Biosynthesis of the Siderophore Desferrioxamine E in Rouxiella badensis SER3 and Its Antagonistic Activity Against Fusarium brachygibbosum
Abstract
1. Introduction
2. Materials and Methods
2.1. Siderophore Production
2.2. Characterizing the Siderophore Produced by Rouxiella badensis SER3
2.3. Confrontation Assays In Vitro
2.4. In Vitro Assays with Commercial Desferrioxamine E
2.5. Desferrioxamine E Cluster Analysis and Phylogeny
2.6. Assays of Gene Expression and Desferrioxamine E Production
2.7. Effect of Direct Interaction of Strain SER3 on the Mycelium of the Pathogen Fusarium brachygibbosum
3. Results
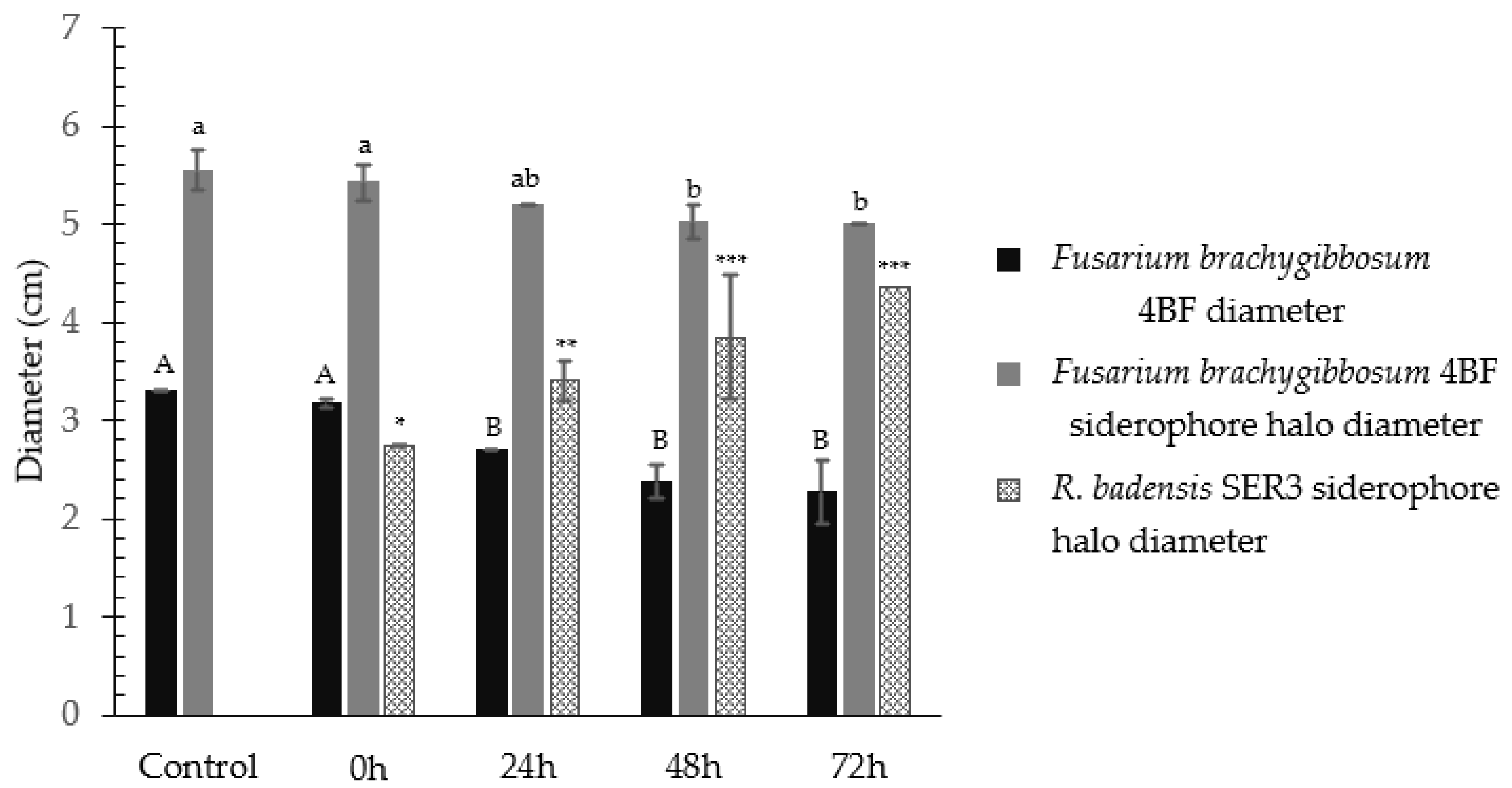
3.1. Production and Characterization of the Rouxiella badensis SER3 Siderophores
3.2. Confrontation Assays
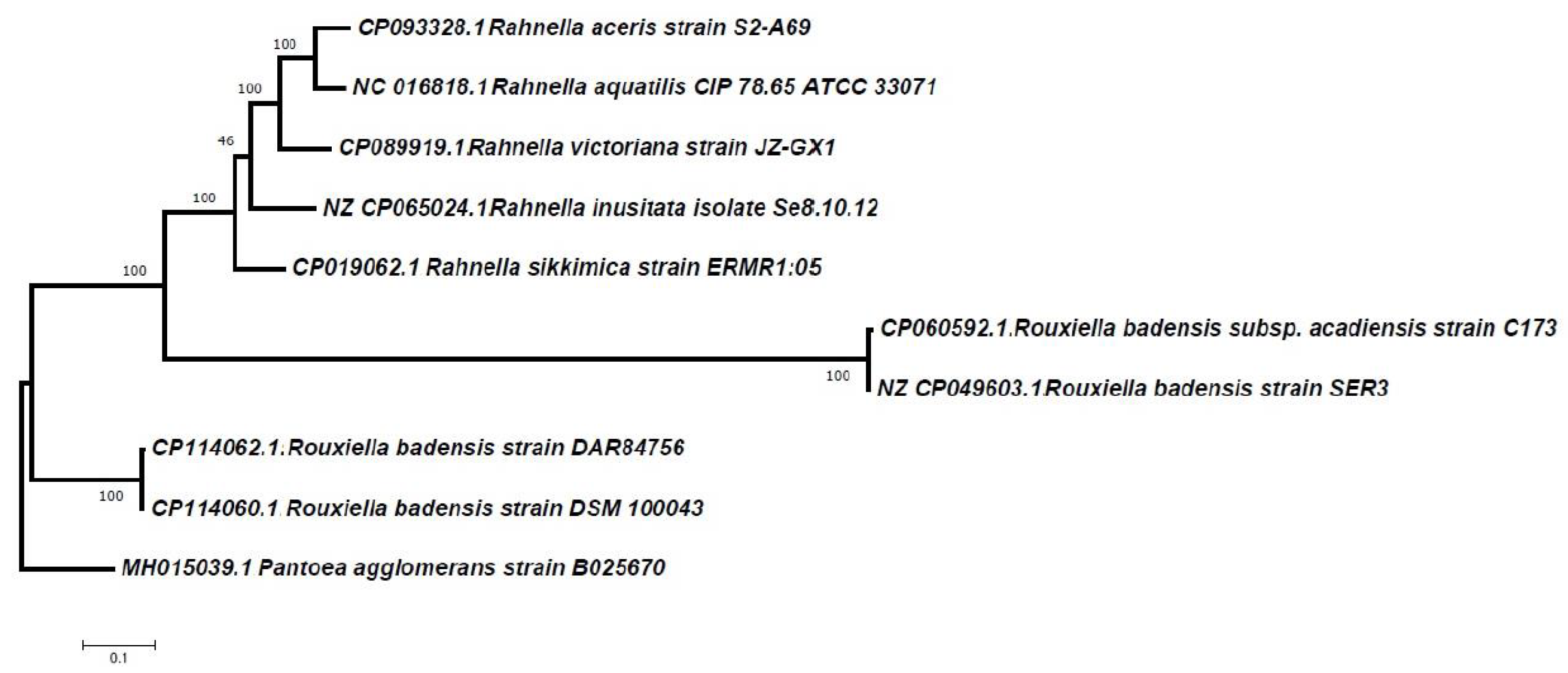
3.3. Desferrioxamine E (Nocardamine) Cluster Analysis and Phylogeny
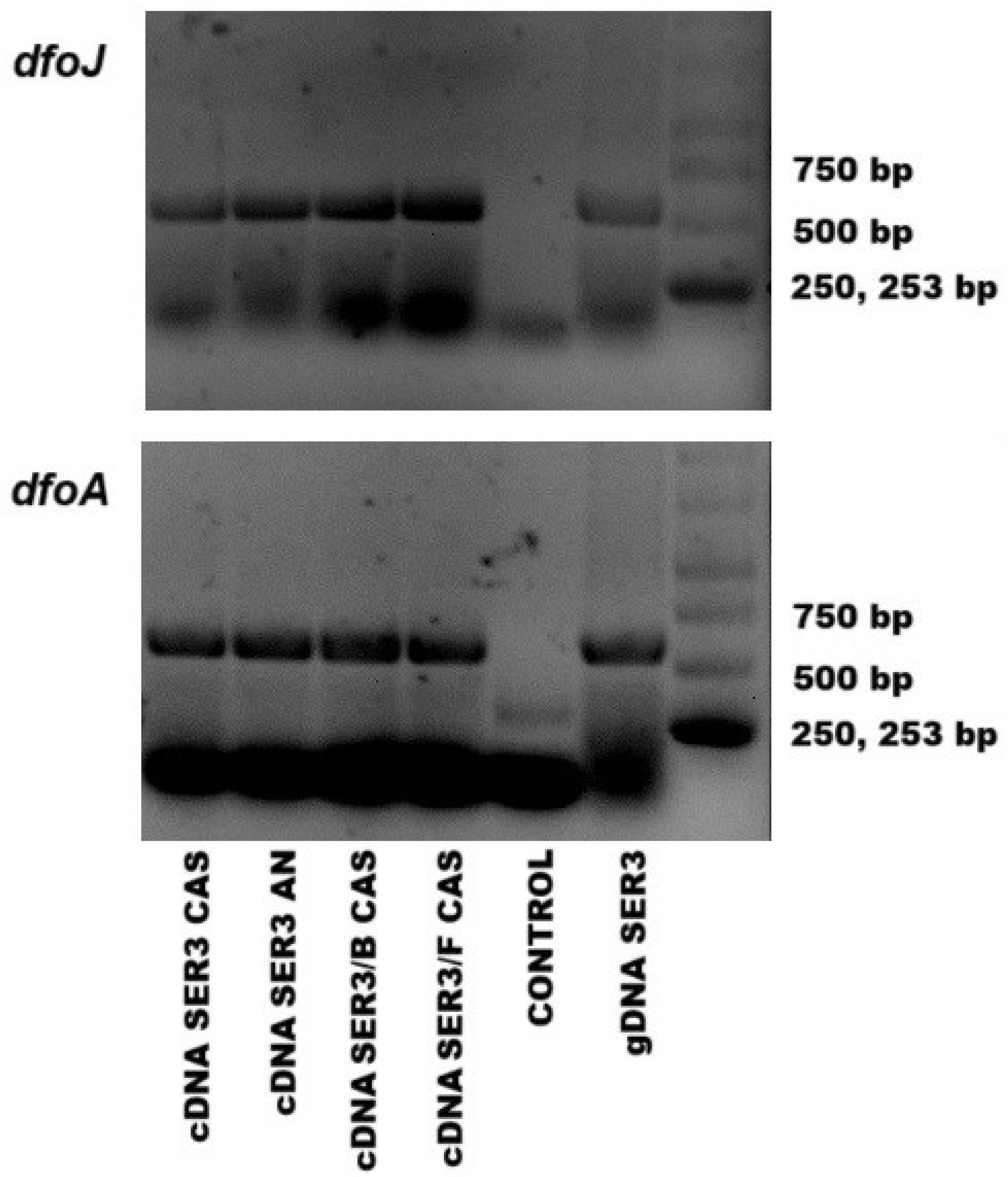
3.4. Gene Expression Assays and Desferrioxamine E Production
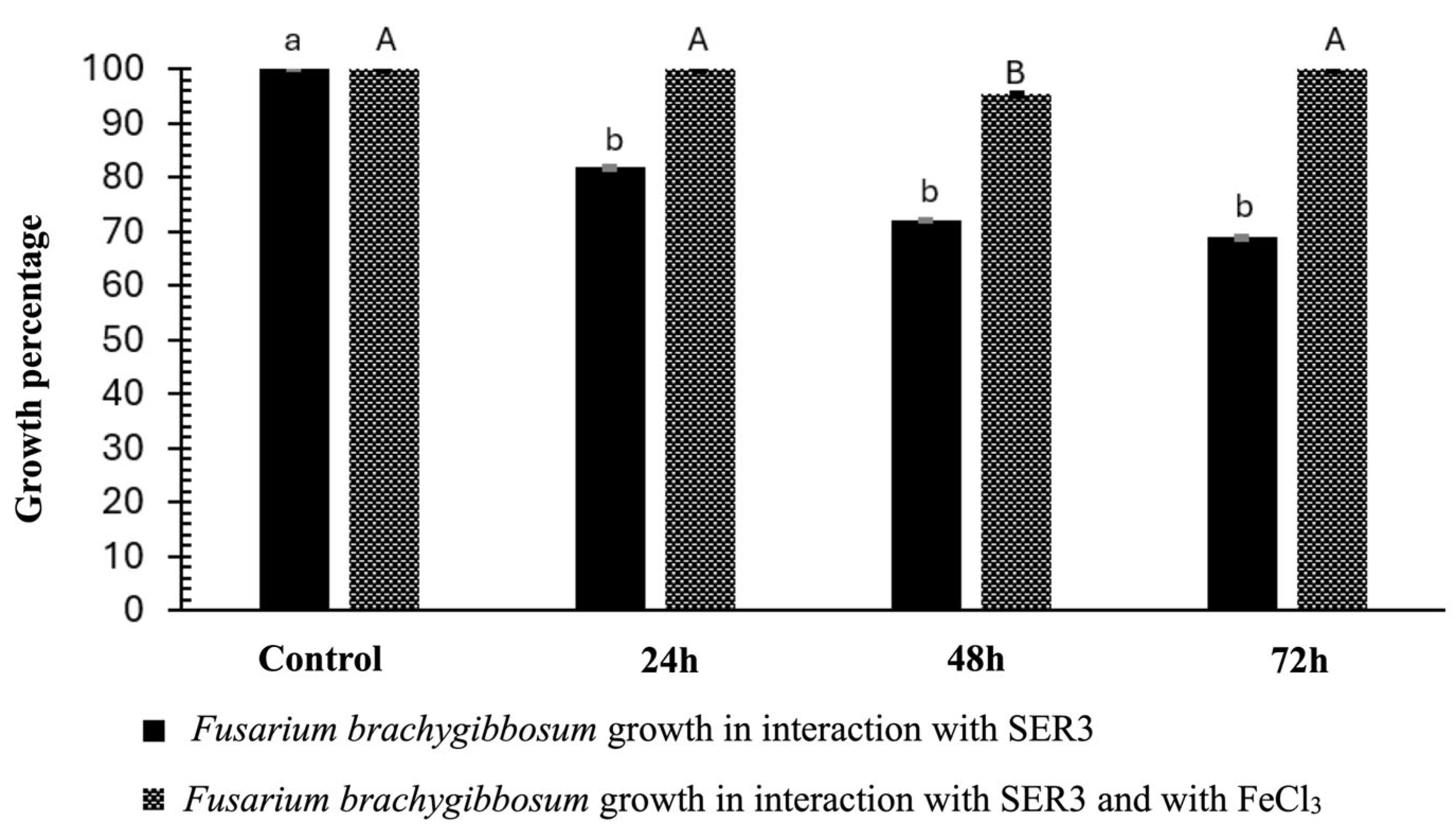
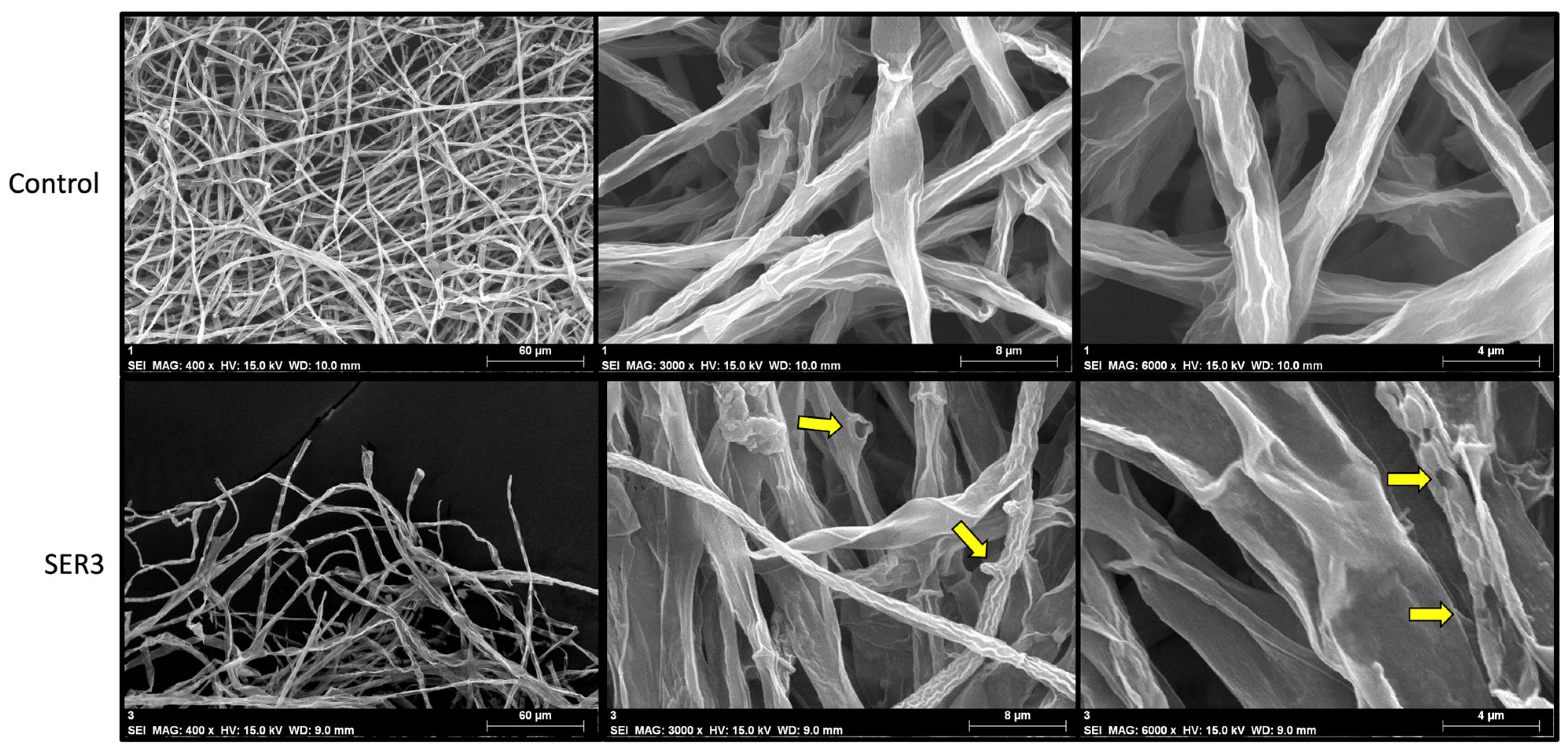
3.5. Effect of Direct Interaction of the SER3 Strain on the Mycelium of the Pathogens Botrytis cinerea and Fusarium brachygibbosum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boiteau, R.M.; Fansler, S.J.; Farris, Y.; Shaw, J.B.; Koppenaal, D.W.; Pasa-Tolic, L.; Jansson, J.K. Siderophore profiling of co-habitating soil bacteria by ultra-high resolution mass spectrometry. Metallomics 2019, 11, 166–175. [Google Scholar] [CrossRef]
- Kramer, J.; Özkaya, Ö.; Kümmerli, R. Bacterial siderophores in community and host interactions. Nat. Rev. Microbiol. 2020, 18, 152–163. [Google Scholar] [CrossRef]
- West, S.A.; Griffin, A.S.; Gardner, A.; Diggle, S.P. Social evolution theory for microorganisms. Nat. Rev. Microbiol. 2006, 4, 597–607. [Google Scholar] [CrossRef]
- Yamanaka, K.; Oikawa, H.; Ogawa, H.-O.; Hosono, K.; Shinmachi, F.; Takano, H.; Sakuda, S.; Beppu, T.; Ueda, K. Desferrioxamine E produced by Streptomyces griseus stimulates growth and development of Streptomyces tanashiensis. Microbiology 2005, 151, 2899–2905. [Google Scholar] [CrossRef]
- Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Singh, R.; Sharma, K.; Vishwakarma, R.K. Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1498–1513. [Google Scholar] [CrossRef]
- Carmona-Hernandez, S.; Reyes-Pérez, J.J.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G.; Cerdan-Cabrera, C.R.; Hernandez-Montiel, L.G. Biocontrol of Postharvest Fruit Fungal Diseases by Bacterial Antagonists: A Review. Agronomy 2019, 9, 121. [Google Scholar] [CrossRef]
- del Carmen Orozco Mosqueda, M.; Pizano, G.S. Bacterias Promotoras del Crecimiento Vegetal; Editorial Fontamara: Coyoacán, Mexico, 2020. [Google Scholar]
- Fischbach, M.A.; Walsh, C.T.; Clardy, J. The evolution of gene collectives: How natural selection drives chemical innovation. Proc. Natl. Acad. Sci. USA 2008, 105, 4601–4608. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.d.C.; Santoyo, G.; Glick, B.R. Recent Advances in the Bacterial Phytohormone Modulation of Plant Growth. Plants 2023, 12, 606. [Google Scholar] [CrossRef]
- Morales-Cedeño, L.R.; Santos-Villalobos, S.d.L.; Santoyo, G. Functional and Genomic Analysis of Rouxiella badensis SER3 as a Novel Biocontrol Agent of Fungal Pathogens. Front. Microbiol. 2021, 12, 709855. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Neilands, J.B. Microbial Iron Compounds. Annu. Rev. Biochem. 1981, 50, 715–731. [Google Scholar] [CrossRef]
- Smits, T.H.M.; Duffy, B. Genomics of iron acquisition in the plant pathogen Erwinia amylovora: Insights in the biosynthetic pathway of the siderophore desferrioxamine E. Arch. Microbiol. 2011, 193, 693–699. [Google Scholar] [CrossRef]
- Blin, K.; Wolf, T.; Chevrette, M.G.; Lu, X.; Schwalen, C.J.; Kautsar, S.A.; Duran, H.G.S.; de Los Santos, E.L.C.; Kim, H.U.; Nave, M.; et al. antiSMASH 4.0—Improvements in chemistry prediction and gene cluster boundary identification. Nucleic Acids Res. 2017, 45, W36–W41. [Google Scholar] [CrossRef]
- Santos-Villalobos, S.d.L.; Barrera-Galicia, G.C.; Miranda-Salcedo, M.A.; Peña-Cabriales, J.J. Burkholderia cepacia XXVI siderophore with biocontrol capacity against Colletotrichum gloeosporioides. World J. Microbiol. Biotechnol. 2012, 28, 2615–2623. [Google Scholar] [CrossRef]
- Karlsson, M.E.; Hellström, M.; Flöhr, A.; Bergstrand, K.-J.; Alsanius, B.W. The power of light: Impact on the performance of biocontrol agents under minimal nutrient conditions. Front. Microbiol. 2023, 14, 1087639. [Google Scholar] [CrossRef]
- Hawkes, C.V.; Connor, E.W. Translating Phytobiomes from Theory to Practice: Ecological and Evolutionary Considerations. Phytobiomes J. 2017, 1, 57–69. [Google Scholar] [CrossRef]
- Gu, S.; Yang, T.; Shao, Z.; Wang, T.; Cao, K.; Jousset, A.; Friman, V.-P.; Mallon, C.; Mei, X.; Wei, Z.; et al. Siderophore-Mediated Interactions Determine the Disease Suppressiveness of Microbial Consortia. mSystems 2020, 5, e00811-19. [Google Scholar] [CrossRef]
- Miethke, M.; Marahiel, M.A. Siderophore-Based Iron Acquisition and Pathogen Control. Microbiol. Mol. Biol. Rev. 2007, 71, 413–451. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Bera, T.; Chakrabarty, A.M. Microbial siderophore—A boon to agricultural sciences. Biol. Control 2020, 144, 104214. [Google Scholar] [CrossRef]
- Lambrese, Y.; Guiñez, M.; Calvente, V.; Sansone, G.; Cerutti, S.; Raba, J.; Sanz, M.I. Production of siderophores by the bacterium Kosakonia radicincitans and its application to control of phytopathogenic fungi. Bioresour. Technol. Rep. 2018, 3, 82–87. [Google Scholar] [CrossRef]
- Nunes, C.A. Biological control of postharvest diseases of fruit. Eur. J. Plant Pathol. 2012, 133, 181–196. [Google Scholar] [CrossRef]
- Hosseini, A.; Saba, M.K.; Watkins, C.B. Microbial Antagonists to Biologically Control Postharvest Decay and Preserve Fruit Quality. Crit. Rev. Food Sci. Nutr. 2023, 7330–7342. [Google Scholar] [CrossRef]
- Verma, S.; Azevedo, L.C.B.; Pandey, J.; Khusharia, S.; Kumari, M.; Kumar, D.; Kaushalendra; Bhardwaj, N.; Teotia, P.; Kumar, A. Microbial Intervention: An Approach to Combat the Postharvest Pathogens of Fruits. Plants 2022, 11, 3452. [Google Scholar] [CrossRef]
- Ram, R.M.; Keswani, C.; Bisen, K.; Tripathi, R.; Singh, S.P.; Singh, H.B. Biocontrol technology: Eco-friendly approaches for sustainable agriculture. In Omics Technologies and Bio-Engineering: Volume 2: Towards Improving Quality of Life; Elsevier: Amsterdam, The Netherlands, 2018; pp. 177–190. [Google Scholar] [CrossRef]
- Soares, E.V. Perspective on the biotechnological production of bacterial siderophores and their use. Appl. Microbiol. Biotechnol. 2022, 106, 3985–4004. [Google Scholar] [CrossRef]
- Lang, A.S.; Buchan, A.; Burrus, V. Interactions and evolutionary relationships among bacterial mobile genetic elements. Nat. Rev. Microbiol. 2025, 23, 423–438. [Google Scholar] [CrossRef]
- Yin, X.; Stotzky, G. Gene Transfer Among Bacteria in Natural Environments; Academic Press Inc.: Cambridge, MA, USA, 1997. [Google Scholar] [CrossRef]




| Region | Type | From | To | Most Similar Known Cluster | Similarity | |
|---|---|---|---|---|---|---|
| Region 1 | NRPS | 10,168 | 71,398 | Turnerbactin | NRP | 38% |
| Region 2 | Siderophore | 873,555 | 885,909 | Desferrioxamine E | Other | 100% |
| Region 3 | Thiopeptide | 1,138,841 | 1,165,158 | O-antigen | Saccharide | 14% |
| Region 4 | Arylpolyene | 1,962,099 | 2,005,722 | Aryl polyenes | Other | 77% |
| Region 5 | T1PKS | 2,289,792 | 2,332,482 | |||
| Region 6 | T3PKS | 2,719,364 | 2,760,449 | Venemycin | Polyketide | 18% |
| Region 7 | NRPS, TransAT-PKS | 3,528,536 | 3,604,918 | Tolaasin I/tolaasin F | NRP: Lipopeptide | 40% |
| Region 8 | Hserlactone | 4,818,682 | 4,839,332 | |||
| Region 9 | Redox-cofactor | 4,905,452 | 4,927,635 | Lankacidin C | NRP+Polyketide | 13% |
| NCBI Accession Number | Gene | Product |
|---|---|---|
| AWD37884.1 | dfoS | Putative siderophore MFS transporter DfoS |
| AWD37885.1 | dfoC | Putative siderophore biosynthesis protein DfoC |
| AWD37886.1 | dfoA | Putative lysine/ornithine N-monooxygenase DfoA |
| AWD37887.1 | dfoJ | Putative pyridoxal-dependent decarboxylase DfoJ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Cedeño, L.R.; de los Santos Villalobos, S.; Loeza-Lara, P.D.; Mitra, D.; Kumar, A.; Orozco-Mosqueda, M.d.C.; Santoyo, G. Biosynthesis of the Siderophore Desferrioxamine E in Rouxiella badensis SER3 and Its Antagonistic Activity Against Fusarium brachygibbosum. Appl. Microbiol. 2025, 5, 91. https://doi.org/10.3390/applmicrobiol5030091
Morales-Cedeño LR, de los Santos Villalobos S, Loeza-Lara PD, Mitra D, Kumar A, Orozco-Mosqueda MdC, Santoyo G. Biosynthesis of the Siderophore Desferrioxamine E in Rouxiella badensis SER3 and Its Antagonistic Activity Against Fusarium brachygibbosum. Applied Microbiology. 2025; 5(3):91. https://doi.org/10.3390/applmicrobiol5030091
Chicago/Turabian StyleMorales-Cedeño, Luzmaria R., Sergio de los Santos Villalobos, Pedro D. Loeza-Lara, Debasis Mitra, Ajay Kumar, Ma. del Carmen Orozco-Mosqueda, and Gustavo Santoyo. 2025. "Biosynthesis of the Siderophore Desferrioxamine E in Rouxiella badensis SER3 and Its Antagonistic Activity Against Fusarium brachygibbosum" Applied Microbiology 5, no. 3: 91. https://doi.org/10.3390/applmicrobiol5030091
APA StyleMorales-Cedeño, L. R., de los Santos Villalobos, S., Loeza-Lara, P. D., Mitra, D., Kumar, A., Orozco-Mosqueda, M. d. C., & Santoyo, G. (2025). Biosynthesis of the Siderophore Desferrioxamine E in Rouxiella badensis SER3 and Its Antagonistic Activity Against Fusarium brachygibbosum. Applied Microbiology, 5(3), 91. https://doi.org/10.3390/applmicrobiol5030091











