Efficacy of Escherichia coli O157:H7 Phage Φ241 in Model Food Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Culture Conditions
2.2. Phage Isolation, Purification, and Propagation
2.3. Model Food Systems
2.4. Efficacy of Phage Infection Against E. coli O157:H7 in Three Model Food Systems
2.5. Phage Infection at MOI of 10 in Cucumber Juice Supplemented with NaCl
2.6. Statistical Analysis
3. Results and Discussion
3.1. pH in Model Food Systems
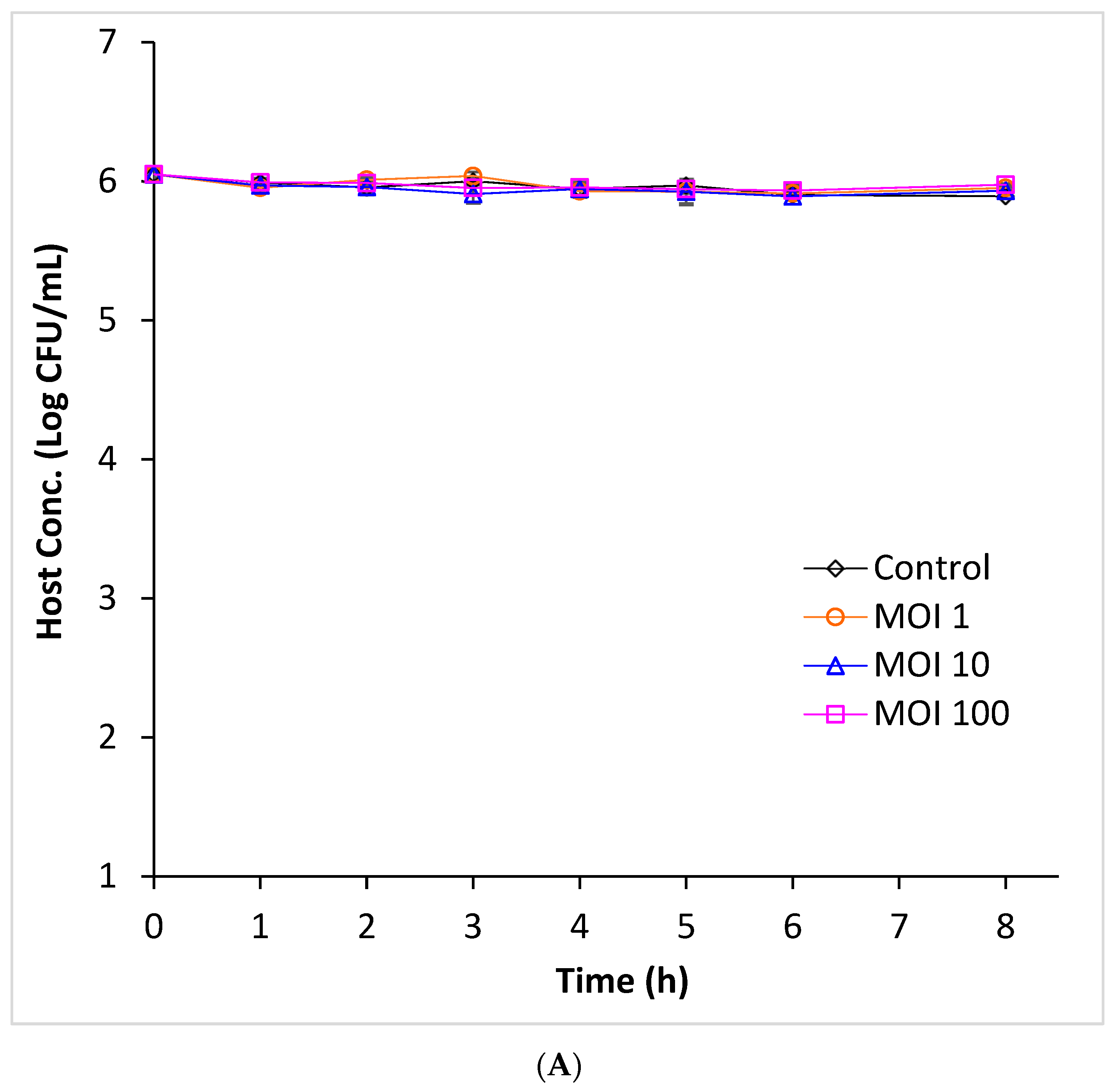
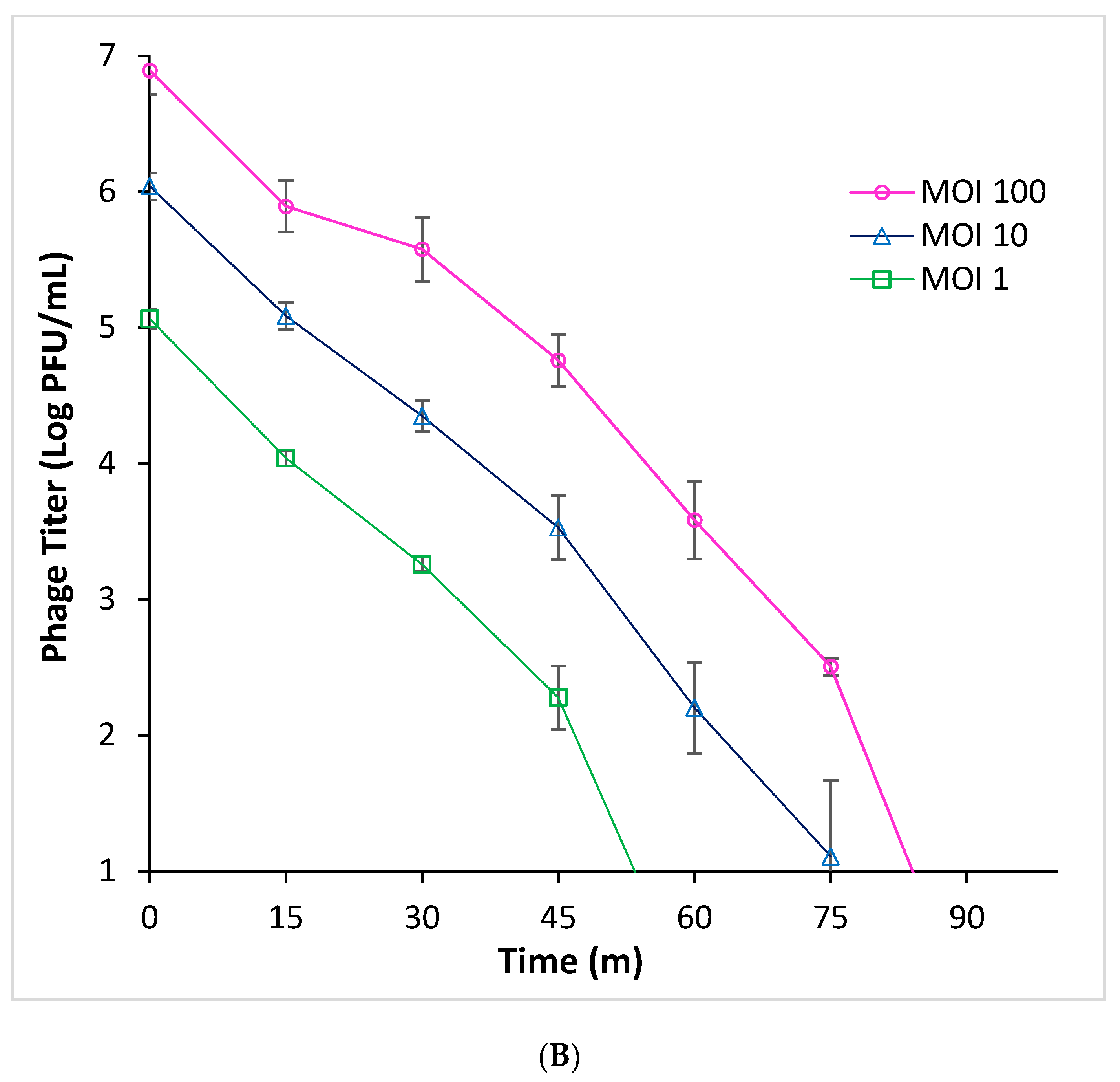
3.2. Efficacy of Phage Infection Against E. coli O157:H7 in Beef Broth
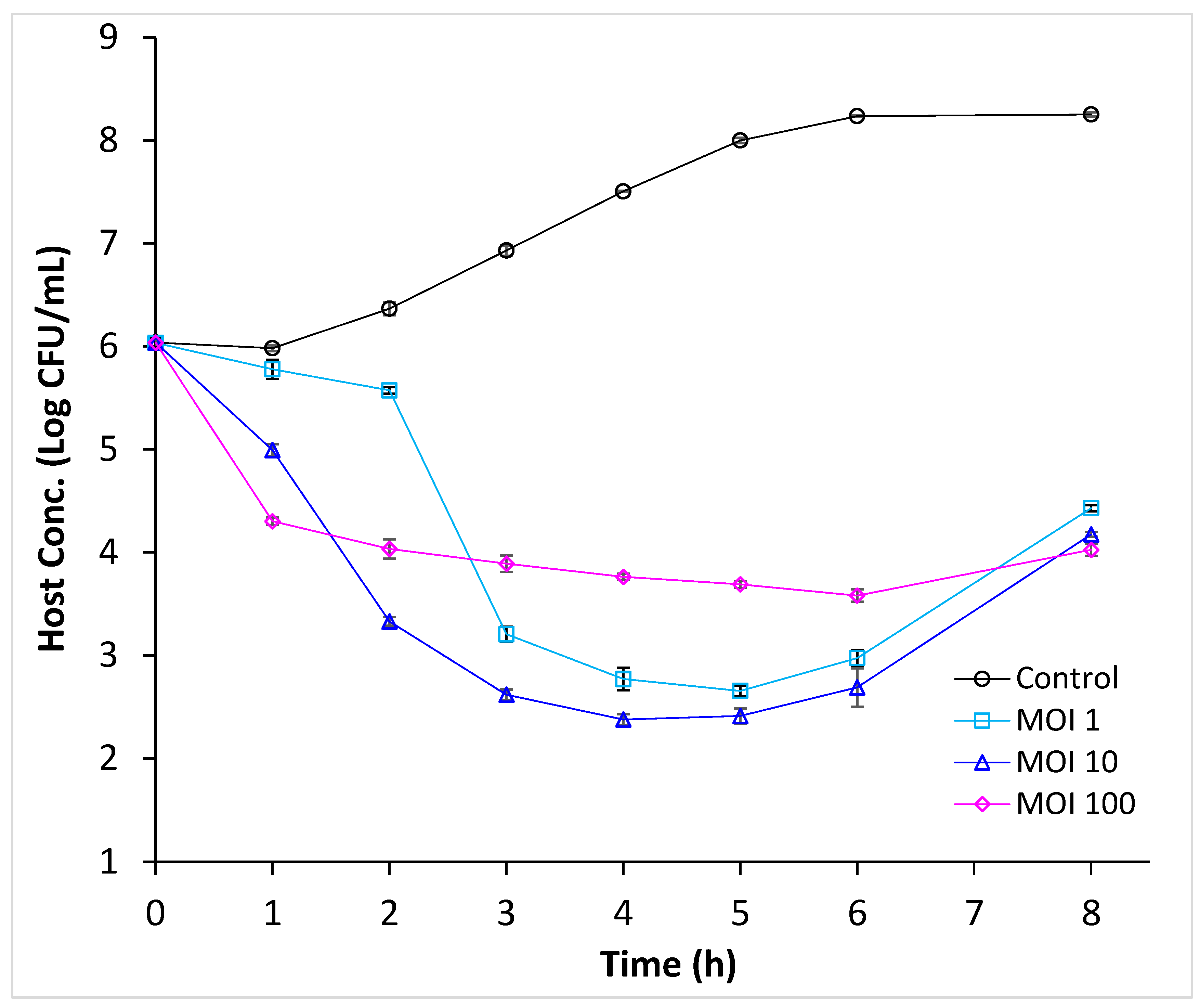
3.3. Efficacy of Phage Infection Against E. coli O157:H7 in Cucumber Juice
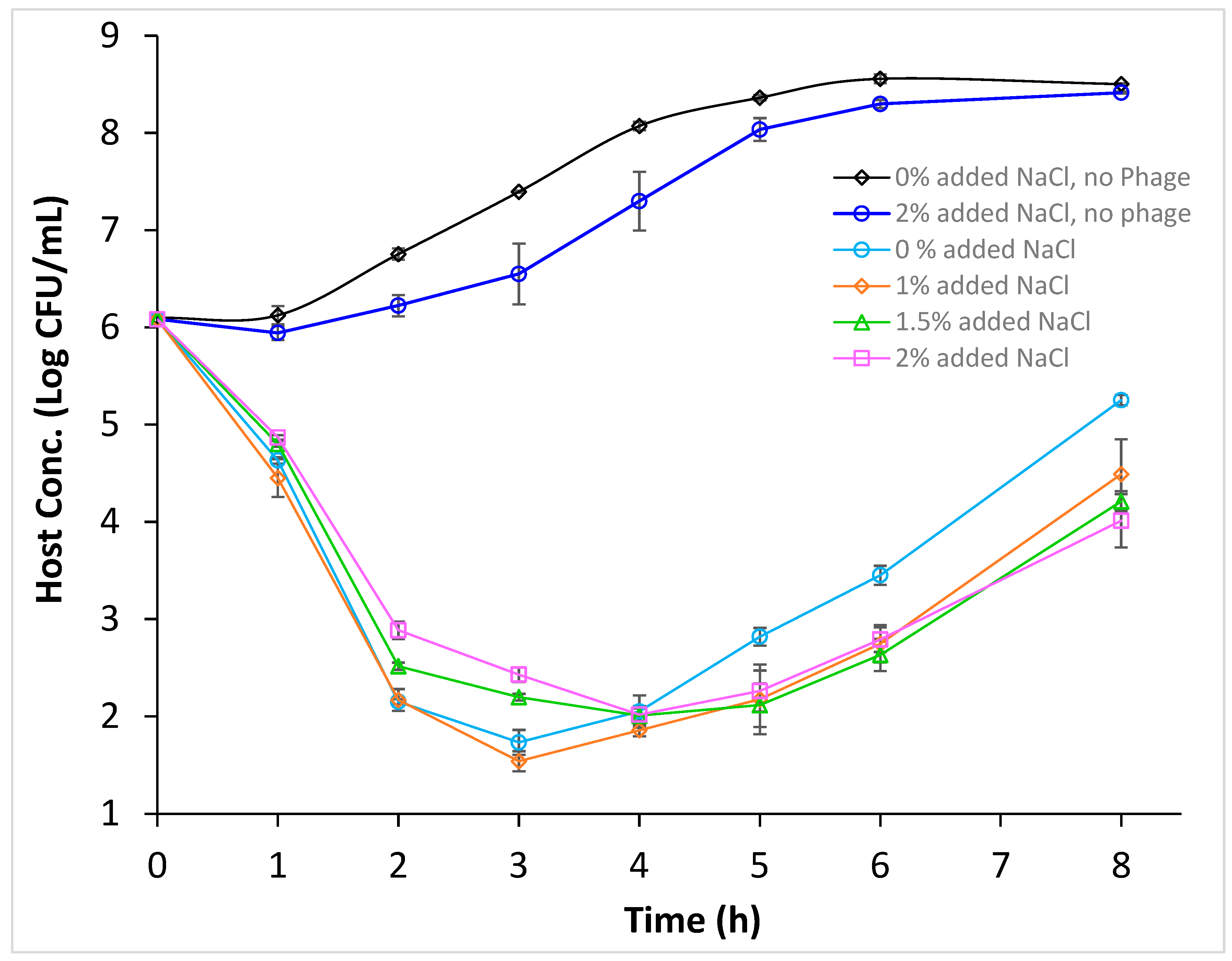
3.4. Efficacy of Phage Infection Against E. coli O157:H7 in Cucumber Juice Supplemented with NaCl
3.5. Efficacy of Phage Infection Against E. coli O157:H7 in Apple Juice
4. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Assefa, A. Prevalence of Escherichia coli O157:H7 in foods of animal origin in Ethiopia: A meta-analysis. Cogent Food Agric. 2019, 5, 1642981. [Google Scholar] [CrossRef]
- World Health Organization. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. 2017. Available online: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/ (accessed on 1 August 2025).
- Singha, S.; Thomas, R.; Viswakarma, J.N.; Gupta, V.K. Foodborne illnesses of Escherichia coli O157 origin and its control measures. J. Food Sci. Technol. 2023, 60, 1274–1283. [Google Scholar] [CrossRef]
- Cleary, T.G. Cytotoxin producing Escherichia coli and the hemolytic uremic syndrome. Pediatr. Clin. N. Am. 1988, 35, 458–501. [Google Scholar] [CrossRef]
- Tarr, P.I. Escherichia coli O157:H7: Clinical, diagnostic, and epidemiological aspects of human infection. Clin. Infect. Dis. 1995, 20, 1–10. [Google Scholar] [CrossRef]
- Nataro, J.P.; Kaper, J.B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 1998, 11, 142–201. [Google Scholar] [CrossRef]
- Rangel, J.M.; Sparling, P.H.; Crowe, C.; Griffin, P.M.; Swerdlow, D.L. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982–2002. Emerg. Infect. Dis. 2005, 11, 603–609. [Google Scholar] [CrossRef]
- Mead, P.S.; Slutsker, L.; Dietz, V.; McCaig, L.F.; Bresee, J.S.; Shapiro, C.; Griffin, P.M.; Tauxe, R.V. Food-related illness and death in the United States. Emerg. Infect. Dis. 1999, 5, 607–625. [Google Scholar] [CrossRef]
- Frenzen, P.D.; Drake, A.; Angulo, F.J. Economic cost of illness due to Escherichia coli O157 infections in the United States. J. Food Prot. 2005, 68, 2623–2630. [Google Scholar] [CrossRef]
- Alhadlaq, M.A.; Aljurayyad, O.I.; Almansour, A.; Al-Akeel, S.I.; Alzahrani, S.O.; Alsalman, S.A.; Yahya, R.; Al-Hindi, R.R.; Hakami, M.A.; Alshahrani, S.D.; et al. Overview of pathogenic Escherichia coli, with a focus on Shiga toxin-producing serotypes, global outbreaks (1982–2024) and food safety criteria. Gut Pathog. 2024, 16, 57. [Google Scholar] [CrossRef]
- Nemati, A.; Dadvar, A.; Eppinger, M.; Karimpour, Z.; Saberi Kakhki, S.; Sabeti Moghaddam Sabzevar, A.; Badouei, M.A.; Gigliucci, F.; Santos, L.F.d.; Nakamura, K.; et al. Shiga Toxin-Producing Escherichia coli (STEC) in developing countries: A 10-year review with global perspective. Microorganisms 2025, 13, 1529. [Google Scholar] [CrossRef]
- Tuttle, J.; Gomez, T.; Doyle, M.P.; Wells, J.G.; Zhao, T.; Tauxe, R.V.; Griffin, P.M. Lessons from a large outbreak of Escherichia coli O157:H7 infections: Insights into the infectious dose and method of widespread contamination of hamburger patties. Epidemiol. Infect. 1999, 122, 185–192. [Google Scholar] [CrossRef]
- Lin, J.; Smith, M.P.; Chapin, K.C.; Baik, H.S.; Bennett, G.N.; Foster, J.W. Mechanisms of acid resistance in enterohemorrhagic Escherichia coli. Appl. Environ. Microbiol. 1996, 62, 3094–3100. [Google Scholar] [CrossRef]
- Jordan, K.N.; Oxford, L.; O’Byrne, C.P. Survival of low-pH stress by Escherichia coli O157:H7: Correlation between alterations in the cell envelope and increased acid tolerance. Appl. Environ. Microbiol. 1999, 65, 3048–3055. [Google Scholar] [CrossRef]
- Price, S.B.; Wright, J.C.; DeGraves, F.J.; Castanie-Cornet, M.; Foster, J.W. Acid resistance systems required for survival of Escherichia coli O157:H7 in the bovine gastrointestinal tract and in apple cider are different. Appl. Environ. Microbiol. 2004, 70, 4792–4799. [Google Scholar] [CrossRef]
- Jones, C.M.; Price, R.E.; Breidt, F. Escherichia coli O157:H7 stationary-phase acid resistance and assessment of aurvival in a model vegetable fermentation system. J. Food Prot. 2020, 83, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Diez-Gonzalez, F.; Russell, J.B. Factors affecting the extreme acid resistance of Escherichia coli O157:H7. Food Microbiol. 1999, 16, 367–374. [Google Scholar] [CrossRef]
- Glass, K.A.; Loeffelholz, J.M.; Ford, J.P.; Doyle, M.P. Fate of Escherichia coli O157:H7 as affected by pH or sodium chloride and in fermented, dry sausage. Appl. Environ. Microbiol. 1992, 58, 2513–2516. [Google Scholar] [CrossRef] [PubMed]
- Nada, H.G.; El-Tahan, A.S.; El-Didamony, G.; Askora, A. Detection of multidrug-resistant Shiga toxin-producing Escherichia coli in some food products and cattle faeces in Al-Sharkia, Egypt: One health menace. BMC Microbiol. 2023, 23, 127. [Google Scholar] [CrossRef]
- Stager, C.; Donovan, D.; Edwards, L.; Pereira, E.; Williams, L.; Freiman, J.; Schwensohn, C.; Gieraltowski, L. Notes from the Field: Multistate Outbreak of Escherichia coli O157:H7 Infections Linked to a National Fast-Food Chain—United States, 2022. Morb. Mortal. Wkly. Rep. 2023, 72, 732–733. [Google Scholar] [CrossRef]
- CDC. Outbreak Linked to Ground Beef. 2022. Available online: https://archive.cdc.gov/www_cdc_gov/ecoli/2022/o157h7-09-22/index.html (accessed on 1 August 2025).
- Thomas, C.M.; Marr, J.H.; Durso, L.M.; Golwalkar, M.; Irving, D.J.; Orejuela, K.; Rasnic, R.; Ripley, D.; Rue, B.; Thomas, L.S.; et al. Notes from the field: Shiga toxin-producing Escherichia coli O157:H7 linked to raw milk consumption associated with a cow-share arrangement—Tennessee, 2022. Morbid Mortal. Weekly Rep. 2023, 72, 469–470. [Google Scholar] [CrossRef]
- Chen, J.C.; Patel, K.; Smith, P.A.; Vidyaprakash, E.; Snyder, C.; Tagg, K.A.; Webb, H.E.; Schroeder, M.N.; Katz, L.S.; Rowe, L.A.; et al. Reoccurring Escherichia coli O157:H7 Strain linked to leafy greens–associated outbreaks, 2016–2019. Emerg. Infect. Dis. 2023, 29, 1895–1899. [Google Scholar] [CrossRef]
- CDC. 2016 E. coli Outbreak LINKED to Alfalfa Sprouts Produced by JACK & the Green Sprouts. 2016. Available online: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/ecoli/2016/o157-02-16/index.html (accessed on 1 August 2025).
- Cody, S.H.; Glynn, M.K.; Farrar, J.A.; Cairns, K.L.; Griffin, P.M.; Kobayashi, J.; Fyfe, M.; Hoffman, R.; King, A.S.; Lewis, J.H.; et al. An outbreak of Escherichia coli O157:H7 infection from unpasteurized commercial apple juice. Ann. Intern. Med. 1999, 130, 202–209. [Google Scholar] [CrossRef]
- Oluwarinde, B.O.; Ajose, D.J.; Abolarinwa, T.O.; Montso, P.K.; Du Preez, I.; Njom, H.A.; Ateba, C.N. Safety properties of Escherichia coli O157:H7 specific bacteriophages: Recent advances for food safety. Foods 2023, 12, 3989. [Google Scholar] [CrossRef] [PubMed]
- Hibstu, Z.; Belew, H.; Akelew, Y.; Mengist, H.M. Phage Therapy: A different approach to fight bacterial infections. Biologics 2022, 6, 173–186. [Google Scholar] [CrossRef]
- McGee, L.W.; Barhoush, Y.; Shima, R.; Hennessy, M. Phage-resistant mutations impact bacteria susceptibility to future phage infections and antibiotic response. Ecol. Evol. 2023, 13, e9712. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, S.D.; Hosseinidoust, Z. Phage therapy with a focus on the human microbiota. Antibiotics 2019, 8, 131. [Google Scholar] [CrossRef]
- Lu, Z.; Breidt, F. Escherichia coli O157:H7 bacteriophage Φ241 isolated from an industrial cucumber fermentation at high acidity and salinity. Front. Microbiol. 2015, 6, 67. [Google Scholar] [CrossRef]
- Lu, Z.; Tseng, T.-T. Complete genome sequence of Escherichia coli O157:H7 phage Φ241. Microbiol. Resour. Announc. 2024, 13, e00106-24. [Google Scholar] [CrossRef]
- Lu, Z.; Marchant, J.; Thompson, S.; Melgarejo, H.; Ignatova, D.; Kopić, S.; Damaj, R.; Trejo, H.; Paramo, R.; Reed, A. Bacteriophages isolated from turkeys infecting diverse Salmonella serovars. Front. Microbiol. 2022, 13, 933751. [Google Scholar] [CrossRef]
- Anonymous. pH of Beef, Chicken, Fish and Pork. 2024. Available online: https://www.pickyourown.org/pH-of-meat-fish.php (accessed on 7 January 2025).
- USDA. Pathogen Modeling Program (PMP) Online. pH of Selected Foods. 2025. Available online: https://pmp.errc.ars.usda.gov/phOfSelectedFoods.aspx (accessed on 1 August 2025).
- Dewi1, D.C.; Mahdi1, C.; Sulistyarti, H.; Aulaniàm, A. Identification of volatile compounds in several meat and bone broth using Solid Phase Micro Extraction-Gas Chromatography Mass Spectrometry (SPME-GCMS) for initial detection of Halal and Non-Halal Food. J. Pure App. Chem. Res. 2023, 12, 65–79. [Google Scholar] [CrossRef]
- Anonymous. What Is the pH of Apple Juice? 2022. Available online: https://www.upthirst.com/what-you-should-know-about-the-ph-of-apple-juice/ (accessed on 18 March 2024).
- Labrie, S.J.; Samson, J.E.; Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010, 8, 317–327. [Google Scholar] [CrossRef]
- Oechslin, F. Resistance development to bacteriophages occurring during bacteriophage therapy. Viruses 2018, 10, 351. [Google Scholar] [CrossRef] [PubMed]
- Dimitriu, T.; Kurilovich, E.; Łapińska, U.; Severinov, K.; Pagliara, S.; Szczelkun, M.D.; Westra, E.R. Bacteriostatic antibiotics promote CRISPR-Cas adaptive immunity by enabling increased spacer acquisition. Cell Host Microbe 2022, 30, 31–40.e5. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi Silva, J.; Storms, Z.; Sauvageau, D. Host receptors for bacteriophage adsorption. FEMS Microbiol. Lett. 2016, 363, fnw002. [Google Scholar] [CrossRef] [PubMed]
- Rostøl, J.T.; Marraffini, L. (Ph)ighting phages: How bacteria resist their parasites. Cell Host Microbe 2019, 25, 184–194. [Google Scholar] [CrossRef]
- Abedon, S.T.; Danis-Wlodarczyk, K.M.; Wozniak, D.J. Phage cocktail development for bacteriophage therapy: Toward improving spectrum of activity breadth and depth. Pharmaceuticals 2021, 14, 1019. [Google Scholar] [CrossRef]
- Kaneko, T.; Osaka, T.; Tsuneda, S. Tailoring effective phage cocktails for long-term lysis of Escherichia coli based on physiological properties of constituent phages. Phage 2023, 4, 128–135. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, W.; Zhong, Q.; Chen, Q.; He, X.; Baker, J.L.; Xiong, K.; Jin, X.; Wang, J.; Hu, F.; et al. Development of a bacteriophage cocktail to constrain the emergence of phage-resistant Pseudomonas aeruginosa. Front. Microbiol. 2020, 11, 327. [Google Scholar] [CrossRef]
- EcoShield™ PX. Available online: https://www.intralytix.com/product/11?e=EcoShield (accessed on 1 August 2025).
- Yang, Z.; Meng, X.; Breidt, F.; Dean, L.L.; Arritt, F.M. Effects of acetic acid and arginine on pH elevation and growth of Bacillus licheniformis in an acidified cucumber juice medium. J. Food Prot. 2015, 78, 728–737. [Google Scholar] [CrossRef]
- Dupree, D.; Price, R.E.; Burgess, B.; Andress, E.L.; Breidt, F. Effects of sodium chloride or calcium chloride concentration on the growth and survival Escherichia coli O157:H7 in model vegetable fermentations. J. Food Prot. 2019, 82, 570–578. [Google Scholar] [CrossRef]
- Anthony, A.; Skinner, C.; Katare, N.; Guydan, D.; Johanningsmeier, S.; Breidt, F. Development of a synthetic medium to investigate the role of buffer capacity and metabolites on the fermentation chemistry of cucumber juice. J. Food Prot. 2025, 88, 100580. [Google Scholar] [CrossRef]
- Lu, Z.; Fleming, H.P.; McFeeters, R.F. Differential glucose and fructose utilization during cucumber juice fermentation. J. Food Sci. 2001, 66, 162–166. [Google Scholar] [CrossRef]
- Lu, Z.; Fleming, H.P.; McFeeters, R.F. Effects of anions and cations on sugar utilization in cucumber juice fermentation. J. Food Sci. 2002, 67, 1155–1161. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, X.; Guo, N. The antimicrobial activities and action-mechanism of tea tree oil against food-borne bacteria in fresh cucumber juice. Microb. Pathog. 2018, 125, 262–271. [Google Scholar] [CrossRef]
- Breidt, F.; Skinner, C. Buffer models for pH and acid changes occurring in cucumber juice fermented with Lactiplantibacillus pentosus and Leuconostoc mesenteroides. J. Food Prot. 2022, 85, 1273–1281. [Google Scholar] [CrossRef]
- Koskella, B.; Brockhurst, M.A. Bacteria-phage coevolution as a driver of ecological and evolutionary processes in microbial communities. FEMS Microbiol. Rev. 2014, 38, 916–931. [Google Scholar] [CrossRef] [PubMed]
- De Sordi, L.; Lourenço, M.; Debarbieux, L. “I will survive”: A tale of bacteriophage-bacteria coevolution in the gut. Gut Microbes 2019, 10, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xiong, X.-S.; Yang, Y.-Y.; Wang, J.-J.; Wang, M.-M.; Tang, J.-W.; Liu, Q.-H.; Wang, L.; Gu, B. Effects of NaCl concentrations on growth patterns, phenotypes associated with virulence, and energy metabolism in Escherichia coli BW25113. Front. Microbiol. 2021, 12, 705326. [Google Scholar] [CrossRef] [PubMed]
- Kargi, F.; Dinçer, A.R. Salt inhibition effects in biological treatment of saline wastewater in RBC. J. Environ. Eng. 1999, 125, 966–971. [Google Scholar] [CrossRef]
- Kan, K.; Chen, J.; Kawamura, S.; Koseki, S. Characteristics of d-Tryptophan as an antibacterial agent: Effect of sodium chloride concentration and temperature on Escherichia coli growth inhibition. J. Food Prot. 2018, 81, 25–30. [Google Scholar] [CrossRef]
- Corrias, F.; Scano, E.; Sarais, G.; Angioni, A. Influence of salting technology on the diffusion of NaCl in swordfish (Xiphias gladius) fillets. Foods 2022, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Elias, M.; Laranjo, M.; Agulheiro-Santos, A.C.; Potes, M.E. The Role of Salt on Food and Human Health. Salt Earth 2020, 19, 1–19. [Google Scholar] [CrossRef]
- Stahl, L.; Frost, V.; Heard, M. Creating a microcosm to examine salinity tolerance of Escherichia coli in beach sand. Winthrop McNair Res. Bull. 2016, 2, 56–61. [Google Scholar]
- USDA. Apples and Oranges Are the Top U.S. Fruit Choices. 2023. Available online: https://www.ers.usda.gov/data-products/chart-gallery/gallery/chart-detail/?chartId=58322 (accessed on 7 January 2025).
- FDA. Introduction to Acid Foods. 2018. Available online: https://www.fdareader.com/blog/2018/12/20/introduction-to-acid-foods (accessed on 18 March 2024).
- Desmarchelier, P.M.; Fegan, N. Enteropathogenic Escherichia coli. In Foodborne Microorganisms of Public Health Significance; Hocking, A.D., Ed.; Australian Institute of Food Sci and Tech, NSW Branch: Sydney, Australia, 2003; pp. 267–310. [Google Scholar]
- Del Rosario, B.A.; Beuchat, L.R. Survival and growth of enterohemorrhagic Escherichia coli O157:H7 in cantaloupe and watermelon. J. Food Prot. 1995, 58, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, C.E.; Broadbent, J.R. External concentration of organic acid anions and pH: Key independent variables for studying how organic acids inhibit growth of bacteria in mildly acidic foods. J. Food Sci. 2009, 74, R12–R15. [Google Scholar] [CrossRef]
- Diez-Gonzalez, F.; Russell, J.B. The ability of Escherichia coli O157:H7 to decrease its intracellular pH and resist the toxicity of acetic acid. Microbiology 1997, 143, 1175–1180. [Google Scholar] [CrossRef]
- Kim, G.H.; Breidt, F.; Fratamico, P.; Oh, D.-H. Acid resistance and molecular characterization of Escherichia coli O157:H7 and different non-O157 Shiga toxin-producing E. coli serogroups. J. Food Sci. 2015, 80, 2257–2264. [Google Scholar] [CrossRef]
- Roering, A.M.; Luchansky, J.B.; Ihnot, A.M.; Ansay, S.E.; Kaspar, C.W.; Ingham, S.C. Comparative survival of Salmonella typhimurium DT104, Listeria monocytogenes, and Escherichia coli O157:H7 in preservative-free apple cider and simulated gastric fluid. Int. J. Food Microbiol. 1999, 46, 263–269. [Google Scholar] [CrossRef]
- Wi, S.M.; Kim, S.K.; Lee, J.B.; Yoon, J.W. Acid tolerance of enterohemorrhagic Escherichia coli O157:H7 strain ATCC 43894 and its relationship with a large virulence plasmid pO157. Vet. Microbiol. 2023, 284, 109833. [Google Scholar] [CrossRef]
- Large, T.M.; Walk, S.T.; Whittam, T.S. Variation in acid resistance among shiga toxin-producing clones of pathogenic Escherichia coli. Appl. Environ. Microbiol. 2005, 71, 2493–2500. [Google Scholar] [CrossRef]
- Peterson, W.L.; Mackowiak, P.A.; Barnett, C.C.; Marling-Cason, M.; Haley, M.L. The human gastric bactericidal barrier: Mechanisms of action, relative antibacterial activity and dietary influences. J. Infect. Dis. 1989, 159, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Conner, D.E.; Kotrola, J.S. Growth and survival of Escherichia coli O157:H7 under acidic conditions. Appl. Environ. Microbiol. 1995, 61, 382–385. [Google Scholar] [CrossRef]
- Deng, Y.; Ryu, J.H.; Beuchat, L.R. Tolerance of acid-adapted and non-adapted Escherichia coli O157:H7 cells to reduced pH as affected by type of acidulant. J. Appl. Microbiol. 1999, 86, 203–210. [Google Scholar] [CrossRef]
- Buchanan, R.L.; Edelson, S.G. pH-dependent stationary-phase acid resistance response of enterohemorrhagic Escherichia coli in the presence of various acidulants. J. Food Prot. 1999, 62, 211–218. [Google Scholar] [CrossRef]
- Breidt, F.; Hayes, J.; McFeeters, R.F. Independent effects of acetic acid and pH on survival of Escherichia coli in simulated acidified pickle products. J. Food Prot. 2004, 67, 12–18. [Google Scholar] [CrossRef]
- Ma, B.; Chen, J.; Zheng, H.; Fang, T.; Ogutu, C.; Li, S.; Han, Y.; Wu, B. Comparative assessment of sugar and malic acid composition in cultivated and wild apples. Food Chem. 2015, 172, 86–91. [Google Scholar] [CrossRef]
- Fleming, H.P.; Kyung, K.H.; Breidt, F. Vegetable fermentations. In Biotechnology: Enzymes, Biomass, Food and Feed, 2nd ed.; Rehm, H.J., Reed, G., Eds.; VCH Publishers, Inc.: New York, NY, USA, 1995; Volume 9, pp. 629–661. [Google Scholar]
- Lu, Z.; Pérez-Díaz, I.M.; Hayes, J.S.; Breidt, F. Bacteriophage ecology in a commercial cucumber fermentation. Appl. Environ. Microbiol. 2012, 78, 8571–8578. [Google Scholar] [CrossRef]
- FDA. What You Need to Know About Juice Safety. 2024. Available online: https://www.fda.gov/food/buy-store-serve-safe-food/what-you-need-know-about-juice-safety (accessed on 1 August 2025).
- FDA. Guidance for Industry: Juice Hazard Analysis Critical Control Point Hazards and Controls Guidance, First Edition. 2004. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-juice-hazard-analysis-critical-control-point-hazards-and-controls-guidance-first#:~:text=The%20juice%20HACCP%20regulation%20includes,the%20hazard%20evaluation%20step%20is (accessed on 1 August 2025).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z. Efficacy of Escherichia coli O157:H7 Phage Φ241 in Model Food Systems. Appl. Microbiol. 2025, 5, 87. https://doi.org/10.3390/applmicrobiol5030087
Lu Z. Efficacy of Escherichia coli O157:H7 Phage Φ241 in Model Food Systems. Applied Microbiology. 2025; 5(3):87. https://doi.org/10.3390/applmicrobiol5030087
Chicago/Turabian StyleLu, Zhongjing. 2025. "Efficacy of Escherichia coli O157:H7 Phage Φ241 in Model Food Systems" Applied Microbiology 5, no. 3: 87. https://doi.org/10.3390/applmicrobiol5030087
APA StyleLu, Z. (2025). Efficacy of Escherichia coli O157:H7 Phage Φ241 in Model Food Systems. Applied Microbiology, 5(3), 87. https://doi.org/10.3390/applmicrobiol5030087




