The Microbiome as a Driver of Insect Physiology, Behavior, and Control Strategies
Abstract
1. Introduction
2. The Burden of Insect Pests and Vectors
3. Composition, Acquisition, and Roles of the Insect Microbiome
4. Microbial Modulation of Chemosensory Physiology
5. Microbiome Effects on Fitness and Vector Competence
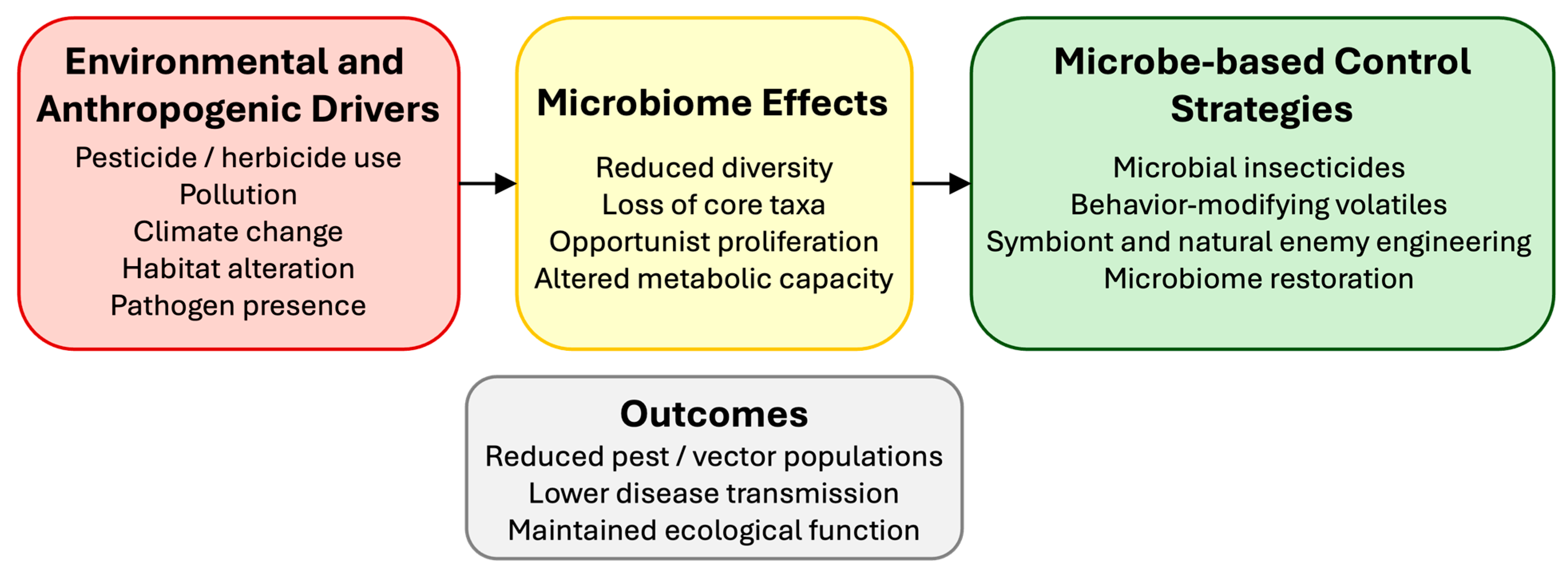
6. Environmental and Anthropogenic Disruption
7. Microbe-Based Insect Control
8. Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| APSE | Acyrthosiphon pisum secondary endosymbiont |
| EPN | Entomopathogenic nematode |
| GABA | Gamma-aminobutyric acid |
| GR | Gustatory receptor |
| IL | Interleukin |
| IPM | Integrated pest management |
| IR | Ionotropic receptor |
| OBP | Odorant binding protein |
| OR | Odorant receptor |
| VOC | Volatile organic compound |
References
- Douglas, A.E. Multiorganismal Insects: Diversity and Function of Resident Microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Engel, P.; Moran, N.A. The Gut Microbiota of Insects-Diversity in Structure and Function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef]
- Holt, J.R.; Cavichiolli de Oliveira, N.; Medina, R.F.; Malacrinò, A.; Lindsey, A.R.I. Insect–Microbe Interactions and Their Influence on Organisms and Ecosystems. Ecol. Evol. 2024, 14, e11699. [Google Scholar] [CrossRef]
- McFall-Ngai, M.; Hadfield, M.G.; Bosch, T.C.G.; Carey, H.V.; Domazet-Lošo, T.; Douglas, A.E.; Dubilier, N.; Eberl, G.; Fukami, T.; Gilbert, S.F.; et al. Animals in a Bacterial World, a New Imperative for the Life Sciences. Proc. Natl. Acad. Sci. USA 2013, 110, 3229–3236. [Google Scholar] [CrossRef]
- Hammer, T.J.; Bowers, M.D. Gut Microbes May Facilitate Insect Herbivory of Chemically Defended Plants. Oecologia 2015, 179, 1–14. [Google Scholar] [CrossRef]
- Motta, E.V.S.; Moran, N.A. The Honeybee Microbiota and Its Impact on Health and Disease. Nat. Rev. Microbiol. 2024, 22, 122–137. [Google Scholar] [CrossRef]
- Qadri, M.; Short, S.; Gast, K.; Hernandez, J.; Wong, A.C.-N. Microbiome Innovation in Agriculture: Development of Microbial Based Tools for Insect Pest Management. Front. Sustain. Food Syst. 2020, 4, 547751. [Google Scholar] [CrossRef]
- Lv, C.; Huang, Y.-Z.; Luan, J.-B. Insect-microbe Symbiosis-Based Strategies Offer a New Avenue for the Management of Insect Pests and Their Transmitted Pathogens. Crop Health 2024, 2, 18. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Xu, L. The Pivotal Roles of Gut Microbiota in Insect Plant Interactions for Sustainable Pest Management. NPJ Biofilms Microbiomes 2023, 9, 66. [Google Scholar] [CrossRef]
- Wei, Y.; Li, H. Editorial: Exploring the Insect Microbiome: The Potential Future Role in Biotechnology Industry. Front. Microbiol. 2022, 13, 1061360. [Google Scholar] [CrossRef]
- Beck, J.J.; Vannette, R.L. Harnessing Insect–Microbe Chemical Communications To Control Insect Pests of Agricultural Systems. J. Agric. Food Chem. 2017, 65, 23–28. [Google Scholar] [CrossRef]
- Davis, T.S.; Crippen, T.L.; Hofstetter, R.W.; Tomberlin, J.K. Microbial Volatile Emissions as Insect Semiochemicals. J. Chem. Ecol. 2013, 39, 840–859. [Google Scholar] [CrossRef]
- Wu, R.; Patocka, J.; Nepovimova, E.; Oleksak, P.; Valis, M.; Wu, W.; Kuca, K. Marine Invertebrate Peptides: Antimicrobial Peptides. Front. Microbiol. 2021, 12, 785085. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, E. Antimicrobial Peptides and Plant Disease Control. FEMS Microbiol. Lett. 2007, 270, 1–11. [Google Scholar] [CrossRef]
- de Souza, W.M.; Weaver, S.C. Effects of Climate Change and Human Activities on Vector-Borne Diseases. Nat. Rev. Microbiol. 2024, 22, 476–491. [Google Scholar] [CrossRef] [PubMed]
- Motta, E.V.S.; Raymann, K.; Moran, N.A. Glyphosate Perturbs the Gut Microbiota of Honey Bees. Proc. Natl. Acad. Sci. USA 2018, 115, 10305–10310. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, C.J.A.; Leroy, B.; Bellard, C.; Roiz, D.; Albert, C.; Fournier, A.; Barbet-Massin, M.; Salles, J.-M.; Simard, F.; Courchamp, F. Massive yet Grossly Underestimated Global Costs of Invasive Insects. Nat. Commun. 2016, 7, 12986. [Google Scholar] [CrossRef]
- Early, R.; Bradley, B.A.; Dukes, J.S.; Lawler, J.J.; Olden, J.D.; Blumenthal, D.M.; Gonzalez, P.; Grosholz, E.D.; Ibañez, I.; Miller, L.P.; et al. Global Threats from Invasive Alien Species in the Twenty-First Century and National Response Capacities. Nat. Commun. 2016, 7, 12485. [Google Scholar] [CrossRef]
- Vector-Borne Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 26 January 2024).
- Belluco, S.; Bertola, M.; Montarsi, F.; Di Martino, G.; Granato, A.; Stella, R.; Martinello, M.; Bordin, F.; Mutinelli, F. Insects and Public Health: An Overview. Insects 2023, 14, 240. [Google Scholar] [CrossRef]
- Gomulski, L.M.; Manni, M.; Carraretto, D.; Nolan, T.; Lawson, D.; Ribeiro, J.M.; Malacrida, A.R.; Gasperi, G. Transcriptional Variation of Sensory-Related Genes in Natural Populations of Aedes Albopictus. BMC Genom. 2020, 21, 547. [Google Scholar] [CrossRef]
- Ryan, S.J.; Carlson, C.J.; Mordecai, E.A.; Johnson, L.R. Global Expansion and Redistribution of Aedes-Borne Virus Transmission Risk with Climate Change. PLoS Negl. Trop. Dis. 2019, 13, e0007213. [Google Scholar] [CrossRef]
- Carlson, C.J.; Albery, G.F.; Merow, C.; Trisos, C.H.; Zipfel, C.M.; Eskew, E.A.; Olival, K.J.; Ross, N.; Bansal, S. Climate Change Increases Cross-Species Viral Transmission Risk. Nature 2022, 607, 555–562. [Google Scholar] [CrossRef]
- Glaser, J.A.; Matten, S.R. Sustainability of Insect Resistance Management Strategies for Transgenic Bt Corn. Biotechnol. Adv. 2003, 22, 45–69. [Google Scholar] [CrossRef]
- Sparks, T.C.; Nauen, R. IRAC: Mode of Action Classification and Insecticide Resistance Management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef]
- Riveron, J.M.; Tchouakui, M.; Mugenzi, L.; Menze, B.D.; Chiang, M.-C.; Wondji, C.S. Insecticide Resistance in Malaria Vectors: An Update at a Global Scale. In Towards Malaria Elimination—A Leap Forward; IntechOpen: London, UK, 2018; ISBN 978-1-78923-551-7. [Google Scholar]
- Bass, C.; Puinean, A.M.; Zimmer, C.T.; Denholm, I.; Field, L.M.; Foster, S.P.; Gutbrod, O.; Nauen, R.; Slater, R.; Williamson, M.S. The Evolution of Insecticide Resistance in the Peach Potato Aphid, Myzus persicae. Insect Biochem. Mol. Biol. 2014, 51, 41–51. [Google Scholar] [CrossRef]
- Ai, S.; Zhang, Y.; Chen, Y.; Zhang, T.; Zhong, G.; Yi, X. Insect-Microorganism Interaction Has Implicates on Insect Olfactory Systems. Insects 2022, 13, 1094. [Google Scholar] [CrossRef]
- Perreau, J.; Patel, D.J.; Anderson, H.; Maeda, G.P.; Elston, K.M.; Barrick, J.E.; Moran, N.A. Vertical Transmission at the Pathogen-Symbiont Interface: Serratia Symbiotica and Aphids. mBio 2021, 12, e00359-21. [Google Scholar] [CrossRef]
- Moran, N.A.; McCutcheon, J.P.; Nakabachi, A. Genomics and Evolution of Heritable Bacterial Symbionts. Annu. Rev. Genet. 2008, 42, 165–190. [Google Scholar] [CrossRef]
- van Tol, S.; Dimopoulos, G. Chapter Nine-Influences of the Mosquito Microbiota on Vector Competence. In Advances in Insect Physiology; Raikhel, A.S., Ed.; Progress in Mosquito Research; Academic Press: Cambridge, MA, USA, 2016; Volume 51, pp. 243–291. [Google Scholar]
- Louie, W.; Coffey, L.L. Microbial Composition in Larval Water Enhances Aedes Aegypti Development but Reduces Transmissibility of Zika Virus. mSphere 2021, 6, e00687-21. [Google Scholar] [CrossRef] [PubMed]
- Akman Gündüz, E.; Douglas, A.E. Symbiotic Bacteria Enable Insect to Use a Nutritionally Inadequate Diet. Proc. Biol. Sci. 2009, 276, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B.; Aksoy, S. Microbiome Influences on Insect Host Vector Competence. Trends Parasitol. 2011, 27, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Benoit, J.B.; Vigneron, A.; Broderick, N.A.; Wu, Y.; Sun, J.S.; Carlson, J.R.; Aksoy, S.; Weiss, B.L. Symbiont-Induced Odorant Binding Proteins Mediate Insect Host Hematopoiesis. eLife 2017, 6, e19535. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Segal, D.; Ringo, J.M.; Hefetz, A.; Zilber-Rosenberg, I.; Rosenberg, E. Commensal Bacteria Play a Role in Mating Preference of Drosophila Melanogaster. Proc. Natl. Acad. Sci. USA 2010, 107, 20051–20056. [Google Scholar] [CrossRef]
- Cirimotich, C.M.; Ramirez, J.L.; Dimopoulos, G. Native Microbiota Shape Insect Vector Competence for Human Pathogens. Cell Host Microbe 2011, 10, 307–310. [Google Scholar] [CrossRef]
- Broderick, N.; Lemaitre, B. Gut-Associated Microbes of Drosophila Melanogaster. Gut Microbes 2012, 3, 307–321. [Google Scholar] [CrossRef]
- Dong, Y.; Manfredini, F.; Dimopoulos, G. Implication of the Mosquito Midgut Microbiota in the Defense against Malaria Parasites. PLoS Pathog. 2009, 5, e1000423. [Google Scholar] [CrossRef]
- Muturi, E.J.; Ramirez, J.L.; Rooney, A.P.; Kim, C.-H. Comparative Analysis of Gut Microbiota of Mosquito Communities in Central Illinois. PLoS Neglected Trop. Dis. 2017, 11, e0005377. [Google Scholar] [CrossRef]
- Lange, C.; Boyer, S.; Bezemer, T.M.; Lefort, M.-C.; Dhami, M.K.; Biggs, E.; Groenteman, R.; Fowler, S.V.; Paynter, Q.; Verdecia Mogena, A.M.; et al. Impact of Intraspecific Variation in Insect Microbiomes on Host Phenotype and Evolution. ISME J. 2023, 17, 1798–1807. [Google Scholar] [CrossRef]
- Cecala, J.M.; Landucci, L.; Vannette, R.L. Seasonal Assembly of Nectar Microbial Communities Across Angiosperm Plant Species: Assessing Contributions of Climate and Plant Traits. Ecol. Lett. 2025, 28, e70045. [Google Scholar] [CrossRef]
- Janson, E.M.; Stireman, J.O.; Singer, M.S.; Abbot, P. Phytophagous Insect-Microbe Mutualisms and Adaptive Evolutionary Diversification. Evolution 2008, 62, 997–1012. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Pérez, S.; Herrera, C.M. Composition, Richness and Nonrandom Assembly of Culturable Bacterial–Microfungal Communities in Floral Nectar of Mediterranean Plants. FEMS Microbiol. Ecol. 2013, 83, 685–699. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Xu, H.; Liu, Y.; Lu, Z. Effects of Host Plant and Insect Generation on Shaping of the Gut Microbiota in the Rice Leaffolder, Cnaphalocrocis Medinalis. Front. Microbiol. 2022, 13, 824224. [Google Scholar] [CrossRef]
- Pirttilä, A.M.; Brusila, V.; Koskimäki, J.J.; Wäli, P.R.; Ruotsalainen, A.L.; Mutanen, M.; Markkola, A.M. Exchange of Microbiomes in Plant-Insect Herbivore Interactions. mBio 2023, 14, e03210-22. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Hayatsu, M.; Hosokawa, T.; Nagayama, A.; Tago, K.; Fukatsu, T. Symbiont-Mediated Insecticide Resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 8618–8622. [Google Scholar] [CrossRef]
- Keesey, I.W.; Koerte, S.; Khallaf, M.A.; Retzke, T.; Guillou, A.; Grosse-Wilde, E.; Buchon, N.; Knaden, M.; Hansson, B.S. Pathogenic Bacteria Enhance Dispersal through Alteration of Drosophila Social Communication. Nat. Commun. 2017, 8, 265. [Google Scholar] [CrossRef]
- Oliver, K.M.; Degnan, P.H.; Burke, G.R.; Moran, N.A. Facultative Symbionts in Aphids and the Horizontal Transfer of Ecologically Important Traits. Annu. Rev. Entomol. 2010, 55, 247–266. [Google Scholar] [CrossRef]
- Feldhaar, H. Bacterial Symbionts as Mediators of Ecologically Important Traits of Insect Hosts. Ecol. Entomol. 2011, 36, 533–543. [Google Scholar] [CrossRef]
- Moran, N.A.; Jarvik, T. Lateral Transfer of Genes from Fungi Underlies Carotenoid Production in Aphids. Science 2010, 328, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Altincicek, B.; Kovacs, J.L.; Gerardo, N.M. Horizontally Transferred Fungal Carotenoid Genes in the Two-Spotted Spider Mite Tetranychus urticae. Biol. Lett. 2012, 8, 253–257. [Google Scholar] [CrossRef]
- Wybouw, N.; Pauchet, Y.; Heckel, D.G.; Van Leeuwen, T. Horizontal Gene Transfer Contributes to the Evolution of Arthropod Herbivory. Genome Biol. Evol. 2016, 8, 1785–1801. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.W.; Chevignon, G.; Oliver, K.M.; Strand, M.R. Culture of an Aphid Heritable Symbiont Demonstrates Its Direct Role in Defence against Parasitoids. Proc. Biol. Sci. 2017, 284, 20171925. [Google Scholar] [CrossRef]
- Oliver, K.M.; Degnan, P.H.; Hunter, M.S.; Moran, N.A. Bacteriophages Encode Factors Required for Protection in a Symbiotic Mutualism. Science 2009, 325, 992–994. [Google Scholar] [CrossRef]
- Dunning Hotopp, J.C.; Clark, M.E.; Oliveira, D.C.S.G.; Foster, J.M.; Fischer, P.; Muñoz Torres, M.C.; Giebel, J.D.; Kumar, N.; Ishmael, N.; Wang, S.; et al. Widespread Lateral Gene Transfer from Intracellular Bacteria to Multicellular Eukaryotes. Science 2007, 317, 1753–1756. [Google Scholar] [CrossRef] [PubMed]
- Dunning Hotopp, J.C.; Slatko, B.E.; Foster, J.M. Targeted Enrichment and Sequencing of Recent Endosymbiont-Host Lateral Gene Transfers. Sci. Rep. 2017, 7, 857. [Google Scholar] [CrossRef]
- Brelsfoard, C.; Tsiamis, G.; Falchetto, M.; Gomulski, L.M.; Telleria, E.; Alam, U.; Doudoumis, V.; Scolari, F.; Benoit, J.B.; Swain, M.; et al. Presence of Extensive Wolbachia Symbiont Insertions Discovered in the Genome of Its Host Glossina Morsitans Morsitans. PLoS Negl. Trop. Dis. 2014, 8, e2728. [Google Scholar] [CrossRef] [PubMed]
- Ravenhall, M.; Škunca, N.; Lassalle, F.; Dessimoz, C. Inferring Horizontal Gene Transfer. PLoS Comput. Biol. 2015, 11, e1004095. [Google Scholar] [CrossRef]
- Ribeiro, P.; Butenko, A.; Linke, D.; Ghanavi, H.R.; Meier, J.I.; Wahlberg, N.; Matos-Maraví, P. Pervasive Horizontal Transmission of Wolbachia in Natural Populations of Closely Related and Widespread Tropical Skipper Butterflies. BMC Microbiol. 2025, 25, 5. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hellemans, S.; Kinjo, Y.; Mikhailova, A.A.; Aumont, C.; Weng, Y.M.; Buček, A.; Husnik, F.; Šobotník, J.; Harrison, M.C.; et al. Horizontal Gene Transfers Are Widespread across Termite Genomes but Do Not Confer Metabolic Innovations. bioRxiv 2025. bioRxiv:2025.03.16.643567. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Zhou, X. The Genomics Revolution Drives a New Era in Entomology. Annu. Rev. Entomol. 2025, 70, 379–400. [Google Scholar] [CrossRef]
- Bourguignon, T.; Lo, N.; Dietrich, C.; Šobotník, J.; Sidek, S.; Roisin, Y.; Brune, A.; Evans, T.A. Rampant Host Switching Shaped the Termite Gut Microbiome. Curr. Biol. 2018, 28, 649–654.e2. [Google Scholar] [CrossRef]
- Almeida, L.G.d.; Moraes, L.A.B.d.; Trigo, J.R.; Omoto, C.; Cônsoli, F.L. The Gut Microbiota of Insecticide-Resistant Insects Houses Insecticide-Degrading Bacteria: A Potential Source for Biotechnological Exploitation. PLoS ONE 2017, 12, e0174754. [Google Scholar] [CrossRef]
- Mondal, S.; Somani, J.; Roy, S.; Babu, A.; Pandey, A.K. Insect Microbial Symbionts: Ecology, Interactions, and Biological Significance. Microorganisms 2023, 11, 2665. [Google Scholar] [CrossRef]
- Chevrette, M.G.; Carlson, C.M.; Ortega, H.E.; Thomas, C.; Ananiev, G.E.; Barns, K.J.; Book, A.J.; Cagnazzo, J.; Carlos, C.; Flanigan, W.; et al. The Antimicrobial Potential of Streptomyces from Insect Microbiomes. Nat. Commun. 2019, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Bérdy, J. Thoughts and Facts about Antibiotics: Where We Are Now and Where We Are Heading. J. Antibiot. 2012, 65, 385–395. [Google Scholar] [CrossRef]
- Blackmon, H.; Ross, L.; Bachtrog, D. Sex Determination, Sex Chromosomes, and Karyotype Evolution in Insects. J. Hered. 2017, 108, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Hurst, G.D.; Jiggins, F.M. Male-Killing Bacteria in Insects: Mechanisms, Incidence, and Implications. Emerg. Infect. Dis. 2000, 6, 329–336. [Google Scholar] [CrossRef]
- Weinert, L.A.; Araujo-Jnr, E.V.; Ahmed, M.Z.; Welch, J.J. The Incidence of Bacterial Endosymbionts in Terrestrial Arthropods. Proc. Biol. Sci. 2015, 282, 20150249. [Google Scholar] [CrossRef] [PubMed]
- Zug, R.; Hammerstein, P. Still a Host of Hosts for Wolbachia: Analysis of Recent Data Suggests That 40% of Terrestrial Arthropod Species Are Infected. PLoS ONE 2012, 7, e38544. [Google Scholar] [CrossRef]
- Teixeira, L.; Ferreira, A.; Ashburner, M. The Bacterial Symbiont Wolbachia Induces Resistance to RNA Viral Infections in Drosophila Melanogaster. PLoS Biol. 2008, 6, e2. [Google Scholar] [CrossRef]
- Bi, J.; Wang, Y. The Effect of the Endosymbiont Wolbachia on the Behavior of Insect Hosts. Insect Sci. 2020, 27, 846–858. [Google Scholar] [CrossRef]
- Miller, W.J.; Ehrman, L.; Schneider, D. Infectious Speciation Revisited: Impact of Symbiont-Depletion on Female Fitness and Mating Behavior of Drosophila Paulistorum. PLoS Pathog. 2010, 6, e1001214. [Google Scholar] [CrossRef]
- Serbus, L.R.; Casper-Lindley, C.; Landmann, F.; Sullivan, W. The Genetics and Cell Biology of Wolbachia-Host Interactions. Annu. Rev. Genet. 2008, 42, 683–707. [Google Scholar] [CrossRef]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master Manipulators of Invertebrate Biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef]
- Khan, M.K.; Rolff, J. Insect Immunity in the Anthropocene. Biol. Rev. 2025, 100, 698–723. [Google Scholar] [CrossRef]
- Khan, S.A.; Kojour, M.A.M.; Han, Y.S. Recent Trends in Insect Gut Immunity. Front. Immunol. 2023, 14, 1272143. [Google Scholar] [CrossRef] [PubMed]
- Malacrinò, A. Host Species Identity Shapes the Diversity and Structure of Insect Microbiota. Mol. Ecol. 2022, 31, 723–735. [Google Scholar] [CrossRef]
- Raymann, K.; Motta, E.V.S.; Girard, C.; Riddington, I.M.; Dinser, J.A.; Moran, N.A. Imidacloprid Decreases Honey Bee Survival Rates but Does Not Affect the Gut Microbiome. Appl. Environ. Microbiol. 2018, 84, e00545-18. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, P.; Duval, P.; Luis, P.; Minard, G.; Valiente Moro, C. Reciprocal Interactions between Anthropogenic Stressors and Insect Microbiota. Environ. Sci. Pollut. Res. Int. 2022, 29, 64469–64488. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, D.; Ren, Q.; Sun, J.; Zhang, L.; Cheng, Y.; Jiang, X. Gut Microbiota Affects Host Fitness of Fall Armyworm Feeding on Different Food Types. Insects 2024, 15, 304. [Google Scholar] [CrossRef]
- Montell, C. A Taste of the Drosophila Gustatory Receptors. Curr. Opin. Neurobiol. 2009, 19, 345–353. [Google Scholar] [CrossRef]
- Rinker, D.C.; Pitts, R.J.; Zwiebel, L.J. Disease Vectors in the Era of next Generation Sequencing. Genome Biol. 2016, 17, 95. [Google Scholar] [CrossRef]
- Gomez-Diaz, C.; Martin, F.; Garcia-Fernandez, J.M.; Alcorta, E. The Two Main Olfactory Receptor Families in Drosophila, ORs and IRs: A Comparative Approach. Front. Cell. Neurosci. 2018, 12, 253. [Google Scholar] [CrossRef]
- Schmidt, H.R.; Benton, R. Molecular Mechanisms of Olfactory Detection in Insects: Beyond Receptors. Open Biol. 2020, 10, 200252. [Google Scholar] [CrossRef]
- Wong, A.C.-N.; Wang, Q.-P.; Morimoto, J.; Senior, A.M.; Lihoreau, M.; Neely, G.G.; Simpson, S.J.; Ponton, F. Gut Microbiota Modifies Olfactory-Guided Microbial Preferences and Foraging Decisions in Drosophila. Curr. Biol. 2017, 27, 2397–2404.e4. [Google Scholar] [CrossRef]
- Slankster, E.; Lee, C.; Hess, K.M.; Odell, S.; Mathew, D. Effect of Gut Microbes on Olfactory Behavior of Drosophila melanogaster Larva. Bios 2019, 90, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mu, X.; Cao, Q.; Shi, Y.; Hu, X.; Zheng, H. Honeybee Gut Lactobacillus Modulates Host Learning and Memory Behaviors via Regulating Tryptophan Metabolism. Nat. Commun. 2022, 13, 2037. [Google Scholar] [CrossRef] [PubMed]
- Vernier, C.L.; Nguyen, L.A.; Gernat, T.; Ahmed, A.C.; Chen, Z.; Robinson, G.E. Gut Microbiota Contribute to Variations in Honey Bee Foraging Intensity. ISME J. 2024, 18, wrae030. [Google Scholar] [CrossRef]
- Chafee, M.E.; Zecher, C.N.; Gourley, M.L.; Schmidt, V.T.; Chen, J.H.; Bordenstein, S.R.; Clark, M.E.; Bordenstein, S.R. Decoupling of Host–Symbiont–Phage Coadaptations Following Transfer Between Insect Species. Genetics 2011, 187, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Leger, L.; McFrederick, Q.S. The Gut-Brain-Microbiome Axis in Bumble Bees. Insects 2020, 11, 517. [Google Scholar] [CrossRef]
- Silva, C.A.; Godoy, L.; Ahumada, M.I.; Carvajal, M.; Chorbadjian, R.A. Potential of an Entomopathogenic Fungus from the Cladosporium cladosporioides Species Complex for Aphid Control: Insights from Biological Parameters and Bioassays. Available online: https://link.springer.com/article/10.1007/s10526-025-10311-7 (accessed on 5 August 2025).
- Parmentier, L.; Meeus, I.; Mosallanejad, H.; de Graaf, D.C.; Smagghe, G. Plasticity in the Gut Microbial Community and Uptake of Enterobacteriaceae (Gammaproteobacteria) in Bombus terrestris Bumblebees’ Nests When Reared Indoors and Moved to an Outdoor Environment. Apidologie 2016, 47, 237–250. [Google Scholar] [CrossRef]
- Dharampal, P.S.; Carlson, C.; Currie, C.R.; Steffan, S.A. Pollen-Borne Microbes Shape Bee Fitness. Proc. Biol. Sci. 2019, 286, 20182894. [Google Scholar] [CrossRef]
- Blot, N.; Veillat, L.; Rouzé, R.; Delatte, H. Glyphosate, but Not Its Metabolite AMPA, Alters the Honeybee Gut Microbiota. PLoS ONE 2019, 14, e0215466. [Google Scholar] [CrossRef]
- Rodpai, R.; Boonroumkaew, P.; Sadaow, L.; Sanpool, O.; Janwan, P.; Thanchomnang, T.; Intapan, P.M.; Maleewong, W. Microbiome Composition and Microbial Community Structure in Mosquito Vectors Aedes aegypti and Aedes albopictus in Northeastern Thailand, a Dengue-Endemic Area. Insects 2023, 14, 184. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Montgomery, B.L.; Popovici, J.; Iturbe-Ormaetxe, I.; Johnson, P.H.; Muzzi, F.; Greenfield, M.; Durkan, M.; Leong, Y.S.; Dong, Y.; et al. Successful Establishment of Wolbachia in Aedes Populations to Suppress Dengue Transmission. Nature 2011, 476, 454–457. [Google Scholar] [CrossRef]
- Walker, T.; Johnson, P.H.; Moreira, L.A.; Iturbe-Ormaetxe, I.; Frentiu, F.D.; McMeniman, C.J.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P.; et al. The wMel Wolbachia Strain Blocks Dengue and Invades Caged Aedes Aegypti Populations. Nature 2011, 476, 450–453. [Google Scholar] [CrossRef]
- Xia, X.; Sun, B.; Gurr, G.M.; Vasseur, L.; Xue, M.; You, M. Gut Microbiota Mediate Insecticide Resistance in the Diamondback Moth, Plutella xylostella (L.). Front. Microbiol. 2018, 9, 25. [Google Scholar] [CrossRef]
- Bruce, T.J.A. Interplay between Insects and Plants: Dynamic and Complex Interactions That Have Coevolved over Millions of Years but Act in Milliseconds. J. Exp. Bot. 2015, 66, 455–465. [Google Scholar] [CrossRef]
- Grunseich, J.M.; Thompson, M.N.; Aguirre, N.M.; Helms, A.M. The Role of Plant-Associated Microbes in Mediating Host-Plant Selection by Insect Herbivores. Plants 2020, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Franco, F.P.; Moura, D.S.; Vivanco, J.M.; Silva-Filho, M.C. Plant-Insect-Pathogen Interactions: A Naturally Complex Ménage à Trois. Curr. Opin. Microbiol. 2017, 37, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Mueller, M.B.; Kalske, A.; Chautá, A. Volatile-Mediated Plant–Plant Communication and Higher-Level Ecological Dynamics. Curr. Biol. 2023, 33, R519–R529. [Google Scholar] [CrossRef] [PubMed]
- Gabrieli, P.; Caccia, S.; Varotto-Boccazzi, I.; Arnoldi, I.; Barbieri, G.; Comandatore, F.; Epis, S. Mosquito Trilogy: Microbiota, Immunity and Pathogens, and Their Implications for the Control of Disease Transmission. Front. Microbiol. 2021, 12, 630438. [Google Scholar] [CrossRef]
- Ganley, J.G.; Pandey, A.; Sylvester, K.; Lu, K.-Y.; Toro-Moreno, M.; Rütschlin, S.; Bradford, J.M.; Champion, C.J.; Böttcher, T.; Xu, J.; et al. A Systematic Analysis of Mosquito-Microbiome Biosynthetic Gene Clusters Reveals Antimalarial Siderophores That Reduce Mosquito Reproduction Capacity. Cell Chem. Biol. 2020, 27, 817–826.e5. [Google Scholar] [CrossRef] [PubMed]
- Asgharian, H.; Chang, P.L.; Mazzoglio, P.J.; Negri, I. Wolbachia Is Not All about Sex: Male-Feminizing Wolbachia Alters the Leafhopper Zyginidia Pullula Transcriptome in a Mainly Sex-Independent Manner. Front. Microbiol. 2014, 5, 430. [Google Scholar] [CrossRef]
- Singh, R.; Linksvayer, T.A. Wolbachia-Infected Ant Colonies Have Increased Reproductive Investment and an Accelerated Life Cycle. J. Exp. Biol. 2020, 223, jeb220079. [Google Scholar] [CrossRef] [PubMed]
- Weeks, A.R.; Velten, R.; Stouthamer, R. Incidence of a New Sex-Ratio-Distorting Endosymbiotic Bacterium among Arthropods. Proc. Biol. Sci. 2003, 270, 1857–1865. [Google Scholar] [CrossRef]
- Provencher, L.M.; Morse, G.E.; Weeks, A.R.; Normark, B.B. Parthenogenesis in the Aspidiotus nerii Complex (Hemiptera: Diaspididae): A Single Origin of a Worldwide, Polyphagous Lineage Associated with Cardinium Bacteria. Ann. Entomol. Soc. Am. 2005, 98, 629–635. [Google Scholar] [CrossRef]
- Hunter, M.S.; Perlman, S.J.; Kelly, S.E. A Bacterial Symbiont in the Bacteroidetes Induces Cytoplasmic Incompatibility in the Parasitoid Wasp Encarsia pergandiella. Proc. Biol. Sci. 2003, 270, 2185–2190. [Google Scholar] [CrossRef]
- Weeks, A.R.; Stouthamer, R. Increased Fecundity Associated with Infection by a Cytophaga-like Intracellular Bacterium in the Predatory Mite, Metaseiulus occidentalis. Proc. Biol. Sci. 2004, 271 (Suppl. S4), S193–S195. [Google Scholar] [CrossRef]
- Kenyon, S.G.; Hunter, M.S. Manipulation of Oviposition Choice of the Parasitoid Wasp, Encarsia pergandiella, by the Endosymbiotic Bacterium Cardinium. J. Evol. Biol. 2007, 20, 707–716. [Google Scholar] [CrossRef]
- Son, J.H.; Weiss, B.L.; Schneider, D.I.; Dera, K.-S.M.; Gstöttenmayer, F.; Opiro, R.; Echodu, R.; Saarman, N.P.; Attardo, G.M.; Onyango, M.; et al. Infection with Endosymbiotic Spiroplasma Disrupts Tsetse (Glossina fuscipes fuscipes) Metabolic and Reproductive Homeostasis. PLoS Pathog. 2021, 17, e1009539. [Google Scholar] [CrossRef]
- Serrato-Salas, J.; Gendrin, M. Involvement of Microbiota in Insect Physiology: Focus on B Vitamins. mBio 2021, 14, e02225-22. [Google Scholar] [CrossRef]
- Kwong, W.K.; Moran, N.A. Gut Microbial Communities of Social Bees. Nat. Rev. Microbiol. 2016, 14, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Cross, K.L.; Leigh, B.A.; Hatmaker, E.A.; Mikaelyan, A.; Miller, A.K.; Bordenstein, S.R. Genomes of Gut Bacteria from Nasonia Wasps Shed Light on Phylosymbiosis and Microbe-Assisted Hybrid Breakdown. mSystems 2021, 6, e01342-20. [Google Scholar] [CrossRef] [PubMed]
- Bordenstein, S.R.; O’Hara, F.P.; Werren, J.H. Wolbachia-Induced Incompatibility Precedes Other Hybrid Incompatibilities in Nasonia. Nature 2001, 409, 707–710. [Google Scholar] [CrossRef]
- Gloder, G.; Bourne, M.E.; Verreth, C.; Wilberts, L.; Bossaert, S.; Crauwels, S.; Dicke, M.; Poelman, E.H.; Jacquemyn, H.; Lievens, B. Parasitism by Endoparasitoid Wasps Alters the Internal but Not the External Microbiome in Host Caterpillars. Anim. Microbiome 2021, 3, 73. [Google Scholar] [CrossRef]
- Gloder, G.; Bourne, M.E.; Cuny, M.A.C.; Verreth, C.; Crauwels, S.; Dicke, M.; Poelman, E.H.; Jacquemyn, H.; Lievens, B. Caterpillar–Parasitoid Interactions: Species-Specific Influences on Host Microbiome Composition. FEMS Microbiol. Ecol. 2024, 100, fiae115. [Google Scholar] [CrossRef]
- Bourne, M.E.; Gloder, G.; Weldegergis, B.T.; Slingerland, M.; Ceribelli, A.; Crauwels, S.; Lievens, B.; Jacquemyn, H.; Dicke, M.; Poelman, E.H. Parasitism Causes Changes in Caterpillar Odours and Associated Bacterial Communities with Consequences for Host-Location by a Hyperparasitoid. PLoS Pathog. 2023, 19, e1011262. [Google Scholar] [CrossRef]
- Wang, J.; Mason, C.J.; Ju, X.; Xue, R.; Tong, L.; Peiffer, M.; Song, Y.; Zeng, R.; Felton, G.W. Parasitoid Causes Cascading Effects on Plant-Induced Defenses Mediated Through the Gut Bacteria of Host Caterpillars. Front. Microbiol. 2021, 12, 708990. [Google Scholar] [CrossRef]
- Mason, C.J.; Ray, S.; Davidson-Lowe, E.; Ali, J.; Luthe, D.S.; Felton, G. Plant Nutrition Influences Resistant Maize Defense Responses to the Fall Armyworm (Spodoptera frugiperda). Front. Ecol. Evol. 2022, 10, 844274. [Google Scholar] [CrossRef]
- Hrček, J.; McLean, A.H.C.; Godfray, H.C.J. Symbionts Modify Interactions between Insects and Natural Enemies in the Field. J. Anim. Ecol. 2016, 85, 1605–1612. [Google Scholar] [CrossRef]
- Hafer-Hahmann, N.; Vorburger, C. Positive Association between the Diversity of Symbionts and Parasitoids of Aphids in Field Populations. Ecosphere 2021, 12, e03355. [Google Scholar] [CrossRef]
- Narayan, K.S.; Vorburger, C.; Hafer-Hahmann, N. Bottom-up Effect of Host Protective Symbionts on Parasitoid Diversity: Limited Evidence from Two Field Experiments. J. Anim. Ecol. 2022, 91, 643–654. [Google Scholar] [CrossRef]
- Ye, Z.; Vollhardt, I.M.G.; Parth, N.; Rubbmark, O.; Traugott, M. Facultative Bacterial Endosymbionts Shape Parasitoid Food Webs in Natural Host Populations: A Correlative Analysis. J. Anim. Ecol. 2018, 87, 1440–1451. [Google Scholar] [CrossRef]
- Lacey, L.A.; Georgis, R. Entomopathogenic Nematodes for Control of Insect Pests above and below Ground with Comments on Commercial Production. J. Nematol. 2012, 44, 218–225. [Google Scholar]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect Pathogens as Biological Control Agents: Back to the Future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef]
- Ortiz-Urquiza, A.; Keyhani, N.O. Action on the Surface: Entomopathogenic Fungi versus the Insect Cuticle. Insects 2013, 4, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Lovett, B.; St Leger, R.J. The Insect Pathogens. Microbiol. Spectr. 2017, 5, FUNK-0001-2016. [Google Scholar] [CrossRef] [PubMed]
- Helander, M.; Saloniemi, I.; Saikkonen, K. Glyphosate in Northern Ecosystems. Trends Plant Sci. 2012, 17, 569–574. [Google Scholar] [CrossRef]
- Kepler, R.M.; Epp Schmidt, D.J.; Yarwood, S.A.; Cavigelli, M.A.; Reddy, K.N.; Duke, S.O.; Bradley, C.A.; Williams, M.M.; Buyer, J.S.; Maul, J.E. Soil Microbial Communities in Diverse Agroecosystems Exposed to the Herbicide Glyphosate. Appl. Environ. Microbiol. 2020, 86, e01744-19. [Google Scholar] [CrossRef] [PubMed]
- Kakumanu, M.L.; Reeves, A.M.; Anderson, T.D.; Rodrigues, R.R.; Williams, M.A. Honey Bee Gut Microbiome Is Altered by In-Hive Pesticide Exposures. Front. Microbiol. 2016, 7, 1255. [Google Scholar] [CrossRef]
- Richter, I.; Büttner, H.; Hertweck, C. Endofungal Bacteria as Hidden Facilitators of Biotic Interactions. ISME J. 2025, 19, wraf128. [Google Scholar] [CrossRef]
- Voulgari-Kokota, A.; Grimmer, G.; Steffan-Dewenter, I.; Keller, A. Bacterial Community Structure and Succession in Nests of Two Megachilid Bee Genera. FEMS Microbiol. Ecol. 2019, 95, fiy218. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.C.; Fruciano, C.; Hildebrand, F.; Al Toufalilia, H.; Balfour, N.J.; Bork, P.; Engel, P.; Ratnieks, F.L.; Hughes, W.O. Gut Microbiota Composition Is Associated with Environmental Landscape in Honey Bees. Ecol. Evol. 2018, 8, 441–451. [Google Scholar] [CrossRef]
- Jaber, L.R.; Ownley, B.H. Can We Use Entomopathogenic Fungi as Endophytes for Dual Biological Control of Insect Pests and Plant Pathogens? Biol. Control. 2018, 116, 36–45. [Google Scholar] [CrossRef]
- Sanahuja, G.; Banakar, R.; Twyman, R.M.; Capell, T.; Christou, P. Bacillus thuringiensis: A Century of Research, Development and Commercial Applications. Plant Biotechnol. J. 2011, 9, 283–300. [Google Scholar] [CrossRef]
- Flury, P.; Vesga, P.; Dominguez-Ferreras, A.; Tinguely, C.; Ullrich, C.I.; Kleespies, R.G.; Keel, C.; Maurhofer, M. Persistence of Root-Colonizing Pseudomonas Protegens in Herbivorous Insects throughout Different Developmental Stages and Dispersal to New Host Plants. ISME J. 2019, 13, 860–872. [Google Scholar] [CrossRef]
- Amelia-Yap, Z.H.; Low, V.L.; Saeung, A.; Ng, F.L.; Chen, C.D.; Hassandarvish, P.; Tan, G.Y.A.; AbuBakar, S.; Azman, A.S. Insecticidal Activities of Streptomyces Sp. KSF103 Ethyl Acetate Extract against Medically Important Mosquitoes and Non-Target Organisms. Sci. Rep. 2023, 13, 4. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Jaronski, S.T. The Production and Uses of Beauveria Bassiana as a Microbial Insecticide. World J. Microbiol. Biotechnol. 2016, 32, 177. [Google Scholar] [CrossRef] [PubMed]
- Campos-Herrera, R. (Ed.) Nematode Pathogenesis of Insects and Other Pests: Ecology and Applied Technologies for Sustainable Plant and Crop Protection; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-18265-0. [Google Scholar]
- Sanad, M.; Sun, J.S.; Shamseldean, M.S.M.; Wang, Y.; Gaugler, R. Superparasitism and Population Regulation of the Mosquito-Parasitic Mermithid Nematodes Romanomermis iyengari and Strelkovimermis spiculatus. J. Nematol. 2017, 49, 316–320. [Google Scholar] [CrossRef]
- Sanad, M.M.; Shamseldean, M.S.M.; Elgindi, A.-E.Y.; Gaugler, R. Host Penetration and Emergence Patterns of the Mosquito-Parasitic Mermithids Romanomermis Iyengari and Strelkovimermis Spiculatus (Nematoda: Mermithidae). J. Nematol. 2013, 45, 30–38. [Google Scholar] [PubMed]
- Slack, J.; Arif, B.M. The Baculoviruses Occlusion-Derived Virus: Virion Structure and Function. Adv. Virus Res. 2007, 69, 99–165. [Google Scholar] [CrossRef] [PubMed]
- Fries, I. Nosema ceranae in European Honey Bees (Apis mellifera). J. Invertebr. Pathol. 2010, 103 (Suppl. S1), S73–S79. [Google Scholar] [CrossRef]
- Jaronski, S.T. Ecological Factors in the Inundative Use of Fungal Entomopathogens. BioControl 2010, 55, 159–185. [Google Scholar] [CrossRef]
- Basit, A.; Haq, I.U.; Hyder, M.; Humza, M.; Younas, M.; Akhtar, M.R.; Ghafar, M.A.; Liu, T.-X.; Hou, Y. Microbial Symbiosis in Lepidoptera: Analyzing the Gut Microbiota for Sustainable Pest Management. Biology 2025, 14, 937. [Google Scholar] [CrossRef]
- Maurice-Lira, J.V.; Pérez-Moreno, J.; Delgadillo-Martínez, J.; Salcedo-Vite, K. Future Perspectives in the Study of Mutualistic Interactions between Insects and Their Microorganisms. Web Ecol. 2025, 25, 39–45. [Google Scholar] [CrossRef]


| Aspect | Microbial Association | Host Insect(s) | Effect | Ref |
|---|---|---|---|---|
| Physiology | Core gut bacteria (S. alvi, G. apicola) | Honeybee (A. mellifera) | Enhances digestion, immune regulation, pathogen defense | [6,116] |
| H. defensa, APSE phages | Aphids (A. pisum) | Provides resistance to parasitoids through phage-encoded toxins | [54,55] | |
| Horizontal gene transfer (fungal carotenoid genes, bacterial pectinases) | Aphids (A. pisum), stick insects (Phasmatodea) | Expands pigment production and plant cell-wall digestion capacities | [51,53] | |
| Behavior | Wolbachia infection | Nasonia spp. (parasitoid wasps) | Alters mate choice and reproductive compatibility | [117,118] |
| C. bombi infection | Bumblebees (Bombus terrestris (Linnaeus) (Hymenoptera: Apidae)) | Reduces sucrose sensitivity and impairs foraging | [92] | |
| Gut bacteria and microbial volatiles | Drosophila melanogaster, mosquitoes | Modulate olfactory preferences, foraging, and host-seeking | [11,87,88] | |
| Control strategies | Wolbachia releases | Aedes aegypti (Linnaeus) (Diptera: Culicidae), Ae. albopictus | Reduces vector competence for dengue, Zika | [98,99] |
| Entomopathogenic nematodes (Steinernematidae, Heterorhabditidae) | Soil- and wood-dwelling insect pests | Rapid host mortality, environmentally safe biocontrol | [128,129] | |
| Entomopathogenic fungi (Beauveria bassiana (Balsamo-Crivelli) Vuillemin (Ascomycota: Cordycipitaceae), Metarhizium anisopliae (Metschnikoff) Sorokin (Ascomycota: Clavicipitaceae)) | Multiple insect pests | Infection through cuticle penetration and immune suppression | [130,131] | |
| Microbial volatiles in lure-and-kill systems | Lepidoptera, Diptera | Attract or repel insects, enhancing integrated pest management (IPM) | [11,12] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Darwish, H.; Tariq, M.; Salama, S.; Hart, T.; Sun, J.S. The Microbiome as a Driver of Insect Physiology, Behavior, and Control Strategies. Appl. Microbiol. 2025, 5, 90. https://doi.org/10.3390/applmicrobiol5030090
Al Darwish H, Tariq M, Salama S, Hart T, Sun JS. The Microbiome as a Driver of Insect Physiology, Behavior, and Control Strategies. Applied Microbiology. 2025; 5(3):90. https://doi.org/10.3390/applmicrobiol5030090
Chicago/Turabian StyleAl Darwish, Hazem, Muqaddasa Tariq, Safiyah Salama, Tia Hart, and Jennifer S. Sun. 2025. "The Microbiome as a Driver of Insect Physiology, Behavior, and Control Strategies" Applied Microbiology 5, no. 3: 90. https://doi.org/10.3390/applmicrobiol5030090
APA StyleAl Darwish, H., Tariq, M., Salama, S., Hart, T., & Sun, J. S. (2025). The Microbiome as a Driver of Insect Physiology, Behavior, and Control Strategies. Applied Microbiology, 5(3), 90. https://doi.org/10.3390/applmicrobiol5030090







