Abstract
There is no doubt that ruminants have the capability to digest lignocellulosic compounds and to utilize them as an absorbable form of energy by tapping into enzymes produced by the microbial population in their rumens. Among the rumens of various ruminants, this study focused on Korean goat rumens because of their unique digestibility of lignocellulosic biomasses. Therefore, a novel Gene12 gene was screened and unmasked from the constructed rumen metagenomic library of a Korean black goat and expressed in a Bacillus megaterium system. The recombinant protein was distinguished as a novel α-L-arabinofuranosidase enzyme from glycosyl hydrolase family 43 (GH43) for its capability to hydrolyze the non-reducing end of α-1,5-L-arabinofuranose linkages in α-L-arabinofuranosyl groups. The enzyme can also break apart α-L-arabinofuranosidic linkages and act synergistically with other hemicellulolytic enzymes to release α-1,2- and α-1,3-L-arabinofuranosyl groups from L-arabinose-comprising polysaccharides. In silico, phylogenetic, and computational analyses proclaimed that the Gene12 gene encodes a novel carbohydrate-active enzyme possessing a V-shaped indentation of the GH43 catalytic and functional domain (carbohydrate-binding module 6). The recombinant Gene12 protein has shared 81% sequence homology with other members of the GH43 family. Enzymic synopses (optimal pH, temperatures, and stability studies) of the recombinant Gene12 enzyme and its substrate specificity (synthetic and natural substrates) profiling were considered. The recombinant Gene12 α-L-arabinofuranosidase works best at pH 6.0 and 40 °C, and it is stable at pH 4.0 to 7.0 at temperatures of 20 to 50 °C. Additionally, 5-blended β-sheets were identified through a tertiary (3D) structure analysis along with the high substrate specificity against p-nitrophenyl-D-arabinofuranoside (pNPA). The highest substrate specificity of pNPA for Gene12 α-L-arabinofuranosidase indicated its confirmation as an exo-type arabinofuronidase. The results thus propose using the Gene12 protein as an exo-mannered GH43 α-L-arabinofuranosidase (EC 3.2.1.55) enzyme.
1. Introduction
Hemicellulose is considered the second most complex carbohydrate polymer in woody and non-woody plants [1]. Dry plants generally contain 23 to 32% hemicellulosic compounds depending on the plant variety [2]. In contrast with cellulose, a linear polymer, hemicelluloses are linear or branched and their backbone contains approximately 500 to 3000 monomer units per chain as opposed to 7000 to 15,000 glucose molecules per polymer [3]. The short-chain and highly branched heterologous polymers of hemicellulose comprised various pentoses (α-L-arabinose and β-D-xylose) and hexoses (β-D-glucose, α-D-galactose, and β-D-mannose). Hemicelluloses are more closely bound with lignin through covalent bonds than celluloses, which are tightly bound with hemicelluloses via non-covalent hydrogen bond interactions contributing to the enhanced strength of plant cell walls [4]. For complete degradation of hemicellulosic compounds, hydrolysis of the backbone xylan and its substitutions by different depolymerizing enzymes such as acetyl xylan esterase (EC 3.1.1.72), feruloyl esterase (EC 3.1.1.73), α-glucuronidase (EC 3.2.1.39), and α-L-arabinofuranosidase (EC 3.2.1.55) is required [5]. The accessory enzyme α-L-arabinofuranosidase is capable of cleaving α-L-arabinofuranosidic linkages to release α-1,2-, α-1,3-, and α-1,5 α-L- arabinofuranosyl groups from L-arabinose comprising polysaccharides and can act synergistically with other hemicellulolytic enzymes for completely degrading the hemicellulose [6].
Ruminants primarily rely on plant-originated substances to comprise major lignocellulosic biomass [7]. However, symbiotic microorganisms (bacteria, fungi, protozoa, and methanogenic archaea) residing in the rumen of ruminants can depolymerize and ferment hemicellulosic compounds into soluble sugars, thereby making the mammalian host consent to gaining energy from the stodgy carbohydrate polymers [8,9]. Therefore, microorganisms residing in the rumen of ruminants can be considered a promising source of different lignocellulolytic enzymes [10]. To date, several studies have revealed the microbe-derived α-L-arabinofuranosidase as a source and their biochemical properties [11,12]. Moreover, another study discovered that α-L-Arabinofuranosidase (PsGH43_12) of the family 43 glycoside hydrolase produced by Pseudopedobacter saltans exhibited biochemical activity in a temperature variety of 35–55 °C with maxima at pH 6.5 [13]. Numerous high-throughput screening procedures have been applied to isolates and have characterized different lignocellulolytic enzymes from different resources [14]. However, due to the anaerobic environment, maintaining a constant pH and temperature in the rumen of ruminants becomes more complex when re-culturing these symbiotic microorganisms within in vitro conditions [15]. Formerly, culture-dependent screening processes were used extensively but these traditional approaches resulted in failures when screening for these rumen microorganisms [10]. Therefore, activity-based metagenomic approaches for isolating and characterizing the novel functional Gene12 gene from unculturable microbial communities of rumens have been considered [8,16].
The optimization of culture conditions (e.g., pH, temperature, inducer concentration, and nutrition source) is necessary for the expression and large-scale production of the α-L-arabinofuranosidase enzyme in industries [17]. The GRAS recognition of Bacillus megaterium for humans and animals furthers its advantages for industrial applications [18]. B. megaterium is also comparatively easy to manipulate and grow in simple media with different carbon sources such as acetate, formate, and many tricarboxylic acid cycle intermediates. Therefore, for over 50 years, B. megaterium has been extensively used as an ideal industrial organism for heterologous expression and secretion of recombinant proteins in downstream processing for industrial-scale fermentation [19,20].
Due to the unique characteristics of the degradation of lignocellulose biomass, the current study focuses primarily on the Korean black goat (Capra hircus coreanae) rumen. As browsing herbivores, black goats prefer to eat herbaceous and woody dicots such as shrub leaves, stems, and forbs. The small size of the black goat rumen necessitates fast digestion of feed particles. Black goats consume relatively high amounts of herbs compared with other large herbivores [5,10,21]. In the present study, an unrecognized novel Gene12 gene from the GH43 family α-L-arabinofuranosidase was identified and biochemically characterized from a fosmid metagenomic library constructed based on microbial communities from Korean black goat rumen [21]. The objective of this study was to use activity-based screening tools to isolate a novel gene (Gene12) from the Korean black goat rumen metagenomic library, to in silico characterize its sequences and domains, and to subsequently express the gene in B. megaterium expression for the production and secretion of recombinant proteins. Additionally, a tertiary (3D) structure of Gene12 α-L-arabinofuranosidase was illustrated based on the generated in silico data. Moreover, the enzymatic characteristics of the recombinant Gene12 protein were analyzed under different pH and temperature ranges and the enzymatic properties were also investigated using various synthetic pNPA substrates.
2. Materials and Methods
2.1. Construction and Screening of Gene12 Gene from Goat Rumen Metagenomic Library
Prior to the experiment, two male and one female eighteen-month-old Korean black goats were raised and fed rice straw and mineral supplements carefully during a 30-day period to enhance the lignocellulolytic adaptation of microorganisms in their rumen. Microbes associated with fiber and liquids in their rumen contents were fractionated immediately after slaughter according to the regulation of the National Institute of Animal Science (Rural Development Administration, Suwon, Republic of Korea; a grant from the Next-Generation BioGreen 21 Program (Project No. PJ011163)). Total microbial community DNA was extracted from their rumens, and a black goat rumen metagenomic fosmid library was constructed as described previously [21]. The Copy ControlTM Fosmid Library Construction Kit (Epicentre, Madison, WI, USA) was used to construct a fosmid library by digestion of isolated DNA with HindIII (R3104, New England Biolabs, Ipswich, MA, USA) and then ligation of the digested 41-kb fragments into the pCC1FOS plasmid. Each clone was screened from the constructed metagenomic fosmid library to identify the α-L-arabinofuranoside hydrolytic activity, as explained in the previous study [22]. Activity-based metagenomic screening successfully identified a single fosmid clone (AD066C18) expressing α-L-arabinofuranosidase movement, and the clone was separated for further study. The DNA of the enzyme-positive fosmid clone was sequenced and assembled via bidirectional approaches using Sanger sequencing and pyrosequencing as described previously [10].
2.2. Cloning, Expression, and Zymographic Analysis
The expressed α-L-arabinofuranosidase gene was identified as the Gene12 gene, and it was subsequently amplified from clone AD066C18 by performing the PCR technique. The PCR products were cloned into plasmid pSPYocH-hp for overexpression. The expression plasmid pSPYocH-hp was carrying a xylose-inducible PxylA promoter and C-terminal His6-tag (Novagen, Darmstadt, Germany) and was used collectively to overexpress the recombinant Gene12 α-L-arabinofuranosidase in the expression host B. megaterium YYBm1 (Mo Bi Tec Inc., Göttingen, Germany).
A zymographic analysis was carried out to confirm the B. megaterium transformant harboring pSPYocH-hp containing the Gene12 gene and the presence of the recombinant Gene12 enzyme after purification by nickel affinity chromatography. The assessment was performed under renaturing conditions by using 15% SDS-PAGE gel containing 2.5 mM p-nitrophenyl-D-arabinofuranoside (pNPA) as described previously [23].
2.3. In Silico Analysis
The construction of a complete metagenomics-insert-DNA sequence was accumulated by combining all DNA fragments using the SeqMan software in Lasergene version 7 (DNASTAR, Madison, WI, USA), as described previously [21]. The online program GeneMark used the advanced heuristic approach to predict the prospective ORFs from the assembled DNA fragments [24]. Functional protein domains were carried out from the ORFs by using the Pfam database (http://pfam.-legacy.xfam.org/null (accessed on 16 November 2022)) [25].
An in silico program (http://insilico.ehu.es/translate (accessed on 27 November 2022)) was applied to translate the nucleotide sequence of the putative Gene12 gene into an amino acid sequence from an enzyme-positive fosmid clone (AD066C18). An algorithmic database the Basic Local Alignment Search Tool for protein (BlastP) (https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 27 November 2022)) was searched to obtain the amino acid similarities between Gene12 and other proteins. An online application tool, Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo (accessed on 11 December 2022)), was used to create multiple amino acid sequence alignments of Gene12 against other similar proteins. The protein database PROSITE on the ExPasy program (http://prosite.expasy.org (accessed on 11 December 2022)) helps to analyze the module structure of Gene12 for searching the conserved domains in the ORFs. The prediction of Mw and pI of the Gene12 protein was achieved by computing their physical and chemical parameters on the ProtParam portal (http://web.expasy.org/protparam (accessed on 11 December 2022)).
2.4. Tertiary (3D) Structure Analysis of Gene12
The building of the Gene12 tertiary structure and the prediction of its function were hypothesized based on homology modeling of the template crystal structure of Gene12 via multiple threading LOMETS (https://zhanglab.ccmb.med.umich.edu/LOMETS (accessed on 17 December 2022)) and the I-TASSER simulation program (http://zhanglab.ccmb.med.umich.edu/I-TASSER (accessed on 23 December 2022)). The protein function of Gene12 was investigated using a BioLiP protein functional database (https://zhanglab.ccmb.med.umich.edu/BioLiP (accessed on 27 December 2022)). For structural analysis, the predicted Gene12 protein model was further visualized using SPDBV 4.0.4 from the Swiss Pdb-Viewer server and submitted to the Verify3D program (http://services.mbi.ucla.edu/Verify_3D (accessed on 27 December 2022)) to evaluate the entire amino acid sequence of the Gene12 protein to obtain information about predicted protein fitness and model validity.
2.5. Induction, Incubation, and Optimization of the Recombinant Gene12 Enzyme
In the present study, B. megaterium growth curve and viable spore population were analyzed every 24 h by using a spread-plating procedure [26]. Optimization of different parameters was carried out in this study to determine the optimal condition from induction time to expression and secretion of the recombinant Gene12 α-L-arabinofuranosidase from B. megaterium. The influence of induction time on enzyme production was observed by inducing the expression of the recombinant Gene12 α-L-arabinofuranosidase by (D)-xylose at various induction intervals (0, 24, 48, 72, 96, 120, and 144 h).
2.6. Heterologous Expression and Purification of the Recombinant Gene12 Protein
The transformant B. megaterium harboring the recombinant Gene12 gene on pSPYocH-hp was cultivated following standard procedure in SOB medium (supplemented with 20 g L−1 of tryptone, 5 g L−1 of yeast extract, 0.5 g L−1 of NaCl, 2.4 g L−1 of MgSO4, and 0.186 g L−1 of KCl in pH 7.0 ± 0.2; Thermo Fisher Scientific, Cleveland, OH, USA) including 10-μg/mL of tetracycline at 37 °C. The recombinant Gene12 α-L-arabinofuranosidase was induced for 96 h by adding 0.5% (w/v) of (D)-xylose after the OD578nm reached 0.3–0.4 at 37 °C. Cell-free supernatant was harvested by centrifugation (4300× g for 10 min), and the overexpressed Gene12 protein was purified from the spent culture supernatants by using gravity-flow column IDA-MiniExcellose (Takara, Tateishi, Japan). To purify the Gene12, 10 mL of the crude cell lysates was loaded onto an IDA-MiniExcellose affinity column and washed three times with 10 mL of equilibration buffer (50 mM Tris-HCl, 0.5 M NaCl, and 20 mM imidazole; pH 8.0). The recombinant proteins were afterward eluted with 3 mL of 250 mM imidazole in the same buffer (50 mM Tris-HCl and 0.5 M NaCl; pH 8.0). Proteins from spent culture supernatants and eluted fractions from the gravity-flow column were analyzed by 15% SDS-PAGE for purity and Mw determination.
2.7. Enzymatic Characterization of Gene12
The synthetic substrate pNPA was used to determine the activity of recombinant Gene12 α-L-arabinofuranosidase. The specific activity of the purified recombinant Gene12 α-L-arabinofuranosidase toward different substrates was determined by measuring reducing sugars or p-nitrophenol released after enzyme reactions as described previously [10,21]. Enzymatic characterization of the recombinant Gene12 depends on finding its reaction system against the synthetic substrate pNPA dissolved in 50 mM sodium citrate-phosphate buffer (pH 6.0). A total volume of 200 μL of the reaction mixture (20 μL of purified enzyme added with 180 μL of preheated 2.5 mM pNPA) was incubated at 40 °C for 20 min in a heating chamber. The addition of 100 μL of cold Na2CO3 (1 M) solution was used to stop the enzymatic reaction, and the activity was read by measuring the absorbance at 405 nm (SpectraMax 190 Molecular Devices, San Jose, CA, USA). One U of Gene12 activity was defined as the quantity of the enzyme liberating, in the reaction mixture, about 1 μmol/min of pNP. The diverse range of pH and temperature effects on the purified Gene12 enzyme was considered to determine its α-L-arabinofuranosidase activity. The optimal pH range of the purified Gene12 α-L-arabinofuranosidase was determined using an enzymatic assay in 50 mM sodium citrate-phosphate buffers (pH 3.0–9.0). Resolving the optimal temperature condition of Gene12, the reaction mixture was incubated at various temperatures (0–70 °C). The results were percentages of the relative activity achieved at either the optimal pH or the temperature.
The pH and thermal stability tests for the Gene12 α-L-arabinofuranosidase enzyme were also considered in this study. In the pH stability study, the purified Gene12 enzyme was pre-incubated at 4 °C for 24 h and assessed on various pH scales (pH 3.0–9.0). For discerning the thermostability of the Gene12 enzyme, the pre-incubated enzyme (1 h in 50 mM sodium citrate-phosphate buffer (pH 6.0)) with the substrate was heated at various temperatures (0–70 °C), and subsequently the remnant α-L-arabinofuranosidase activity was measured. A substrate specificity test of the Gene12 enzyme was also conducted using different substrates including BWX, pNPA, pNPG, and pNPX (all Sigma-Aldrich, St Louis, MO, USA). The enzyme activity of each substrate was assessed by measuring the reducing sugar or pNP at 540 or 405 nm, respectively [27].
2.8. Identification of Gene12 in GenBank
The recombinant Gene12 gene was deposited in the GenBank database under the accession number MW291614.
2.9. Data Analysis
All experiments were executed in triplicate, and statistical analyses were carried out with the SPSS software v26.0 (SPSS Inc., Chicago, IL, USA). This study presented all values as the means ± SD of three independent experiments (n = 3). The statistical analysis involved ANOVA, followed by Tukey’s post hoc test using the SPSS to establish significant differences from the control group, and the values greater than 0.05 (p < 0.05) were accounted for as significant.
3. Results and Discussion
3.1. Construction and Screening of Gene12 Gene from Goat Rumen Metagenomic Library
To date, several α-L-arabinofuranosidases have been identified that belong to diverse glycoside hydrolase families; characterized; and documented from different sources, including plant, yeast, fungi, and bacteria [28], but insignificant numbers of α-L-arabinofuranosidases (EC 3.2.1.55) have been reported to belong to the GH43 family in the CAZy database [29]. Bacterial species residing in ruminants’ gastrointestinal tracts (GITs) play a significant role in their nutrition.
A wide range of high-throughput assays have been conducted on isolates, and various lignocellulolytic enzymes have been identified from various sources. Numerous investigations have been engaged to discover and exemplify lignocellulosic enzymes like α-L-arabinofuranosidase from different sources; however, culture-dependent traditional approaches have not been optimally successful in screening the lignocellulosic enzymes from microbial communities of rumen [16]. Therefore, activity-based metagenomic approaches have been used in this study for screening and characterizing Gene12 α-L-arabinofuranosidase from the uncultivable rumen microbes [21]. This study is considered one of the metagenomic-based endeavors for screening and characterizing a novel CAZyme α-L-arabinofuranosidase from an unrevealed lignocellulosic compound hydrolyzing bacteria inhabiting the Korean black goat rumen and additionally expressed heterologously in B. megaterium.
3.2. Cloning, Expression, and Zymographic Analysis
In total, 115,200 clones in a fosmid metagenomic library were prepared from the Korean goat rumen microbial DNA. Around a 31 kbp insert size was found after performing a restriction analysis with NotI. The sum of 13 clones was identified to express α-L-arabinofuranosidase activities during the screening of the metagenomic library. Among the expressed clones, the clone AD066C18 comprising the Gene12 gene showed the highest clear regions and hydrolysis ability on α-1, 3-linked L-arabinofuranosyl groups to release arabinose residues from L-arabinose-containing polysaccharides. The Gene12 gene amplified from the clone AD066C18 was successfully inserted into the expression vector pSPYocH-hp and overexpressed in B. megaterium. The zymographic investigation confirmed that a single band with the identical Mw (~32 kDa) of Gene12 α-L-arabinofuranosidase indicated the active band (Figure 1).
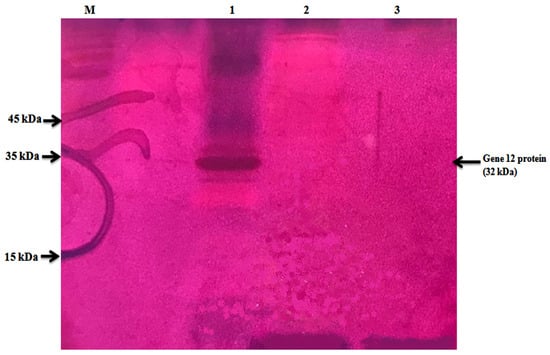
Figure 1.
Zymogram analysis of spent bacterial culture supernatants from the B. megaterium transformant harboring pSPYocH-hp containing Gene12 gene, B. megaterium transformant harboring a parental expression plasmid (pSPYocH-hp), and B. megaterium host strain. Lane M: protein size marker; lane 1: transformant harboring pSPYocH-hp containing Gene12 gene; lane 2: B. megaterium transformant harboring pSPYocH-hp plasmid; lane 3: B. megaterium as host strain.
3.3. In Silico Analysis
In clone AD066C18, a 945 bp ORF was recognized as a Gene12 gene that encoded a protein consisting of 299 amino acid sequences and bluntly carried a catalytic domain of the glycosyl hydrolase family 43 (GH43; CAZy database (http://www.cazy.org/) (accessed on 30 December 2022)). The amino-acid-sequence-based CAZy database has reported 17,988 GH43 enzyme producers categorized into 37 different subfamilies, making GH43 one of the colossal GH families, where the bacterial sequences dominated (17,286).
Based on the module structures they have searched through on the PROSITE database, the recombinant Gene12 protein possessed three amino acid residues identified at positions 12 (Asp12), 68 (Glu68), and 50 (Asp50) in the GH43 domain, potentially playing a catalytic role during arabinofuranoside hydrolysis. Additionally, the recombinant Gene12 also had three possible ligand binding sites. The putative domain structure of the Gene12 protein is demonstrated in Figure 2A. The conceptual Mw and pI of Gene12 protein were estimated to be 32 kDa and 4.74, respectively. The Gene12 protein enclosed 21 positively charged (Arg and Lys) and 36 negatively charged (Asp and Glu) amino acid residues. The instability index was projected to be 27.26, expecting Gene12 protein stability. The review of the Gene12 amino acid sequence with other previously reported homologous proteins in the GenBank database exhibited that the Gene12 protein shared an 81% sequence similarity with the two GH43 family α-L-arabinofuranosidase proteins from cow ruminal uncultured bacterium contigs 27 (AHF24624.1) and 52 (AHF25089.1), respectively (Table 1). Amino acid sequence alignments between Gene12 enzymes and other homologous proteins are exhibited in Figure 2B.
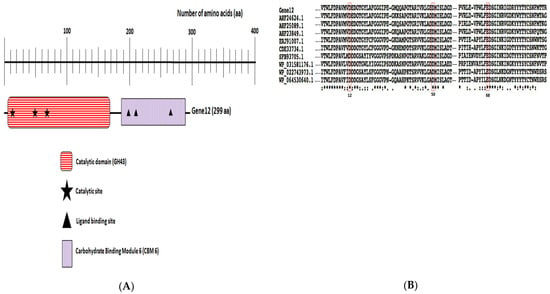
Figure 2.
In silico analysis. (A) In silico analysis of Gene12 gene product. Domain structure of Gene12 protein displays its GH43 domain; (B) multiple amino acid alignment of the GH43 domain of Gene12 enzyme with other closely related GH43 enzymes. Conserved amino acids are marked with different symbols: significant similarity of amino acid residues (asterisk), highly identical (colon), and moderately identical (circle) [29]. Putative catalytic residues of the GH43 domain are indicated in red boxes. Gene12 is aligned with nine others homologous GH43 proteins from the following microorganisms: uncultured bacterium contig27 (AHF24624.1), uncultured bacterium contig52 (AHF25089.1), uncultured bacterium contig15 (AHF23849.1), R. callidus ATCC 27760 (ERJ91007.1), Ruminococcus sp. CAG:403 (CDE33734.1), Butyrivibrio sp. YAB3001 (SFB93705.1), Lachnospiraceae bacterium AC2028 (WP_031581176.1), Clostridium saccharobutylicum (WP_022743973.1), and C. saccharobutylicum (WP_064530640.1).

Table 1.
Comparison of amino acid sequence identifies between Gene12 enzyme and other homologous GH43 proteins.
3.4. Tertiary (3D) Structure Analysis of Gene12
Initiation, implication, and constant upgrading of algorithms in computer programming have accelerated the quick prediction and construction of the protein’s 3D structures and analysis of the biochemical functions based on their simple amino acid sequences [30]. The predicted molecular 3D structure of the Gene12 protein was assembled based on the tertiary template protein structure (PDB ID 5GLK and 3C7F; (http://www.rcsb.org/pdb) (accessed on 10 January 2023)) with the I-TASSER and SPDBV 4.0.4 platform (Figure 3). Crystallography studies of the proteins from the GH43 family confirmed that it possesses a catalytic 5-blended β-propeller domain, usually forming a long V-shaped indentation and partially bounded at one end, with a single extended substrate-binding surface diagonally facing the propeller [31]. The predicted 3D structure of the Gene12 protein identified that its catalytic domain has 5-blended β-sheets making a long V-shaped groove from the outer edge. Additionally, the Gene12 protein also has three putative catalytic carboxylate residues such as Asp12 as a nucleophile, Glu68 as a proton donor, and Asp50 playing the role of a pKa modulator for a proton donor while also keeping the proton donor and substrate in the correct positions within the protein structure. GH43 is one of the largest and most diverse GH families, where the bacterial sequences dominate. Historically, GH43 was a member of the “GH-F” clan, and this family’s functional and biochemical studies have successfully characterized 217 members against synthetic or natural substrates assigned into 11 different EC numbers [29]. To date, all 39 subfamilies of GH43 have been functionally characterized and at most 25 subfamilies (1, 2, 9, 10, 11, 12, 13, 15, 16, 17, 18, 19, 20, 21, 22, 23, 25, 26, 27, 29, 33, 34, 35, 36, and 39) showed α-L-arabinofuranosidase activities (EC 3.2.1.55) [32]. Moreover, GH43 members are among the most abundant in the GH families and are characterized by multifunctional enzymes including β-xylosidase/α-L-arabinofuranosidase/β-1,4-lactase/α-1,6-raffinase/α-1,6-stachyase/β-galactosidase/α-1,4-glucosidase from a cow rumen microbial consortium, as well as the trifunctional exo-β-xylosidase/endoxylanase/α-L-arabinofuranosidase [33]. The bioinformatics analysis in this study revealed that the recombinant Gene12 protein possessed a GH43 catalytic domain including a functional carbohydrate-binding module 6, which can functionally demonstrate the binding of amorphous cellulose and/or β-1,4-xylan substrates [29].
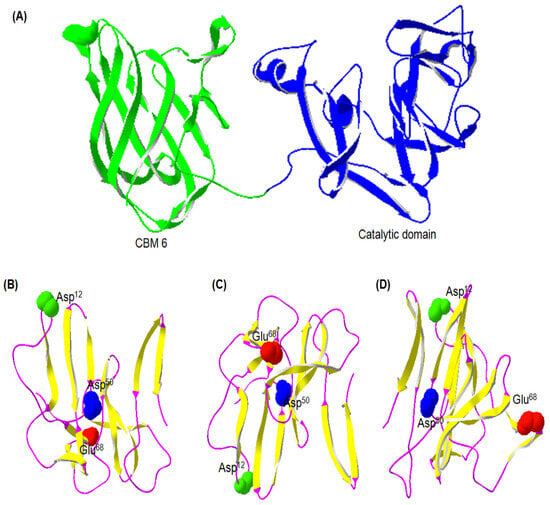
Figure 3.
Predicted tertiary (3D) structure of the Gene12 protein. (A) Overall structure of Gene12, where the CBM 6 and catalytic domain are displayed in green and blue, respectively. Different views of catalytic domain: (B) top, (C) bottom, and (D) lateral. Unique regions are illustrated in different colors: β-sheet (yellow) and random coil (magenta). Catalytic active residues are also illustrated in their own unique colors: nucleophilic base Asp12 (green), general acid or proton donor Glu68 (red), and acid pKa modulator Asp50 (blue).
Unlike other GH families, the members from the GH43 family have three essential catalytic residues for activity, making them distinct from the classical organization of an active site (one proton donor and one nucleophile) in other GH family inverting enzymes. The third carboxylate in the GH43 family members is catalytically active for the pKa modulation of the proton donor (general acid) and responsible for the appropriate positioning of both the proton donor and substrates [33]. The functional role of these three amino acid residues in the GH43 family was verified previously by site-directed mutagenesis studies [6].
The predicted 3D model of the Gene12 protein exhibited entire amino acid scores above zero in the profile, which indicated the superiority of protein fitness and justified the Gene12 tertiary (3D) protein model. All the collected in silico data for the 3D structure of Gene12 protein in this study validate and strongly support the link-up with the GH43 family.
3.5. Induction, Incubation, and Optimization of the Recombinant Gene12 Enzyme
The optimal temperature for growth of the B. megaterium transformant harboring pSPYocH-hp vector containing the Gene12 gene was found to be at 37 °C and the best growth of the B. megaterium strain was observed with an incubation time of 24 h. The detection limit for B. megaterium was 2.0 log CFU/mL (Figure 4A). After observing different time points, the influences of the induction time and inducer concentration were established on α-L-arabinofuranosidase enzyme production. The maximum enzyme activity (0.30 U/mL spent bacterial culture supernatant) was obtained after induction of 0.5% (w/v) (D)-xylose as the carbon source for 96 h (Figure 4B). After that, the enzyme activity decreased sharply until 144 h.
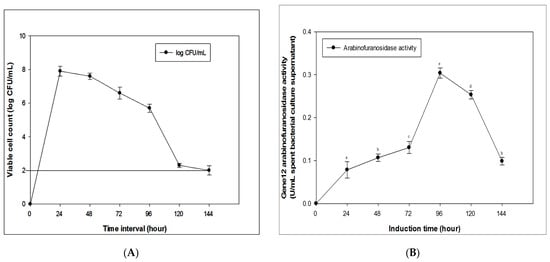
Figure 4.
Induction, incubation, and optimization of the recombinant Gene12 enzyme. (A) Growth curve of B. megaterium. The detection limit for B. megaterium was indicated in 2.0 log CFU/mL. (B) Effect of induction times (0 to 144 h) on Gene12 α-L-arabinofuranosidase activity after induction with 0.5% xylose.
3.6. Heterologous Expression and Purification of the Recombinant Gene12 Protein
The putative α-L-arabinofuranosidase Gene12 gene was implanted successfully into the hp-shuttle vector expression system (pSPYocH-hp) and overexpressed in B. megaterium YYBm1. The recombinant Gene12 α-L-arabinofuranosidase enzyme was purified by nickel affinity chromatography, and the purified fraction was visualized as a single band of about 32 kDa in the SDS-PAGE gel, and a single active protein band also emerged at the same Mw in the zymography analysis (Figure 5).
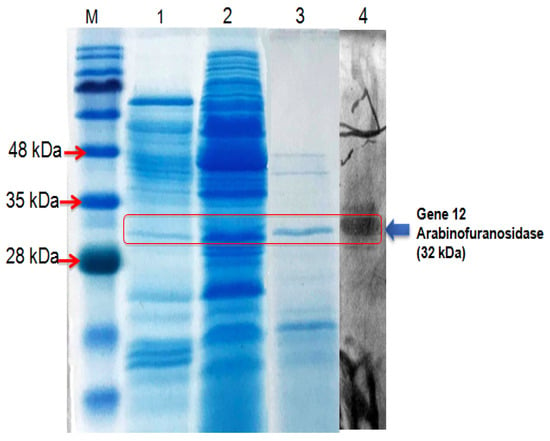
Figure 5.
SDS-PAGE analysis of the Gene12 α-L-arabinofuranosidase. Lane M: protein marker; lane 1: spent culture before induction; lane 2: spent culture after induction; lane 3: purified Gene12 α-L-arabinofuranosidase; lane 4: zymography of Gene12.
3.7. Enzymatic Characterization of Gene12
The ruminal fluid can vary in dietary conditions based on different pH (5.0–7.0) and body temperature (38–41 °C) ranges, respectively [21]. The purified recombinant Gene12 enzyme was optimized at different pH and temperature ranges in a 50 mM sodium citrate-phosphate buffer. The optimal pH and temperature for the Gene12 enzyme were 6.0 and 40 °C, respectively (Figure 6A,B). Gene12 retained its considerable enzymatic activity (above 40%) at a pH range of 4.0 to 7.0 (Figure 6C). In this study, the recombinant Gene12 α-L-arabinofuranosidase exhibited the maximum relative enzymatic activity and consistent stability at the optimal reaction environment close to the optimal temperature and pH of all rumen. The considerable strength for Gene12 α-L-arabinofuranosidase activity was enumerated at the optimal pH and temperature, and the enzyme retained its activity above 40% detected at temperatures from 20 to 50 °C after 1 h incubation. The enzymatic activity of Gene12 drastically decreased at temperatures below 10 °C and above 50 °C (Figure 6D).
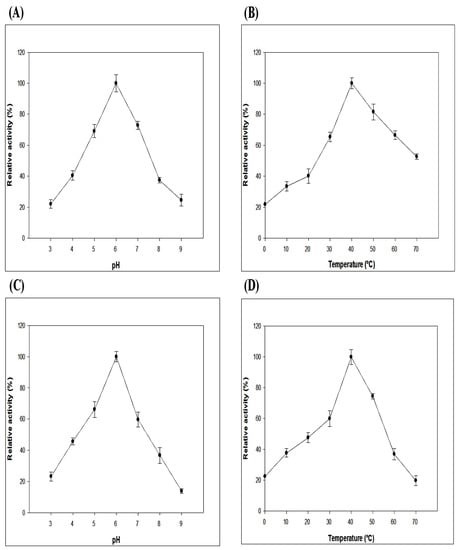
Figure 6.
Enzymatic activity and stability profiles of Gene12 at different pH and temperature values. (A) Optimal pH of the Gene12 enzyme. Enzymatic activity of Gene12 was measured using pNPA at a range of pH 3.0 to 9.0. (B) Optimal temperature of the Gene12 enzyme. The effect of temperature on Gene12 enzyme at a range of 0 to 70 °C using pNPA at pH 6.0 in 50 mM sodium citrate-phosphate buffer. (C) pH stability of the Gene12 enzyme. The relative activity was measured after 24 h incubation of Gene12 at 4 °C at the indicated pH range. (D) Thermostability of the Gene12 enzyme. The residual α-L-arabinofuranosidase activity was measured at the indicated temperature after 1 h in 50 mM sodium citrate-phosphate buffer at pH 6.0. The highest activities were defined as 100% in all tests. The experiments performed in triplicates were repeated independently three times.
GH43 enzymes have various substrate specificities including α-L-arabinofuranosidase, β-xylosidase, xylanase, arabinanase, galactan 1,3-β-galactosidase, exo-α-1,5-L-arabinofuranosidase, α-1,2-L-arabinofuranosidase, exo-α-1,5-L-arabinanase, and β-1,3-xylosidase (GH43; CAZy database (http://www.cazy.org/) (accessed on 30 December 2022)). The purified Gene12 enzyme (GH43) α-L-arabinofuranosidase was also assayed for various synthetic and natural substrates (Table 2). The Gene12 enzyme exhibited higher enzymatic activity against pNPA (75.41 U/mg), while traceable activity (>4 U/mg) was found against pNPX, BWX, and pNPG. The computational analysis revealed that the recombinant Gene12 protein possessed a GH43 catalytic domain, including a functional sugar-binding protein CBM 6 (Figure 3A), which is common in 6% of all GH43 family members [34]. The CBM 6 can functionally demonstrate the binding of amorphous cellulose and β-1, 4-xylan substrates. Through the presence of the CBM 6 module in the Gene12, some activities towards pNPX, BWX, and pNPG were exhibited in this study. However, like most exo-type α-L-arabinofuranosidase containing genes, the recombinant Gene12 enzyme showed the highest enzymatic activity against pNPA [35].

Table 2.
Substrate specificities of novel Gene12 enzyme against various substrates.
Enzymatic characterization and the present properties of Gene12 α-L-arabinofuranosidase suggest a possible significant role in the degradation of lignocelluloses in black goat rumen environments. In addition, the Gene12 enzyme can synergistically work with other lignocellulolytic enzymes for numerous potential applications. It can be applicable in diverse industries for improving the kneading and clarification process of fruit and vegetable juices, enhancing the flavor of wine, increasing the shelf life of bread by delaying staling, enriching the soluble dietary fiber, enriching the digestion of animal feeds, improving the bio-bleaching process in paper and pulp industries, and producing fermentable sugars for biofuel industries [2,36].
4. Conclusions
This study successfully isolated and biochemically characterized a novel Gene12 α-L-arabinofuranosidase derived from uncultured rumen bacteria of a Korean black goat, and the protein was effectively expressed in a heterologous host B. megaterium. In silico, phylogenetic, and computational analyses exposed the relationship and attachment of the Gene12 gene with the GH43 family and its predicted tertiary protein structure. The recombinant Gene12 α-L-arabinofuranosidase optimally functioned at pH 6.0 at 40 °C and was enzymatically stable in a wide-ranging pH from 4.0 to 7.0 at 20 to 50 °C. The highest substrate specificity of Gene12 α-L-arabinofuranosidase based solely on pNPA indicated its confirmation as an exo-type enzyme of arabinofuranosidase.
Author Contributions
Conceptualization, S.H.T.; methodology, S.H.T.; software, S.H.T. and M.A.; validation, S.H.T. and M.A.; formal analysis, S.H.T.; investigation, S.H.T. and M.A.; resources, M.A.; data curation, S.H.T.; writing—original draft preparation, S.H.T.; writing—review and editing, S.H.T. and M.A.; visualization, S.H.T. and M.A.; supervision, S.H.T.; project administration, S.H.T.; funding acquisition, S.H.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data are contained within the article.
Acknowledgments
We are grateful for the excellent support during experiments at the Food Microbiology Lab at Chung-Ang University, Republic of Korea.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ANOVA, analysis of variance; BWX, beechwood xylan; CAZy, carbohydrate-active enzyme database; CBM, carbohydrate-binding module; CFU, colony-forming unit; DNA, deoxyribonucleic acid; GH43, glycosyl hydrolase family 43; GI, gastrointestinal; GRAS, generally recognized as safe; Mw, molecular weight; NCBI, National Center for Biotechnology Information; OD, optical density; ORF, open reading frames; PCR, polymerase chain reaction; PDB, protein data bank; pI, isoelectric point; pNP, p-nitrophenol; pNPA, p-nitrophenyl-D-arabinofuranoside; pNPG, p-nitrophenyl-D-glucopyranoside; pNPX, p-nitrophenyl-D-xylopyranoside; SD, standard deviation; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; SOB, super optimal broth; SPSS, statistical package for the social sciences; U, unit; 3D, three-dimensional.
References
- Rytioja, J.; Hildén, K.; Falco, M.D.; Zhou, M.; Aguilar-Pontes, M.V.; Sietiö, O.M.; Tsang, A.; Vries, R.P.; Mäkelä, M.R. The Molecular Response of the White-Rot Fungus Dichomitus Squalens to Wood and Non-Woody Biomass as Examined by Transcriptome and Exoproteome Analyses. Environ. Microbiol. 2017, 19, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- Toushik, S.H.; Lee, K.-T.; Lee, J.-S.; Kim, K.-S. Functional Applications of Lignocellulolytic Enzymes in the Fruit and Vegetable Processing Industries. J. Food Sci. 2017, 82, 585–593. [Google Scholar] [CrossRef]
- Zhang, M.; Buekens, A.; Li, X. Dioxins from Biomass Combustion: An Overview. Waste Biomass Valorization 2017, 8, 1–20. [Google Scholar] [CrossRef]
- Zeng, Y.; Himmel, M.E.; Ding, S.-Y. Visualizing Chemical Functionality in Plant Cell Walls. Biotechnol. Biofuels 2017, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Sae-Lee, R.; Boonmee, A. Newly Derived Gh43 Gene from Compost Metagenome Showing Dual Xylanase and Cellulase Activities. Folia Microbiol. 2014, 59, 409–417. [Google Scholar] [CrossRef]
- Ahmed, S.; Luis, A.S.; Bras, J.L.A.; Ghosh, A.; Gautam, S.; Gupta, M.N.; Fontes, C.M.G.A.; Goyal, A. A Novel A-L-Arabinofuranosidase of Family 43 Glycoside Hydrolase (Ct43araf) from Clostridium thermocellum. PLoS ONE 2013, 8, e73575. [Google Scholar] [CrossRef]
- Souza, S.P.; Seabra, J.E.A.; Nogueira, L.A.H. Feedstocks for Biodiesel Production: Brazilian and Global Perspectives. Biofuels 2018, 9, 455–478. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Q.; Kong, F.; Yang, Y.; Wu, D.; Mishra, S.; Li, Y. Exploring the Goat Rumen Microbiome from Seven Days to Two Years. PLoS ONE 2016, 11, e0154354. [Google Scholar] [CrossRef]
- Ferrer, M.; Ghazi, A.; Beloqui, A.; Vieites, J.M.; Lopez-Cortes, N.; Marín-Navarro, J.; Nechitaylo, T.Y.; Guazzaroni, M.E.; Polaina, J.; Waliczek, A.; et al. Functional Metagenomics Unveils a Multifunctional Glycosyl Hydrolase from the Family 43 Catalysing the Breakdown of Plant Polymers in the Calf Rumen. PLoS ONE 2012, 7, e38134. [Google Scholar] [CrossRef]
- Lee, K.-T.; Toushik, S.H.; Baek, J.-Y.; Kim, J.-E.; Lee, J.-S.; Kim, K.-S. Metagenomic Mining and Functional Characterization of a Novel Kg51 Bifunctional Cellulase/Hemicellulase from Black Goat Rumen. J. Agric. Food Chem. 2018, 66, 9034–9041. [Google Scholar] [CrossRef]
- Thakur, A.; Sharma, K.; Goyal, A. a-L-Arabinofuranosidase: A Potential Enzyme for the Food Industry. Green Bio-Process. Enzym. Ind. Food Process. 2018, 3, 229. [Google Scholar] [CrossRef]
- Pisalwar, P.; Fernandes, A.; Tribhuvan, D.; Gite, S.; Ahmed, S. Arabinofuranosidases. In Glycoside Hydrolases; Academic Press: Cambridge, MA, USA, 2023; pp. 187–211. [Google Scholar]
- Thakur, A.; Sharma, K.; Jamaldheen, S.B.; Goyal, A. Molecular Characterization, Regioselective and Synergistic Action of First Recombinant Type III α-l-arabinofuranosidase of Family 43 Glycoside Hydrolase (Ps GH43_12) from Pseudopedobacter saltans. Mol. Biotechnol. 2020, 62, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, X.; Zhang, Y.; Yu, Z.; Zhang, T.; Dai, X.; Pan, X.; Jing, R.; Yan, Y.; Liu, Y.; et al. Genomic Insights into the Phylogeny and Biomass-Degrading Enzymes of Rumen Ciliates. ISME J. 2022, 16, 2775–2787. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.; Crispie, F.; Lewis, E.; Reid, M.; O’Toole, P.W.; Cotter, P.D. The Rumen Microbiome: A Crucial Consideration When Optimising Milk and Meat Production and Nitrogen Utilisation Efficiency. Gut Microbes 2019, 10, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Silva-Portela, R.C.B.; Carvalho, F.M.; Pereira, C.P.M.; de Souza-Pinto, N.C.; Modesti, M.; Fuchs, R.P.; Agnez-Lima, L.F. Exomeg1: A New Exonuclease from Metagenomic Library. Sci. Rep. 2016, 6, 19712. [Google Scholar] [CrossRef]
- Xu, B.; Dai, L.; Zhang, W.; Yang, Y.; Wu, Q.; Li, J.; Tang, X.; Zhou, J.; Ding, J.; Han, N.; et al. Characterization of a Novel Salt-, Xylose- and Alkali-Tolerant Gh43 Bifunctional Β-Xylosidase/A-L-Arabinofuranosidase from the Gut Bacterial Genome. J. Biosci. Bioeng. 2019, 128, 429–437. [Google Scholar] [CrossRef]
- Williams, A.; Gedeon, K.S.; Vaidyanathan, D.; Yu, Y.; Collins, C.H.; Dordick, J.S.; Linhardt, R.J.; Koffas, M.A.G. Metabolic Engineering of Bacillus Megaterium for Heparosan Biosynthesis Using Pasteurella Multocida Heparosan Synthase, Pmhs2. Microb. Cell Factories 2019, 18, 132. [Google Scholar] [CrossRef] [PubMed]
- Grage, K.; McDermott, P.; Rehm, B.H.A. Engineering Bacillus Megaterium for Production of Functional Intracellular Materials. Microb. Cell Factories 2017, 16, 211. [Google Scholar] [CrossRef] [PubMed]
- Vary, P.S.; Biedendieck, R.; Fuerch, T.; Meinhardt, F.; Rohde, M.; Deckwer, W.D.; Jahn, D. Bacillus Megaterium-from Simple Soil Bacterium to Industrial Protein Production Host. Appl. Microbiol. Biotechnol. 2007, 76, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-H.; Lee, K.-T.; Baek, J.-Y.; Kim, M.-J.; Kwon, M.-R.; Kim, Y.-J.; Park, M.-R.; Ko, H.; Lee, J.-S.; Kim, K.-S. Isolation and Characterization of a Novel Glycosyl Hydrolase Family 74 (Gh74) Cellulase from the Black Goat Rumen Metagenomic Library. Folia Microbiol. 2017, 62, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Teather, R.M.; Wood, P.J. Use of Congo Red-Polysaccharide Interactions in Enumeration and Characterization of Cellulolytic Bacteria from the Bovine Rumen. Appl. Environ. Microbiol. 1982, 43, 777–780. [Google Scholar] [CrossRef]
- Yadav, P.; Maharjan, J.; Korpole, S.; Prasad, G.S.; Sahni, G.; Bhattarai, T.; Sreerama, L. Production, Purification, and Characterization of Thermostable Alkaline Xylanase from Anoxybacillus Kamchatkensis Nastpd13. Front. Bioeng. Biotechnol. 2018, 6, 65. [Google Scholar] [CrossRef]
- Zhu, W.; Lomsadze, A.; Borodovsky, M. Ab Initio Gene Identification in Metagenomic Sequences. Nucleic Acids Res. 2010, 38, e132. [Google Scholar] [CrossRef] [PubMed]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam Protein Families Database in 2019. Nucleic Acids Res. 2018, 47, D427–D432. [Google Scholar] [CrossRef]
- Zhang, C.; Li, B.; Jadeja, R.; Hung, Y.C. Effects of Electrolyzed Oxidizing Water on Inactivation of Bacillus Subtilis and Bacillus Cereus Spores in Suspension and on Carriers. J. Food Sci. 2016, 81, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Bao, L.; Chang, L.; Zhou, Y.; Lu, H. Biochemical and Kinetic Characterization of Gh43 Β-D-Xylosidase/A-L-Arabinofuranosidase and Gh30 A-L-Arabinofuranosidase/Β-D-Xylosidase from Rumen Metagenome. J. Ind. Microbiol. Biotechnol. 2012, 39, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Kozlova, L.V.; Gorshkov, O.V.; Mokshina, N.E.; Gorshkova, T.A. Differential Expression of A-L-Arabinofuranosidases During Maize (Zea Mays L.) Root Elongation. Planta 2015, 241, 1159–1172. [Google Scholar] [CrossRef]
- Mewis, K.; Lenfant, N.; Lombard, V.; Henrissat, B. Dividing the Large Glycoside Hydrolase Family 43 into Subfamilies: A Motivation for Detailed Enzyme Characterization. Appl. Environ. Microbiol. 2016, 82, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-Tasser Suite: Protein Structure and Function Prediction. Nat. Methods 2015, 12, 7–8. [Google Scholar] [CrossRef]
- Jitonnom, J.; Hannongbua, S. Theoretical Study of the Arabinan Hydrolysis by an Inverting Gh43 Arabinanase. Mol. Simul. 2018, 44, 631–637. [Google Scholar] [CrossRef]
- Kaneko, S.; Fujimoto, Z. Applications. In Glycoside Hydrolases; Academic Press: Cambridge, MA, USA, 2023; pp. 165–186. [Google Scholar] [CrossRef]
- Limsakul, P.; Phitsuwan, P.; Waeonukul, R.; Pason, P.; Tachaapaikoon, C.; Poomputsa, K.; Kosugi, A.; Ratanakhanokchai, K. A Novel Multifunctional Arabinofuranosidase/Endoxylanase/Β-Xylosidase GH43 Enzyme From Paenibacillus Curdlanolyticus B-6 and its Synergistic Action To Produce Arabinose And Xylose From Cereal Arabinoxylan. Appl. Environ. Microbiol. 2021, 87, e01730-21. [Google Scholar] [CrossRef] [PubMed]
- Vandermarliere, E.; Bourgois, T.M.; Winn, M.D.; Van Campenhout, S.; Volckaert, G.; Delcour, J.A.; Strelkov, S.V.; Rabijns, A.; Courtin, C.M. Structural Analysis of a Glycoside Hydrolase Family 43 Arabinoxylan Arabinofuranohydrolase in Complex with Xylotetraose Reveals a Different Binding Mechanism Compared with Other Members of the Same Family. Biochem. J. 2009, 418, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Pieczywek, P.M.; Cybulska, J.; Zdunek, A. An Atomic Force Microscopy Study on the Effect of Β-Galactosidase, A-L-Rhamnosidase and A-L-Arabinofuranosidase on the Structure of Pectin Extracted from Apple Fruit Using Sodium Carbonate. Int. J. Mol. Sci. 2020, 21, 4064. [Google Scholar] [CrossRef] [PubMed]
- Stoffels, G.; Nes, I.F.; Guomundsdottir, A. Isolation and Properties of a Bacteriocin-Producing Carnobacterium Piscicola Isolated from Fish. J. Appl. Bacteriol. 1992, 73, 309–316. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).