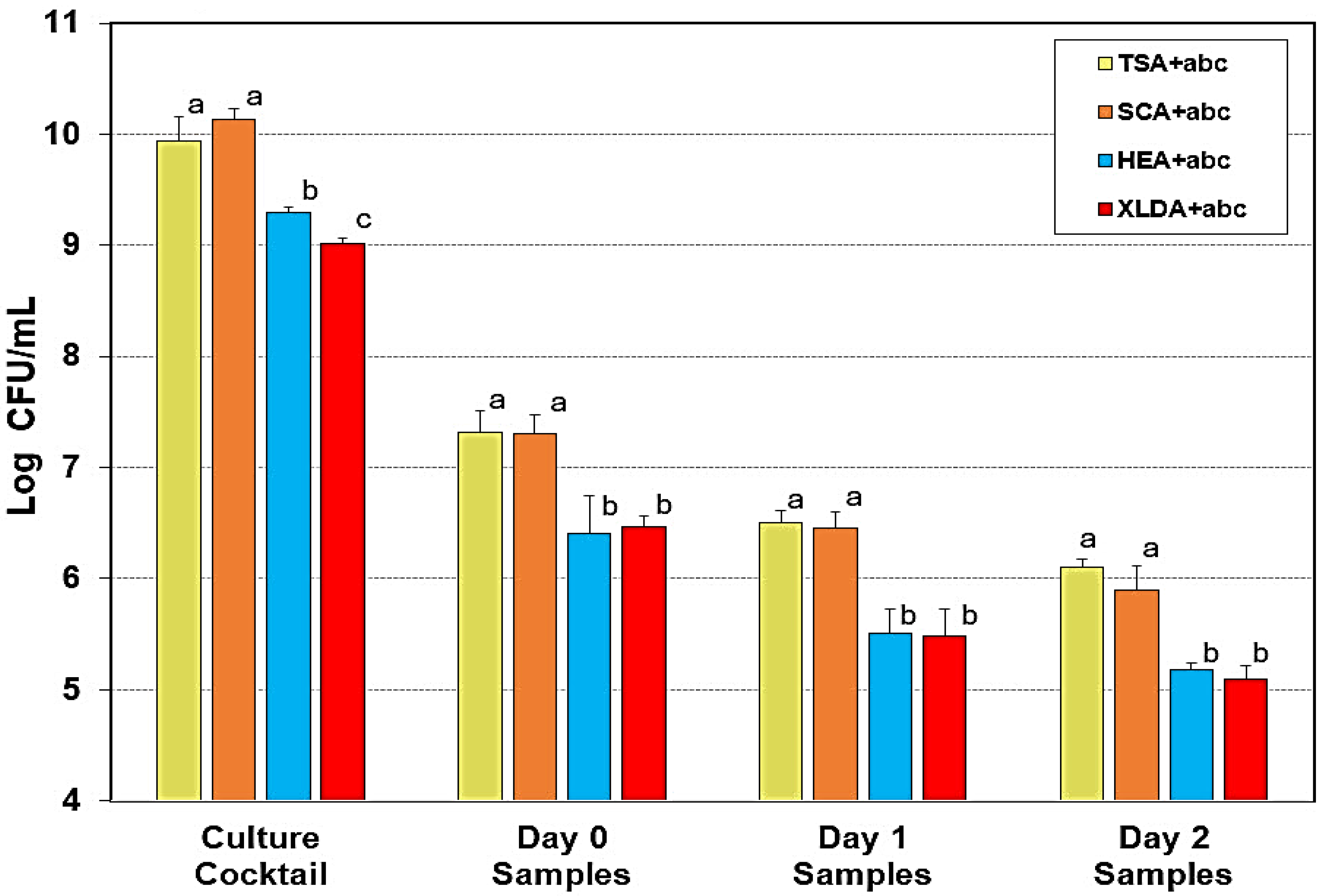
3.1. Comparison of Selective Media for Quantitative Enumeration of Salmonella Challenge Cultures After Droëwors Processing
3.2. Biltong Processing: Comparison of Acid Adapted and Non Adapted Salmonella Challenge Cultures
3.2.1. Salmonella Enterica Typhimurium (Serovar 1,4,[5],12:i:-)
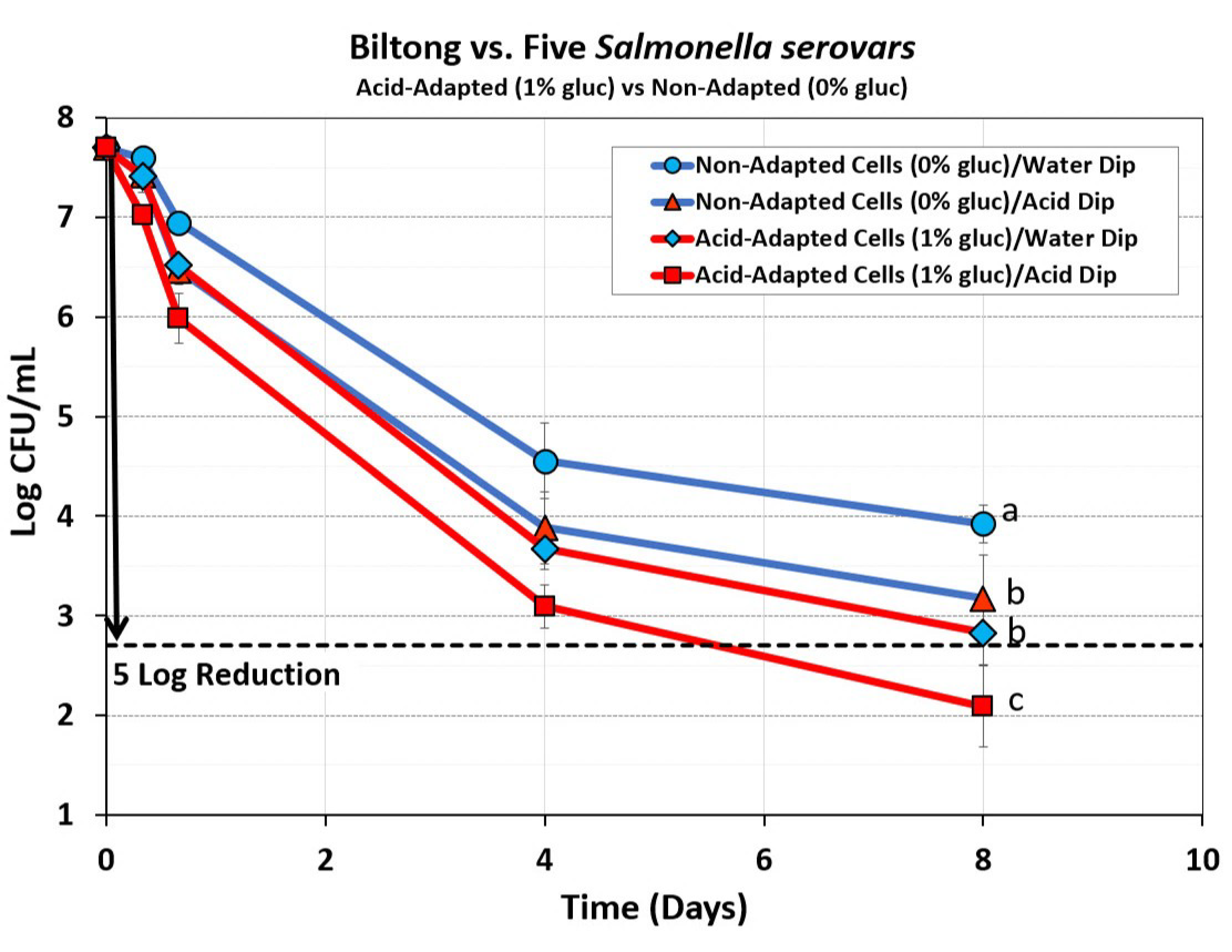
3.2.2. Mixture of Five Salmonella Serovars: Acid Adapted (1% Glucose) vs. Non Adapted (0% Glucose)
3.2.3. Mixture of Five Salmonella Serovars: Acid Adapted (1% Glucose) vs. Non Adapted (1% Glucose + Buffer)
3.3. Droëwors Processing: Comparison of Acid Adapted and Non Adapted Salmonella Challenge Cultures
Mixture of Five Salmonella Serovars: Comparison of Acid Adapted (1% Glucose) vs. Non-Adapted (0% Glucose) During Droëwors Processing
3.4. Temperature, Relative Humidiay, and pH of Biltong and Droewors During Processing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA-FSIS. FSIS Compliance Guideline for Meat and Poultry Jerky Produced by Small and Very Small Establishments; U.S. Food Safety and Inspection Service: Washington, DC, USA, 2014; pp. 1–54.
- Karolenko, C.E.; Bhusal, A.; Nelson, J.L.; Muriana, P.M. Processing of biltong (dried beef) to achieve USDA-FSIS 5-log reduction of Salmonella without a heat lethality step. Microorganisms 2020, 8, 791. [Google Scholar] [CrossRef] [PubMed]
- USDA-FSIS. FSIS Ready-to-Eat Fermented, Salt-Cured, and Dried Products Guideline. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/documents/FSIS-GD-2023-0002.pdf (accessed on 18 April 2025).
- Karolenko, C.E.; Bhusal, A.; Gautam, D.; Muriana, P.M. Selenite cystine agar for enumeration of inoculated Salmonella serovars recovered from stressful conditions during antimicrobial validation studies. Microorganisms 2020, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.W.; Hall, H.K. Inducible pH homeostasis and the acid tolerance response of Salmonella typhimurium. J. Bacteriol. 1991, 173, 5129–5135. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.W.; Hall, H.K. Adaptive acidification tolerance response of Salmonella typhimurium. J. Bacteriol. 1990, 172, 771–778. [Google Scholar] [CrossRef]
- Gorden, J.; Small, P.L. Acid resistance in enteric bacteria. Infect. Immun. 1993, 61, 364–367. [Google Scholar] [CrossRef]
- Merrell, D.S.; Camilli, A. Acid tolerance of gastrointestinal pathogens. Curr. Opin. Microbiol. 2002, 5, 51–55. [Google Scholar] [CrossRef]
- Berry, E.D.; Cutter, C.N. Effects of acid adaptation of Escherichia coli O157:H7 on efficacy of acetic acid spray washes to decontaminate beef carcass tissue. Appl. Environ. Microbiol. 2000, 66, 1493–1498. [Google Scholar] [CrossRef]
- Davidson, P.M.; Harrison, M.A. Resistance and adaptation to food antimicrobials, sanitizers, and other process controls. Food Technol.-Champaign Chic. 2002, 56, 69–78. [Google Scholar]
- Slabbert, R.S. Acid tolerance and organic acid susceptibility of selected food-borne pathogens. Interim Interdiscip. J. 2013, 12, 42–50. [Google Scholar]
- Samelis, J.; Ikeda, J.S.; Sofos, J.N. Evaluation of the pH-dependent, stationary-phase acid tolerance in Listeria monocytogenes and Salmonella Typhimurium DT104 induced by culturing in media with 1% glucose: A comparative study with Escherichia coli O157:H7. J. Appl. Microbiol. 2003, 95, 563–575. [Google Scholar] [CrossRef]
- Fratamico, P.M. Tolerance to Stress and Ability of Acid-Adapted and Non–Acid-Adapted Salmonella enterica Serovar Typhimurium DT104 To Invade and Survive in Mammalian Cells In Vitro. J. Food Prot. 2003, 66, 1115–1125. [Google Scholar] [CrossRef]
- Calicioglu, M.; Sofos, J.N.; Samelis, J.; Kendall, P.A.; Smith, G.C. Effect of acid adaptation on inactivation of Salmonella during drying and storage of beef jerky treated with marinades. Int. J. Food Microbiol. 2003, 89, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Wilde, S.; Jørgensen, F.; Campbell, A.; Rowbury, R.; Humphrey, T. Growth of Salmonella enterica Serovar Enteritidis PT4 in media containing glucose results in enhanced RpoS-independent heat and acid tolerance but does not affect the ability to survive air-drying on surfaces. Food Microbiol. 2000, 17, 679–686. [Google Scholar] [CrossRef]
- Ryu, J.-H.; Deng, Y.; Beuchat, L.R. Behavior of Acid-Adapted and Unadapted Escherichia coli O157:H7 When Exposed to Reduced pH Achieved with Various Organic Acids. J. Food Prot. 1999, 62, 451–455. [Google Scholar] [CrossRef]
- National Advisory Committee on the Microbiological Criteria for Foods. Parameters for determining inoculated pack/challenge study protocols. J. Food Prot. 2010, 73, 140–203. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.; Lucia, L.M.; Kemp, G.K.; Acuff, G.R. Reduction of Escherichia coli O157:H7 and Salmonella Typhimurium on Beef Carcass Surfaces Using Acidified Sodium Chlorite. J. Food Prot. 1999, 62, 580–584. [Google Scholar] [CrossRef]
- Cutter, C.N.; Siragusa, G.R. Efficacy of Organic Acids Against Escherichia coli O157:H7 Attached to Beef Carcass Tissue Using a Pilot Scale Model Carcass Washer1. J. Food Prot. 1994, 57, 97–103. [Google Scholar] [CrossRef]
- Dickson, J.S.; Anderson, M.E. Microbiological Decontamination of Food Animal Carcasses by Washing and Sanitizing Systems: A Review. J. Food Prot. 1992, 55, 133–140. [Google Scholar] [CrossRef]
- Castillo, A.; Lucia, L.M.; Goodson, K.J.; Savell, J.W.; Acuff, G.R. Comparison of Water Wash, Trimming, and Combined Hot Water and Lactic Acid Treatments for Reducing Bacteria of Fecal Origin on Beef Carcasses. J. Food Prot. 1998, 61, 823–828. [Google Scholar] [CrossRef]
- Álvarez-Ordóñez, A.; Fernández, A.; Bernardo, A.; López, M. Acid adaptation sensitizes Salmonella enterica serovar Typhimurium to osmotic and oxidative stresses. Arch. Lebensmittelhyg 2010, 61, 148–152. [Google Scholar] [CrossRef]
- Leyer, G.J.; Johnson, E.A. Acid adaptation sensitizes Salmonella Typhimurium to hypochlorous acid. Appl. Environ. Microbiol. 1997, 63, 461–467. [Google Scholar] [CrossRef]
- Lianou, A.; Nychas, G.E.; Koutsoumanis, K.P. Variability in the adaptive acid tolerance response phenotype of Salmonella enterica strains. Food Microbiol. 2017, 62, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Wesche, A.M.; Gurtler, J.B.; Marks, B.P.; Ryser, E.T. Stress, sublethal injury, resuscitation, and virulence of bacterial foodborne pathogens. J. Food Prot. 2009, 72, 1121–1138. [Google Scholar] [CrossRef] [PubMed]
- Gruzdev, N.; Pinto, R.; Sela, S. Effect of desiccation on tolerance of Salmonella enterica to multiple stresses. Appl. Environ. Microbiol. 2011, 77, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Gavriil, A.; Thanasoulia, A.; Skandamis, P.N. Sublethal concentrations of undissociated acetic acid may not always stimulate acid resistance in Salmonella enterica sub. enterica serovar Enteritidis Phage Type 4: Implications of challenge substrate associated factors. PLoS ONE 2020, 15, e0234999. [Google Scholar] [CrossRef]
- Greenacre, E.J.; Lucchini, S.; Hinton, J.C.; Brocklehurst, T.F. The lactic acid-induced acid tolerance response in Salmonella enterica serovar Typhimurium induces sensitivity to hydrogen peroxide. Appl. Environ. Microbiol. 2006, 72, 5623–5625. [Google Scholar] [CrossRef]
- Juneja, V.K.; Hwang, C.A.; Friedman, M. Thermal inactivation and postthermal treatment growth during storage of multiple Salmonella serotypes in ground beef as affected by sodium lactate and oregano oil. J. Food Sci. 2010, 75, M1–M6. [Google Scholar] [CrossRef]
- Carpenter, C.E.; Smith, J.V.; Broadbent, J.R. Efficacy of washing meat surfaces with 2% levulinic, acetic, or lactic acid for pathogen decontamination and residual growth inhibition. Meat Sci. 2011, 88, 256–260. [Google Scholar] [CrossRef]
- Juneja, V.K.; Eblen, B.S.; Marks, H.M. Modeling non-linear survival curves to calculate thermal inactivation of Salmonella in poultry of different fat levels. Int. J. Food Microbiol. 2001, 70, 37–51. [Google Scholar] [CrossRef]
- Juneja, V.K.; Yadav, A.S.; Hwang, C.A.; Sheen, S.; Mukhopadhyay, S.; Friedman, M. Kinetics of thermal destruction of Salmonella in ground chicken containing trans-cinnamaldehyde and carvacrol. J. Food Prot. 2012, 75, 289–296. [Google Scholar] [CrossRef]
- Gavai, K.; Karolenko, C.; Muriana, P.M. Effect of biltong dried beef processing on the reduction of Listeria monocytogenes, E. coli O157:H7, and Staphylococcus aureus, and the contribution of the major marinade components. Microorganisms 2022, 10, 1308. [Google Scholar] [CrossRef]
- Karolenko, C.; Muriana, P. Quantification of process lethality (5-log reduction) of Salmonella and salt concentration during sodium replacement in biltong marinade. Foods 2020, 9, 1570. [Google Scholar] [CrossRef] [PubMed]
- Karolenko, C.E.; Wilkinson, J.; Muriana, P.M. Evaluation of various lactic acid bacteria and generic E. coli as potential nonpathogenic surrogates for in-plant validation of biltong dried beef processing. Microorganisms 2022, 10, 1648. [Google Scholar] [CrossRef]
- Soyer, Y.; Moreno Switt, A.; Davis, M.A.; Maurer, J.; McDonough, P.L.; Schoonmaker-Bopp, D.J.; Dumas, N.B.; Root, T.; Warnick, L.D.; Gröhn, Y.T.; et al. Salmonella enterica serotype 4,5,12:i:-, an emerging Salmonella serotype that represents multiple distinct clones. J. Clin. Microbiol. 2009, 47, 3546–3556. [Google Scholar] [CrossRef]
- Leyer, G.J.; Johnson, E.A. Acid adaptation induces cross-protection against environmental stresses in Salmonella typhimurium. Appl. Environ. Microbiol. 1993, 59, 1842–1847. [Google Scholar] [CrossRef]
- Leyer, G.J.; Johnson, E.A. Acid adaptation promotes survival of Salmonella spp. in cheese. Appl. Environ. Microbiol. 1992, 58, 2075–2080. [Google Scholar] [CrossRef]
- Calicioglu, M.; Sofos, J.N.; Samelis, J.; Kendall, P.A.; Smith, G.C. Inactivation of acid-adapted and nonadapted Escherichia coli O157:H7 during drying and storage of beef jerky treated with different marinades. J. Food Prot. 2002, 65, 1394–1405. [Google Scholar] [CrossRef]
- Adhikari, P.; Muriana, P. Air-Dried Beef: Comparison of Acid-Adapted and Non-Adapted Salmonella Serovars in Process Validation to Achieve 5-log Reduction. In Proceedings of the International Association of Food Protection, Cleveland, OH, USA, 27–30 July 2025. [Google Scholar]




| Processing Step | Biltong | Droëwors | ||
|---|---|---|---|---|
| Position During Processing | pH | S.D. | pH | S.D. |
| Raw Beef: Day 0 | 5.60 | 0.03 | 5.60 | 0.03 |
| Post-Marination: Day 0 | 5.04 | 0.03 | 4.74 * | 0.14 |
| Drying: Day 2 | 5.19 | 0.07 | 4.97 | 0.07 |
| Drying: Day 4 | 5.20 | 0.06 | 5.05 | 0.04 |
| Drying: Day 6 | 5.26 | 0.03 | 5.06 | 0.15 |
| Drying: Day 8 | 5.20 | 0.03 | 5.08 | 0.04 |
| Drying: Day10 | 5.22 | 0.03 | 5.08 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adhikari, P.; Karolenko, C.E.; Wilkinson, J.; Muriana, P.M. Acid Adaptation Leads to Sensitization of Salmonella Challenge Cultures During Processing of Air-Dried Beef (Biltong, Droëwors). Appl. Microbiol. 2025, 5, 106. https://doi.org/10.3390/applmicrobiol5040106
Adhikari P, Karolenko CE, Wilkinson J, Muriana PM. Acid Adaptation Leads to Sensitization of Salmonella Challenge Cultures During Processing of Air-Dried Beef (Biltong, Droëwors). Applied Microbiology. 2025; 5(4):106. https://doi.org/10.3390/applmicrobiol5040106
Chicago/Turabian StyleAdhikari, Pratikchhya, Cailtin E. Karolenko, Jade Wilkinson, and Peter M. Muriana. 2025. "Acid Adaptation Leads to Sensitization of Salmonella Challenge Cultures During Processing of Air-Dried Beef (Biltong, Droëwors)" Applied Microbiology 5, no. 4: 106. https://doi.org/10.3390/applmicrobiol5040106
APA StyleAdhikari, P., Karolenko, C. E., Wilkinson, J., & Muriana, P. M. (2025). Acid Adaptation Leads to Sensitization of Salmonella Challenge Cultures During Processing of Air-Dried Beef (Biltong, Droëwors). Applied Microbiology, 5(4), 106. https://doi.org/10.3390/applmicrobiol5040106








