16S rRNA Analysis of Electrogenic Bacterial Communities from Soil Microbial Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sampling
2.2. Microbial Fuel Cell Assembly
2.3. Electricity and Electrogenic Bacteria Measurements
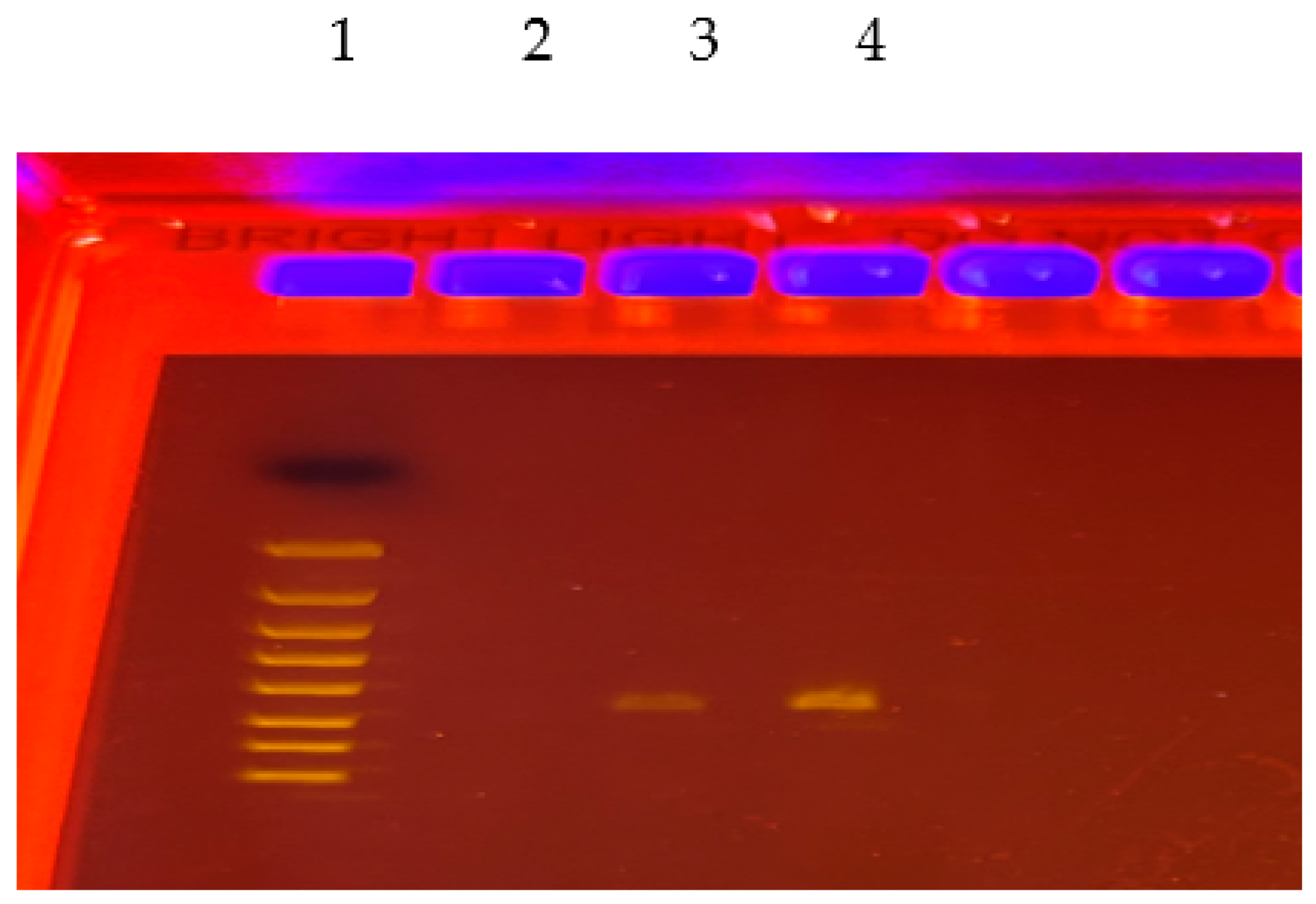
2.4. DNA Extraction and PCR Analysis of Bacterial 16S rRNA Genes in SMFC Samples
2.5. DNA Sequencing Analysis of 16S rRNA Genes in SMFC Samples
2.6. Analysis of Cellulase Genes in SMFC Samples
3. Results
3.1. Electricity Generation and Electrogenic Bacteria by SMFCs
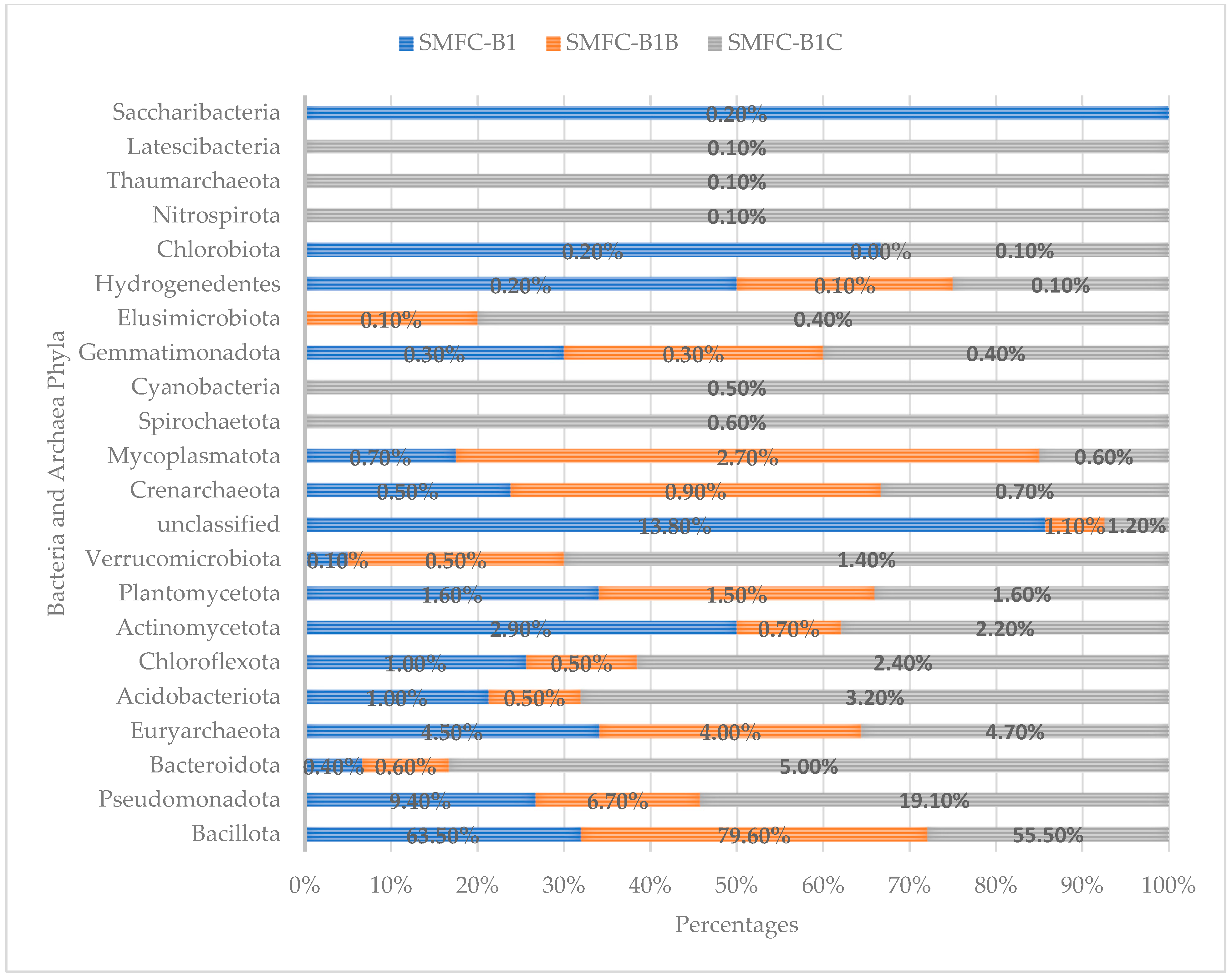
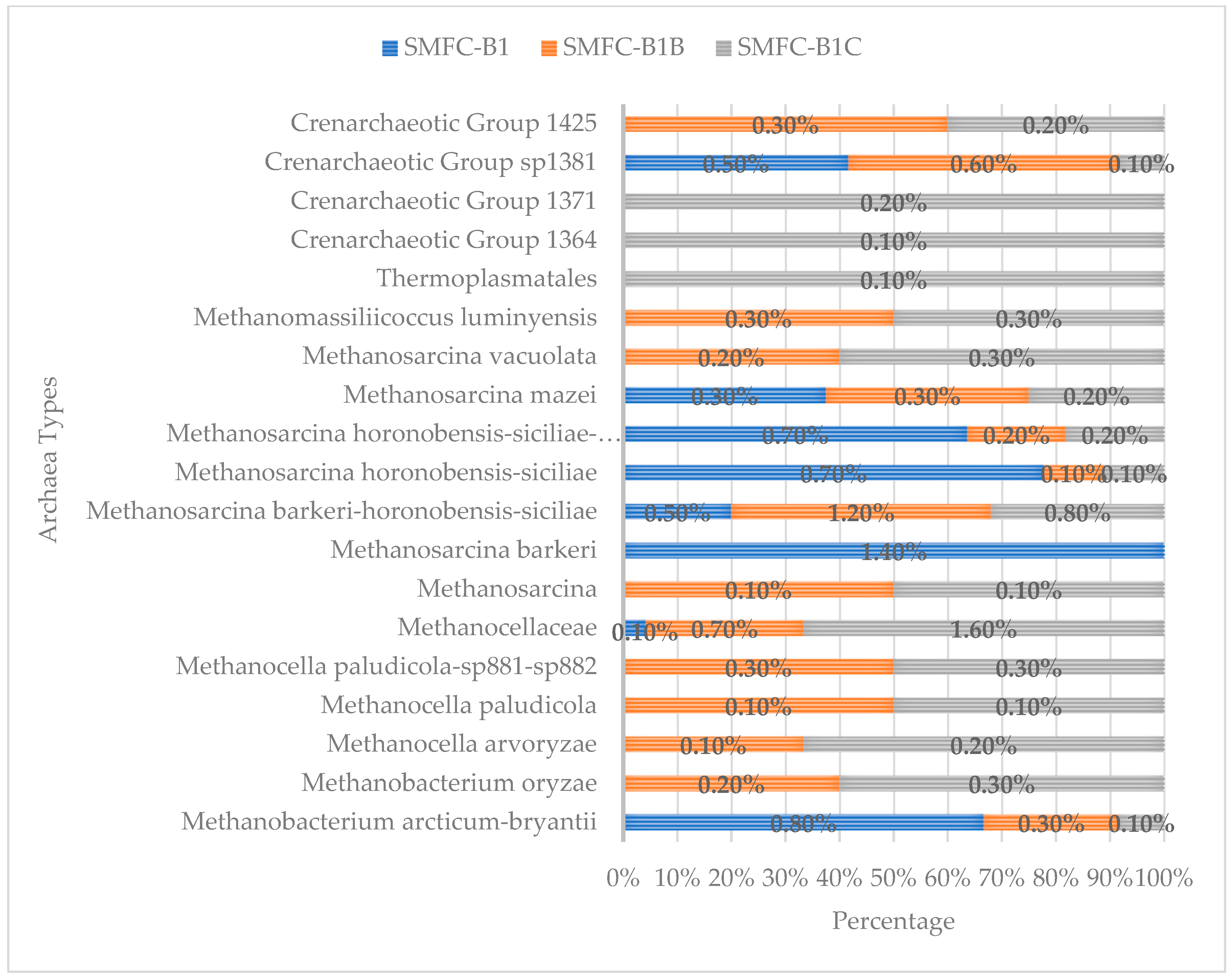
3.2. Diversity, Bacteria and Archaea Phyla in SMFCs with High Electrical Output
3.3. Distribution of Bacteria and Archaea in SMFC with High Electrical Output
3.4. PCR Detection and Quantification of Cellulase Genes in SMFCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Falkowski, P.G.; Fenchel, T.; Delong, E.F. The microbial engines that drive Earth’s biogeochemical cycles. Science 2008, 320, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacteria communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.E.; Zhang, E.; Terauds, A.; Ji, M.; Kong, W.; Ferrari, B.C. Soil microbiomes with the genetic capacity for atmospheric chemosynthesis are widespread across the poles and are associated with moisture, carbon, and nitrogen limitation. Front. Microbiol. 2020, 11, 1936. [Google Scholar] [CrossRef] [PubMed]
- Bickel, S.; Or, D. Soil bacterial diversity mediated by microscale aqueous-phase processes across biomes. Nat. Commun. 2020, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-Gonzalez, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A global atlas of the dominant bacteria found in soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, C.; Luo, Y. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef] [PubMed]
- Llado, S.; Lopez-Mondejar, R.; Baldrian, P. Forest soil bacteria: Diversity, involvement in ecosystem processes, and response to global change. Microbiol. Mol. Biol. Rev. 2017, 81, e00063-16. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, L.; Kulko, M.; Kim, R.; Jashari, T.; Choe, T. 16S rRNA analysis of electrogenic bacterial communities in microbial fuel cells developed from temperate soils. BIOS 2020, 91, 9–20. [Google Scholar] [CrossRef]
- Ruijven, B.J.; De Cian, E.; Wing, I.S. Amplification of future energy demand growth due to climate change. Nat. Commun. 2019, 10, 2762. [Google Scholar] [CrossRef]
- Al-Ghussain, L. Global warming: Review on driving forces and mitigation. Environ. Prog. Sustain. Energy 2019, 38, 13–21. [Google Scholar]
- Wang, J.; Ren, K.; Zhu, Y.; Huang, J.; Liu, S. A review of recent advances in microbial fuel cells: Preparation, operation, and application. BioTech 2022, 11, 44. [Google Scholar] [CrossRef]
- Rabaey, K.; Boon, N.; Siciliano, S.D.; Verhaege, M.; Verstraete, W. Biofuel cells select for microbial consortia that self-mediate electron transfer. Appl. Environ. Microbiol. 2004, 70, 5373–5382. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.R.; Holmes, D.E.; Tender, L.M.; Lovley, D.R. Electrode-reducing microorganisms that harvest energy from marine sediments. Science 2002, 295, 483–485. [Google Scholar] [CrossRef]
- Dunaj, S.J.; Vallino, J.J.; Hines, M.E.; Gay, M.; Kobyljanec, C.; Rooney-Varga, J.N. Relationships between soil organic matter, nutrients, bacterial community structure, and the performance of microbial fuel cells. Environ. Sci. Technol. 2012, 46, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, Z.; Li, H.B.; Su, J.Q.; Zhao, F.; Zhu, Y.G. Bacterial community composition at anodes of microbial fuel cells for paddy soils: The effects of soil properties. J. Soils Sediments 2015, 15, 926–936. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X.; Ren, Y.; Wang, X.; Jian, C. Electricity generation from Taihu Lake cyanobacteria by sediment microbial fuel cells. J. Chem. Technol. Biotechnol. 2012, 87, 1567–1573. [Google Scholar] [CrossRef]
- Kaur Dhillon, S.; Dziegielowski, J.; Patit Paban, P.; Di Lorenzo, M. Functionalized graphite felt anodes for enhanced power generation in membrane-less soil microbial fuel cells. R. Soc. Chem. Sustain. 2023, 1, 310–325. [Google Scholar] [CrossRef]
- Nandy, A.; Farkas, D.; Pepio-Tarrega, B.; Martinez-Crespiera, S.; Borras, E.; Avignone-Rossa, C.; Di Lorenzo, C. Influence of carbon-based cathodes on biofilm composition and electrochemical performance in soil microbial fuel cells. Environ. Sci. Ecotechnol. 2023, 16, 100276. [Google Scholar] [CrossRef] [PubMed]
- Uria-Molto, N.; Costa, R.D.; Nunziata, C.; Santiago, S.; Guirado, G.; Munoz-Berbel, X.; Kowalski, L. Self-contained and integral microbial fuel cells as portable and sustainable energy sources for low-power field devices. Electr. J. Biotechnol. 2022, 57, 44–51. [Google Scholar] [CrossRef]
- Greenman, J.; Thorn, R.; Willey, N.; Ieropoulos, I. Energy harvesting from plants using hybrid microbial fuel cells; potential applications and future exploitation. Front. Bioeng. Biotechnol. 2024, 12, 1276176. [Google Scholar] [CrossRef]
- Ishii, S.; Shimoyama, T.; Hotta, Y.; Watanabe, K. Characterization of a filamentous biofilm community established in a cellulose-fed microbial cell. BMC Microbiol. 2008, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Dziegielowski, J.; Mascia, M.; Metcalfe, B.; Di Lorenzo, M. Voltage evolution and electrochemical behavior of soil microbial fuel cells operated in different quality soils. Sustain. Energy Technol. Assess. 2023, 56, 103071. [Google Scholar]
- Hodgson, D.M.; Smith, A.; Dahale, S.; Stratford, J.P.; Li, J.V.; Gruning, A.; Bushell, M.E.; Marchesi, J.R.; Avignone Rossa, C. Segregation of the anodic microbial communities in a microbial fuel cell cascade. Fontiers Microbiol. 2016, 7, 699. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Mu, H.; Liu, W.; Zhang, R.; Guo, J.; Xian, M.; Liu, H. Electrigens in the anode of microbial fuel cells: Pure cultures versus mixed communities. Microb. Cell Factories 2019, 18, 39. [Google Scholar] [CrossRef]
- Rumora, A.; Hopkins, L.; Yim, K.; Baykus, M.F.; Martinez, L.; Jimenez, L. Detection and characterization of electrogenic bacteria from soils. BioTech 2023, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, L.; Jashari, T.; Vasquez, J.; Zapata, S.; Bochis, J.; Kulko, M.; Ellman, V.; Gardner, M.; Choe, T. Real-Time PCR detection of Burkholderia cepacia in pharmaceutical products contaminated with low levels of bacterial contamination. PDA J. Pharm. Sci. Technol. 2018, 72, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2012, 41, e1. [Google Scholar] [CrossRef]
- Jimenez, L.; Vazquez, S.; Turku, A.; Pincus, L. PCR detection, cloning, and genetic identification of microbial cellulases in soils. BIOS 2022, 93, 31–48. [Google Scholar] [CrossRef]
- Simeon, M.I.; Freitag, R. Influence of electrode spacing and fed-batch operation on the maximum performance trend of a soil microbial fuel cell. Int. J. Hydrogen Energy 2022, 47, 12304–12316. [Google Scholar] [CrossRef]
- Simeon, M.I.; Weig, A.; Freitag, R. Optimization of soil microbial fuel cell for sustainable bio-electricity production: Combined effects of electrode material, electrode spacing, and substrate feeding frequency on power generation and microbial community diversity. Biotechnol. Biofuels Bioprod. 2022, 15, 124. [Google Scholar] [CrossRef]
- Wang, Y.; Gallagher, L.A.; Andrade, P.A.; Liu, A.; Humphreys, I.R.; Tukarslan, S.; Cutler, K.J.; Arrieta-Ortiz, M.L.; Li, Y.; Radey, M.C.; et al. Genetic manipulation of Patescibacteria provides mechanistic insights into microbial dark matter and the epibiotic lifestyle. Cell 2023, 186, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Francioli, D.; Lentendu, G.; Lewin, S.; Kolb, S. DNA metabarcoding for the characterization of terrestrial microbiota-pitfalls and solutions. Microorganisms 2021, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, H.; Li, N.; Fahad Sardar, M.; Song, T.; Zhu, H.; Xing, X.; Zhu, C. Dynamics of a bacterial community in the anode and cathode of microbial fuel cells under sulfadiazine pressure. Int. J. Environ. Res. Public Health 2022, 19, 6253. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wei, J.; Liang, P.; Huang, X. Electricity generation and microbial community changes in microbial fuel cells packed with different anodic materials. Bioresour. Technol. 2011, 102, 10886–10891. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.B.; Zhong, W.H.; Han, C.; Deng, H. Characterization of electricity generated by soil in microbial fuel cells and the isolation of soil source exoelectrogenic bacteria. Front. Microbiol. 2016, 7, 1776. [Google Scholar] [CrossRef] [PubMed]
- Xiao Min, L.; Cheng, K.Y.; Selvam, A.; Wong, J.W.C. Bioelectricity production from acidic food waste leachate using microbial fuel cells: Effect of inocula. Process Biochem. 2013, 48, 283–288. [Google Scholar]
- Feng, S.; Tan, C.H.; Constancias, F.; Kohli, G.S.; Cohen, Y.; Rice, S.A. Predation by Bdellovibrio bacteriovorus significantly reduced viability and alters the microbial community composition of activated sludge flocs and granules. FEMS Microbiol. Ecol. 2017, 93, 1093. [Google Scholar] [CrossRef] [PubMed]
- Johnke, J.; Fraune, S.; Bosch, T.C.G.; Hentschel, U.; Schulenburg, H. Bdellovibrio and other like organisms are predictors of microbiome diversity in distinct host groups. Microb. Ecol. 2020, 79, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.E.; Nevin, K.P.; Snoeyenbos-West, O.L.; Woodward, T.L.; Strickland, J.N.; Lovely, D.R. Protozoan grazing reduced the current output of microbial cells. Bioresour. Technol. 2015, 193, 8–14. [Google Scholar] [CrossRef]
- Yamasaki, R.; Maeda, T.; Wood, T.K. Electron carriers increase electricity production in methane microbial fuel cells that reverse methanogenesis. Biotechnol. Biofuels 2018, 11, 211. [Google Scholar] [CrossRef]
- MacAnulty, M.J.; Poosarla, V.G.; Kim, K.Y.; Jasso-Chavez, R.; Logan, B.E.; Wood, T.K. Electricity from methane by reversing methanogenesis. Nat. Commun. 2017, 8, 15419. [Google Scholar] [CrossRef] [PubMed]
- Barbato, R.A.; Foley, K.L.; Toro-Zapata, J.A.; Jines, R.M.; Reynolds, C.M. The power of soil microbes: Sustained power production in terrestrial microbial fuel cells under various temperature regimes. Appl. Soil Ecol. 2017, 109, 14–22. [Google Scholar] [CrossRef]
- Guerrero-Cruz, S.; Vaksmaa, A.; Horn, M.A.; Niemann, H.; Pijuan, M.; Ho, A. Methanotrophs: Discoveries, environmental relevance, and a perspective on current and future applications. Front. Microbiol. 2021, 12, 678057. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Lee, J.; Neufeld, J.D.; Park, J.; Rittmann, B.E.; Lee, H.S. Anaerobic oxidation of methane coupled with extracellular electron transfer to electrodes. Sci. Rep. 2017, 7, 5099. [Google Scholar] [CrossRef]
- Ouboter, H.T.; Mesman, R.; Sleutels, T.; Postma, J.; Wissink, M.; Jetten, M.S.M.; Ter Heijne, A.; Berbern, R.; Welte, C.U. Mechanisms of extracellular electron transfer in anaerobic methanotrophic archaea. Nat. Commun. 2024, 15, 1477. [Google Scholar] [CrossRef]

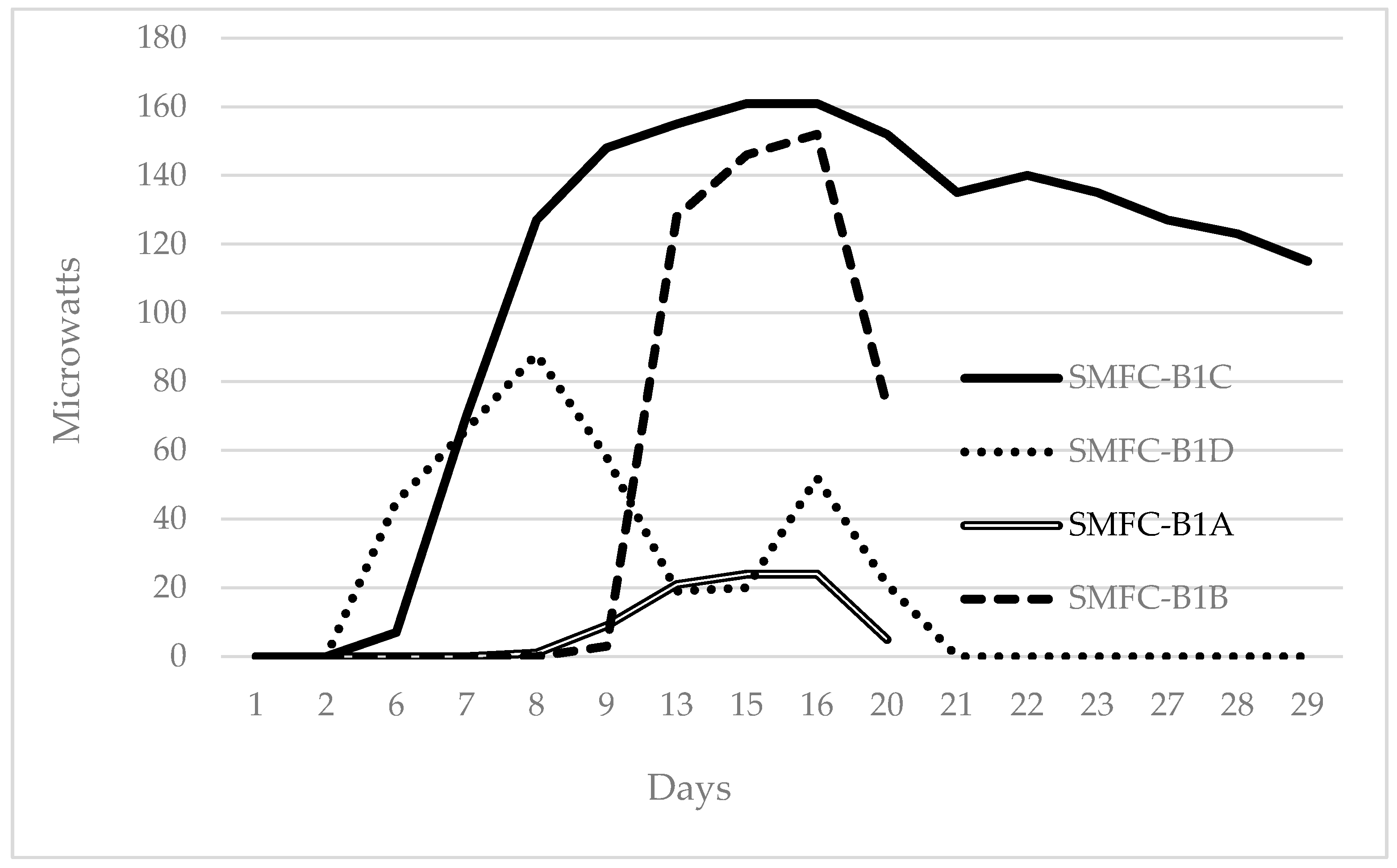
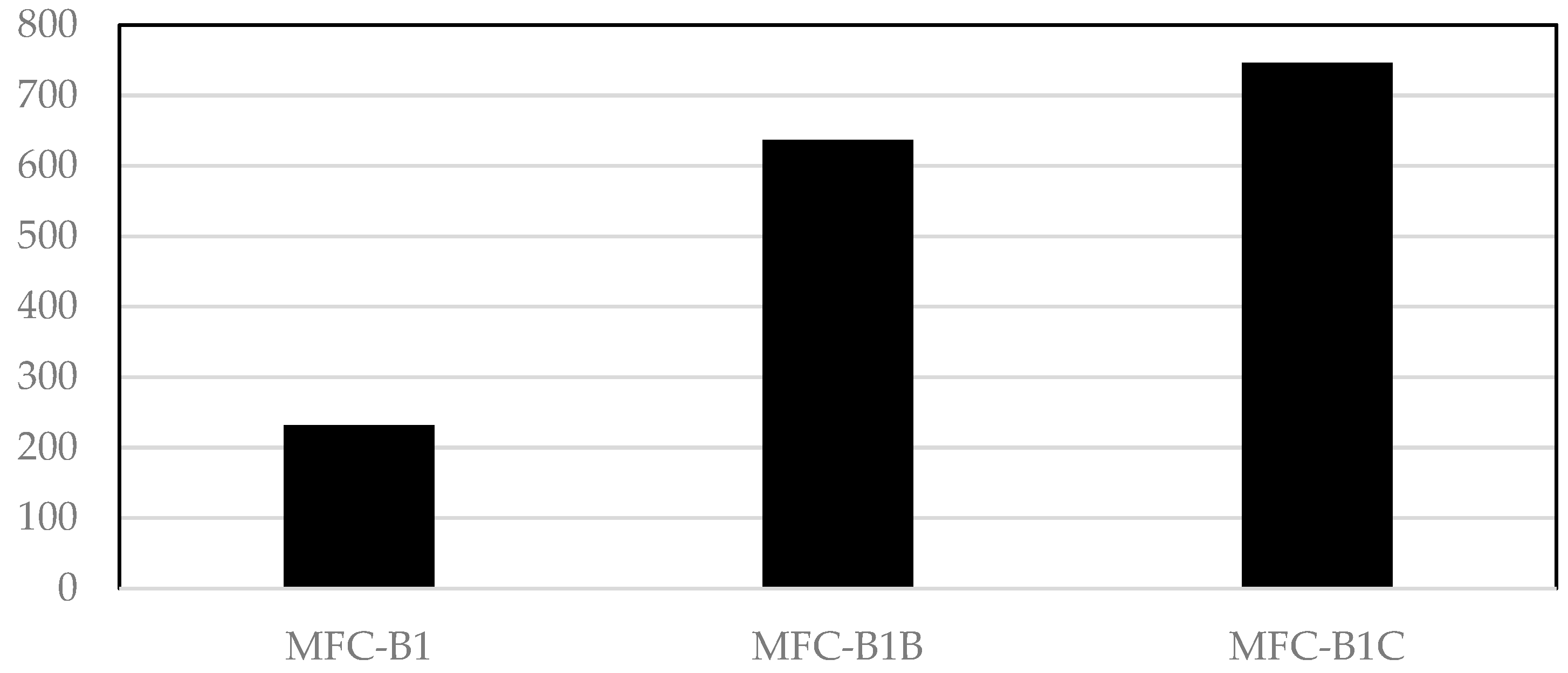
| Name | S | Microwatts | H | Microwatts |
|---|---|---|---|---|
| SMFC1 | 0 | 0 | 0 | 0 |
| SMFC2 | 5 | 6 | 7 | 20 |
| SMFC3 | 3 | 13 | 12 | 80 |
| SMFC-B1 | 1 | 7 | 14 | 143 |
| SMFC-B2A | 11 | 2 | 26 | 50 |
| SMFC-B2B | 11 | 24 | 21 | 31 |
| SMFC-B1A | 8 | 1 | 16 | 24 |
| SMFC-B1B | 9 | 3 | 16 | 152 |
| SMFC-CT | 1 | 15 | 1 | 15 |
| SMFC-AT | 0 | 0 | 0 | 0 |
| SMFC-B1C | 6 | 7 | 14 | 161 |
| SMFC-B1D | 6 | 45 | 8 | 88 |
| SMFC-T1 | 0 | 0 | 0 | 0 |
| SMFC-T4 | 1 | 2 | 7 | 111 |
| Name | EBS | EBH |
|---|---|---|
| SMFC1 | 0 | 0 |
| SMFC2 | 1.37 × 108 | 4.33 × 108 |
| SMFC3 | 2.71 × 108 | 1.67 × 109 |
| SMFC-B1 | 1.51 × 108 | 2.99 × 109 |
| SMFC-B2A | 5.43 × 107 | 1.06 × 109 |
| SMFC-B2B | 5.08 × 108 | 6.53 × 108 |
| SMFC-B1A | 3.70 × 107 | 5.15 × 108 |
| SMFC-B1B | 8.14 × 107 | 3.17 × 109 |
| SMFC-CT | 3.19 × 108 | 3.19 × 108 |
| SMFC-AT | 0 | 0 |
| SMFC-B1C | 1.55 × 108 | 3.37 × 109 |
| SMFC-B1D | 9.49 × 108 | 1.84 × 109 |
| SMFC-T1 | 0 | 0 |
| SMFC-T4 | 4.71 × 107 | 2.31 × 109 |
| Phylum, Class | Order, Family | Genus | Percent |
|---|---|---|---|
| Unclassified | NM * | NM * | 13.8 |
| Bacillota, Clostridia | Clostridiales, Heliobacteriaceae | NM * | 8.4 |
| Bacillota, Clostridia | Clostridiales, Ruminococcaceae | NM * | 3.1 |
| Bacillota, Clostridia | Clostridiales, Caldicoprobacteraceae | Caldicoprobacter | 3.0 |
| Bacillota, | NM * | NM * | 2.6 |
| Bacillota, Clostridia | Clostridiales, Lachnospiraceae | Mobilitalea | 2.2 |
| Bacillota, Clostridia | Clostridiales, Ruminococcaceae | NM * | 2.0 |
| Pseudomonadota, Alphaproteobacteria | Rhodospirillales, Rhodospirillaceae | Magnetospirillum | 1.7 |
| Bacillota | NM * | NM * | 1.7 |
| Bacillota, Clostridia | Clostridiales, Ruminococcaceae | NM * | 1.6 |
| Phylum, Class | Order, Family | Genus | Percent |
|---|---|---|---|
| Bacillota, Clostridia | Clostridiales, Ruminococcaceae | NM * | 6.7 |
| Bacillota, Clostridia | Clostridiales, Gracilibacteraceae | Gracilibacter | 6.3 |
| Bacillota, Clostridia | Clostridiales, Heliobacteriaceae | NM * | 5.0 |
| Bacillota, Clostridia | Clostridiales, Ruminococcaceae | NM * | 3.8 |
| Bacillota, NM * | NM * | NM * | 2.5 |
| Bacillota, NM * | NM * | NM * | 2.2 |
| Bacillota, NM * | NM * | NM * | 2.1 |
| Bacillota, NM * | NM * | NM * | 2.1 |
| Bacillota, Clostridia | Clostridiales, Ruminococcaceae | NM * | 2.0 |
| Bacillota, Clostridia | Clostridiales, Ruminococcaceae | NM * | 2.0 |
| Phylum, Class | Order, Family | Genus | Percent |
|---|---|---|---|
| Pseudomonadota, Alphaproteobacteria | Rhodocyclales, Rhodocyclaceae | Azospira | 6.8 |
| Bacillota, Clostridia | Clostridiales, Lachnospiraceae | Mobilitalea | 6.7 |
| Bacillota, Clostridia | Clostridiales, Gracilibacteraceae | Gracilibacter | 3.5 |
| Bacillota, Clostridia | Clostridiales, NM * | NM * | 3.5 |
| Pseudomonadota, Deltaproteobacteria | Bdellovibrionalles, Bdellovibrionaceae | Bdellovibrio | 3.2 |
| Bacillota, Clostridia | Clostridiales, Christensenellaceae | NM * | 2.6 |
| Bacillota, Clostridia | NM * | NM * | 2.3 |
| Bacillota, Clostridia | Clostridiales, NM * | NM * | 2.3 |
| Bacillota, Clostridia | Clostridiales, Ruminococcaceae | NM * | 2.0 |
| Bacillota, Clostridia | Clostridiales, Ruminococcaceae | NM * | 1.9 |
| Sample | DNA Concentration Micrograms/mL |
|---|---|
| SMFC-B1 | 1.59 |
| SMFC-B1B | 1.95 |
| SMFC-B1C | 4.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rumora, A.; Hopkins, L.; Yim, K.; Baykus, M.F.; Martinez, L.; Jimenez, L. 16S rRNA Analysis of Electrogenic Bacterial Communities from Soil Microbial Fuel Cells. Appl. Microbiol. 2024, 4, 918-933. https://doi.org/10.3390/applmicrobiol4020062
Rumora A, Hopkins L, Yim K, Baykus MF, Martinez L, Jimenez L. 16S rRNA Analysis of Electrogenic Bacterial Communities from Soil Microbial Fuel Cells. Applied Microbiology. 2024; 4(2):918-933. https://doi.org/10.3390/applmicrobiol4020062
Chicago/Turabian StyleRumora, Ana, Liliana Hopkins, Kayla Yim, Melissa F. Baykus, Luisa Martinez, and Luis Jimenez. 2024. "16S rRNA Analysis of Electrogenic Bacterial Communities from Soil Microbial Fuel Cells" Applied Microbiology 4, no. 2: 918-933. https://doi.org/10.3390/applmicrobiol4020062
APA StyleRumora, A., Hopkins, L., Yim, K., Baykus, M. F., Martinez, L., & Jimenez, L. (2024). 16S rRNA Analysis of Electrogenic Bacterial Communities from Soil Microbial Fuel Cells. Applied Microbiology, 4(2), 918-933. https://doi.org/10.3390/applmicrobiol4020062





