Abstract
Background and Aims: Parkinson’s disease (PD) is a multifaceted disease that can cause symptoms in multiple body systems, including the gastrointestinal (GI) tract. Fecal microbiota transplantation (FMT) is a proposed treatment to address dysregulation in the microbiome, with neurologic and GI symptoms as theoretic effects. The complex relationship between gut dysbiosis and PD symptoms suggests that FMT may have a role in therapy. Methods: A total of 124 articles with information pertaining to PD and FMT were reviewed. PD adult patients with neuromotor or GI symptoms who received FMT with moderate levels of evidence were examined. Data using self-reporting symptom scales were compared at baseline and following FMT. Results: An overall improvement was seen in patients’ neuromotor or GI symptoms, when compared to the baseline after FMT. After FMT, the patients reported improved constipation and decreased time to stool. Overall satisfaction differed between groups receiving differing routes of FMT administration and the severity of their baseline symptoms. Conclusions: FMT shows promising utility for PD patients with neuromotor and/or GI symptoms. Our results are limited by need to evaluate routes of administration, the chronicity of inflammation, and acidity’s role in colonization and efficacy. FMT has been illustrated as a well-tolerated non-pharmacologic treatment method for PD with refractory symptoms.
1. Introduction
Parkinson’s disease (PD) is a chronic and progressive nervous system disorder characterized by motor symptoms, such as bradykinesia, tremors, and rigidity, as well as non-motor symptoms, including constipation, sleep disturbances, cognitive deficits, depression, and other psychiatric ailments. These symptoms often worsen over time and can significantly impact the daily lives of affected individuals [1]. Interestingly, many PD patients report experiencing constipation symptoms years before the diagnosis of PD or the onset of neurologic symptoms. This observation suggests that there may be a connection between the gastrointestinal (GI) tract and the development and progression of PD [2].
The gut microbiome, which refers to the diverse community of microorganisms residing in the GI tract, has emerged as a potential contributor to the pathogenesis of various diseases, including neurologic disorders such as PD. Alterations in the gut microbiome can lead to changes in the gut–brain axis, a bidirectional communication pathway between the gut and the central nervous system, which may influence the progression of neurologic diseases [2]. This raises the question of whether targeting changes in the gut microbiome could have an impact on the symptoms and progression of PD [3].
Fecal microbiota transplantation (FMT) has been proposed as a therapeutic approach to address dysregulation in the gut microbiome of PD patients, with potential effects on both their neurologic symptoms and GI motility. FMT involves transferring fecal material from a healthy donor to the recipient, with the goal of restoring a more balanced and diverse microbiota composition. The safety of FMT has been extensively studied and validated in the treatment of conditions such as recurrent Clostridium difficile infection, inflammatory bowel disease, hepatic encephalopathy, autoimmune diseases, mood disorders, and obesity [4]. Although there is currently no standardized protocol for FMT procedures, a donor stool is typically obtained from a stool bank or a donor of the recipient’s choice and rigorously screened for potential pathogens. The delivery method of FMT can vary among different medical centers and may include colonoscopies, esophagogastroduodenoscopies, or the administration of capsules.
Given the intricate relationship between gut dysbiosis and neuromotor symptoms in PD, many have explored the potential therapeutic role of FMT in this patient population. Several studies have investigated the link between the gut microbiota and PD. Researchers have noted that PD patients exhibit alterations in the composition of their gut microbiomes compared to those of healthy individuals. Specifically, PD patients tend to have a reduced amount of certain beneficial bacteria, such as Prevotellaceae, and increased levels of pro-inflammatory bacteria, including Ralstonia [5,6]. These findings suggest that components of the intestinal microbiota may contribute to the progression and severity of PD.
In addition, constipation is a common non-motor symptom in PD, and it significantly affects the quality of life of patients. Our review also examined the impact of FMT on constipation symptoms. The majority of studies reported a significant improvement in constipation following FMT treatment. This improvement was associated with changes in the gut microbiota composition, suggesting that the restoration of a more balanced and less inflammatory gut microbiome may alleviate constipation symptoms in PD patients [7,8].
In conclusion, our systematic review aims to provide preliminary evidence supporting the potential non-pharmacologic therapeutic role of FMT in improving both neurologic and constipation symptoms in PD patients. Current evidence appears to support the positive impact of FMT on motor symptoms, particularly in the early stages of the disease, and shows FMT’s promise in alleviating constipation.
2. Materials and Methods
2.1. Search Strategy
We performed a comprehensive literature search of the PubMed and Cochrane databases using variations of the keywords “fecal microbiota transplant”, “fmt”, and “Parkinsons” to identify original studies published from FMT’s inception to 30 January 2023. Results were limited to human studies published in English. There were a total of 124 studies for our review. See Supplemental File S1 for detailed search terms.
2.2. Eligibility Criteria
Inclusion criteria were as follows: (1) FMT treatment; (2) patients with diagnosed Parkinson’s disease; (3) patients of any sex; (4) baseline motor symptoms as well as motor symptom grading after FMT treatment OR baseline GI symptoms as well as GI symptom grading after FMT.
Exclusion criteria were as follows: (1) non-human studies; (2) non-English studies; (3) studies with low-quality evidence.
2.3. Quality Assessment
A series of quality assessment tools developed by US National Heart Lung and Blood Institute (NHLBI) of National Institutes of Health (NIH) (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 25 March 2023) was used to determine methodological quality and risk of bias for case series. A set of question items with Yes/No answer options were used, with a “Yes” counting as a score of 1 and a “No” as a score of 0. In the tool used for case series, there were a total of 9 questions. A score of 7–9 corresponds to good quality, while scores of 4–6 and 1–3 indicate moderate and poor quality, respectively [7].
In the final selection stage, studies with at least moderate level of evidence were included. One case report was reviewed but excluded in the final stage due to its low quality level. Quality appraisal was performed by the two following authors (T.V. and T.L.). If there was any disagreement, a senior reviewer (A.S.H.) evaluated the article and achieved consensus through discussion. See Supplemental Table S1 for quality assessment scores for each study. See Supplemental Table S2 for a summary of excluded studies. The study selection process by Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) is shown in Figure 1.
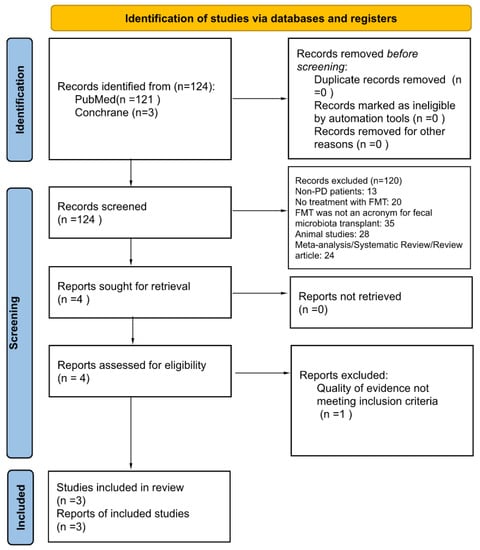
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses [8].
2.4. Study Outcomes
The primary outcome of this study was the improvement in neurologic symptoms of PD after FMT. Parkinsonism-related neurologic symptoms were reported using Movement Disorders Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) parts II and III, Hoehn and Yahr (H-Y) staging scale, and Non-Motor Symptoms Scale (NMSS). We reported p-values from derived t-tests across the three different studies, with statistical significance determined using a p-value less than 0.05 [9]. MDS-UPDRS is the current benchmark scale for PD evaluation with higher scores indicating higher severity of symptoms; part II assesses motor aspects of experiences of daily living and includes 13 different items, while part III assesses the motor exam and includes 33 scores from separate body parts on both sides [10]. H-Y stages are used to determine a patient’s functional status on a scale from 1 to 5, with 1 being only with unilateral involvement and stage 5 being bound to bed or wheelchair unless aided [11]. Finally, the NMSS rates non-motor symptoms, such as sexual function, mood, and sleep, with higher scores correlating to severe symptoms [12].
The secondary outcome was clinical success of FMT in improving GI symptoms in PD patients, primarily constipation. GI symptom scores were reported using Patient Assessment of Constipation Quality of Life (PAC-QOL), Wexner Constipation Scoring System (WCSS), and Bristol Stool Form Scale (BSFS) ratings. The PAC-QOL evaluates patients’ quality of life with constipation. It is a questionnaire that is categorized into physical discomfort, psychosocial discomfort, treatment satisfaction, and worries and discomfort; higher scores indicate more negative effects on quality of life [13]. The WCSS measures severity of constipation on 32-point scale, rating 8 categories, such as minutes in lavatory to pain with evacuation, from 0 to 2 or 4, with scores closer to 32 indicating increased severity of constipation [14]. Finally, the BSFS is a well-known tool to categorize stool into 1 of 7 categories depending on its morphology, with 1 being hard and pellet-like and 7 being entirely liquid [15].
2.5. Study Selection and Data Extraction
A total of 121 articles were retrieved on initial search. Two authors (T.V. and T.L.) independently reviewed these titles and abstracts, after which four articles were deemed relevant with patient data. Full texts were then reviewed by the following authors (T.V. and T.L.), after which three remaining studies fulfilled complete eligibility criteria. In cases of disagreement, a senior reviewer (A.S.H.) arbitrated the final decision for inclusion. Study selection process by Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement is detailed in Figure 1. Summary of included studies is shown in Table 1, while excluded articles are listed in Supplemental Table S1. IRB review was not required as all data were extracted from published literature, and no patient intervention was directly performed.

Table 1.
Summary of studies included.
3. Results
Through a multi-database systematic literature search, we found a total of three studies, all case series, describing the use of FMT in adult PD patients. A total of 32 Parkinson’s patients were included in this review, all of whom had formally been diagnosed with PD by a healthcare professional and had some regimen for the treatment of PD-related symptoms. Summary of included studies included in Table 2. In Kuai’s [16] case series, patients with baseline constipation were recruited to assess the efficacy and safety of FMT treatment for PD-related GI dysfunction. The study also noted significant improvement in oral-cecal transit time (OCTT) after FMT and a correction with small intestinal overgrowth as measured with the lactulose breath test. While Bacteroides was the most prominent genus before FMT, this study found that there was a significant decrease after FMT treatment. Faecalibacterium, noted for its anti-inflammatory T-regs [19], also saw a significant increase to being the most prevalent genus. Segal’s [17] study reported average improvements in mean motor and nonmotor scores, but a statistical analysis was not performed due to the small sample size (Table 3 and Table 4). In Xue’s [18] case series, neuromotor symptoms refractory to other medical treatments showed a significant improvement overall, while GI symptoms were not investigated (Table 3).

Table 2.
Summary of patient populations and their baseline scores before FMT.

Table 3.
A summary of the patients’ neuro-motor symptoms at baseline then at follow-up in the included studies.

Table 4.
A summary of the patients’ gastrointestinal symptoms at baseline then at follow-up in the included studies.
4. Discussion
Previous investigations suggest that gut dysbiosis may play a role in the progression and severity of PD as well as other neuromotor-related diseases. We conducted the first systematic review to evaluate the efficacy and safety of fecal microbiota transplantation (FMT) in treating neurologic and gastrointestinal (GI) symptoms in PD patients. FMT appears to be effective and safe in treating the neurologic symptoms of PD. Several studies have compared the microbial flora of PD patients and that of non-PD patients and found that overall, the PD patients had lower amounts of Prevotellaceae [5] and higher amounts of proinflammatory short-chain fatty acids producing Ralstonia [6]. Furthermore, neuromotor symptoms, such as tremors and gait instability, were more severe in PD patients with higher concentrations of Enterobacteriaceae [5], suggesting some degree of progression related to intestinal microbiota components. While the existing evidence is promising, it is important to note that the studies included in our systematic review had some limitations.
The sample sizes were relatively small in most of the studies, and there was heterogeneity in the FMT protocols used, including variations in donor selection, the preparation of the fecal material, and delivery methods. Additionally, the follow-up durations varied, making it difficult to assess the long-term effects of FMT in PD patients. Further research is necessary to establish optimal FMT protocols, determine long-term effects, and address the underlying mechanisms of FMT in PD. If future studies can confirm the efficacy and safety of FMT, this could potentially revolutionize the treatment approach for PD by targeting the gut microbiome and its influence on neurologic symptoms and GI dysfunction. Therefore, larger randomized controlled trials with standardized protocols are needed to confirm the efficacy and safety of FMT in PD.
In a clinical study by Dupont [20] published outside of the data collection period of this review, patients with PD were treated with oral multidose FMT. In support of this study, an overall significant increase in intestinal microbial diversity was noted, associated with a reduction in constipation, improved motility, and improved transit times. Similarly, Dupont noted that there was a subjective improvement in patients’ neurologic symptoms after FMT; however, this was transient and objective.
4.1. Neurologic Symptoms
For our primary outcome in this study, we found improvements in patients’ neurologic symptoms of PD after FMT across all studies. However, we did note some variability in score progression throughout the varying follow-up periods. This appears to be related to the severity and chronicity of the baseline disease. Those with a longstanding and more severe neurologic disease had a greater improvement in their scores compared to those with shorter, less severe disease; however, the overall trend showed improving scores among all patients. One possible explanation may be related to the abundance of pro-inflammatory bacteria found in higher concentrations in PD patients. In example, studies have shown that PD patients with higher concentrations of Enterobacteriaceae and Ralstonia, which produce short-chain fatty acids associated with inflammation, tend to experience more severe neuromotor symptoms, such as tremors and gait instability [5]. These induced inflammatory states within the GI tract may lead to an increased amount of alpha-synuclein delivery from the GI tract to the central nervous system. One leading theory is that in PD, misfolded alpha-synuclein arises from the GI tract, more so in increased states of inflammation, and is transported to the central nervous system via the vagal system [21]. This theory finds roots in previously discoveries by Braak [22]. Braak noted that alpha-synuclein was found in substantia nigra biopsies in patients with PD. He noted that these aggregates may proceed caudo-rostrally, which might explain why increased alpha-synuclein aggregates have been found in biopsy samples of PD patients in the colon, esophagus, small intestine, and up to the substantia nigra [23,24]. Thus, it would follow that those with more a severe and longer neurologic disease would likely have more inflammation and dysbiosis and therefore benefit more from a GI intervention. Furthermore, while the severity of disease and its progression are not always related to one’s age, age does appear to be one of the stronger predictive values in PD. Older individuals also tend to have decreased adaptive immunity; thus, they may mount a smaller response to foreign flora, have more time for transplants to flourish in their GI tract, and ultimately experience more of an effect on their nervous system [21]. In Xue’s study, patients who received FMT via a colonoscopy had decreased NMSS and UPDRS III scores and more satisfactory symptomatic relief even up to and beyond 24 months. Those who received FMT via a nasointestinal tube reported unsatisfactory results, especially in terms of non-motor symptoms. This effect may be related to the location of colonization of bacteria into areas of differing pHs and the colonizing bacteria’s ability to survive in the local environment. In Xue’s [18] study, the patients received transplants in the more alkalotic jejunum via a nasojejunal tube or in the ascending colon via a colonoscopy. The varying pHs of different sections of the GI tract may affect the efficacy of FMT and warrants further investigation into anti-inflammatory bacterial strains, their viability in different host environments, and the possible role of treatment with acid-suppressing or acid-stimulating substances prior to FMT treatment.
4.2. Gastrointestinal Symptoms
For our secondary outcome, we evaluated self-reported changes in constipation after treatment with FMT. We found an overall improvement in GI symptoms related to PD, such as constipation, leading to an overall reported improvement in patients’ quality of life. Constipation is a prevalent and debilitating complication of Parkinson’s disease, can be present in any stage of the disease, and often presents before the usual bradykinesia or rigidity. As dysmotility worsens, transit and absorption are also affected, making medical treatments of symptoms less readily absorbed and therefore less effective [24]. In these studies, there was a steady improvement in patient-reported constipation scales at different follow-up points. This may be related to the severity of constipation and baseline flora. The decreased transit time in those with more severe disease may lead to the FMT being able to be retained in the colon for more time, allowing those with a slower transit time to have more FMT exposure time. Notably, there have also been genetic correlates between PD and autoimmune and inflammatory diseases such as Crohn’s disease [21]. It has been observed in other FMT studies that recipients’ microbiomes often return to baseline sometime after receiving FMT [25]. Whether this is due to the resumption of a regular diet, competition with more established flora, or the lifespan of the FMT is unknown. This return to baseline may explain why those with a shorter disease duration and less chronically established flora did not report as much improvement as those with severe dysbiosis and a more pro-inflammatory environment. Therefore, in future studies, providing more timed and spaced-out doses over longer periods may lead to significant and prolonged symptomatic improvements. In Xue’s [18] study, the patients’ reported satisfaction and length of satisfaction appeared to be dependent on the FMT delivery method: a colonoscopy versus a naso-intestinal tube. The more alkaline nature of the jejunum may prevent effective bacterial colonization or decrease longevity compared to the large intestine; thus, colonic sampling may be indicated to identify the currently unknown strains of bacteria that are beneficial for treating PD symptoms [26] as well as the environment in which they thrive. Further randomized controlled trials or comparative analyses are needed to evaluate the efficacy of FMT based on the delivery method.
4.3. Safety
The use of FMT has been established as safe overall, with adverse effects generally being mild and self-resolving; it has even been used in immune-compromised groups [26]. In a meta-analysis of 1149 patients by Green and colleagues [27], FMT was found to be well tolerated and safe. The reported rates of adverse events (AEs) were similar between the FMT intervention and control groups. While its safety and efficacy have been well demonstrated, there are limitations to the use of FMT for the treatment of PD. In regards to its safety profile, there have been no deaths reported in any FMT recipients in the studies that were reviewed, and the single serious adverse event (SAE) was reported as such due to need for hospitalization. This patient had recurrent episodic vasovagal syncope 24 h after FMT that lasted for 8 h, which required hospital observation but was ultimately self-resolving. Most adverse events were mild, including abdominal pain, nausea, and flatulence, as shown in Table 5. While having an overall low-risk profile, we would recommend the continuation of the disease screening of donors.

Table 5.
A summary of adverse events.
4.4. Limitations
There is no standardized treatment for Parkinson’s disease. The limitations of this review are mainly due to the small amount of data available on the use of FMT in this population. In the included studies, the patients’ baseline symptoms were not measured while off of home PD medications, nor were the medication regimens of patients controlled or standardized. Due to PD’s multisystem impairment, treatments are tailored to the individual. Thus, the regimens of PD patients can be complex, have potential medication interactions, and create confounding variables. Although carbidopa-levodopa is the primary therapy and is effective against bradykinesia, it does not always control gait, speech, or postural reflex impairments [28,29,30]. While many patients in this study were treated with carbidopa-levodopa, the dosage as well as time on this medication were not reported and thus may confound their baseline neurologic complaints or lead to more reported symptomatology. In this study, the included patients had diseases with varying times of onset and progressive courses. Without controlling for the onset of disease, the chronicity of symptoms may contribute to confounding reports of symptoms relief. Due to its lack of standardization, the FMT delivery method as well as the dose in the included studies varied. As noted in the discussion section, the location of FMT delivery may alter the colonization response via differing transit times as well as differing environmental pHs. Thus, the differing delivery methods, locations, non-standardized microbiota amounts, and the lack of protocolled pre- and post-FMT treatments limit this study and offer routes for future studies.
5. Conclusions
In summation, FMT is a promising tool for the treatment for motor and non-motor symptoms related to PD. Due to being its infancy in regards to its utility in treating PD, FMT may be difficult for PD patients to access or find insurance coverage for and requires further investigations to determine its validity, treatment dosage, and scheduling. Because of its novelty, evidence for the use of FMT in PD patients is scarce. However, as it lacks drug–drug interactions, has shown significant improvements in patient-reported symptoms, and might be able to increase absorption of other symptomatic treatments, it warrants further investigation, as the limited available data do show promise.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/applmicrobiol3030067/s1, File S1: Search Terms Utilized, Table S1: Average quality assessment score for each study, Table S2: Excluded studies.
Author Contributions
Conceptualization, T.V., T.L. and A.S.H.; methodology, T.V. and A.S.H.; data retrieval, T.V. and T.L.; writing—original draft preparation, T.V.; writing—review and editing, K.M.T. and A.S.H.; supervision, A.S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data supporting the statements can be found on PubMed, Cochrane, and Google Scholar.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Armstrong, M.J.; Okun, M.S. Diagnosis and treatment of Parkinson disease: A review. Jama 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-M.; Huang, H.-L.; Zhou, Y.-L.; Zhao, H.-L.; Xu, J.; Shou, D.-W.; Liu, Y.-D.; Zhou, Y.-J.; Nie, Y.-Q. Fecal Microbiota Transplantation: A New Therapeutic Attempt from the Gut to the Brain. Gastroenterol. Res. Pract. 2021, 2021, 6699268. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.W.; Gao, L.; Stastka, P.; Cheney, M.C.; Mahabamunuge, J.; Soto, M.T.; Ford, C.B.; Bryant, J.A.; Henn, M.R.; Hohmann, E.L. Fecal microbiota transplantation for the improvement of metabolism in obesity: The FMT-TRIM double-blind placebo-controlled pilot trial. PLoS Med. 2020, 17, e1003051. [Google Scholar] [CrossRef] [PubMed]
- Scheperjans, F.; Aho, V.; Pereira, P.A.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 2015, 30, 350–358. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Green, S.J.; Engen, P.A.; Voigt, R.M.; Naqib, A.; Forsyth, C.B.; Mutlu, E.; Shannon, K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015, 30, 1351–1360. [Google Scholar] [CrossRef]
- American College of Cardiology. American Heart Association Task Force on Practice Guidelines, Obesity Expert Panel, 2013 Expert panel report: Guidelines (2013) for the management of overweight and obesity in adults. Obesity 2014, 22 (Suppl. S2), S41–S410. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Di Leo, G.; Sardanelli, F. Statistical significance: P value, 0.05 threshold, and applications to radiomics—Reasons for a conservative approach. Eur. Radiol. Exp. 2020, 4, 18. [Google Scholar] [CrossRef]
- Rodriguez-Blazquez, C.; Rojo-Abuin, J.M.; Alvarez-Sanchez, M.; Arakaki, T.; Bergareche-Yarza, A.; Chade, A.; Garretto, N.; Gershanik, O.; Kurtis, M.M.; Martinez-Castrillo, J.C.; et al. The MDS-UPDRS Part II (motor experiences of daily living) resulted useful for assessment of disability in Parkinson’s disease. Park. Relat. Disord. 2013, 19, 889–893. [Google Scholar] [CrossRef]
- Clarke, C.E.; Patel, S.; Ives, N.; Rick, C.E.; Woolley, R.; Wheatley, K.; Walker, M.F.; Zhu, S.; Kandiyali, R.; Yao, G.; et al. Clinical Effectiveness and Cost-Effectiveness of Physiotherapy and Occupational Therapy versus no Therapy in Mild to Moderate Parkinson’s Disease: A Large Pragmatic Randomised Controlled Trial (PD REHAB); NIHR Journals Library: Southampton, UK, 2016; Health Technology Assessment, No. 20.63. Appendix 8, Hoehn and Yahr Stages. Available online: https://www.ncbi.nlm.nih.gov/books/NBK379751/ (accessed on 1 April 2023).
- Martinez-Martin, P.; Chaudhuri, K.R.; Rojo-Abuin, J.M.; Rodriguez-Blazquez, C.; Alvarez-Sanchez, M.; Arakaki, T.; Bergareche-Yarza, A.; Chade, A.; Garretto, N.; Gershanik, O.; et al. Assessing the non-motor symptoms of Parkinson’s disease: MDS-UPDRS and NMS Scale. Eur. J. Neurol. 2015, 22, 37–43. [Google Scholar] [CrossRef]
- Nikjooy, A.; Jafari, H.; Saba, M.A.; Ebrahimi, N.; Mirzaei, R. Patient Assessment of Constipation Quality of Life Questionnaire: Translation, Cultural Adaptation, Reliability, and Validity of the Persian Version. Iran. J. Med. Sci. 2018, 43, 261–268. [Google Scholar]
- Frattini, J.C.; Nogueras, J.J. Slow Transit Constipation: A Review of a Colonic Functional Disorder. Clin. Colon Rectal Surg. 2008, 21, 146–152. [Google Scholar] [CrossRef]
- Chumpitazi, B.P.; Self, M.M.; Czyzewski, D.I.; Cejka, S.; Swank, P.R.; Shulman, R.J. Bristol Stool Form Scale Reliability and Agreement Decreases When Determining Rome III Stool Form Designations. Neurogastroenterol. Motil. 2016, 28, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Kuai, X.-Y.; Yao, X.-H.; Xu, L.-J.; Zhou, Y.-Q.; Zhang, L.-P.; Liu, Y.; Pei, S.-F.; Zhou, C.-L. Evaluation of fecal microbiota transplantation in Parkinson’s disease patients with constipation. Microb. Cell Factories 2021, 20, 98. [Google Scholar] [CrossRef] [PubMed]
- Segal, A.; Zlotnik, Y.; Moyal-Atias, K.; Abuhasira, R.; Ifergane, G. Fecal microbiota transplant as a potential treatment for Parkinson’s disease—A case series. Clin. Neurol. Neurosurg. 2021, 207, 106791. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.-J.; Yang, X.-Z.; Tong, Q.; Shen, P.; Ma, S.-J.; Wu, S.-N.; Zheng, J.-L.; Wang, H.-G. Fecal microbiota transplantation therapy for Parkinson’s disease: A preliminary study. Medicine 2020, 99, e22035. [Google Scholar] [CrossRef]
- Touch, S.; Godefroy, E.; Rolhion, N.; Danne, C.; Oeuvray, C.; Straube, M.; Galbert, C.; Brot, L.; Salgueiro, I.A.; Chadi, S.; et al. Human CD4+CD8α+ Tregs induced by Faecalibacterium prausnitzii protect against intestinal inflammation. JCI Insight. 2022, 7, e154722. [Google Scholar] [CrossRef]
- DuPont, H.L.; Suescun, J.; Jiang, Z.-D.; Brown, E.L.; Essigmann, H.T.; Alexander, A.S.; DuPont, A.W.; Iqbal, T.; Utay, N.S.; Newmark, M.; et al. Fecal microbiota transplantation in Parkinson’s disease—A randomized repeat-dose, placebo-controlled clinical pilot study. Front. Neurol. 2023, 14, 1104759. [Google Scholar] [CrossRef]
- Warnecke, T.; Schäfer, K.-H.; Claus, I.; Del Tredici, K.; Jost, W.H. Gastrointestinal involvement in Parkinson’s disease: Pathophysiology, diagnosis, and management. npj Park. Dis. 2022, 8, 31. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K. Neuropathological Staging of Brain Pathology in Sporadic Parkinson’s disease: Separating the Wheat from the Chaff. J. Park. Dis. 2017, 7 (Suppl. S1), S71–S85. [Google Scholar] [CrossRef] [PubMed]
- Reichmann, H. Premotor Diagnosis of Parkinson’s Disease. Neurosci. Bull. 2017, 33, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.K.; Wall, E.S.; Robinson, C.D.; Guillemin, K.; Eisen, J.S. Enteric nervous system modulation of luminal pH modifies the microbial environment to promote intestinal health. PLoS Pathog. 2022, 18, e1009989. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.R.; Ihunnah, C.; Fischer, M.; Khoruts, A.; Surawicz, C.; Afzali, A.; Aroniadis, O.; Barto, A.; Borody, T.; Giovanelli, A.; et al. Fecal Microbiota Transplant for Treatment of Clostridium difficile Infection in Immunocompromised Patients. Am. J. Gastroenterol. 2014, 109, 1065–1071. [Google Scholar] [CrossRef]
- Green, J.E.; Davis, J.A.; Berk, M.; Hair, C.; Loughman, A.; Castle, D.; Athan, E.; Nierenberg, A.A.; Cryan, J.F.; Jacka, F.; et al. Efficacy and safety of fecal microbiota transplantation for the treatment of diseases other than Clostridium difficile infection: A systematic review and meta-analysis. Gut Microbes 2020, 12, 1–25. [Google Scholar] [CrossRef]
- Miyasaki, J.M.; Martin, W.; Suchowersky, O.; Weiner, W.J.; Lang, A.E. Practice parameter: Initiation of treatment for Parkinson’s disease: An evidence-based review: Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2002, 58, 11–17. [Google Scholar] [CrossRef]
- Goetz, C.G.; Poewe, W.; Rascol, O.; Sampaio, C. Evidence-based medical review update: Pharmacological and surgical treatments of Parkinson’s disease: 2001 to 2004. Mov. Disord. 2005, 20, 523–539. [Google Scholar] [CrossRef]
- Ooijevaar, R.E.; Terveer, E.M.; Verspaget, H.W.; Kuijper, E.J.; Keller, J.J. Clinical Application and Potential of Fecal Microbiota Transplantation. Annu. Rev. Med. 2019, 70, 335–351. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).