Development of a Chicken Gastrointestinal Tract (GIT) Simulation Model: Impact of Cecal Inoculum Storage Preservation Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents/Chemicals and Apparatus
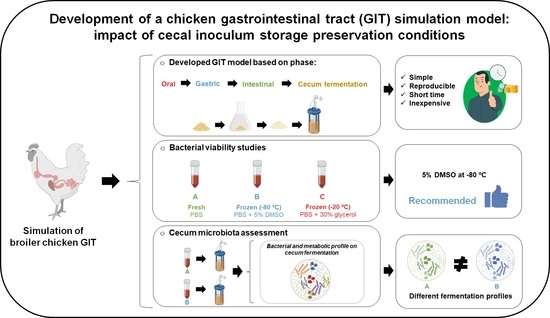
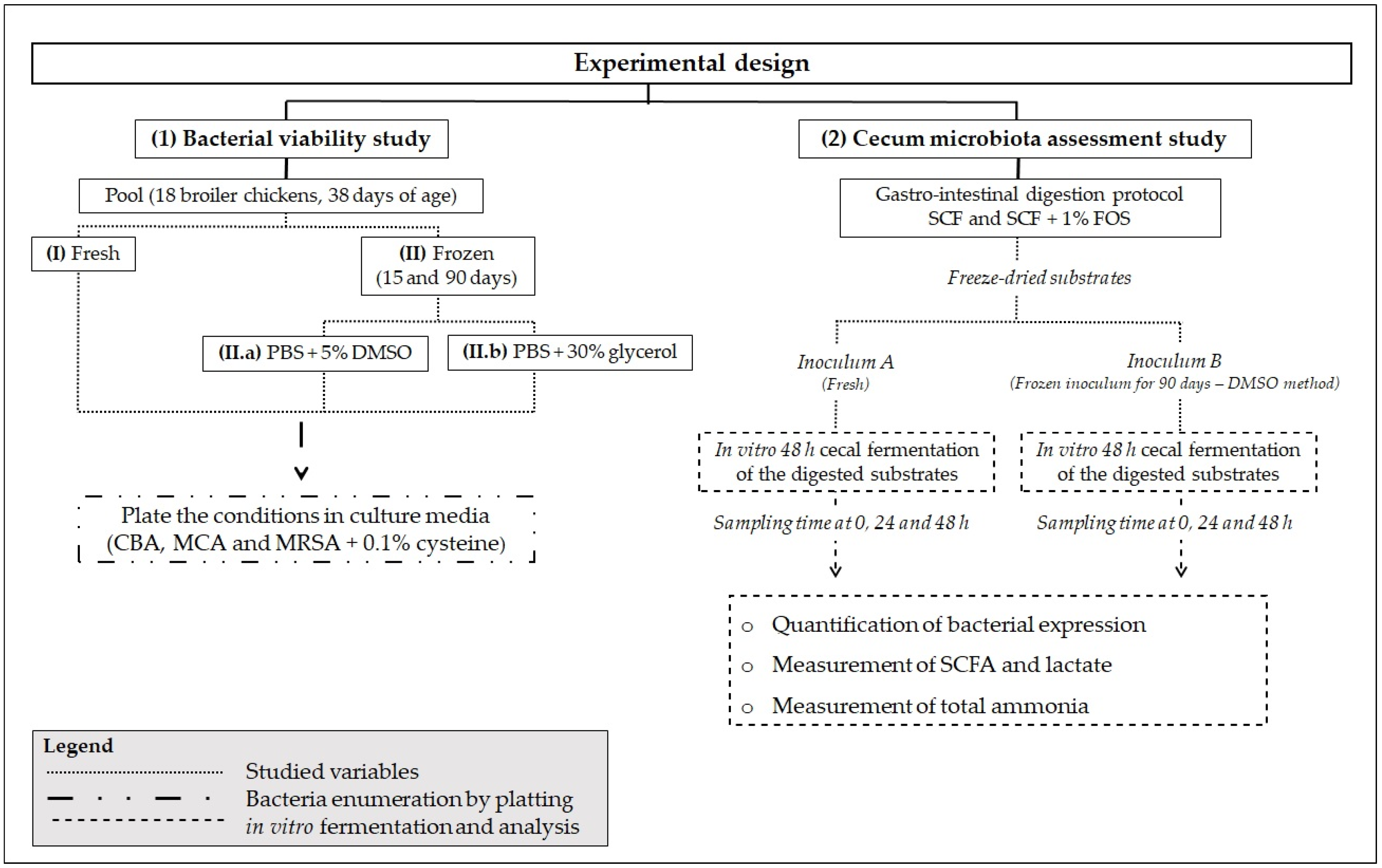
2.2. Study Experimental Design and Objectives
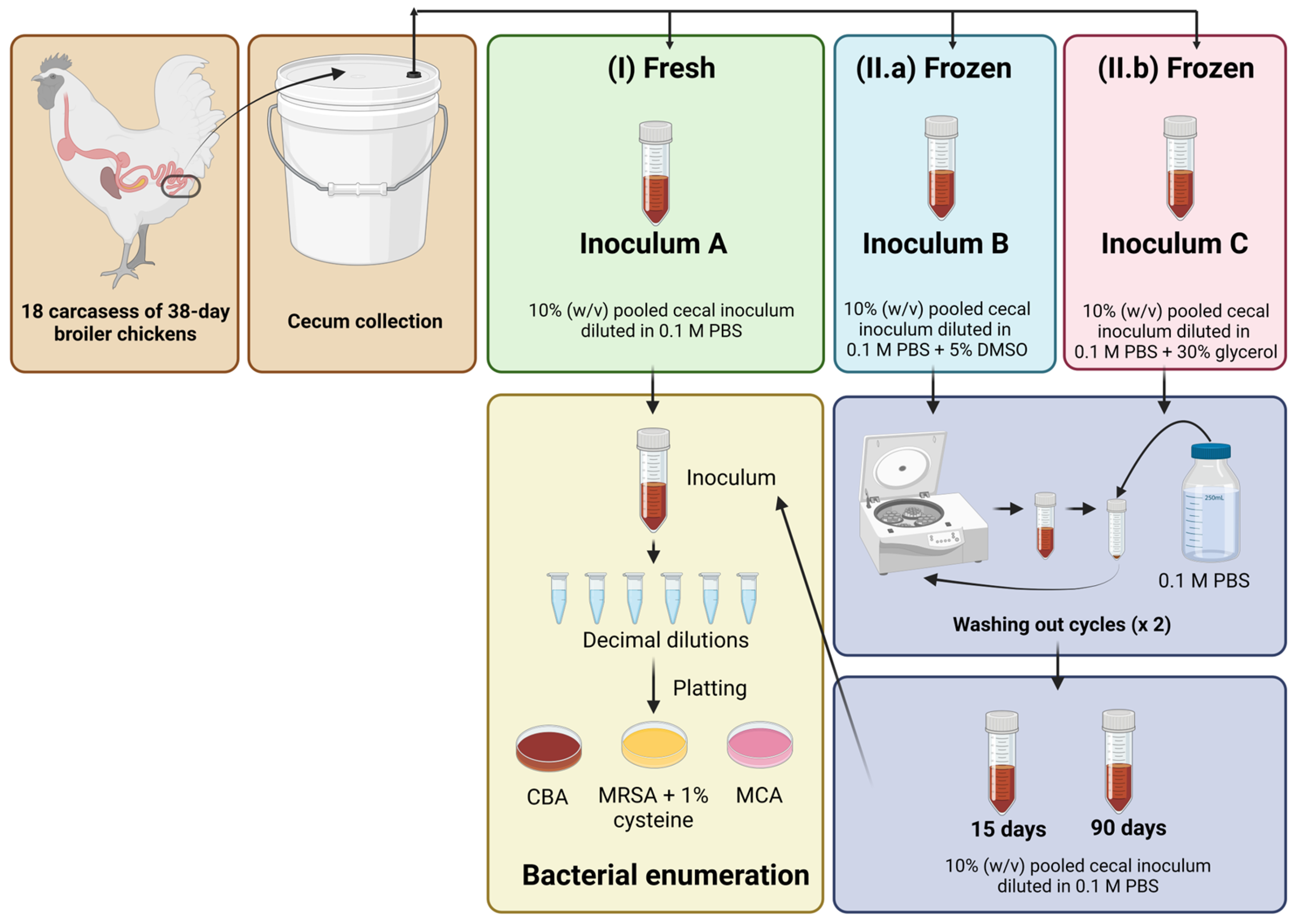
2.3. Bacterial Viability Study
2.3.1. Fresh and Frozen Cecal Inoculum Preparation
2.3.2. Bacterial Enumeration by Culture-Dependent Methods
2.4. Cecum Microbiota Assessment
2.4.1. SCF
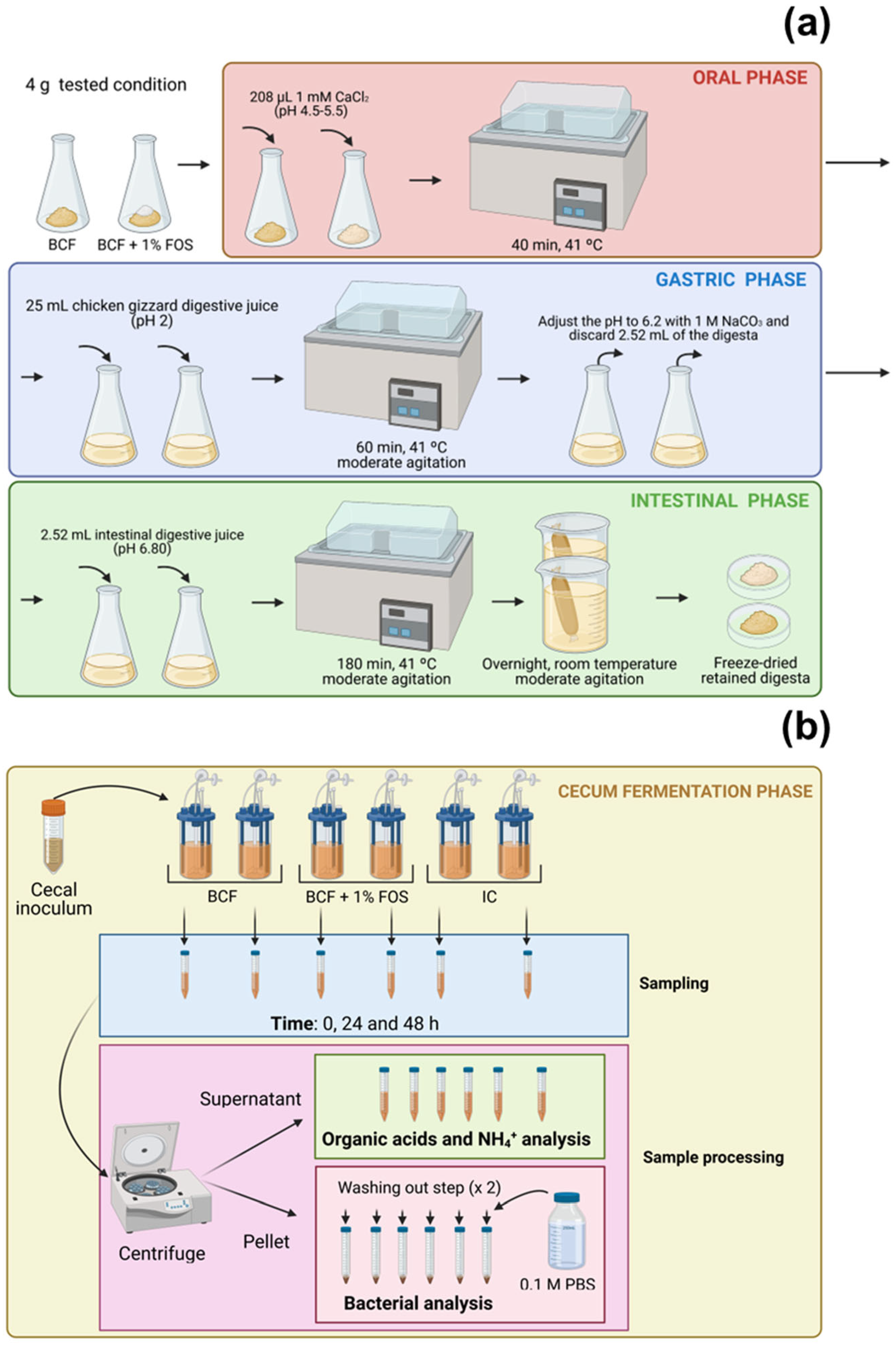
2.4.2. Chicken GIT Simulation Model
Oral Phase
Gastric Phase
Intestinal Phase
Cecum Fermentation
2.4.3. Bacterial Enumeration by Culture-Independent Methods
2.4.4. Determination of Organic Acids Produced during In Vitro Fermentation
2.4.5. Measurement of Total Ammonia Nitrogen Concentration
2.5. Statistical Analysis
| Primer | Target Organisms | Genomic DNA Standard | Media Broth | Media Agar | Incubation Conditions | Sequence (5′-3′) | Amplicon Size (bp) | Reference |
|---|---|---|---|---|---|---|---|---|
| Firm | Firmicutes | Lactobacillus gasseri DSM 20077 | MRSB + 0.1% (w/v) cysteine | MRSA + 0.1% (w/v) cysteine | Anaerobic 37 °C 2 days | F: ATG TGGTTTAATTCGAAGCA | 126 | [39] |
| R: AGCTGACGACAACCATGCAC | ||||||||
| Lac | Lactobacillus group | F: CACCGCTACACATGGAG | 341 | [40,41] | ||||
| R: AGCAGTAGGGAATCTTCCA | ||||||||
| Bdt | Bacteroidetes | Bacteroides intestinalis DSM 17393 | TSB + 5% (v/v) DSB | CBA + 5% (v/v) DSB | F: CATGTGGTTTAATTCGATGAT | 126 | [39] | |
| R: AGCTGACGACAACCATGCAG | ||||||||
| Bac | Bacteroides | F: GAAGGTCCCCCACATTG | 103 | [42] | ||||
| R: CGCKACTTGGCTGGTTCAG | ||||||||
| Bif | Bifidobacterium | Bifidobacterium animalis ssp. lactis BB-12 DSM 15954 | MRSB + 0.1% (w/v) cysteine | MRSA + 0.1% (w/v) cysteine | F: CGCGTCYGGTGTGAAAG | 244 | [43] | |
| R: CCCCACATCCAGCATCCA | ||||||||
| Enb | Enterobacteriaceae family | Salmonella enteritidis subsp. enterica ATCC 13076 | MHB | MCA | F: CATTGACGTTACCCGCAGAAGAAGC | 195 | [44] | |
| R: CTCTACGAGACTCAAGCTTGC |
3. Results
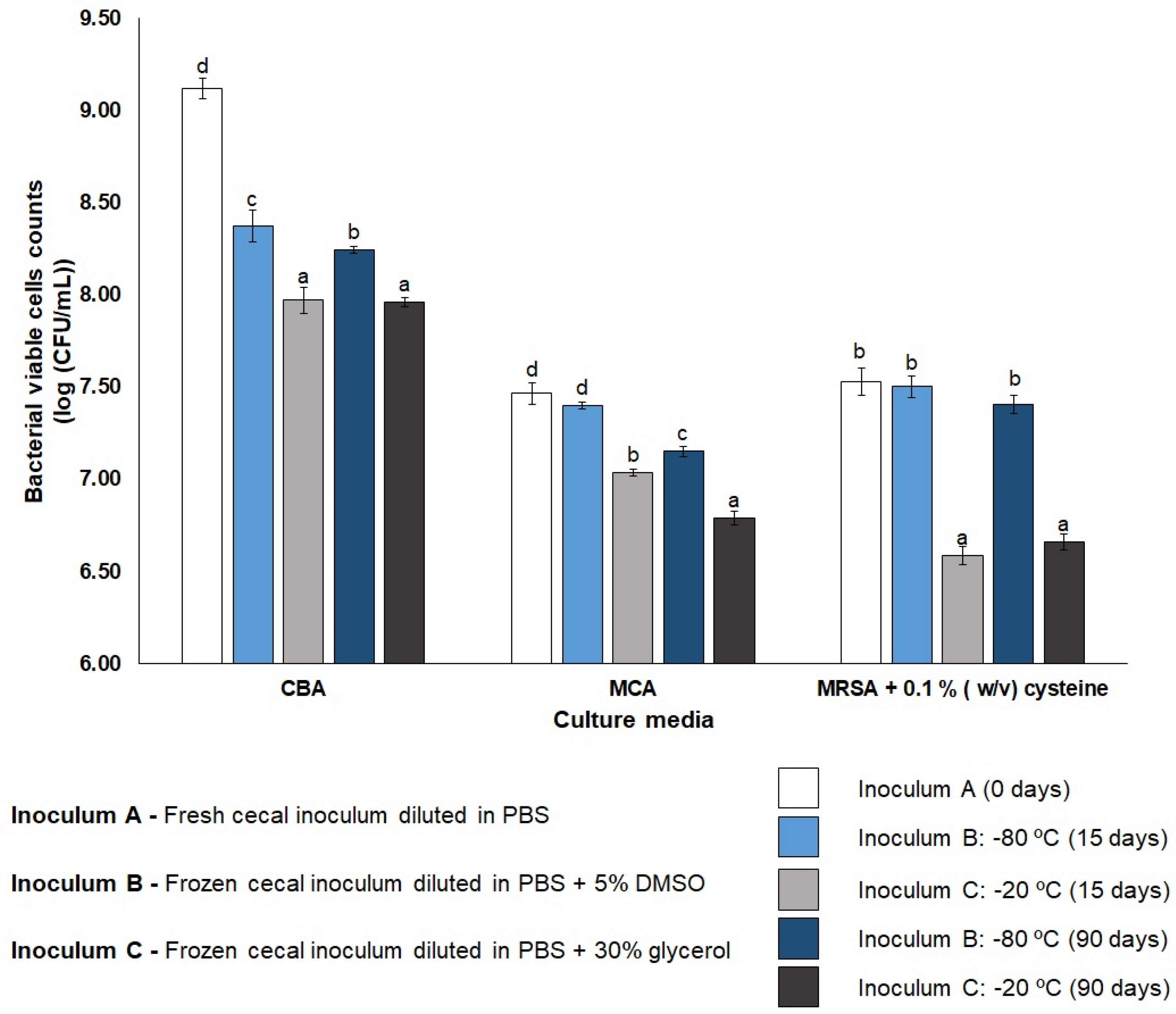
3.1. Cecal Inoculum Viability
3.2. DMSO Wash-Out Confirmation
3.3. Chicken GIT Model Development
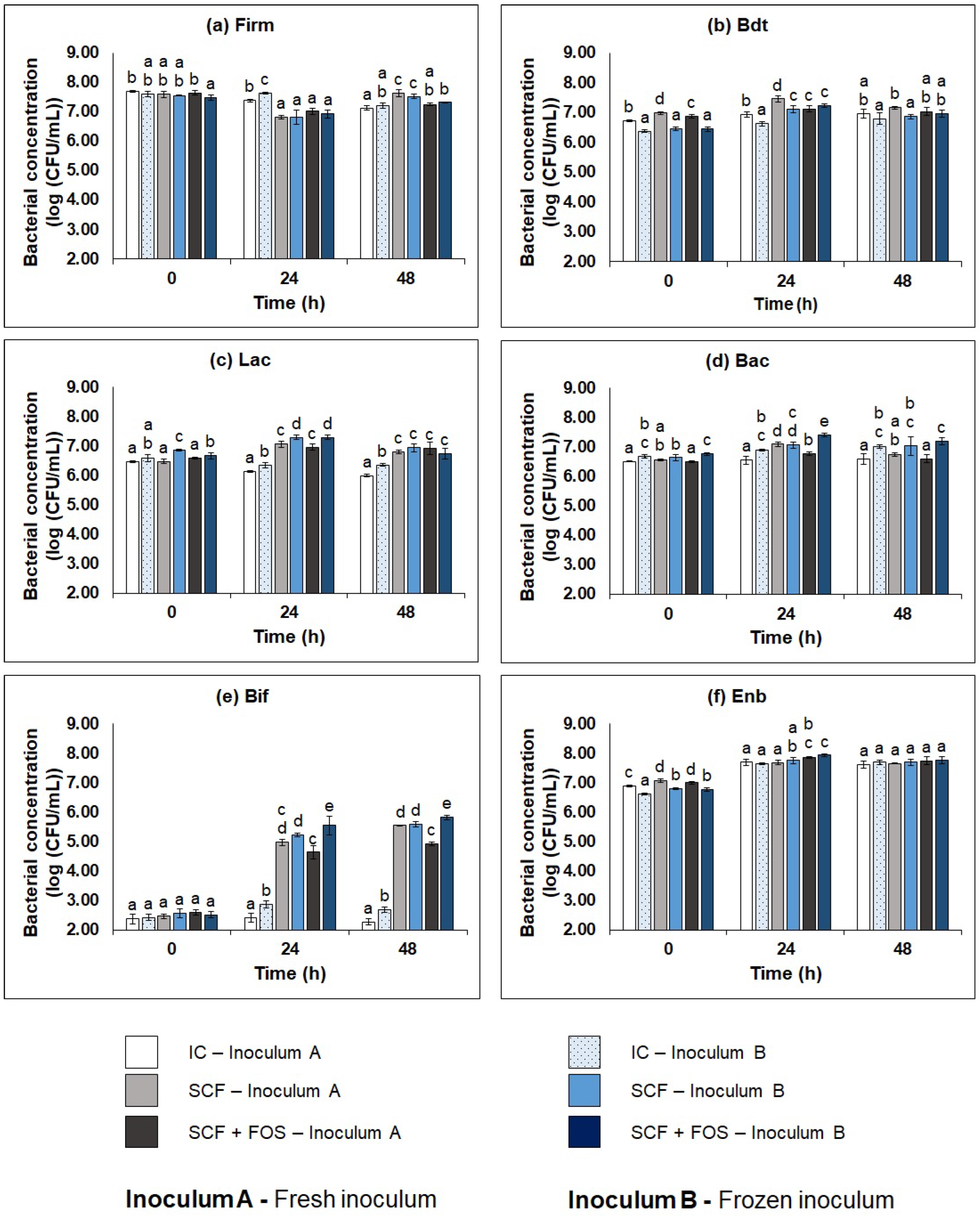
3.3.1. Bacterial Profile of the Cecum Fermentations
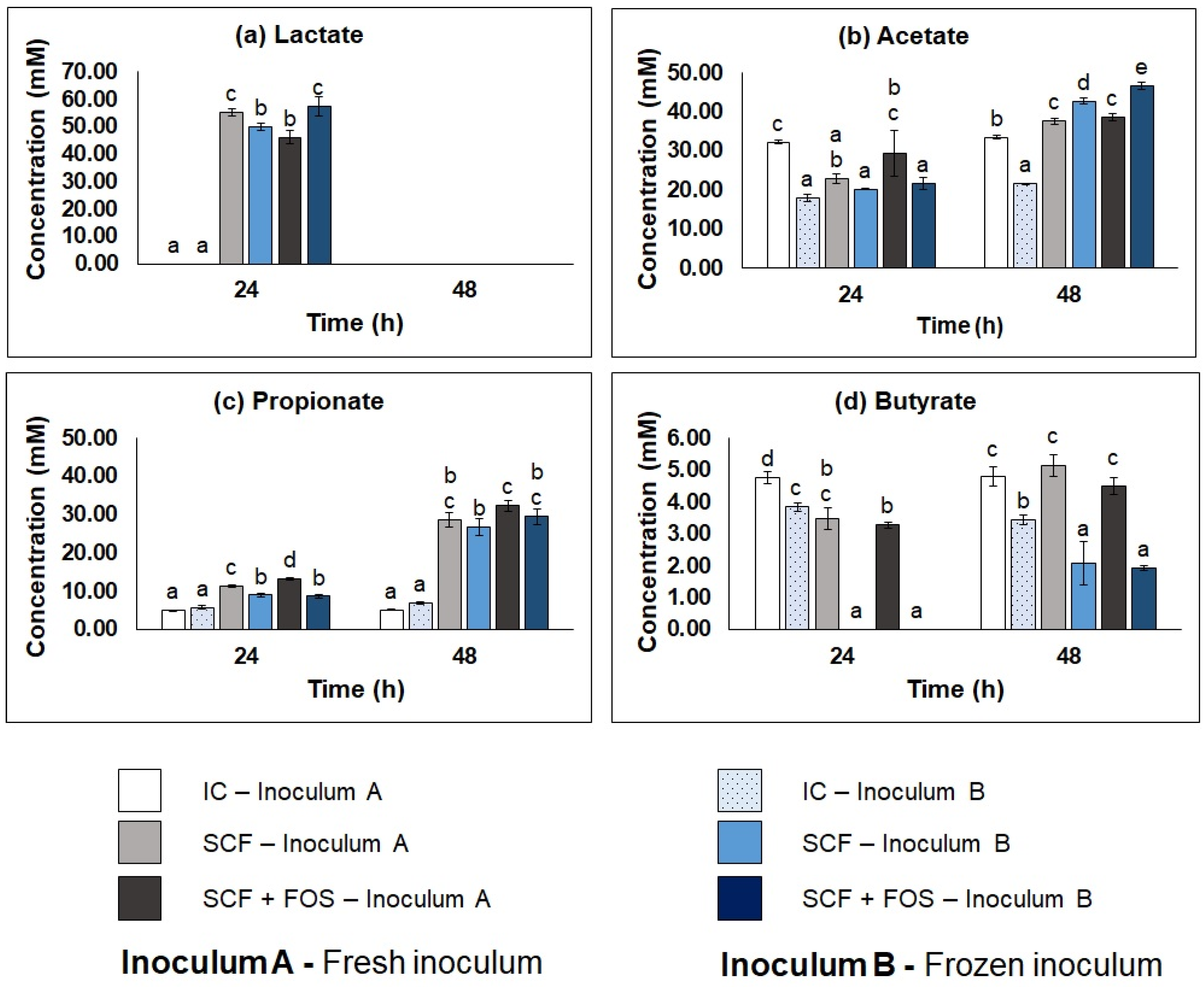
3.3.2. Metabolic Profile of Cecum Fermentations
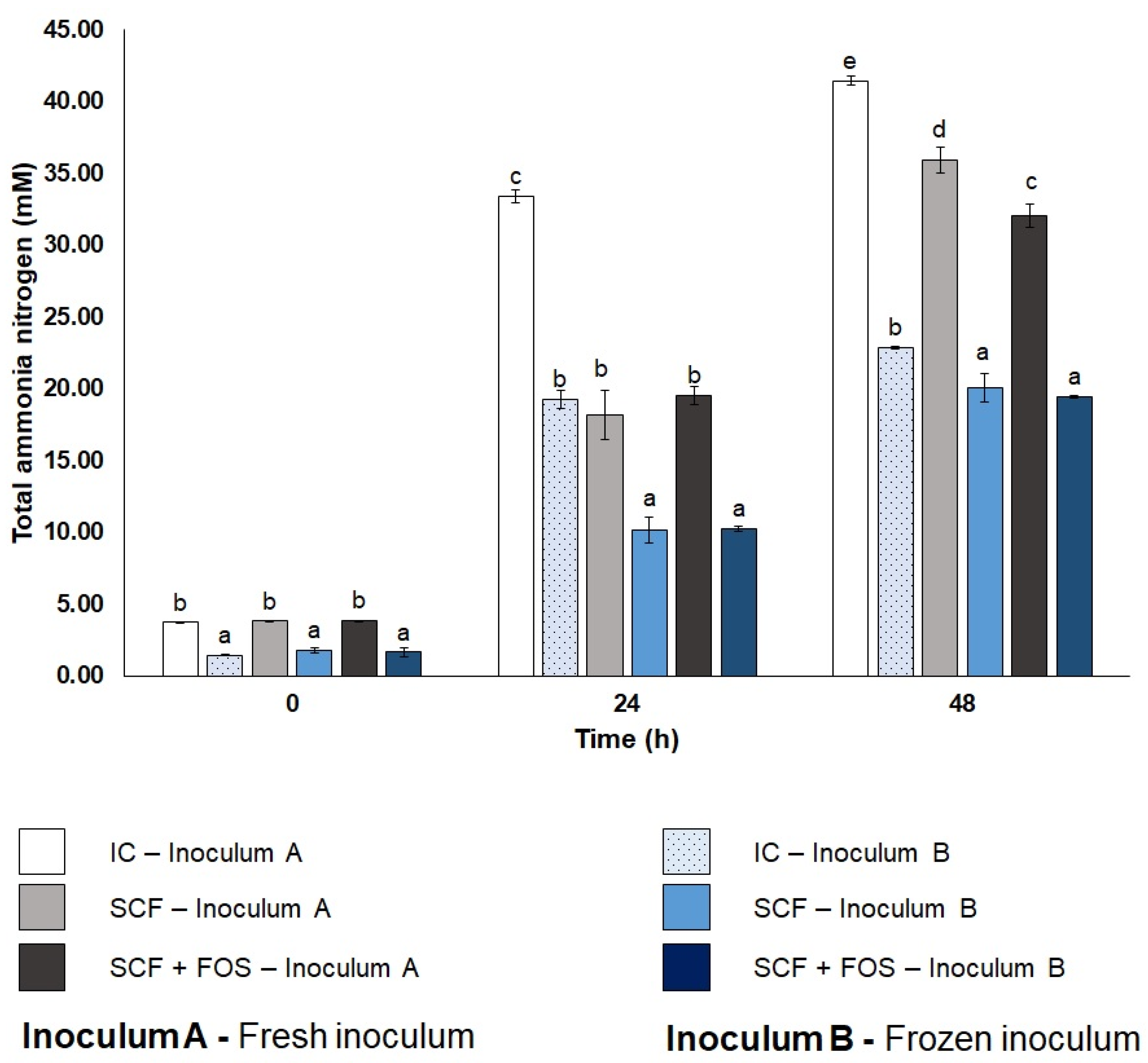
3.3.3. Total Ammonia Nitrogen Profile of Cecum Fermentations
4. Discussion
4.1. Chicken Inoculum Preservation Method Impact on Bacteria Viability
4.2. Gastrointestinal Model and Cecum Microbiota Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Reagents/Chemicals Used in This Study
- Acetic acid glacial (Sigma, St. Louis, MO, USA);
- Agarose—electrophoresis grade (Nzytech, Lisbon, Portugal);
- Ammonium chloride—NH4Cl (Mettler Toledo, Urdorf, Switzerland);
- Bile porcine- B8631 (Sigma, St. Louis, MO, USA);
- Bile salts (Sigma, St. Louis, MO, USA);
- Butyric acid (Sigma, St. Louis, MO, USA);
- Calcium chloride—CaCl2 (Merck KGaA, Darmstadt, Germany);
- Calcium chloride hexahydrate—CaCl2(H2O)6 (Sigma, St. Louis, MO, USA);
- Defibrinated sheep blood (Oxoid Limited, Basingstoke, UK);
- DMSO (Sigma-Aldrich, St. Louis, MO, USA);
- Dipotassium hydrogen phosphate—K2HPO4 (Honeywell Fluka, Seelze, Germany);
- DL-lactic acid (Sigma, St. Louis, MO, USA);
- FOS from chicory root (Megazyme, Bray, Ireland);
- Glycerol—analytic grade (Fisher Scientific, Loughborough, UK);
- GRS Universal Ladder (Grisp, Porto, Portugal);
- Microbial DNA-free water (Quiagen, Hilden, Germany);
- Hemin (Sigma, St. Louis, MO, USA);
- Hydrochloric acid—HCl (Honeywell Fluka, Seelze, Germany);
- L-cysteine HCl (Sigma-Aldrich, St. Louis, MO, USA);
- Magnesium sulfate—MgSO4 (Honeywell Fluka, Seelze, Germany);
- Magnesium sulfate heptahydrate—MgSO4(H2O)7 (Sigma, St. Louis, MO, USA);
- NZYSpeedy qPCR Green Master Mix (2×) (Nzytech, Lisbon, Portugal);
- Pancreatin from the porcine pancreas—P7545 (Sigma, St. Louis, MO, USA);
- Pepsin from porcine gastric mucose powder—P7000 (Sigma, St. Louis, MO, USA);
- Peptone from animal tissue (Sigma, St. Louis, MO, USA);
- Phosphate buffered saline (Dulbecco A)—PBS (Oxoid Limited, Basingstoke, UK);
- Potassium dihydrogen phosphat—eKH2PO4 (Merck KGaA, Darmstadt, Germany);
- Propionic acid (Sigma, St. Louis, MO, USA);
- Resazurin sodium salt (Sigma, St. Louis, MO, USA);
- Sodium chloride—NaCl (Honeywell Fluka, Seelze, Germany);
- Sodium hydrogen carbonate—NaHCO3 (Panreac, Barcelona, Spain);
- Sodium hydroxide—NaOH (LabChem, Zelienople, USA);
- Soja 115 INT2—complete feed for broiler chickens (Sorgal S.A., Aveiro, Portugal);
- Sulfuric acid—H2SO4 (Honeywell Fluka, Seelze, Germany);
- TAE Buffer 50× solution—RNAse free solution (Nzytech, Lisbon, Portugal);
- Tween 80 (Sigma, St. Louis, MO, USA);
- Vitamin K1 (Sigma, St. Louis, MO, USA);
- Yeast extract (Sigma, St. Louis, MO, USA).
Appendix A.2. Culture Media Used in This Study
- Columbia agar base—CBA (Liofilchem, Roseto degli Abruzzi, Italy);
- de Man, Rogosa, and Sharpe agar—MRSA (Biokar Diagnostics, Allonne, France);
- MacConkey agar—MCA (Biolife, Milan, Italy);
- Mueller–Hinton broth—MHB (Biokar Diagnostics, Allonne, France);
- Tryptic soy broth—TSB (Biokar Diagnostics, Allonne, France).
Appendix A.3. Apparatus Used in This Study
- 1 kDa molecular weight cut-off regenerated cellulose dialysis tubing Spectra/Por® 6 (Spectrum, NB, USA);
- Agilent 1260 II series HPLC (Agilent, Santa Clara, CA, USA);
- Alpha 2-4 LSC plus model (Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany)
- Anaerobic cabinet, Whitley A35 workstation (Don Whitley Scientific, Bingley, UK);
- Biometra Compact Multi-Wide (Analytik-Jena, Jena, Germany);
- Clifton NE4-22P digital water circulator (Nickel-Electro Ltd., Weston-super-Mare, UK);
- CryoCube® F570n freezer (Eppendorf, Hamburg, Germany);
- FerMac 260 pH controller (Electrolab Biotech Ltd., Gloucestershire, UK);
- Ion-exclusion Aminex HPX-87H column (Biorad, Hercules, CA, USA);
- Heraeus™ Megafuge™ 16R Centrifuge (Thermo Fischer Scientific, Waltham, MA, USA);
- Mixwel® laboratory blender (Alliance Bio Expertise Guipry, France);
- MR Hei-Tec magnetic stirrer (Heidolph Instruments GmbH & CO. KG, Schwabach, Germany);
- MST magnetic stirrer (Velp Scientifica, Usmate Velate, Italy);
- OxoidTM AnaeroGenTM 2.5 L sachet (Thermo Fischer Scientific, Waltham, MA, USA);
- OxoidTM AnaeroJarTM 2.5 L (Thermo Fischer Scientific, Waltham, MA, USA);
- pH sensor 405-DPAS-SC-K8S/200 (Mettler Toledo, Urdorf, Switzerland);
- PureLink™ Microbiome DNA Purification Kit (Thermo Fisher Scientific, Waltham, MA, USA);
- qTower3 G (Analytik-Jena, Jena, Germany);
- Qubit 4 fluorometer (Thermo Fisher Scientific, Waltham, MA, USA);
- Qubit® dsDNA HS assay Kit (Thermo Fisher Scientific, Waltham, MA, USA);
- Reax top vortex (Heidolph Instruments GmbH & CO. KG, Schwabach, Germany);
- Refrigerator Beko RSNE445E33WN (Beko, Istanbul, Turkey);
- Sension+ 9663 ammonium ion selective electrode (ISE) (Hach, Loveland, CO, USA);
- SevenCompact pH meter (Mettler Toledo, Urdorf, Switzerland);
- SW22 shaking water bath (Julabo GmbH, Seelbach, Germany);
- Tamper-proof specimen 1-L containers (Sigma, St. Louis, MO, USA);
- UVP ChemStudio imagers (Analytik-Jena, Jena, Germany).
References
- Borda-Molina, D.; Seifert, J.; Camarinha-Silva, A. Current Perspectives of the Chicken Gastrointestinal Tract and Its Microbiome. Comput. Struct. Biotechnol. J. 2018, 16, 131–139. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Qattan, S.Y.A.; Batiha, G.E.; Khafaga, A.F.; Abdel-Moneim, A.E.; Alagawany, M. Probiotics in Poultry Feed: A Comprehensive Review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1835–1850. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, J.M.D.; Casanova, N.A.; Miyakawa, M.E.F. Microbiota, Gut Health and Chicken Productivity: What Is the Connection? Microorganisms 2019, 7, 374. [Google Scholar] [CrossRef]
- Pascual, A.; Pauletto, M.; Trocino, A.; Birolo, M.; Dacasto, M.; Giantin, M.; Bordignon, F.; Ballarin, C.; Bortoletti, M.; Pillan, G.; et al. Effect of the Dietary Supplementation with Extracts of Chestnut Wood and Grape Pomace on Performance and Jejunum Response in Female and Male Broiler Chickens at Different Ages. J. Anim. Sci. Biotechnol. 2022, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Ebling, P.D.; Ribeiro, A.M.L.; Trevizan, L.; da Silva, I.C.M.; Kessler, A.M.; Rubin, L.L. Effect of Different Dietary Concentrations of Amino Acids on the Performance of Two Different Broiler Strains. Braz. J. Poult. Sci. 2013, 15, 339–346. [Google Scholar] [CrossRef][Green Version]
- Baéza, E.; Arnould, C.; Jlali, M.; Chartrin, P.; Gigaud, V.; Mercerand, F.; Durand, C.; Meteau, K.; Le Bihan-Duval, E.; Berri, C. Influence of Increasing Slaughter Age of Chickens on Meat Quality, Welfare, and Technical and Economic Results. J. Anim. Sci. 2012, 90, 2003–2013. [Google Scholar] [CrossRef]
- Augère-Granier, M.-L. The EU Poultry Meat and Egg Sector: Main Features, Challenges and Prospects; European Parliamentary Research Service (EPRS): Brussels, Belgium, 2019; pp. 1–20. [Google Scholar] [CrossRef]
- Milanovic, S. Literature Review on the Influence of Milling and Pelleting on Nutritional Quality, Physical Characteristics, and Production Cost of Pelleted Poultry Feed. Master’s Thesis, Norwegian University of Life Sciences, Ås, Norway, 2018. [Google Scholar]
- Raza, A.; Bashir, S.; Tabassum, R. An Update on Carbohydrases: Growth Performance and Intestinal Health of Poultry. Heliyon 2019, 5, e01437. [Google Scholar] [CrossRef]
- Yadav, S.; Jha, R. Strategies to Modulate the Intestinal Microbiota and Their Effects on Nutrient Utilization, Performance, and Health of Poultry. J. Anim. Sci. Biotechnol. 2019, 10, 2. [Google Scholar] [CrossRef]
- Te Pas, M.F.W.; Borg, R.; Buddiger, N.J.H.; Wood, B.J.; Rebel, J.M.J.; van Krimpen, M.M.; Calus, M.P.L.; Park, J.; Schokker, D. Regulating Appetite in Broilers for Improving Body and Muscle Development—A Review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1819–1834. [Google Scholar] [CrossRef]
- Saeed, M.; Yatao, X.; Rehman, Z.U.; Arain, M.A.; Soomro, R.N.; Abd El-Hack, M.E.; Bhutto, Z.A.; Abbasi, B.; Dhama, K.; Sarwar, M. Nutritional and Healthical Aspects of Yacon (Smallanthus sonchifolius) for Human, Animals and Poultry. Int. J. Pharmacol. 2017, 13, 361–369. [Google Scholar] [CrossRef]
- Biswas, A.; Mohan, N.; Raza, M.; Mir, N.A.; Mandal, A. Production Performance, Immune Response and Blood Biochemical Parameters in Broiler Chickens Fed Diet Incorporated with Prebiotics. J. Anim. Physiol. Anim. Nutr. 2019, 103, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chen, C.; Indugu, N.; Werlang, G.O.; Singh, M.; Kim, W.K.; Thippareddi, H. Effect of Antibiotic Withdrawal in Feed on Chicken Gut Microbial Dynamics, Immunity, Growth Performance and Prevalence of Foodborne Pathogens. PLoS ONE 2018, 13, e0192450. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalaifah, H.S. Benefits of Probiotics and/or Prebiotics for Antibiotic-Reduced Poultry. Poult. Sci. 2018, 97, 3807–3815. [Google Scholar] [CrossRef] [PubMed]
- Ricke, S.C.; Lee, S.I.; Kim, S.A.; Park, S.H.; Shi, Z. Prebiotics and the Poultry Gastrointestinal Tract Microbiome. Poult. Sci. 2020, 99, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Solis-Cruz, B.; Hernandez-Patlan, D.; Hargis, B.M.; Tellez, G. Use of Prebiotics as an Alternative to Antibiotic Growth Promoters in the Poultry Industry. In Prebiotics and Probiotics-Potential Benefits in Nutrition and Health; IntechOpen: London, UK, 2019. [Google Scholar]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Asare, P.T.; Greppi, A.; Pennacchia, A.; Brenig, K.; Geirnaert, A.; Schwab, C.; Stephan, R.; Lacroix, C. In Vitro Modeling of Chicken Cecal Microbiota Ecology and Metabolism Using the PolyFermS Platform. Front. Microbiol. 2021, 12, 780092. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Q.; Yang, Y.; Guo, A. Biological Function of Short-Chain Fatty Acids and Its Regulation on Intestinal Health of Poultry. Front. Vet. Sci. 2021, 8, 736739. [Google Scholar] [CrossRef]
- de Carvalho, N.M.; Oliveira, D.L.; Saleh, M.A.D.; Pintado, M.E.; Madureira, A.R. Importance of Gastrointestinal in Vitro Models for the Poultry Industry and Feed Formulations. Anim. Feed. Sci. Technol. 2020, 271, 114730. [Google Scholar] [CrossRef]
- Meimandipour, A.; Shuhaimi, M.; Hair-Bejo, M.; Azhar, K.; Kabeir, B.M.; Rasti, B.; Yazid, A.M. In Vitro Fermentation of Broiler Cecal Content: The Role of Lactobacilli and PH Value on the Composition of Microbiota and End Products Fermentation. Lett. Appl. Microbiol. 2009, 49, 415–420. [Google Scholar] [CrossRef]
- Forssten, S.D.; Morovic, W.; Nurminen, P. An in Vitro Model of the Chicken Gastrointestinal Tract with Special Emphasis to the Cecal Microbiota. Poult. Sci. 2023, 102, 102654. [Google Scholar] [CrossRef]
- Oost, M.J.; Velkers, F.C.; Kraneveld, A.D.; Venema, K. Development of the in Vitro Cecal Chicken ALIMEntary TRact MOdel-2 to Study Microbiota Composition and Function. Front. Microbiol. 2021, 12, 726447. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Kumar, S.; Oakley, B.; Kim, W.K. Chicken Gut Microbiota: Importance and Detection Technology. Front. Vet Sci. 2018, 5, 254. [Google Scholar] [CrossRef] [PubMed]
- Diether, N.E.; Willing, B.P. Microbial Fermentation of Dietary Protein: An Important Factor in Diet–Microbe–Host Interaction. Microorganisms 2019, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, N.M.; Oliveira, D.L.; Saleh, M.A.D.; Pintado, M.; Madureira, A.R. Preservation of Human Gut Microbiota Inoculums for In Vitro Fermentations Studies. Fermentation 2021, 7, 14. [Google Scholar] [CrossRef]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The Estimation of the Bactericidal Power of the Blood. Epidemiol. Infect. 1938, 38, 732–749. [Google Scholar] [CrossRef]
- de Carvalho, N.M.; Oliveira, D.L.; Costa, C.M.; Pintado, M.E.; Madureira, A.R. Applied Microbiology in Poultry Industry: From Nutrition to the Gut Microbiota. In Proceedings of the Book of Abstracts of the 73rd Annual Meeting of the European Federation of Animal Science, Porto, Portugal, 5–9 September 2022; p. 568. [Google Scholar]
- Martinez-Haro, M.; Taggart, M.A.; Green, A.J.; Mateo, R. Avian Digestive Tract Simulation to Study the Effect of Grit Geochemistry and Food on Pb Shot Bioaccessibility. Environ. Sci. Technol. 2009, 43, 9480–9486. [Google Scholar] [CrossRef] [PubMed]
- Bean, T.G.; Arnold, K.E.; Lane, J.; Pietravalle, S.; Boxall, A.B.A. An in Vitro Method for Determining the Bioaccessibility of Pharmaceuticals in Wildlife. Environ. Toxicol. Chem. 2016, 35, 2349–2357. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Śliżewska, K. The Role of Probiotics, Prebiotics and Synbiotics in Animal Nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [CrossRef]
- Mills, D.J.S.; Tuohy, K.M.; Booth, J.; Buck, M.; Crabbe, M.J.C.; Gibson, G.R.; Ames, J.M. Dietary Glycated Protein Modulates the Colonic Microbiota towards a More Detrimental Composition in Ulcerative Colitis Patients and Non-ulcerative Colitis Subjects. J. Appl. Microbiol. 2008, 105, 706–714. [Google Scholar] [CrossRef]
- de Carvalho, N.M.; Walton, G.E.; Poveda, C.G.; Silva, S.N.; Amorim, M.; Madureira, A.R.; Pintado, M.E.; Gibson, G.R.; Jauregi, P. Study of In Vitro Digestion of Tenebrio Molitor Flour for Evaluation of Its Impact on the Human Gut Microbiota. J. Funct. Foods 2019, 59, 101–109. [Google Scholar] [CrossRef]
- Duggett, N. High-Throughput Sequencing of the Chicken Gut Microbiome. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2016. [Google Scholar]
- de Carvalho, N.M.; Oliveira, D.L.; Costa, C.M.; Pintado, M.; Madureira, A.R. Can Supplemented Skim Milk (SKM) Boost Your Gut Health? Fermentation 2022, 8, 126. [Google Scholar] [CrossRef]
- Fangueiro, J.F.; de Carvalho, N.M.; Antunes, F.; Mota, I.F.; Pintado, M.E.; Madureira, A.R.; Costa, P.S. Lignin from Sugarcane Bagasse as a Prebiotic Additive for Poultry Feed. Int. J. Biol. Macromol. 2023, 239, 124262. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Mao, Y.; Kang, Z.; Luo, Q. Application of an Ammonium Ion-Selective Electrode for the Real-Time Measurement of Ammonia Nitrogen Based on PH and Temperature Compensation. Measurement 2019, 137, 98–101. [Google Scholar] [CrossRef]
- Queipo-Ortuño, M.I.; Seoane, L.M.; Murri, M.; Pardo, M.; Gomez-Zumaquero, J.M.; Cardona, F.; Casanueva, F.; Tinahones, F.J. Gut Microbiota Composition in Male Rat Models under Different Nutritional Status and Physical Activity and Its Association with Serum Leptin and Ghrelin Levels. PLoS ONE 2013, 8, e65465. [Google Scholar] [CrossRef] [PubMed]
- Heilig, H.G.H.J.; Zoetendal, E.G.; Vaughan, E.E.; Marteau, P.; Akkermans, A.D.L.; de Vos, W.M. Molecular Diversity of Lactobacillus Spp. and Other Lactic Acid Bacteria in the Human Intestine as Determined by Specific Amplification of 16S Ribosomal DNA. Appl. Environ. Microbiol. 2002, 68, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Hertel, C.; Tannock, G.W.; Lis, C.M.; Munro, K.; Hammes, W.P. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella Species in Human Feces by Using Group-Specific PCR Primers and Denaturing Gradient Gel Electrophoresis. Appl. Environ. Microbiol. 2001, 67, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Effect of Inulin on the Human Gut Microbiota: Stimulation of Bifidobacterium Adolescentis and Faecalibacterium Prausnitzii. Br. J. Nutr. 2008, 101, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Delroisse, J.-M.; Boulvin, A.-L.; Parmentier, I.; Dauphin, R.D.; Vandenbol, M.; Portetelle, D. Quantification of Bifidobacterium Spp. and Lactobacillus Spp. in Rat Fecal Samples by Real-Time PCR. Microbiol. Res. 2008, 163, 663–670. [Google Scholar] [CrossRef]
- Bartosch, S.; Fite, A.; Macfarlane, G.T.; McMurdo, M.E.T. Characterization of Bacterial Communities in Feces from Healthy Elderly Volunteers and Hospitalized Elderly Patients by Using Real-Time PCR and Effects of Antibiotic Treatment on the Fecal Microbiota. Appl. Environ. Microbiol. 2004, 70, 3575–3581. [Google Scholar] [CrossRef]
- Shang, Y.; Kumar, S.; Thippareddi, H.; Kim, W.K. Effect of Dietary Fructooligosaccharide (FOS) Supplementation on Ileal Microbiota in Broiler Chickens. Poult. Sci. 2018, 97, 3622–3634. [Google Scholar] [CrossRef]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tang, H.; Chen, P.; Xie, H.; Tao, Y. Demystifying the Manipulation of Host Immunity, Metabolism, and Extraintestinal Tumors by the Gut Microbiome. Signal Transduct. Target. Ther. 2019, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Kralik, P.; Ricchi, M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef]
- Pan, D.; Yu, Z. Intestinal Microbiome of Poultry and Its Interaction with Host and Diet. Gut Microbes 2013, 5, 108–119. [Google Scholar] [CrossRef]
- Rehman, H.U.; Vahjen, W.; Awad, W.A.; Zentek, J. Indigenous Bacteria and Bacterial Metabolic Products in the Gastrointestinal Tract of Broiler Chickens. Arch. Anim. Nutr. 2007, 61, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, Colonic Fermentation, and Gastrointestinal Health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Tunkala, B.Z.; DiGiacomo, K.; Alvarez Hess, P.S.; Dunshea, F.R.; Leury, B.J. Impact of Rumen Fluid Storage on In Vitro Feed Fermentation Characteristics. Fermentation 2023, 9, 392. [Google Scholar] [CrossRef]
- Bircher, L.; Schwab, C.; Geirnaert, A.; Lacroix, C. Cryopreservation of Artificial Gut Microbiota Produced with In Vitro Fermentation Technology. Microb. Biotechnol. 2018, 11, 163–175. [Google Scholar] [CrossRef]
- Njokweni, S.G.; Weimer, P.J.; Botes, M.; van Zyl, W.H. Effects of Preservation of Rumen Inoculum on Volatile Fatty Acids Production and the Community Dynamics during Batch Fermentation of Fruit Pomace. Bioresour. Technol. 2021, 321, 124518. [Google Scholar] [CrossRef]
- Achá, S.J.; Kühn, I.; Mbazima, G.; Colque-Navarro, P.; Möllby, R. Changes of Viability and Composition of the Escherichia Coli Flora in Faecal Samples during Long Time Storage. J. Microbiol. Methods 2005, 63, 229–238. [Google Scholar] [CrossRef]
- Whaley, D.; Damyar, K.; Witek, R.P.; Mendoza, A.; Alexander, M.; Lakey, J.R.T. Cryopreservation: An Overview of Principles and Cell-Specific Considerations. Cell Transpl. 2021, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Belanche, A.; Palma-Hidalgo, J.M.; Nejjam, I.; Serrano, R.; Jiménez, E.; Martín-García, I.; Yáñez-Ruiz, D.R. In Vitro Assessment of the Factors That Determine the Activity of the Rumen Microbiota for Further Applications as Inoculum. J. Sci. Food Agric. 2019, 99, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Yu, L.; Xu, T.; Zhu, N. Microbiota and Metabolome Responses in the Cecum and Serum of Broiler Chickens Fed with Plant Essential Oils or Virginiamycin. Sci. Rep. 2020, 10, 5382. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [PubMed]
- Maki, J.J.; Klima, C.L.; Sylte, M.J.; Looft, T. The Microbial Pecking Order: Utilization of Intestinal Microbiota for Poultry Health. Microorganisms 2019, 7, 376. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Wang, J.; Yu, L.; Zhang, Q.; Chen, K.; Liu, B. Modulation of Growth Performance and Intestinal Microbiota in Chickens Fed Plant Extracts or Virginiamycin. Front. Microbiol. 2019, 10, 1333. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Luo, L.; Wang, X.; Wen, Q.; Zhou, L.; Wu, K. Characterization of the Cecal Microbiome Composition of Wenchang Chickens before and after Fattening. PLoS ONE 2019, 14, e0225692. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; De Los Reyes-gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and Their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Lei, F.; Yin, Y.; Wang, Y.; Deng, B.; Yu, H.D.; Li, L.; Xiang, C.; Wang, S.; Zhu, B.; Wang, X. Higher-Level Production of Volatile Fatty Acids In Vitro by Chicken Gut Microbiotas than by Human Gut Microbiotas as Determined by Functional Analyses. Appl. Environ. Microbiol. 2012, 78, 5763–5772. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.A.; Bird, A.R. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients 2015, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Naseem, S.; King, A.J. Ammonia Production in Poultry Houses Can Affect Health of Humans, Birds, and the Environment—Techniques for Its Reduction during Poultry Production. Environ. Sci. Pollut. Res. 2018, 25, 15269–15293. [Google Scholar] [CrossRef] [PubMed]
- Swelum, A.A.; El-Saadony, M.T.; Abd El-Hack, M.E.; Ghanima, M.M.A.; Shukry, M.; Alhotan, R.A.; Hussein, E.O.S.; Suliman, G.M.; Ba-Awadh, H.; Ammari, A.A. Ammonia Emissions in Poultry Houses and Microbial Nitrification as a Promising Reduction Strategy. Sci. Total Environ. 2021, 781, 146978. [Google Scholar] [CrossRef]
| Culture Media | Incubation Conditions | Target Bacterial Groups |
|---|---|---|
| CBA with 5% (v/v) defibrinated sheep blood (DSB) | Anaerobic 37 °C for 72 h | Total anaerobic bacteria |
| MCA | Gram-negative and enteric bacteria | |
| MRSA with 0.1% (w/v) cysteine | Lactic acid bacteria (LAB) and Bifidobacterium |
| Analytical constituents (%) | Protein | 19.40 |
| Fat | 5.50 | |
| Fiber | 2.60 | |
| Ash | 4.10 | |
| Calcium | 0.80 | |
| Phosphorous | 0.60 | |
| Sodium | 0.15 | |
| Lysine | 1.15 | |
| Methionine | 0.50 | |
| Additives (per kg) | Vitamin A | 12,000 IU |
| Vitamin D3 | 2000 IU | |
| Vitamin E (All-rac-α-tocopheryl acetate) | 12 mg | |
| Biotin | 0.09 mg | |
| Potassium iodide | 0.70 mg | |
| Copper | 8.00 mg | |
| Manganese | 100 mg | |
| Zinc | 60 mg | |
| Selenium | 0.20 mg | |
| Iron | 21.30 mg | |
| Canthaxanthin | 4 mg | |
| Ethyl ester of beta-apo-8′ carotenoic acid | 14 mg | |
| Coccidiostats and histomonostats (per kg) | Narasine | 70 mg |
| Amino acids and analogs (per kg) | Hydroxy analogue of methionine | 1 g |
| Digestibility enhancer (per kg) | Endo-1,4-beta-xylanase | 40 U |
| Endo-1,3(4)-beta-glucanase | 35 U | |
| Endo-1,4-beta-glucanase | 135 U | |
| Antioxidants (per kg) | Butylated hydroxytoluene (BHT) | 120 mg |
| Anti-caking (per kg) | Sepiolite | 0.20 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Carvalho, N.M.; Costa, C.M.; Castro, C.; Saleh, M.A.D.; Pintado, M.E.; Oliveira, D.L.; Madureira, A.R. Development of a Chicken Gastrointestinal Tract (GIT) Simulation Model: Impact of Cecal Inoculum Storage Preservation Conditions. Appl. Microbiol. 2023, 3, 968-992. https://doi.org/10.3390/applmicrobiol3030066
de Carvalho NM, Costa CM, Castro C, Saleh MAD, Pintado ME, Oliveira DL, Madureira AR. Development of a Chicken Gastrointestinal Tract (GIT) Simulation Model: Impact of Cecal Inoculum Storage Preservation Conditions. Applied Microbiology. 2023; 3(3):968-992. https://doi.org/10.3390/applmicrobiol3030066
Chicago/Turabian Stylede Carvalho, Nelson Mota, Célia Maria Costa, Cláudia Castro, Mayra Anton Dib Saleh, Manuela Estevez Pintado, Diana Luazi Oliveira, and Ana Raquel Madureira. 2023. "Development of a Chicken Gastrointestinal Tract (GIT) Simulation Model: Impact of Cecal Inoculum Storage Preservation Conditions" Applied Microbiology 3, no. 3: 968-992. https://doi.org/10.3390/applmicrobiol3030066
APA Stylede Carvalho, N. M., Costa, C. M., Castro, C., Saleh, M. A. D., Pintado, M. E., Oliveira, D. L., & Madureira, A. R. (2023). Development of a Chicken Gastrointestinal Tract (GIT) Simulation Model: Impact of Cecal Inoculum Storage Preservation Conditions. Applied Microbiology, 3(3), 968-992. https://doi.org/10.3390/applmicrobiol3030066