Evaluation of Solid-State Fermentation Conditions from Pineapple Peel Waste for Release of Bioactive Compounds by Aspergillus niger spp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining Pineapple Peel Waste and Reagents
2.2. Physicochemical Properties of Pineapple Peel Waste
2.3. Proximate Analysis of Pineapple Peel Waste
2.4. Evaluation of Fungal Strains with Invasive Capacity on Pineapple Peel Waste
2.5. Quantification of Polyphenolic Compounds
2.6. Evaluation of Solid-State Fermentation (SSF) Conditions Using Pineapple Peel Waste for the Release of Bioactive Compounds
2.7. Analysis of the Polyphenolic Content of the Fermentation Extracts by RP-HPLC-ESI-MS
2.8. Determination of Antioxidant Capacity
3. Results
3.1. Physicochemical Characterization of Pineapple Peel
3.2. Evaluation of Fungal Strains with Invasive Growth Capacity
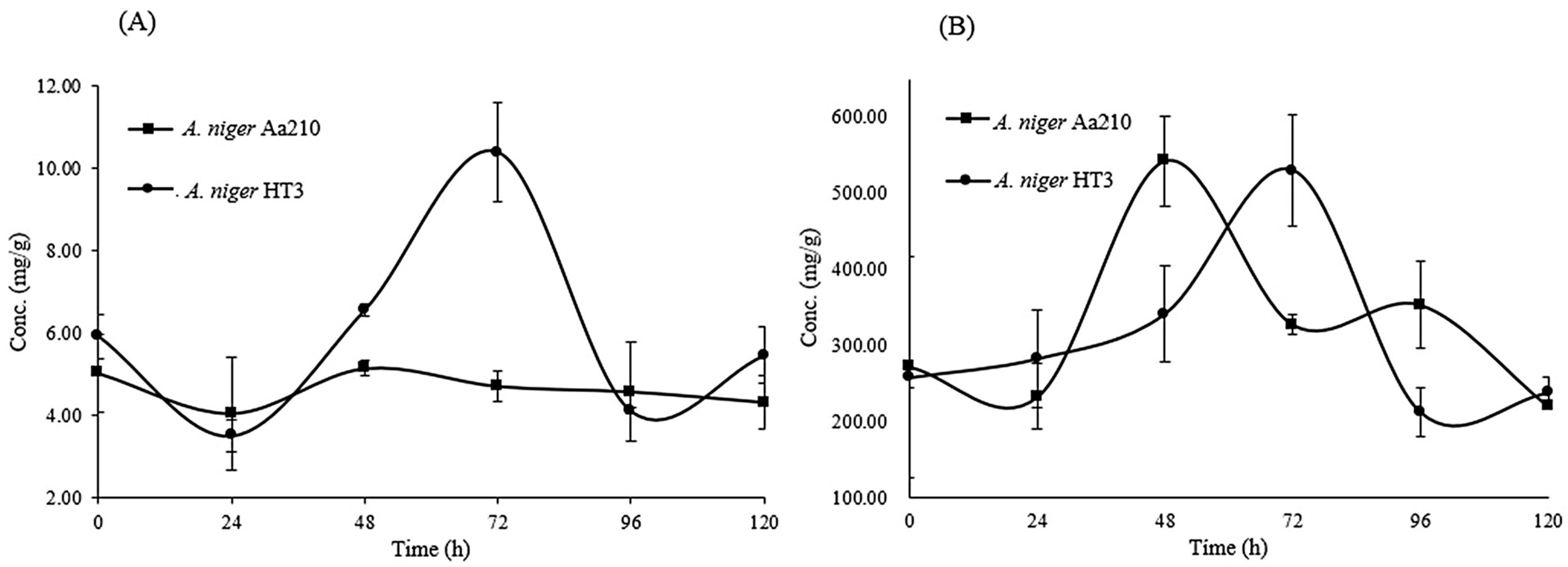
3.3. Quantification of Tannins from Fermentation Kinetics
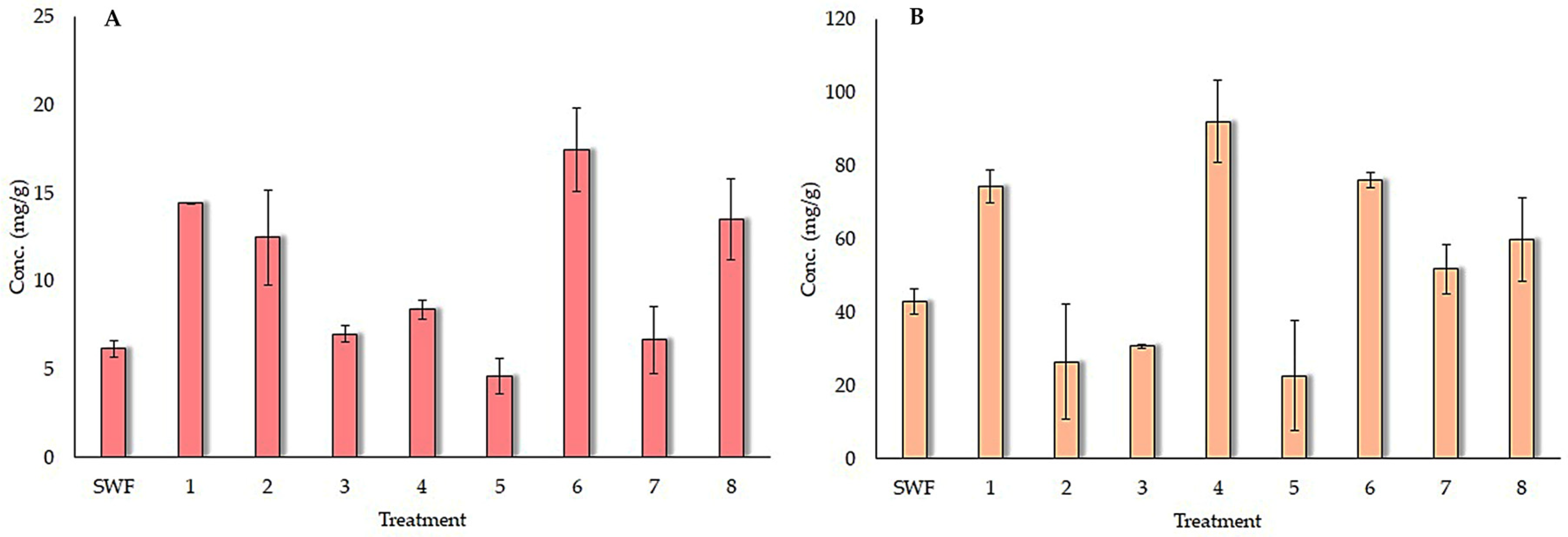
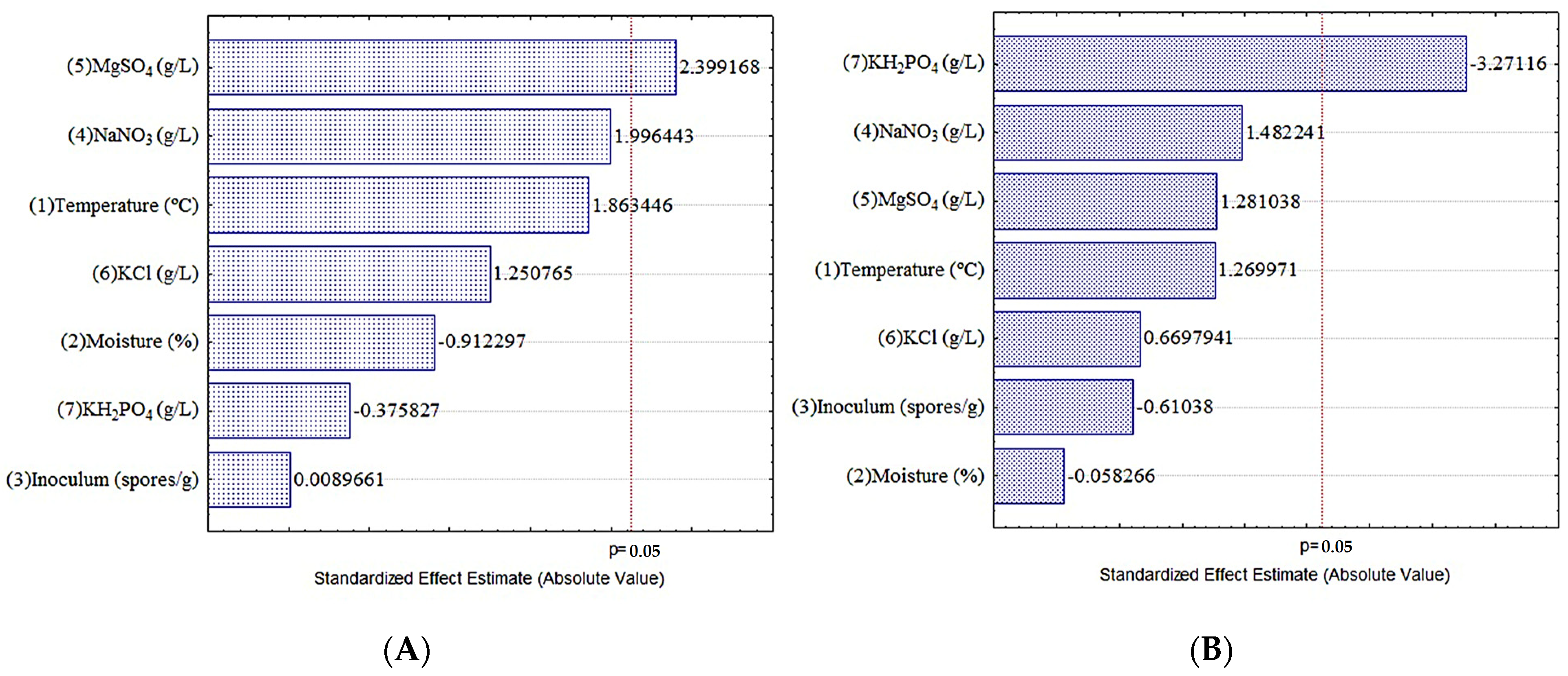
3.4. Evaluation of the SSF Conditions for the Release of HT and CT
3.5. Identification of HT and CT by HPLC-MS
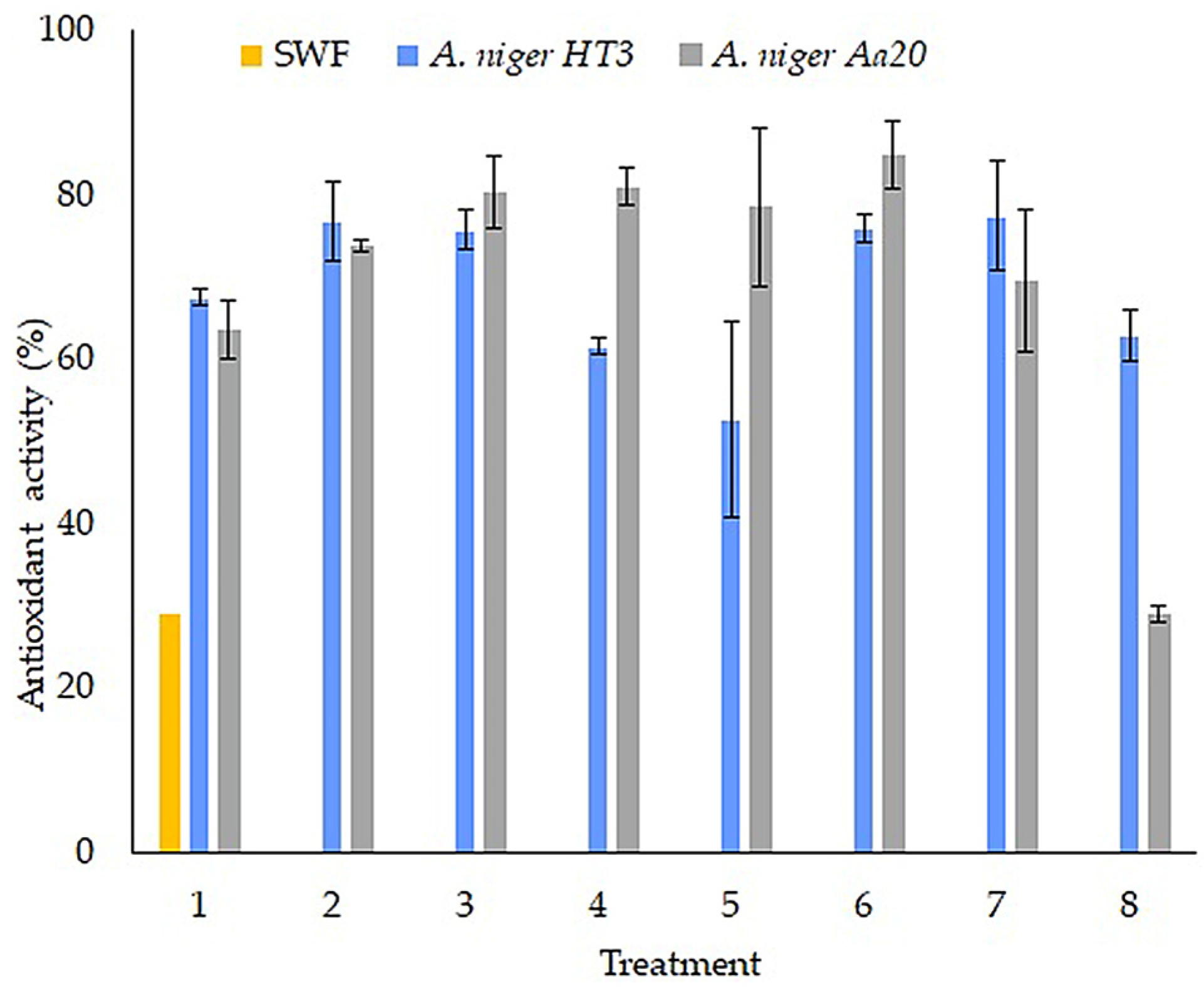
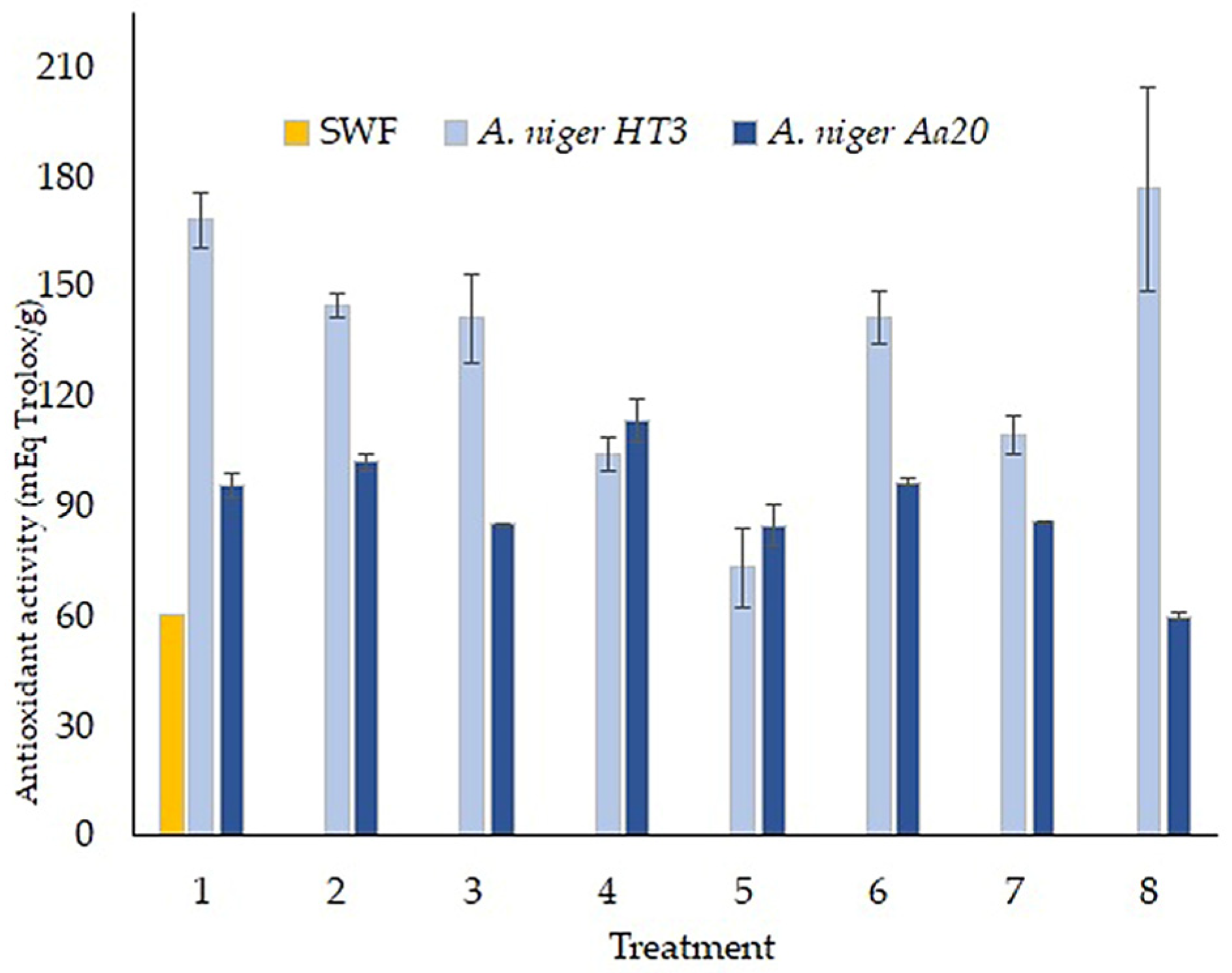
3.6. Antioxidant Activity of the Fermentation Extracts
4. Discussion
4.1. Physicochemical Characterization
4.2. Adaptation of Aspergillus Strains in Fermentation Kinetics
4.3. Release of Tannins by Solid-State Fermentation
4.4. Evaluation of Antioxidant Activity in Fermentation Extracts
4.5. Identification of Polyphenolic Compounds by HPLC-MS
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teshome, E.; Teka, T.A.; Nandasiri, R.; Rout, J.R.; Harouna, D.V.; Astatkie, T.; Urugo, M.M. Fruit By-Products and Their Industrial Applications for Nutritional Benefits and Health Promotion: A Comprehensive Review. Sustainability 2023, 15, 7840. [Google Scholar] [CrossRef]
- Tu, Q.; Liu, S.; Li, Y.; Zhang, L.; Wang, Z.; Yuan, C. The effects of regions and the wine aging periods on the condensed tannin profiles and the astringency perceptions of Cabernet Sauvignon wines. Food Chem. X 2022, 15, 100409. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Islam, N.; Faruk, O.; Ashaduzzaman; Dungani, R. Review on tannins: Extraction processes, applications and possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Sharma, K.P. Tannin degradation by phytopathogen’s tannase: A Plant’s defense perspective. Biocatal. Agric. Biotechnol. 2019, 21, 101342. [Google Scholar] [CrossRef]
- Muanda, F.N.; Dicko, A.; Soulimani, R. Assessment of polyphenolic compounds, in vitro antioxidant and anti-inflammation properties of Securidaca longepedunculata root barks. Comptes Rendus Biol. 2010, 333, 663–669. [Google Scholar] [CrossRef] [PubMed]
- FAO. FAOSTAT Database. Available online: http://www.fao.org/faostat (accessed on 3 June 2024).
- Hamzah, A.F.A.; Hamzah, M.H.; Man, H.C.; Jamali, N.S.; Siajam, S.I.; Ismail, M.H. Recent Updates on the conversion of pineapple waste (Ananas comosus) to value-added products, future perspectives and challenges. Agronomy 2021, 11, 2221. [Google Scholar] [CrossRef]
- Prado, K.S.; Spinacé, M.A. Isolation and characterization of cellulose nanocrystals from pineapple crown waste and their potential uses. Int. J. Biol. Macromol. 2019, 122, 410–416. [Google Scholar] [CrossRef]
- Casabar, J.T.; Ramaraj, R.; Tipnee, S.; Unpaprom, Y. Enhancement of hydrolysis with Trichoderma harzianum for bioethanol production of sonicated pineapple fruit peel. Fuel 2020, 279, 118437. [Google Scholar] [CrossRef]
- Mamani, D.C.; Nole, K.S.O.; Montoya, E.E.C.; Huiza, D.A.M.; Alta, R.Y.P.; Vitorino, H.A. Minimizing organic waste generated by pineapple crown: A simple process to obtain cellulose for the preparation of recyclable containers. Recycling 2020, 5, 24. [Google Scholar] [CrossRef]
- Hamidin, N.A.S.; Abdullah, S.; Nor, F.H.M.; Hadibarata, T. Isolation and identification of natural green and yellow pigments from pineapple pulp and peel. Mater. Today Proc. 2022, 63, S406–S410. [Google Scholar] [CrossRef]
- Li, T.; Shen, P.; Liu, W.; Liu, C.; Liang, R.; Yan, N.; Chen, J. Major polyphenolics in pineapple peels and their antioxidant interactions. Int. J. Food Prop. 2014, 17, 1805–1817. [Google Scholar] [CrossRef]
- Sun, G.-M.; Zhang, X.-M.; Soler, A.; Marie-Alphonsine, P. Nutritional composition of pineapple (Ananas comosus (L.) Merr.). In Nutritional Composition of Fruit Cultivars; Simmonds, M.S.J., Preedy, V.R., Eds.; Elsevier: New York, NY, USA, 2016; pp. 609–637. [Google Scholar]
- Elss, S.; Preston, C.; Hertzig, C.; Heckel, F.; Richling, E.; Schreier, P. Aroma profiles of pineapple fruit (Ananas comosus [L.] Merr.) and pineapple products. LWT-Food Sci. Technol. 2005, 38, 263–274. [Google Scholar] [CrossRef]
- Zampar, G.G.; Zampar, I.C.; Beserra da Silva de Souza, S.; da Silva, C.; Barros, B.C.B. Effect of solvent mixtures on the ultrasound-assisted extraction of compounds from pineapple by-product. Food Biosci. 2022, 50, 102098. [Google Scholar] [CrossRef]
- Sepúlveda, L.; Romaní, A.; Aguilar, C.N.; Teixeira, J. Valorization of pineapple waste for the extraction of bioactive compounds and glycosides using autohydrolysis. Innov. Food Sci. Emerg. Technol. 2018, 47, 38–45. [Google Scholar] [CrossRef]
- Wong-Paz, J.E.; Aguilar-Zárate, P.; Veana, F.; Muñiz-Márquez, D.B. Impact of green extraction technologies to obtain bioactive compounds from citrus fruit residues. TIP Rev. Espec. Cienc. Quím.-Biol. 2020, 23, 1–11. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Martinović, J.; Perković, G.; Bucić-Kojić, A. Bioconversion of Grape Pomace with Rhizopus oryzae under Solid-State Conditions: Changes in the Chemical Composition and Profile of Phenolic Compounds. Microorganisms 2023, 11, 956. [Google Scholar] [CrossRef] [PubMed]
- Handa, C.L.; de Lima, F.S.; Guelfi, M.F.G.; da Silva Fernandes, M.; Georgetti, S.R.; Ida, E.I. Parameters of the fermentation of soybean flour by Monascus purpureus or Aspergillus oryzae on the production of bioactive compounds and antioxidant activity. Food Chem. 2019, 271, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Buenrostro-Figueroa, J.; Ascacio-Valdés, A.; Sepúlveda, L.; De la Cruz, R.; Prado-Barragán, A.; Aguilar-González, M.A.; Rodríguez, R.; Aguilar, C.N. Potential use of different agroindustrial by-products as supports for fungal ellagitannase production under solid-state fermentation. Food Bioprod. Process. 2014, 92, 376–382. [Google Scholar] [CrossRef]
- Polania-Rivera, A.M.; Toro, C.R.; Londoño, L.; Bolivar, G.; Ascacio, J.A.; Aguilar, C.N. Bioprocessing of pineapple waste biomass for sustainable production of bioactive compounds with high antioxidant activity. J. Food Meas. Charact. 2023, 17, 586–606. [Google Scholar] [CrossRef]
- Selvanathan, Y.; Masngut, N. Optimization of process factor and characterization of vinegar-like beverage production via spontaneous fermentation from pineapple peel waste. LWT 2023, 182, 114818. [Google Scholar] [CrossRef]
- Redmile-Gordon, M.; Armenise, E.; White, R.; Hirsch, P.; Goulding, K. A comparison of two colorimetric assays, based upon Lowry and Bradford techniques, to estimate total protein in soil extracts. Soil Biol. Biochem. 2013, 67, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Púa, A.L.; Barreto, G.E.; Zuleta, J.L.; Herrera, O.D. Análisis de Nutrientes de la Raíz de la Malanga (Colocasia esculenta Schott) en el Trópico Seco de Colombia. Inf. Tecnol. 2019, 30, 69–76. [Google Scholar] [CrossRef]
- Andrade-Damián, M.F.; Muñiz-Márquez, D.B.; Wong-Paz, J.E.; Veana-Hernández, F.; Reyes-Luna, C.; Aguilar-Zárate, P. Exploratory study of pigment extraction from Curcuma longa L. by slid-state fermentation using five fungal strains. Mex. J. Biotechnol. 2019, 4, 1–11. [Google Scholar] [CrossRef]
- Zaki, N.A.M.; Alwi, H.; Hashib, S.A.; Ibrahim, U.K.; Jai, J. Microwave drying characteristics and quality of Ananas comosus peel, core and pulp. Mater. Today Proc. 2023, 87, 8–12. [Google Scholar] [CrossRef]
- Palacios, C.E.; Nagai, A.; Torres, P.; Rodrigues, J.A.; Salatino, A. Contents of tannins of cultivars of sorghum cultivated in Brazil, as determined by four quantification methods. Food Chem. 2020, 337, 127970. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Herrera, R.; Alvarez-Pérez, O.B.; Ventura-Sobrevilla, J.; Ascacio-Valdés, A.; Aguilar-Gonzalez, M.A.; Buenrostro-Figueroa, J.; Aguilar, C.N. Pomegranate peel polyphenols as an antioxidant additive for the development and characterization of a new active pectin edible film. eFood 2023, 4, e115. [Google Scholar] [CrossRef]
- Mala, T.; Piayura, S.; Itthivadhanapong, P. Characterization of dried pineapple (Ananas comosus L.) peel powder and its application as a novel functional food ingredient in cracker product. Future Foods 2024, 9, 100322. [Google Scholar] [CrossRef]
- Buenrostro-Figueroa, J.; Velázquez, M.; Flores-Ortega, O.; Ascacio-Valdés, J.; Huerta-Ochoa, S.; Aguilar, C.; Prado-Barragán, L. Solid state fermentation of fig (Ficus carica L.) by-products using fungi to obtain phenolic compounds with antioxidant activity and qualitative evaluation of phenolics obtained. Process. Biochem. 2017, 62, 16–23. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzmán, N.; Ascacio-Valdés, J.; Serna-Cock, L.; dos Santos Correia, M.T.; Contreras-Esquivel, J.C.; Aguilar, C.N. Solid-state fermentation with Aspergillus niger to enhance the phenolic contents and antioxidative activity of Mexican mango seed: A promising source of natural antioxidants. LWT 2019, 112, 108236. [Google Scholar] [CrossRef]
- Selani, M.M.; Brazaca, S.G.C.; dos Santos Dias, C.T.; Ratnayake, W.S.; Flores, R.A.; Bianchini, A. Characterisation and potential application of pineapple pomace in an extruded product for fibre enhancement. Food Chem. 2014, 163, 23–30. [Google Scholar] [CrossRef]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Bonafe, E.G.; Suzuki, R.M.; Souza, N.E.; Matsushita, M.; Visentainer, J.V. Proximate Composition, Mineral Contents and Fatty Acid Composition of the Different Parts and Dried Peels of Tropical Fruits Cultivated in Brazil. J. Braz. Chem. Soc. 2016, 28, 308–318. [Google Scholar] [CrossRef]
- Huang, C.W.; Lin, I.J.; Liu, Y.; Mau, J.L. Composition, enzyme and antioxidant activities of pineapple. Int. J. Food Prop. 2021, 24, 1244–1251. [Google Scholar] [CrossRef]
- Sanchez Pardo, M.E.S.; Cassellis, M.E.R.; Escobedo, R.M.; García, E.J. Chemical Characterisation of the Industrial Residues of the Pineapple (Ananas comosus). J. Agric. Chem. Environ. 2014, 3, 53–56. [Google Scholar] [CrossRef]
- Aparecida Damasceno, K.; Alvarenga Gonçalves, C.A.; Dos Santos Pereira, G.; Lacerda Costa, L.; Bastianello Cam-pagnol, P.C.; Leal De Almeida, P.; Arantes-Pereira, L. Development of Cereal Bars Containing Pineapple Peel Flour (Ananas comosus L. Merril). J. Food Qual. 2016, 39, 417–424. [Google Scholar] [CrossRef]
- Hassan, A.; Othman, Z.; Siriphanich, J. Pineapple (Ananas comosus L. Merr.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Zakaria, N.A.; Rahman, R.A.; Zaidel, D.N.A.; Dailin, D.J.; Jusoh, M. Microwave-assisted extraction of pectin from pineapple peel. Malays. J. Fundam. Appl. Sci. 2021, 17, 33–38. [Google Scholar] [CrossRef]
- Diaz-Vela, J.; Totosaus, A.; Cruz-Guerrero, A.E.; de Lourdes Pérez-Chabela, M. In vitroevaluation of the fermentation of added-value agroindustrial by-products: Cactus pear (Opuntia ficus-indica L.) peel and pineapple (Ananas comosus) peel as functional ingredients. Int. J. Food Sci. Technol. 2013, 48, 1460–1467. [Google Scholar] [CrossRef]
- Paz-Arteaga, S.L.; Ascacio-Valdes, J.A.; Aguilar, C.N.; Cadena-Chamorro, E.; Serna-Cock, L.; Aguilar-Gonzalez, M.A.; Ramirez-Guzman, N.; Torres-Leon, C. Bioprocessing of pineapple waste for sustainable production of bioactive compounds using solid-state fermentation. Innov. Food Sci. Emerg. Technol. 2023, 85, 103313. [Google Scholar] [CrossRef]
- Correia, R.T.; McCue, P.; Magalhães, M.M.; Macêdo, G.R.; Shetty, K. Production of phenolic antioxidants by the solid-state bioconversion of pineapple waste mixed with soy flour using Rhizopus oligosporus. Process. Biochem. 2004, 39, 2167–2172. [Google Scholar] [CrossRef]
- Rajan, M.; Santana Andrade, J.K.; Chagas Barros, R.G.; Farias Lima Guedes, T.J.; Narain, N. Enhancement of polyphenolics and antioxidant activities of jambolan (Syzygium cumini) fruit pulp using solid state fermentation by Aspergillus niger and A. flavus. Biocatal. Agric. Biotechnol. 2023, 47, 102589. [Google Scholar] [CrossRef]
- Polania, A.M.; Londoño, L.; Ramírez, C.; Bolívar, G. Influence of Ultrasound Application in Fermented Pineapple Peel on Total Phenolic Content and Antioxidant Activity. Fermentation 2022, 8, 314. [Google Scholar] [CrossRef]
- Chiet, C.H.; Zulkifli, R.M.; Hidayat, T.; Yaakob, H. Bioactive compounds and antioxidant activity analysis of Malaysian pineapple cultivars. AIP Conf. Proc. 2014, 1589, 398–399. [Google Scholar] [CrossRef]
- Brito, T.B.N.; Lima, L.R.S.; Santos, M.C.B.; Moreira, R.F.A.; Cameron, L.C.; Fai, A.E.C.; Ferreira, M.S.L. Antimicrobial, antioxidant, volatile and phenolic profiles of cabbage-stalk and pineapple-crown flour revealed by GC-MS and UPLC-MSE. Food Chem. 2021, 339, 127882. [Google Scholar] [CrossRef] [PubMed]
- Larios-Cruz, R.; Buenrostro-Figueroa, J.; Prado-Barragán, A.; Rodríguez-Jasso, R.M.; Rodríguez-Herrera, R.; Montañez, J.C.; Aguilar, C.N. Valorization of Grapefruit By-Products as Solid Support for Solid-State Fermentation to Produce Antioxidant Bioactive Extracts. Waste Biomass-Valorization 2019, 10, 763–769. [Google Scholar] [CrossRef]
- Erskine, E.; Ozkan, G.; Lu, B.; Capanoglu, E. Effects of Fermentation Process on the Antioxidant Capacity of Fruit Byproducts. ACS Omega 2023, 8, 4543–4553. [Google Scholar] [CrossRef]
- Banerjee, S.; Ranganathan, V.; Patti, A.; Arora, A. Valorisation of pineapple wastes for food and therapeutic applications. Trends Food Sci. Technol. 2018, 82, 60–70. [Google Scholar] [CrossRef]


| Treatments | Temperature (°C) | Humidity (%) | Inoculum (spores/g) | NaNO3 | MgSO4 | KCl | KH2PO4 |
|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | 1 | 1 | 1 | −1 |
| 2 | 1 | −1 | −1 | −1 | −1 | 1 | 1 |
| 3 | −1 | 1 | −1 | −1 | 1 | −1 | 1 |
| 4 | 1 | 1 | −1 | 1 | −1 | −1 | −1 |
| 5 | −1 | −1 | 1 | 1 | −1 | −1 | 1 |
| 6 | 1 | −1 | 1 | −1 | 1 | −1 | −1 |
| 7 | −1 | 1 | 1 | −1 | −1 | 1 | −1 |
| 8 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Factors | Levels | ||||||
| +1 | −1 | ||||||
| Temperature (°C) | 30 | 25 | |||||
| Humidity (%) | 80 | 70 | |||||
| Inoculum (spores/g) | 1 × 107 | 1 × 106 | |||||
| NaNO3 (g/L) | 15.6 | 7.65 | |||||
| MgSO4 (g/L) | 3.04 | 1.52 | |||||
| KCl (g/L) | 3.04 | 1.52 | |||||
| KH2PO4 (g/L) | 6.08 | 3.04 | |||||
| Unfermented Sample | ||||
|---|---|---|---|---|
| No. | Molecular weight | Chemical formula | Molecule | Family |
| 1 | 341.0 | C15H18O9 | Caffeic acid 4-O-glucoside | Hydroxycinnamic acids |
| 2 | 314.9 | C16H12O7 | Rhamnetin | Methoxyflavonols |
| 3 | 389.0 | C20H22O8 | Resveratrol 3-O-glucoside | Stilbenes |
| 4 | 322.9 | Gallic acid 3-O-gallate | Hydroxybenzoic acids | |
| 5 | 252.9 | C15H10O4 | 7,4′-Dihydroxyflavone | Flavones |
| 6 | 352.8 | C16H18O9 | 1-Caffeoylquinic acid | Hydroxycinnamic acids |
| 7 | 370.8 | C20H20O7 | Sinensetin | Methoxyflavones |
| 8 | 336.8 | C16H18O8 | 3-p-Coumaroylquinic acid | Hydroxycinnamic acids |
| 9 | 622.8 | C28H32O16 | Isorhamnetin 3-O-glucoside 7-O-rhamnoside | Methoxyflavonols |
| 10 | 414.9 | C21H20O9 | Daidzin | Isoflavones |
| Treatment 4 of A. niger Aa20 strain | ||||
| No. | Molecular weight | Chemical formula | Molecule | Family |
| 1 | 368.8 | C21H20O6 | Curcumin | Curcuminoids |
| 352.9 | C16H18O9 | 1-Caffeoylquinic acid | Hydroxycinnamic acids | |
| 2 | 359.8 | C20H24O6 | Lariciresinol | Lignans |
| 3 | 366.8 | C17H20O9 | 3-Feruloylquinic acid | Methoxycinnamic acids |
| 4 | 380.5 | C15H10O10S | Quercetin 3′-sulfate | Flavonols |
| 5 | 300.8 | C15H10O7 | Quercetin | Flavonols |
| Treatment 6 of A. niger HT3 strain | ||||
| No. | Molecular weight | Chemical formula | Molecule | Family |
| 1 | 358.6 | C20H24O6 | Lariciresinol | Lignans |
| 2 | 352.9 | C16H18O9 | 1-Caffeoylquinic acid | Hydroxycinnamic acids |
| 3 | 283.9 | C16H12O5 | Methylgalangin | Methoxyflavonols |
| 4 | 306.8 | C15H14O7 | (+)-Gallocatechin | Catechins |
| 5 | 256.7 | C15H12O4 | Pinocembrin | Flavanones |
| 6 | 311.9 | C13H12O9 | Caffeoyl tartaric acid | Hydroxycinnamic acids |
| 7 | 588.9 | C39H58O4 | Schottenol ferulate | Methoxycinnamic acids |
| 8 | 600.9 | C7H6O5 | Gallagic acid | Hydroxybenzoic acids |
| 9 | 289.0 | C15H14O6 | (+)-Catechin | Catechins |
| 10 | 286.6 | C15H11O6+ | Cyanidin | Anthocyanins |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casas-Rodríguez, A.D.; Ascacio-Valdés, J.A.; Dávila-Medina, M.D.; Medina-Morales, M.A.; Londoño-Hernández, L.; Sepúlveda, L. Evaluation of Solid-State Fermentation Conditions from Pineapple Peel Waste for Release of Bioactive Compounds by Aspergillus niger spp. Appl. Microbiol. 2024, 4, 934-947. https://doi.org/10.3390/applmicrobiol4020063
Casas-Rodríguez AD, Ascacio-Valdés JA, Dávila-Medina MD, Medina-Morales MA, Londoño-Hernández L, Sepúlveda L. Evaluation of Solid-State Fermentation Conditions from Pineapple Peel Waste for Release of Bioactive Compounds by Aspergillus niger spp. Applied Microbiology. 2024; 4(2):934-947. https://doi.org/10.3390/applmicrobiol4020063
Chicago/Turabian StyleCasas-Rodríguez, A. Danitza, Juan A. Ascacio-Valdés, Miriam Desirée Dávila-Medina, Miguel A. Medina-Morales, Liliana Londoño-Hernández, and Leonardo Sepúlveda. 2024. "Evaluation of Solid-State Fermentation Conditions from Pineapple Peel Waste for Release of Bioactive Compounds by Aspergillus niger spp." Applied Microbiology 4, no. 2: 934-947. https://doi.org/10.3390/applmicrobiol4020063
APA StyleCasas-Rodríguez, A. D., Ascacio-Valdés, J. A., Dávila-Medina, M. D., Medina-Morales, M. A., Londoño-Hernández, L., & Sepúlveda, L. (2024). Evaluation of Solid-State Fermentation Conditions from Pineapple Peel Waste for Release of Bioactive Compounds by Aspergillus niger spp. Applied Microbiology, 4(2), 934-947. https://doi.org/10.3390/applmicrobiol4020063









