Microwave-Assisted Dried Cells of the Fungus Arthrinium malaysianum as a Potential Biomaterial with Sustainable Bioremediation of Toxic Heavy Metals
Abstract
1. Introduction
| Sl. No. | Name of the Fungi | % Cr(VI) Removal | References |
|---|---|---|---|
| 1 | A. niger (live biomass) | 48.7 | [37] |
| 2 | P. Lilacinus (live biomass) | 100 | |
| 3 | P. spp. (live biomass) | 100 | |
| 4 | A. awamori (dead biomass) | 29 | |
| 5 | A. spp. (live biomass) | 68 | |
| 6 | A. flavus (live biomass) | 99.2 | |
| 7 | A. niger (dead biomass) | 100 | |
| 8 | P. chrysosporium (live biomass) | 98.5 | |
| 9 | R. oryzae (live biomass) | 91.15 | |
| 10 | T. clypeatus (dead biomass) | 84.5 | [38] |
| 11 | A. malaysianum (dead and microwave dried biomass) | 100 | Present study |
2. Materials and Methods
2.1. Materials
2.2. Functionalized Biomass Preparation
2.3. Efficacy of Cr(VI) Biosorption by Microwave-Assisted Heat-Dried Fungal Biosorbent
2.4. Different Interactions of Chromium with Oven-Dried Biomass of A. malaysianum: Various Investigations with Biophysical and Microscopic Studies
2.5. (FTIR) Fourier Transform Infrared Spectroscopy
2.6. X-Ray Photoelectron Spectra Analysis
2.7. FESEM-EDX Analysis
2.8. Performance of Statistical Analysis
3. Results
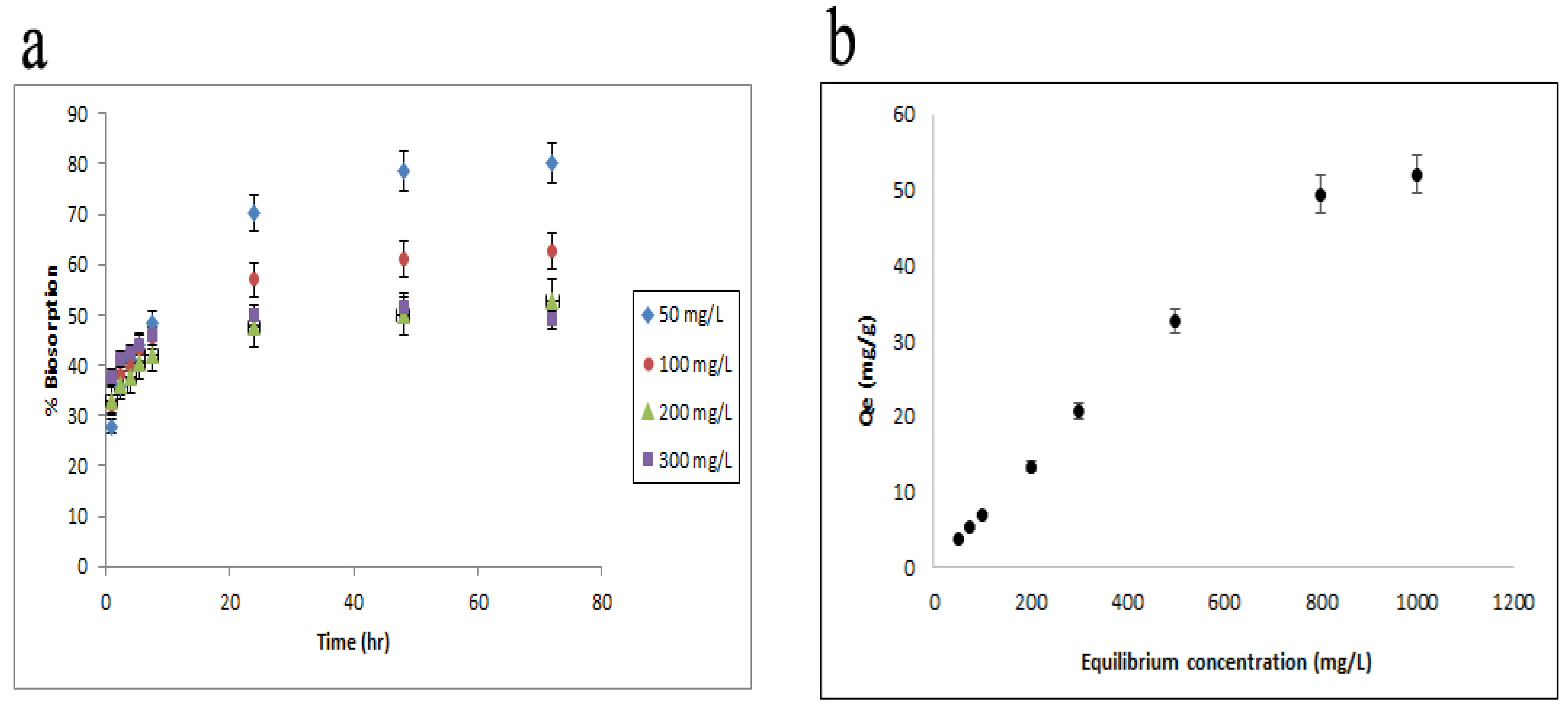
3.1. Mode of Adsorption Shown by Equilibrium Isotherm Studies
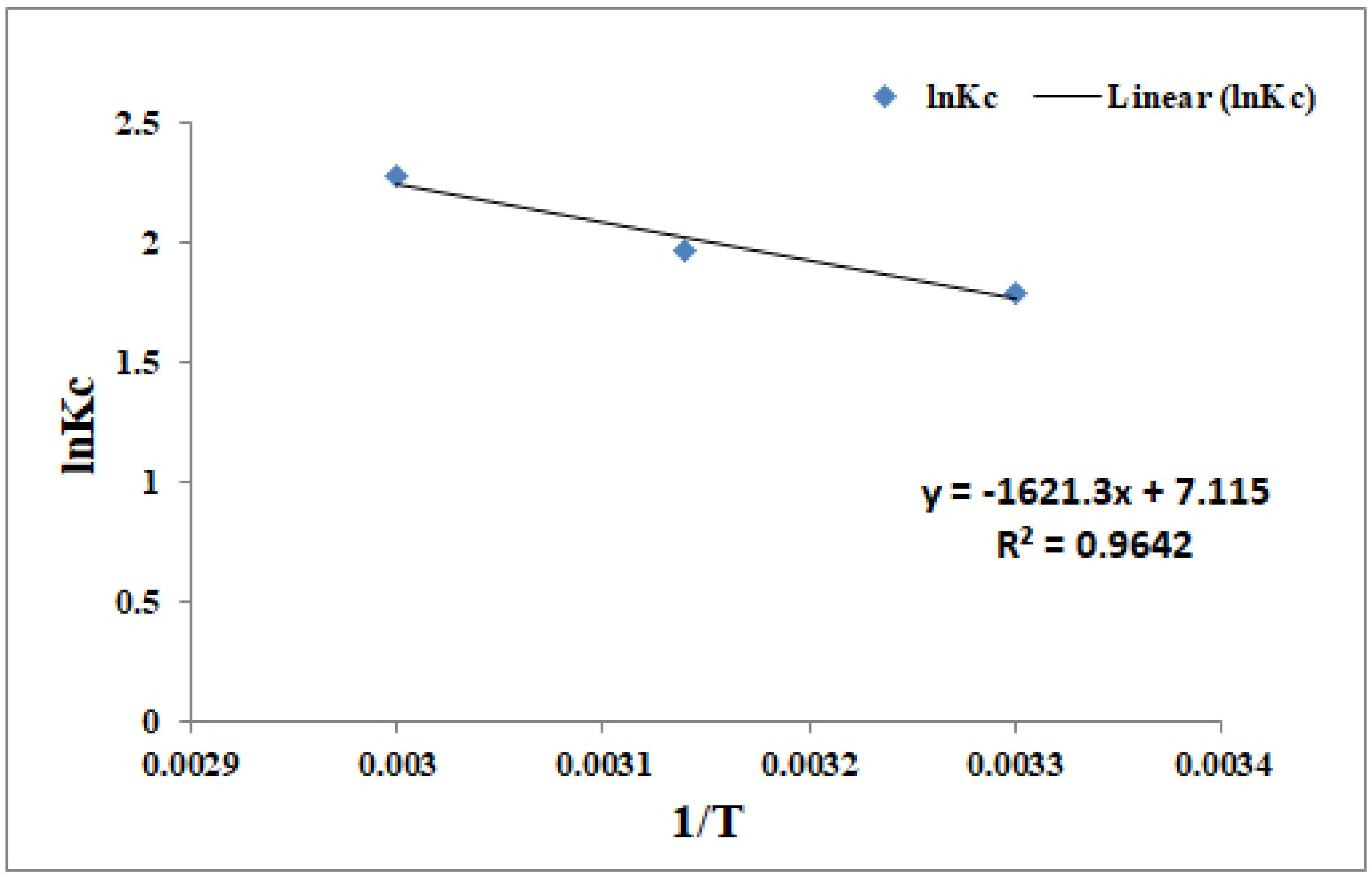
3.2. Endothermic and Entropy Driven Is the Biosorption of Cr(IV)
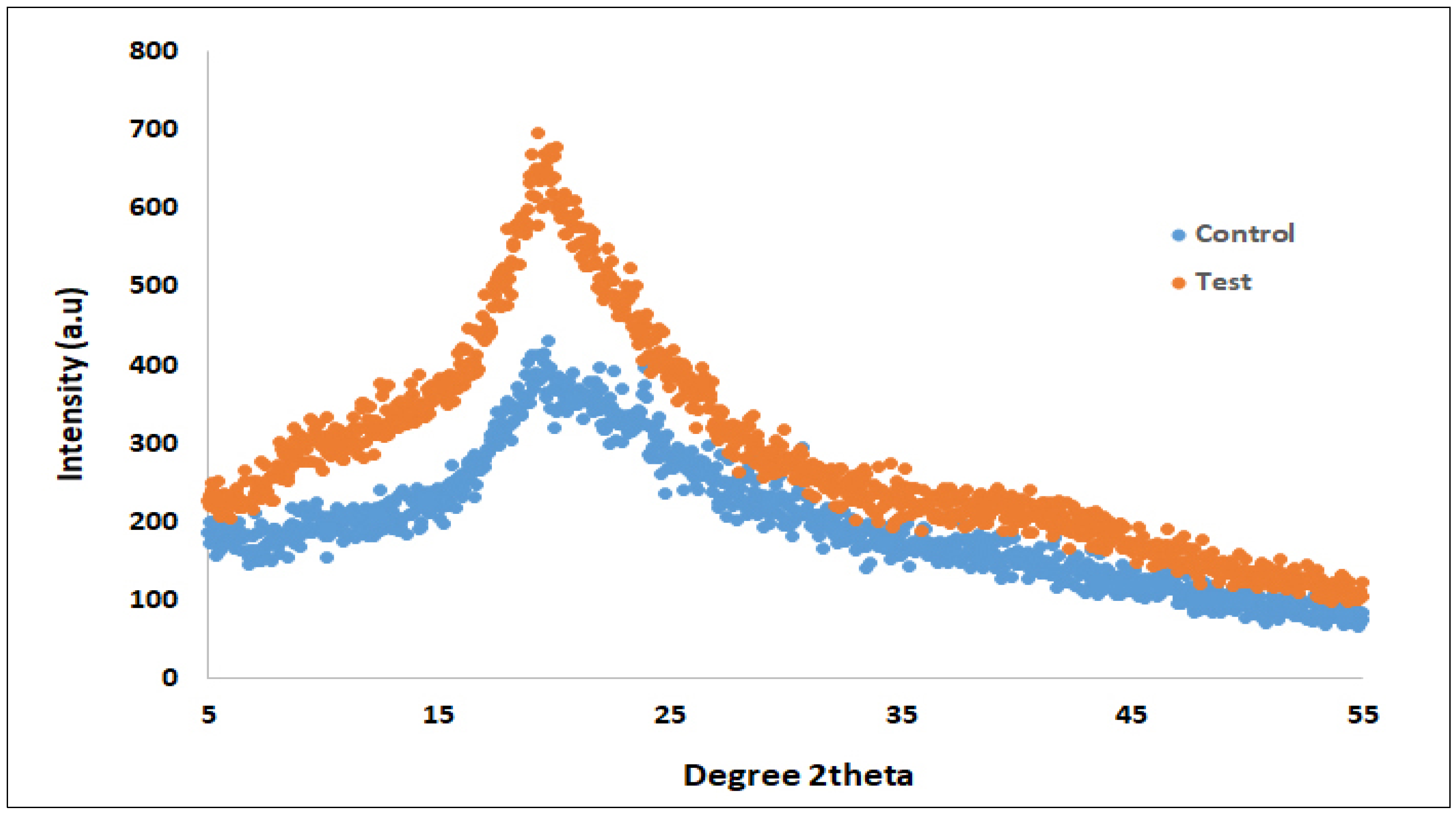
3.3. A Change in Structural Integrity Revealed by X-Ray Diffraction Studies
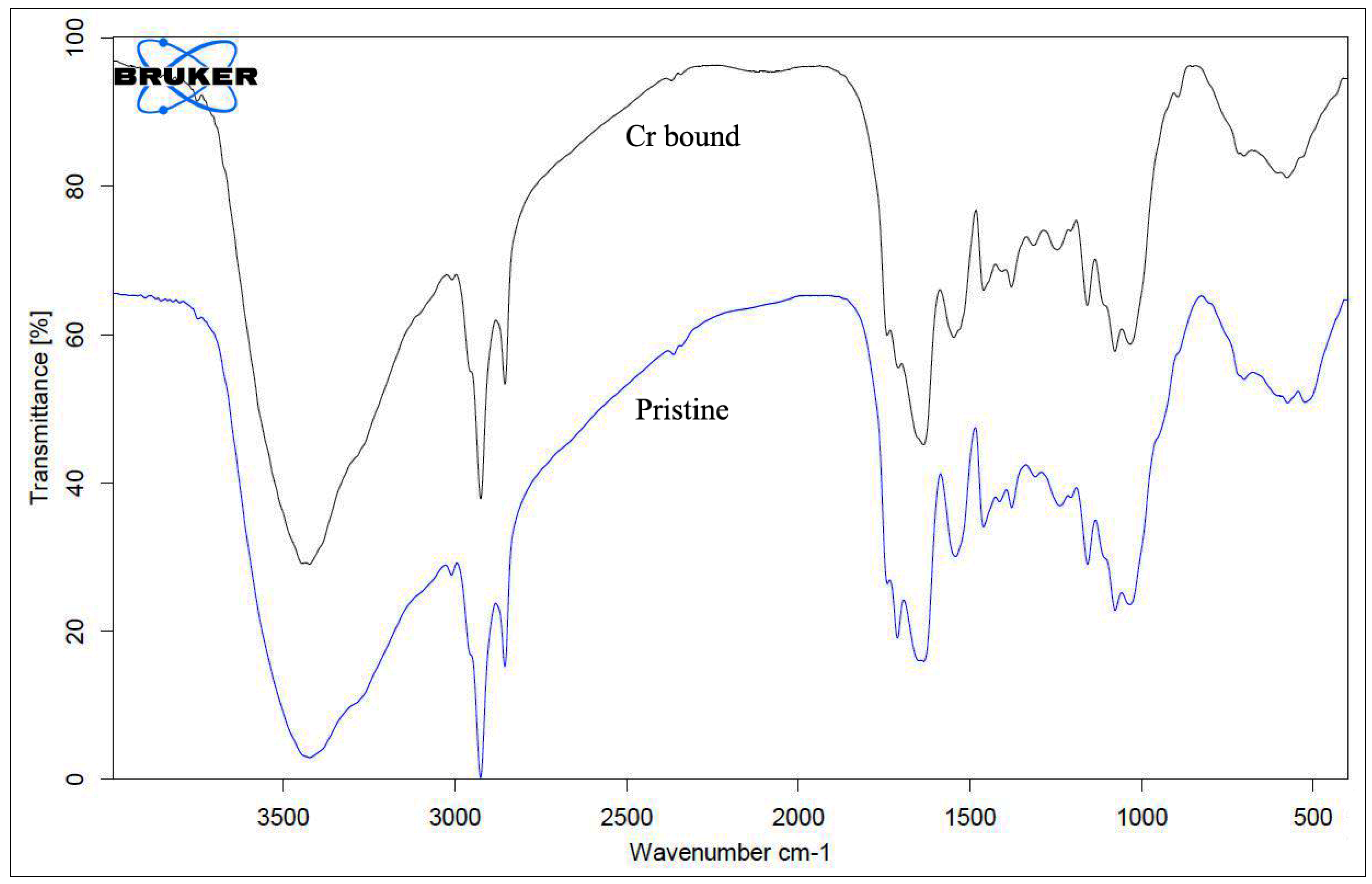
3.4. Occurrence of Functional Groups on Fungal Biomass Surface as Revealed by FTIR Analysis
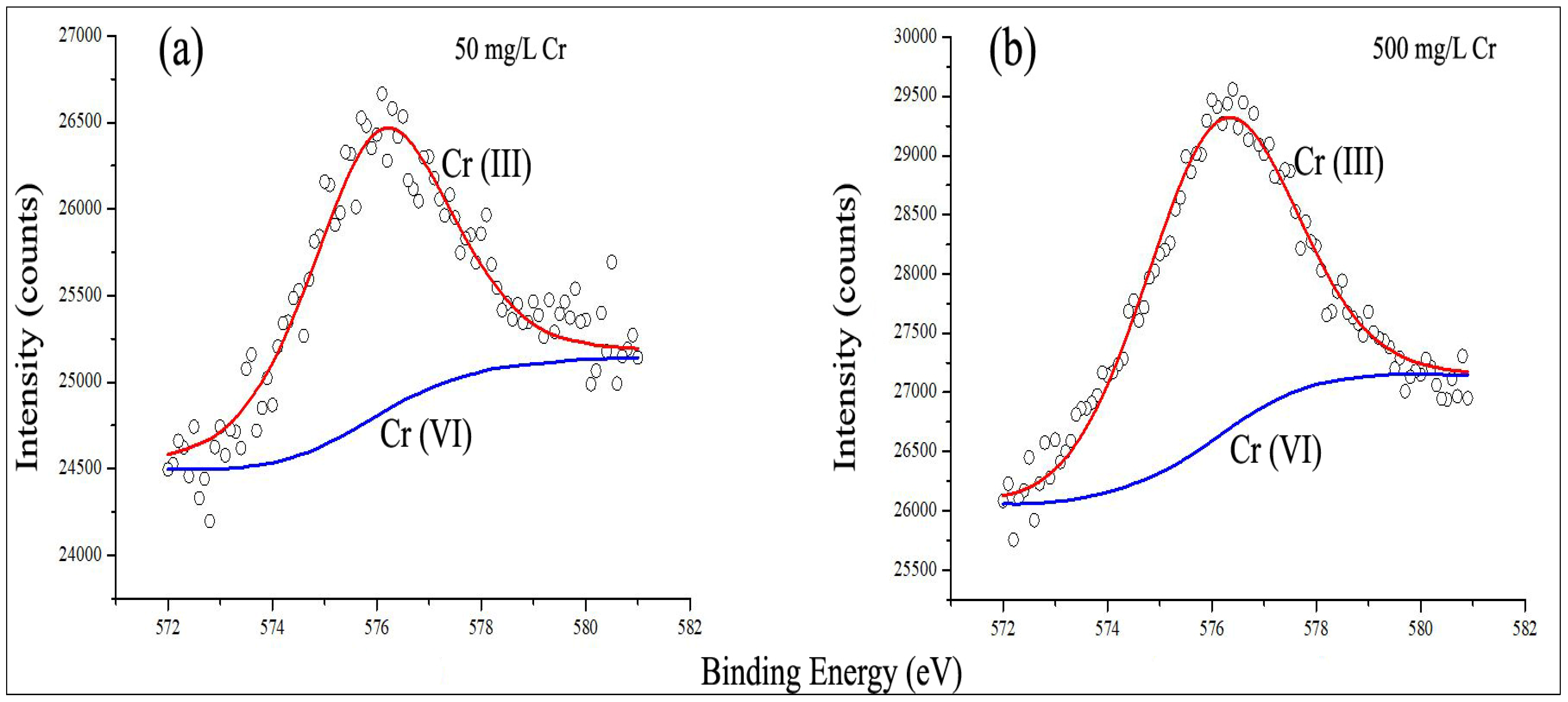
3.5. Existence of Two Types of Species of Chromium [Cr(III) and Cr(VI)] and Formation of Reduced Cr(III) from Cr(VI) as Confirmed with XPS Studies
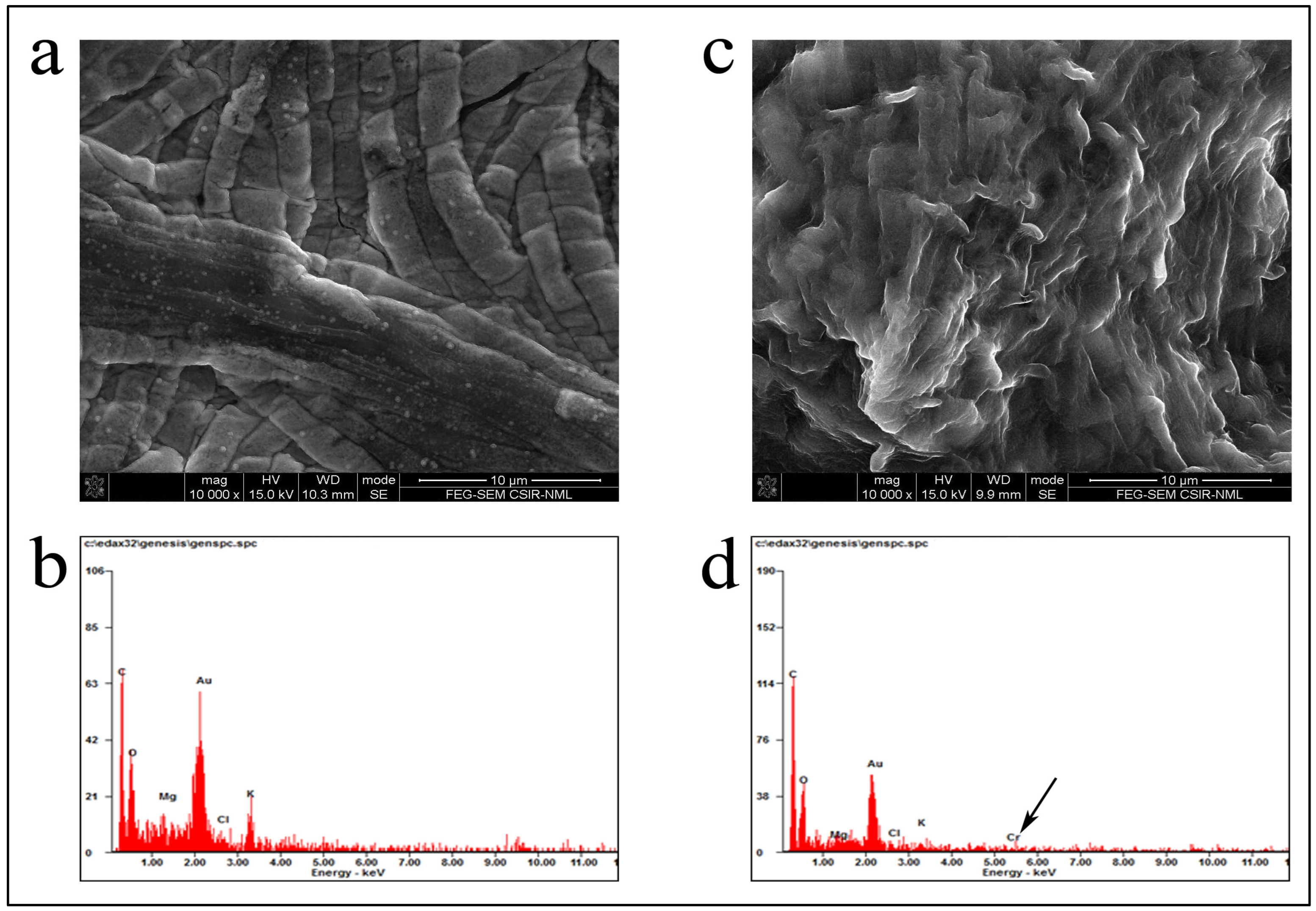
3.6. Cellular Ultrastructure Changes Were Apparent from EDX and FESEM Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Vidu, R.; Matei, E.; Predescu, A.M.; Alhalaili, B.; Pantilimon, C.; Tarcea, C.; Predescu, C. Removal of heavy metals from wastewaters: A challenge from current treatment methods to nanotechnology applications. Toxics 2020, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Raji, Z.; Karim, A.; Karam, A.; Khalloufi, S. Adsorption of heavy metals: Mechanisms, kinetics, and applications of various adsorbents in wastewater remediation—A review. Waste 2023, 1, 775–805. [Google Scholar] [CrossRef]
- Netzahuatl-Muñoz, A.R.; Cristiani-Urbina, M.D.C.; Cristiani-Urbina, E. Chromium biosorption from Cr(VI) aqueous solutions by Cupressus lusitanica bark: Kinetics, equilibrium and thermodynamic studies. PLoS ONE 2015, 10, e0137086. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, S.; Zhang, H.; Wang, X. Removal behaviors and mechanisms of hexavalent chromium from aqueous solution by cephalosporin residue and derived chars. Bioresour. Technol. 2017, 238, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.R.; Roy, S.R.; Ganguly, A.; Ghosh, R.; Majumder, S.; Dasgupta, A.; Das, R.; Kumar, A.; Naskar, A.; Majumder, R. Biosorption of Acid dye by Jackfruit Leaf Powder: Isotherm, kinetics and Response surface methodology studies. Jebas 2022, 10, 254–265. [Google Scholar] [CrossRef]
- Abigail, M.; Samuel, M.S.; Chidambaram, R. Isotherm modelling, kinetic study and optimization of batch parameters using response surface methodology for effective removal of Cr(VI) using fungal biomass. PLoS ONE 2015, 10, e0116884. [Google Scholar]
- Tang, H.; Xiang, G.; Xiao, W.; Yang, Z.; Zhao, B. Microbial mediated remediation of heavy metals toxicity: Mechanisms and future prospects. Front. Plant Sci. 2024, 15, 1420408. [Google Scholar] [CrossRef] [PubMed]
- Abd Elnabi, M.K.; Elkaliny, N.E.; Elyazied, M.M.; Azab, S.H.; Elkhalifa, S.A.; Elmasry, S.; Mouhamed, M.S.; Shalamesh, E.M.; Alhorieny, N.A.; Abd Elaty, A.E.; et al. Toxicity of heavy metals and recent advances in their removal: A review. Toxics 2023, 11, 580. [Google Scholar] [CrossRef]
- Acar, F.; Malkoc, E. The removal of chromium (VI) from aqueous solutions by Fagus orientalis L. Bioresour. Technol. 2004, 94, 13–15. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Sun, Y.; Wu, Z.; Li, T.; Hu, Y.; Su, D.; Lv, J.; Li, G.; Zhang, Z.; et al. Transformation and immobilization of chromium by arbuscular mycorrhizal fungi as revealed by SEM–EDS, TEM–EDS, and XAFS. Environ. Sci. Technol. 2015, 49, 14036–14047. [Google Scholar] [CrossRef]
- Staszak, K.; Regel-Rosocka, M. Removing heavy metals: Cutting-edge strategies and advancements in biosorption technology. Materials 2024, 17, 1155. [Google Scholar] [CrossRef]
- Samuel, M.S.; Chidambaram, R. Hexavalent chromium biosorption studies using Penicillium griseofulvum MSR1 a novel isolate from tannery effluent site: Box-Behnken optimization, equilibrium, kinetics and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 2015, 49, 156–164. [Google Scholar]
- Ramamurthy, G.; Ramalingam, B.; Katheem, M.F.; Sastry, T.P.; Inbasekaran, S.; Thanveer, V.; Jayaramachandran, S.; Das, S.K.; Mandal, A.B. Total elimination of polluting chrome shavings, chrome, and dye exhaust liquors of tannery by a method using keratin hydrolysate. ACS Sustain. Chem. Eng. 2015, 3, 1348–1358. [Google Scholar]
- Sharma, P.; Bihari, V.; Agarwal, S.K.; Verma, V.; Kesavachandran, C.N.; Pangtey, B.S.; Mathur, N.; Singh, K.P.; Srivastava, M.; Goel, S.K. Groundwater contaminated with hexavalent chromium [Cr(VI)]: A health survey and clinical examination of community inhabitants (Kanpur, India). PLoS ONE 2012, 7, e47877. [Google Scholar] [CrossRef]
- Aftab, K.; Iqbal, S.; Khan, M.R.; Busquets, R.; Noreen, R.; Ahmad, N.; Kazimi, S.G.T.; Karami, A.M.; Al Suliman, N.M.S.; Ouladsmane, M. Wastewater-irrigated vegetables are a significant source of heavy metal contaminants: Toxicity and health risks. Molecules 2023, 28, 1371. [Google Scholar] [CrossRef]
- Li, Z.H.; Li, P.; Randak, T. Evaluating the toxicity of environmental concentrations of waterborne chromium (VI) to a model teleost, Oncorhynchus mykiss: A comparative study of in vivo and in vitro. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 402–407. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, M.; Lan, J.; Huang, Y.; Zhang, J.; Huang, S.; Yang, Y.; Ru, J. Water quality degradation due to heavy metal contamination: Health impacts and eco-friendly approaches for heavy metal remediation. Toxics 2023, 11, 828. [Google Scholar] [CrossRef]
- Hkiri, N.; Olicón-Hernández, D.R.; Pozo, C.; Chouchani, C.; Asses, N.; Aranda, E. Simultaneous Heavy Metal-Polycyclic Aromatic Hydrocarbon Removal by Native Tunisian Fungal Species. J. Fungi 2023, 9, 299. [Google Scholar] [CrossRef]
- Ronda, A.; Della Zassa, M.; Martín-Lara, M.; Calero, M.; Canu, P. Combustion of a Pb (II)-loaded olive tree pruning used as biosorbent. J. Hazard. Mater. 2016, 308, 285–293. [Google Scholar] [CrossRef]
- Sounthararajah, D.; Loganathan, P.; Kandasamy, J.; Vigneswaran, S. Adsorptive removal of heavy metals from water using sodium titanate nanofibres loaded onto GAC in fixed-bed columns. J. Hazard. Mater. 2015, 28, 306–316. [Google Scholar] [CrossRef]
- Kim, N.; Park, M.; Park, D. A new efficient forest biowaste as biosorbent for removal of cationic heavy metals. Bioresour. Technol. 2015, 175, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Ramrakhiani, L.; Halder, A.; Majumder, A.; Mandal, A.K.; Majumdar, S.; Ghosh, S. Industrial waste derived biosorbent for toxic metal remediation: Mechanism studies and spent biosorbent management. Chem. Eng. J. 2017, 308, 1048–1064. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, T.; Wang, F. Microbial-based heavy metal bioremediation: Toxicity and eco-friendly approaches to heavy metal decontamination. Appl. Sci. 2023, 13, 8439. [Google Scholar] [CrossRef]
- Alam, M.Z.; Ahmad, S. Chromium removal through biosorption and bioaccumulation by bacteria from tannery effluents contaminated soil. Clean–Soil Air Water 2011, 39, 226–237. [Google Scholar] [CrossRef]
- Das, S.K.; Guha, A.K. Biosorption of hexavalent chromium by Termitomyces clypeatus biomass: Kinetics and transmission electron microscopic study. J. Hazard. Mater 2009, 167, 685–691. [Google Scholar] [CrossRef]
- Bishnoi, N.R.; Kumar, R.; Kumar, S.; Rani, S.J. Biosorption of Cr(III) from aqueous solution using algal biomass Spirogyra spp. J. Hazard. Mater. 2007, 145, 142–147. [Google Scholar] [CrossRef]
- Yang, L.; Chen, J.P. Biosorption of hexavalent chromium onto raw and chemically modified Sargassum sp. Bioresour. Technol. 2008, 99, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, R.; Philip, L.; Chandraraj, K.J. Biosorption of chromium species by aquatic weeds: Kinetics and mechanism studies. Hazard. Mater. 2008, 152, 100–112. [Google Scholar] [CrossRef]
- Kang, S.Y.; Lee, J.U.; Kim, K.W. Biosorption of Cr(III) and Cr(VI) onto the cell surface of Pseudomonas aeruginosa. Biochem. Eng. J. 2007, 36, 54–58. [Google Scholar] [CrossRef]
- Salvadori, M.R.; Nascimento, C.A.O.; Corrêa, B. Nickel oxide nanoparticles film produced by dead biomass of filamentous fungus. Sci. Rep. 2014, 4, 6404. [Google Scholar] [CrossRef]
- Shen, L.; Xia Jl He, H.; Nie, Z.Y.; Qiu, G.Z. Biosorption mechanism of Cr(VI) onto cells of Synechococcus sp. J. Cent. South Univ. Technol. 2007, 14, 157–162. [Google Scholar] [CrossRef]
- Wei, W.; Wang, Q.; Li, A.; Yang, J.; Ma, F.; Pi, S.; Wu, D. Biosorption of Pb (II) from aqueous solution by extracellular polymeric substances extracted from Klebsiella sp. J1: Adsorption behavior and mechanism assessment. Sci. Rep. 2016, 6, 31575. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, P.; Ibáñez, A.B.; Bauer, S.; Glassman, S.I.; Szaro, T.M.; Bruns, T.D.; Taylor, J.W. Fungi isolated from Miscanthus and sugarcane: Biomass conversion, fungal enzymes, and hydrolysis of plant cell wall polymers. Biotechnol. Biofuels 2015, 8, 38. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chandrababunaidu, M.M.; Panda, A.; Khowala, S.; Tripathy, S. Tricking Arthrinium malaysianum into producing industrially important enzymes under 2-deoxy D-glucose treatment. Front. Microbiol. 2016, 7, 596. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Groenewald, J.Z. A phylogenetic re-evaluation of Arthrinium. IMA Fungus 2013, 4, 133–154. [Google Scholar] [CrossRef]
- Majumder, R.; Banik, S.P.; Ramrakhiani, L.; Khowala, S.J. Bioremediation by alkaline protease (AkP) from edible mushroom Termitomyces clypeatus: Optimization approach based on statistical design and characterization for diverse applications. Chem. Technol. Biotechnol. 2015, 90, 1886–1896. [Google Scholar] [CrossRef]
- Kuanar, A.; Kabi, S.K.; Rath, M.; Dhal, N.K.; Bhuyan, R.; Das, S.; Kar, D. A Comparative Review on Bioremediation of Chromium by Bacterial, Fungal, Algal and Microbial Consortia. Geomicrobiol. J. 2022, 39, 515–530. [Google Scholar] [CrossRef]
- Ramrakhiani, L.; Majumder, R.; Khowala, S. Removal of hexavalent chromium by heat inactivated fungal biomass of Termitomyces clypeatus: Surface characterization and mechanism of biosorption. Chem. Eng. J. 2011, 171, 1060–1068. [Google Scholar] [CrossRef]
- Sanchez-Hachair, A.; Hofmann, A. Hexavalent chromium quantification in solution: Comparing direct UV–visible spectrometry with 1, 5-diphenylcarbazide colorimetry. C. R. Chim. 2018, 21, 890–896. [Google Scholar] [CrossRef]
- Cárdenas González, J.F.; Acosta Rodríguez, I.; Terán Figueroa, Y.; Lappe Oliveras, P.; Martínez Flores, R.; Rodríguez Pérez, A.S. Biotransformation of Chromium (VI) via a reductant activity from the fungal strain Purpureocillium lilacinum. J. Fungi 2021, 7, 1022. [Google Scholar] [CrossRef]
- Chatterjee, S.; De, R.; Gupta, A. Activated charcoal mediated purification of yellow sodium sulphate: A green process to utilize a hazardous by-product of the leather chemical industry. RSC Adv. 2016, 6, 53651–53656. [Google Scholar] [CrossRef]
- Li, M.H.; Gao, X.Y.; Li, C.; Yang, C.L.; Fu, C.A.; Liu, J.; Wang, R.; Chen, L.X.; Lin, J.Q.; Liu, X.M.; et al. Isolation and identification of chromium reducing Bacillus Cereus species from chromium-contaminated soil for the biological detoxification of chromium. Int. J. Environ. Res. Public Health 2020, 17, 2118. [Google Scholar] [CrossRef]
- McCracken, K.; Angus, S.; Reynolds, K.; Yoon, J. Multimodal imaging and lighting bias correction for improved μPAD-based water quality monitoring via smartphones. Sci. Rep. 2016, 6, 27529. [Google Scholar] [CrossRef]
- Kholisa, B.; Matsena, M.; Chirwa, E.M. Evaluation of Cr(VI) reduction using indigenous bacterial consortium isolated from a municipal wastewater sludge: Batch and kinetic studies. Catalysts 2021, 11, 1100. [Google Scholar] [CrossRef]
- Xu, F.; Liu, X.; Chen, Y.; Zhang, K.; Xu, H. Self-assembly modified-mushroom nanocomposite for rapid removal of hexavalent chromium from aqueous solution with bubbling fluidized bed. Sci. Rep. 2016, 6, 26201. [Google Scholar] [CrossRef] [PubMed]
- Sathvika, T.; Rajesh, V.; Rajesh, N. Microwave assisted immobilization of yeast in cellulose biopolymer as a green adsorbent for the sequestration of chromium. Chem. Eng. J. 2015, 279, 38–46. [Google Scholar] [CrossRef]
- Sebastian, J.; Rouissi, T.; Brar, S.K.; Hegde, K.; Verma, M. Microwave-assisted extraction of chitosan from Rhizopus oryzae NRRL 1526 biomass. Carbohydr. Polym. 2019, 219, 431–440. [Google Scholar] [CrossRef]
- Bhatt, A.S.; Sakaria, P.L.; Vasudevan, M.; Pawar, R.R.; Sudheesh, N.; Bajaj, H.C.; Mody, H.M. Adsorption of an anionic dye from aqueous medium by organoclays: Equilibrium modeling, kinetic and thermodynamic exploration. RSC Adv. 2012, 2, 8663–8671. [Google Scholar] [CrossRef]
- Ma, J.; Yang, M.; Yu, F.; Zheng, J. Water-enhanced removal of ciprofloxacin from water by porous graphene hydrogel. Sci. Rep. 2015, 5, 13578. [Google Scholar] [CrossRef] [PubMed]
- Samanta, T.; Sinha, S.; Mukherjee, M. Effect of added salt on swelling dynamics of ultrathin films of strong polyelectrolytes. Polymer 2016, 97, 285–294. [Google Scholar] [CrossRef]
- Das, S.K.; Mukherjee, M.; Guha, A.K. Interaction of chromium with resistant strain Aspergillus versicolor: Investigation with atomic force microscopy and other physical studies. Langmuir 2008, 24, 8643–8650. [Google Scholar] [CrossRef] [PubMed]
- Parandhaman, T.; Pentela, N.; Ramalingam, B.; Samanta, D.; Das, S.K. Metal nanoparticle loaded magnetic-chitosan microsphere: Water dispersible and easily separable hybrid metal nano-biomaterial for catalytic applications. ACS Sustain. Chem. Eng. 2017, 5, 489–501. [Google Scholar] [CrossRef]
- Baker, P.W.; Ito, K.; Watanabe, K. Marine prosthecate bacteria involved in the ennoblement of stainless steel. Environ. Microbiol. 2003, 5, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, L.; Tripathy, S.; Nayar, S. Biomimetic matrix mediated room temperature synthesis and characterization of nano-hydroxyapatite towards targeted drug delivery. RSC Adv. 2016, 6, 62556–62571. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Sun, Y.; Wu, Z.; Li, T.; Hu, Y.; Lv, J.; Li, G.; Zhang, Z.; Zhang, J.; et al. Chromium immobilization by extra-and intraradical fungal structures of arbuscular mycorrhizal symbioses. J. Hazard. Mater. 2016, 316, 34–42. [Google Scholar] [CrossRef]
- Naskar, A.; Guha, A.K.; Mukherjee, M.; Ray, L. Adsorption of nickel onto Bacillus cereus M116: A mechanistic approach. Sep. Sci. Technol. 2016, 51, 427–438. [Google Scholar] [CrossRef]
- Qi, X.; Li, L.; Tan, T.; Chen, W.; Smith, R.L., Jr. Adsorption of 1-butyl-3-methylimidazolium chloride ionic liquid by functional carbon microspheres from hydrothermal carbonization of cellulose. Environ. Sci. Technol. 2013, 47, 2792–2798. [Google Scholar] [CrossRef]
- Duan, F.; Chen, C.; Wang, G.; Yang, Y.; Liu, X.; Qin, Y. Efficient adsorptive removal of dibenzothiophene by graphene oxide-based surface molecularly imprinted polymer. RSC Adv. 2014, 4, 1469–1475. [Google Scholar] [CrossRef]
- Arıca, M.Y.; Bayramoğlu, G. Cr(VI) biosorption from aqueous solutions using free and immobilized biomass of Lentinussajor-caju: Preparation and kinetic characterization. Colloids Surf. A Physicochem. Eng. Asp. 2005, 253, 203–211. [Google Scholar] [CrossRef]
- Cárdenas-González, J.F.; Acosta-Rodríguez, I. Hexavalent chromium removal by a Paecilomyces sp. fungal strain isolated from environment. Bioinorg. Chem Appl. 2010, 2010, 676243. [Google Scholar] [CrossRef]
- Deepa KKSathishkumar, M.; Binupriya, A.R.; Murugesan, G.S.; Swaminathan, K.; Yun, S.E. Sorption of Cr(VI) from dilute solutions and wastewater by live and pretreated biomass of Aspergillus flavus. Chemosphere 2006, 62, 833–840. [Google Scholar] [CrossRef]
- Kavita, B.; Limbachia, J.; Keharia, H. Hexavalent chromium sorption by biomass of chromium tolerant Pythium sp. J. Basic Microbiol. 2011, 51, 173–182. [Google Scholar] [CrossRef]
- Khambhaty, Y.; Mody, K.; Basha, S.; Jha, B. Kinetics, equilibrium and thermodynamic studies on biosorption of hexavalent chromium by dead fungal biomass of marine Aspergillus niger. Chem. Eng. J 2009, 145, 489–495. [Google Scholar] [CrossRef]
- Kumar, R.; Bishnoi, N.R.; Bishnoi, K. Biosorption of chromium (VI) from aqueous solution and electroplating wastewater using fungal biomass. Chem. Eng. J 2008, 135, 202–208. [Google Scholar] [CrossRef]
- Liu, T.; Li, H.; Deng, L. The optimum conditions, thermodynamical isotherm and mechanism of hexavalent chromium removal by fungal biomass of Mucor racemosus. World J. Microbiol. Biotechnol. 2007, 23, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Mungasavalli, D.P.; Viraraghavan, T.; Jin, Y.C. Biosorption of chromium from aqueous solutions by pretreated Aspergillus niger: Batch and column studies. Colloids Surf. A Physicochem. Eng. Asp. 2007, 301, 214–223. [Google Scholar] [CrossRef]
- Park, D.; Yun, Y.S.; Jo, J.H.; Park, J.M. Mechanism of hexavalent chromium removal by dead fungal biomass of Aspergillus niger. Water Res. 2005, 39, 533–540. [Google Scholar] [CrossRef]
- Sanghi, R.; Sankararamakrishnan, N.; Dave, B.C. Fungal bioremediation of chromates: Conformational changes of biomass during sequestration, binding, and reduction of hexavalent chromium ions. J. Hazard. Matter. 2009, 169, 1074–1080. [Google Scholar] [CrossRef]
- Say, R.; Yilmaz, N.; Denizli, A. Removal of chromium (VI) ions from synthetic solutions by the fungus Penicillium purpurogenum. Eng. Life Sci. 2004, 4, 276–280. [Google Scholar] [CrossRef]
- Shroff, K.A.; Vaidya, V.K. Effect of pre-treatments on the biosorption of chromium (VI) ions by the dead biomass of Rhizopus arrhizus. J. Chem. Technol. Biotechnol. 2012, 87, 294–304. [Google Scholar] [CrossRef]
- Tewari, N.; Vasudevan, P.; Guha, B. Study on biosorption of Cr(VI) by Mucor hiemalis. Biochem. Eng. J 2005, 23, 185–192. [Google Scholar] [CrossRef]
- Ahluwalia, S.S.; Goyal, D. Removal of Cr(VI) from aqueous solution by fungal biomass. Eng. Life Sci 2010, 10, 480–485. [Google Scholar] [CrossRef]
- Kumar, S.; Koh, J. Physiochemical, optical and biological activity of chitosan-chromone derivative for biomedical applications. Int. J. Mol. Sci 2012, 13, 6102–6116. [Google Scholar] [CrossRef] [PubMed]
- Majumder, R.; Sheikh, L.; Naskar, A.; Mukherjee, M.; Tripathy, S. Depletion of Cr(VI) from aqueous solution by heat dried biomass of a newly isolated fungus Arthrinium malaysianum: A mechanistic approach. Sci. Rep. 2017, 7, 11254. [Google Scholar] [CrossRef] [PubMed]
- Fagundez, J.L.; Netto, M.S.; Dotto, G.L.; Salau, N.P. A new method of developing ANN-isotherm hybrid models for the determination of thermodynamic parameters in the adsorption of ions Ag+, Co2+ and Cu2+ onto zeolites ZSM-5, HY, and 4A. J. Environ. Chem. Eng. 2021, 9, 106126. [Google Scholar] [CrossRef]
- Vigdorowitsch, M.; Pchelintsev, A.; Tsygankova, L.; Tanygina, E. Freundlich isotherm: An adsorption model complete framework. Appl. Sci. 2021, 11, 8078. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.J. Biosorption isotherms, kinetics and thermodynamics. Sep. Purif. Technol. 2008, 61, 229–242. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, A.; Li, Z.; Wang, J.; Zhang, S. Influence of anionic structure on the dissolution of chitosan in 1-butyl-3-methylimidazolium-based ionic liquids. Green Chem. 2011, 13, 3446–3452. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Yun, Y.S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef]
- Chakravarty, S.; Mohanty, A.; Sudha, T.N.; Upadhyay, A.K.; Konar, J.; Sircar, J.K.; Madhukar, A.; Gupta, K.K. Removal of Pb (II) ions from aqueous solution by adsorption using bael leaves Aegle marmelos. J. Hazard. Mater. 2010, 173, 502–509. [Google Scholar] [CrossRef]
- Das, S.K.; Guha, A.K. Biosorption of chromium by Termitomyces clypeatus. Colloids Surf. B Biointerfaces 2007, 60, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Hsu, N.H.; Wang, S.L.; Liao, Y.H.; Huang, S.T.; Tzou, Y.M.; Huang, Y.M. Removal of hexavalent chromium from acidic aqueous solutions using rice straw-derived carbon. J. Hazard. Mater. 2009, 171, 1066–1070. [Google Scholar] [CrossRef]
- Hsu, N.H.; Wang, S.L.; Lin, Y.C.; Sheng, G.D.; Lee, J.F. Reduction of Cr(VI) by crop-residue-derived black carbon. Environ. Sci. Technol. 2009, 43, 8801–8806. [Google Scholar] [CrossRef] [PubMed]
- Fuks, H.; Kaczmarek, S.; Bosacka, M. EPR and IR investigations of some chromium (III) phosphate (V) compounds. Rev. Adv. Mater. Sci. 2010, 23, 57–63. [Google Scholar]
- Vieira, R.S.; Oliveira, M.L.M.; Guibal, E.; Rodríguez-Castellón, E.; Beppu, M.M. Copper, mercury and chromium adsorption on natural and crosslinked chitosan films: An XPS investigation of mechanism. Colloids Surf. A Physicochem. Eng. Asp 2011, 374, 108–114. [Google Scholar] [CrossRef]
| Isotherm Models | Test Parameters | Values |
|---|---|---|
| Langmuir | Qm (mg g−1) | 102.310 |
| Kl (L mg−1) | 1.08 × 10−3 | |
| χ2 | 6.24 | |
| R2 | 0.9807 | |
| Freundlich | KF (L g−1) | 0.370 |
| n | 1.014 | |
| χ2 | 12.13 | |
| R2 | 0.9535 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy Chowdhury, S.; Das, A.; Ghosh, S.; Chatterjee, S.; Majumder, R. Microwave-Assisted Dried Cells of the Fungus Arthrinium malaysianum as a Potential Biomaterial with Sustainable Bioremediation of Toxic Heavy Metals. Appl. Microbiol. 2025, 5, 55. https://doi.org/10.3390/applmicrobiol5020055
Roy Chowdhury S, Das A, Ghosh S, Chatterjee S, Majumder R. Microwave-Assisted Dried Cells of the Fungus Arthrinium malaysianum as a Potential Biomaterial with Sustainable Bioremediation of Toxic Heavy Metals. Applied Microbiology. 2025; 5(2):55. https://doi.org/10.3390/applmicrobiol5020055
Chicago/Turabian StyleRoy Chowdhury, Swagata, Arpita Das, Sanmitra Ghosh, Saptarshi Chatterjee, and Rajib Majumder. 2025. "Microwave-Assisted Dried Cells of the Fungus Arthrinium malaysianum as a Potential Biomaterial with Sustainable Bioremediation of Toxic Heavy Metals" Applied Microbiology 5, no. 2: 55. https://doi.org/10.3390/applmicrobiol5020055
APA StyleRoy Chowdhury, S., Das, A., Ghosh, S., Chatterjee, S., & Majumder, R. (2025). Microwave-Assisted Dried Cells of the Fungus Arthrinium malaysianum as a Potential Biomaterial with Sustainable Bioremediation of Toxic Heavy Metals. Applied Microbiology, 5(2), 55. https://doi.org/10.3390/applmicrobiol5020055







