Microalgae, in Spatial Assessment of the Drainage Basin, Influences on the Ecosystem of Lake Agmon, Israel
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of Study Area
2.2. Collecting of Chemical Data
2.3. Sampling and Analysing of Biological Data
3. Results
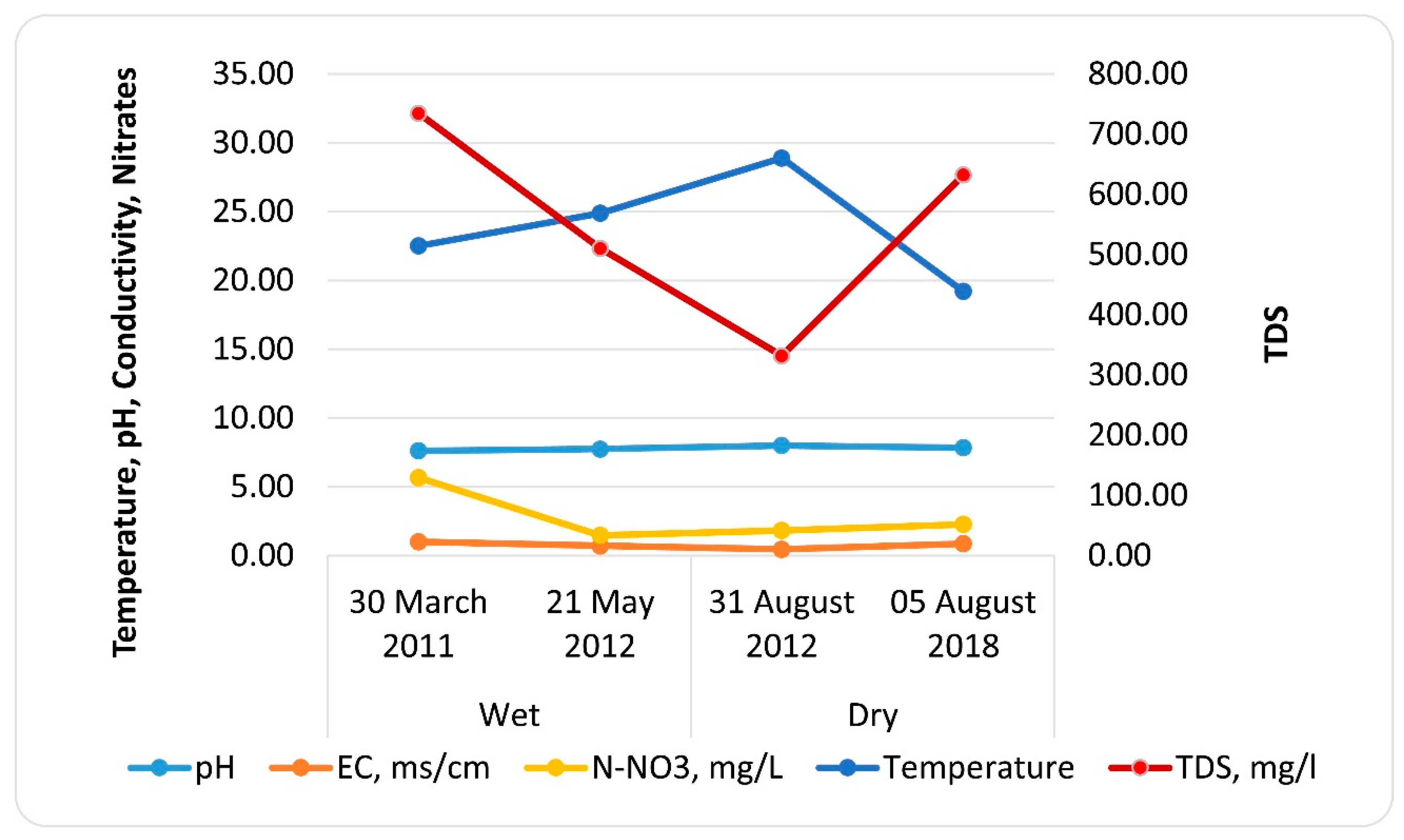
3.1. Chemical Variables
3.2. Microalgae Data
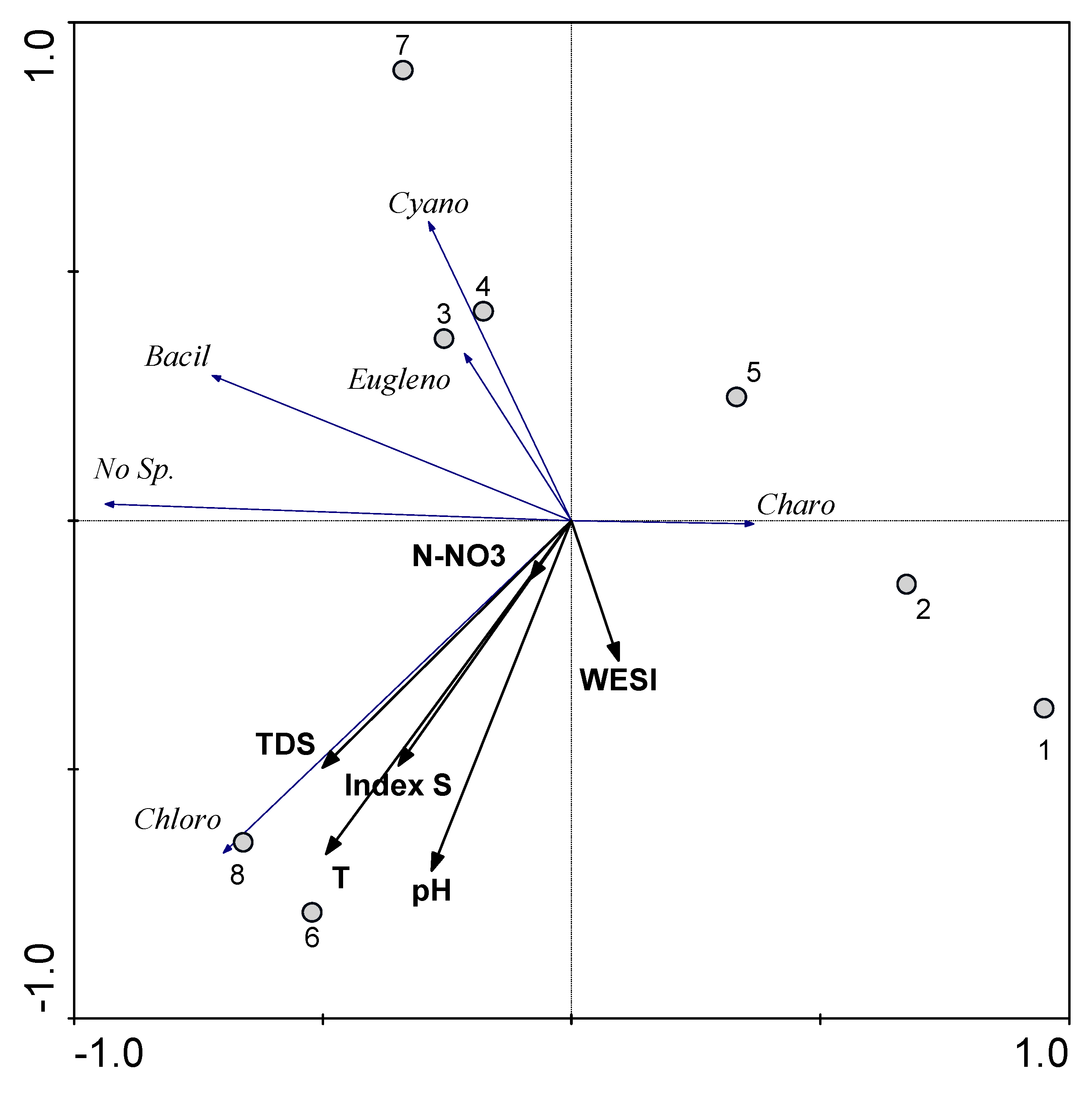
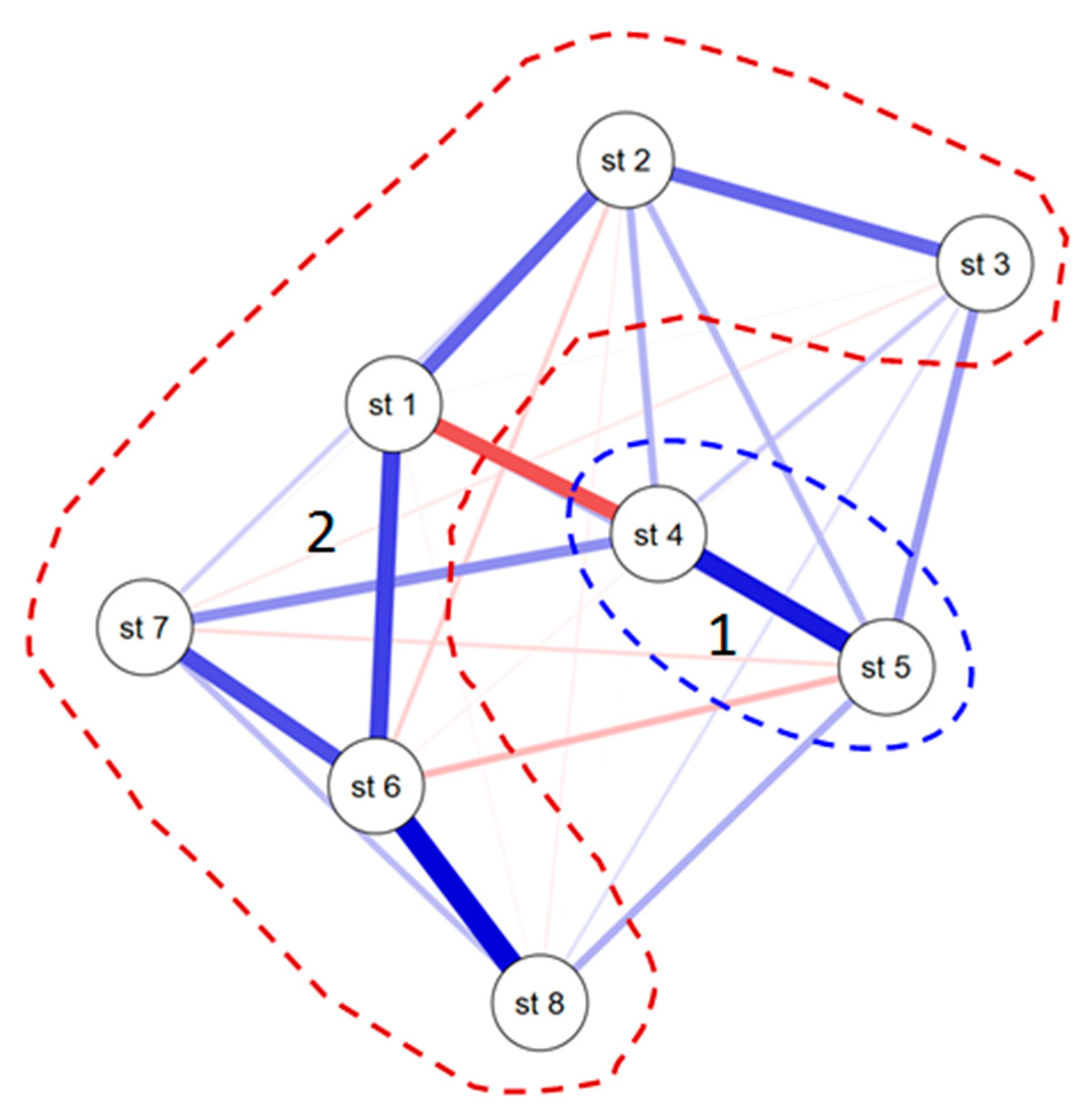
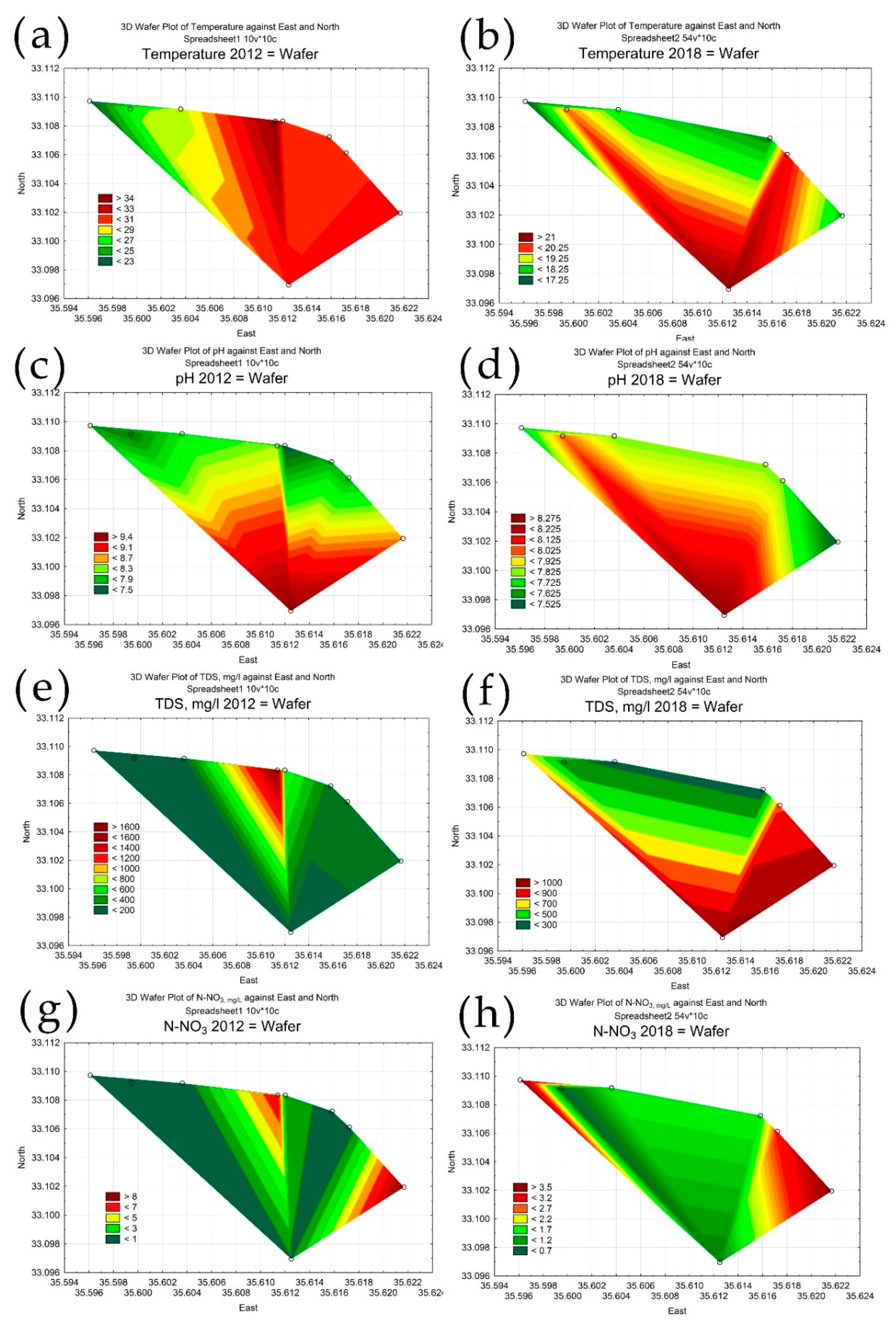
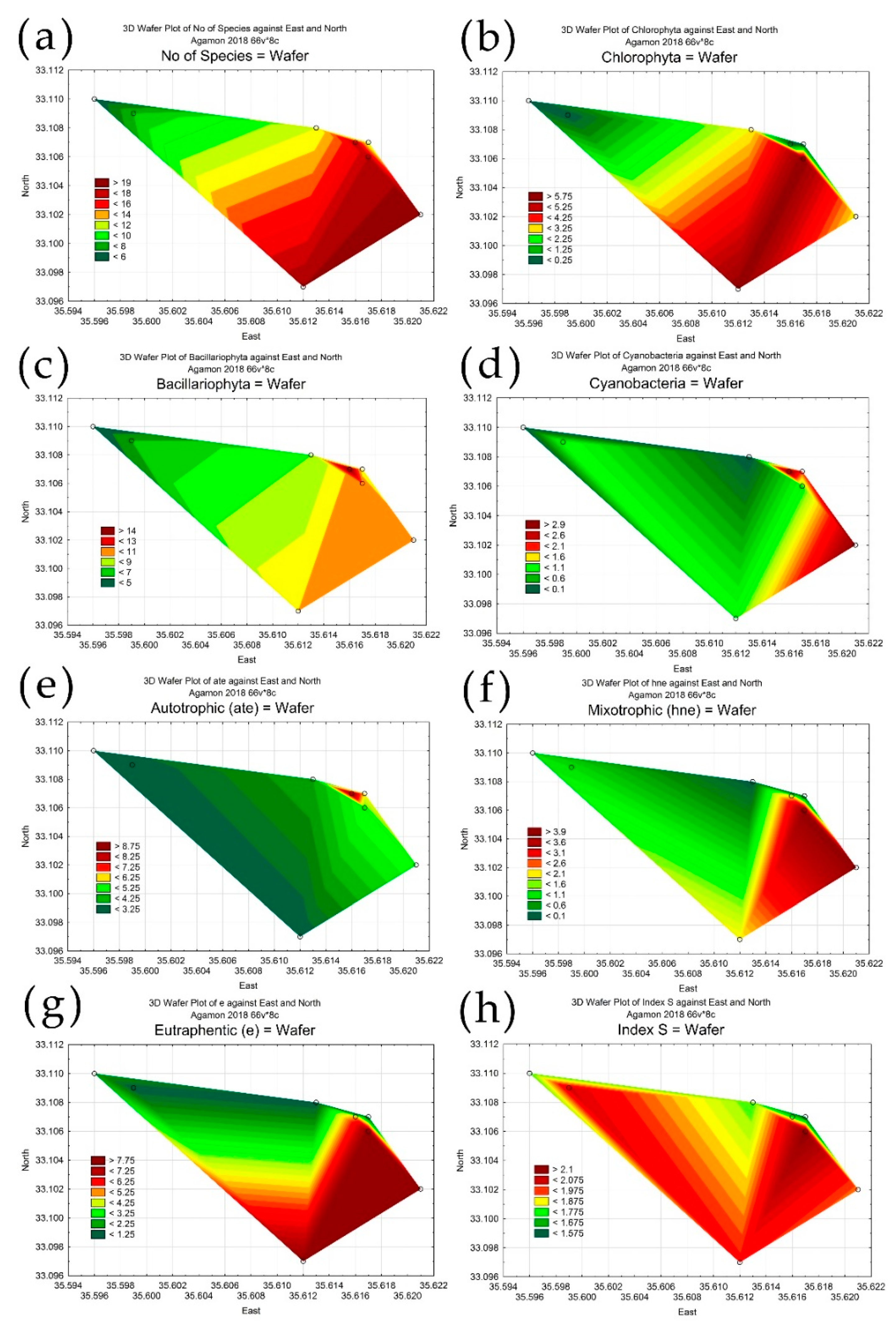
3.3. Statistical Mapping
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Taxa | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Hab | Tem | OXY | D | S | SAP | HAL | pH | AUT-HET | TRO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacillariophyta | ||||||||||||||||||
| Achnanthidium kranzii (Lange-Bertalot) Round & Bukhtiyarova 1996 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Achnanthidium minutissimum (Kützing) Czarnecki 1994 | 0 | 1 | 0 | 4 | 4 | 3 | 1 | 3 | P-B | eterm | st-str | es | 0.95 | b-m | i | ind | ate | e |
| Amphora ovalis (Kützing) Kützing 1844 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | B | temp | st-str | sx | 1.5 | b | i | alf | ate | e |
| Aulacoseira granulata (Ehrenberg) Simonsen 1979 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | P-B | temp | st-str | es | 2.0 | b | i | alf | ate | e |
| Cocconeis pediculus Ehrenberg 1838 | 0 | 0 | 2 | 2 | 2 | 1 | 1 | 0 | B | temp | st-str | sx | 1.8 | a-b | i | alf | ate | me |
| Cocconeis placentula Ehrenberg 1838 | 0 | 1 | 2 | 0 | 0 | 1 | 0 | 0 | P-B | temp | st-str | es | 1.35 | o | i | alf | ate | me |
| Craticula cuspidata (Kutzing) D.G.Mann 1990 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | B | temp | st-str | es | 2.45 | a | i | alf | - | me |
| Craticula halophila (Grunow) D.G.Mann 1990 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | B | temp | st-str | es | 3.0 | a | mh | alf | ate | e |
| Cyclotella meneghiniana Kützing 1844 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 4 | P-B | temp | st-str | sp | 2.8 | a | hl | alf | hne | e |
| Decussiphycus placenta (Ehrenberg) Guiry & Gandhi 2019 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | P-B,aer | cool | st | sx | 0.4 | x-o | i | alf | ats | ot |
| Diatoma vulgaris Bory 1824 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | P-B | temp | st-str | sx | 2.2 | b | i | alf | ate | me |
| Encyonema minutum (Hilse) D.G.Mann 1990 | 0 | 0 | 0 | 6 | 6 | 0 | 0 | 2 | B | temp | st-str | sx | 1.2 | o | i | ind | ate | m |
| Encyonema muelleri (Hustedt) D.G.Mann 1990 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | B | temp | st-str | sx | 1.2 | o | i | ind | ate | o-e |
| Encyonema silesiacum (Bleisch) D.G.Mann 1990 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | B | temp | st | es | - | - | i | alb | hne | - |
| Eunotia pectinalis (Kützing) Rabenhorst 1864 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | - | st-str | sx | 0.3 | x | i | acf | ate | m |
| Gomphonella angustata Rabenhorst 1853 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | B | temp | st-str | es | 1.3 | o | i | ind | ats | om |
| Gomphonella olivacea (Hornemann) Rabenhorst 1853 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | B | - | str | - | 1.2 | o | i | ind | ats | m |
| Gomphonella subtile Ehrenberg, 1838 | 0 | 0 | 3 | 0 | 2 | 0 | 2 | 0 | B | - | st-str | es | 1.0 | o | i | ind | ats | om |
| Gomphonema parvulum (Kützing) Kützing 1849 | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 5 | B | temp | st-str | es | 2.35 | b | i | ind | hne | om |
| Gyrosigma kuetzingii (Grunow) Cleve 1894 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | - | - | - | - | - | - | - | - | - | e |
| Melosira varians C.Agardh 1827 | 0 | 0 | 0 | 2 | 0 | 0 | 3 | 0 | P-B | temp | st-str | es | 2.1 | b | hl | ind | hne | me |
| Navicula cryptocephala Kützing 1844 | 1 | 1 | 4 | 3 | 5 | 1 | 1 | 2 | P-B | temp | st-str | es | 2.1 | b | i | ind | ate | o-e |
| Navicula salinarum Grunow 1880 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | P-B | - | st-str | - | 2.1 | b | mh | ind | ate | me |
| Navicula vulpina Kützing 1844 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | B | temp | st-str | - | 2.0 | b | i | ind | ats | me |
| Nitzschia flexa Schumann 1862 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | - | 1.8 | o-a | oh | alf | - | - |
| Nitzschia palea (Kützing) W.Smith 1856 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | P-B | temp | st-str | sp | 2.8 | a-o | i | ind | hce | he |
| Nitzschia paleacea (Grunow) Grunow 1881 | 0 | 0 | 4 | 0 | 0 | 0 | 2 | 0 | P-B | temp | st-str | es | 2.2 | b | i | alf | hce | e |
| Nitzschia recta Hantzsch ex Rabenhorst 1862 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | B | temp | st-str | es | 1.5 | o-b | i | alf | ate | om |
| Nitzschia subacicularis Hustedt 1922 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | B | temp | st-str | es | 2.0 | b | i | alf | ats | o-e |
| Nitzschia tropica Hustedt 1949 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | P-B | temp | - | - | - | - | i | alf | - | - |
| Nupela impexiformis (Lange-Bertalot) Lange-Bertalot 1999 | 0 | 0 | 0 | 0 | 6 | 0 | 1 | 0 | B | - | - | es | - | - | - | ind | - | - |
| Paraplaconeis placentula (Ehrenberg) Kulikovskiy & Lange-Bertalot 2012 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | B | temp | st-str | sx | 1.5 | o-b | i | alf | ate | e |
| Prestauroneis crucicula (W.Smith) Genkal & Yarushina 2017 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | - | - | - | - | - | - | - | - |
| Sellaphora arvensis (Hustedt) C.E.Wetzel & L.Ector 2015 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | B | eterm | st-str | sx | 1.9 | o-a | hl | ind | ate | me |
| Sellaphora pupula (Kützing) Mereschkovsky 1902 | 1 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | B | - | - | es | - | - | hl | acf | hne | - |
| Staurosira construens Ehrenberg 1843 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | P-B | temp | st-str | sx | 1.3 | o | i | alf | ats | me |
| Staurosira subsalina (Hustedt) Lange-Bertalot 2004 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | P-B | - | st-str | es | 1.3 | o | hl | alf | ate | me |
| Staurosirella pinnata (Ehrenberg) D.M.Williams & Round 1988 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | P-B | temp | st-str | es | 1.2 | o | hl | alf | ate | o-e |
| Surirella librile (Ehrenberg) Ehrenberg 1845 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | P-B | temp | st-str | - | 2.1 | b | i | alf | ate | e |
| Charophyta | ||||||||||||||||||
| Closterium moniliferum Ehrenberg ex Ralfs 1848 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | P-B | - | st-str | - | 2.1 | b | i | ind | - | me |
| Spirogyra sp. | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | - | - | - | - | - | - | alf | - | e |
| Chlorophyta | ||||||||||||||||||
| Ankistrodesmus falcatus (Corda) Ralfs 1848 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | P-B | - | st-str | - | 2.3 | b | hb | - | - | e |
| Carteria fritschii H.Takeda 1916 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Characium pringsheimii A.Braun 1855 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | B | - | - | - | - | - | - | - | - | - |
| Desmodesmus communis (E.Hegewald) E.Hegewald 2000 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 3 | P-B | - | st | - | 2.0 | o-a | - | - | - | e |
| Monoraphidium convolutum (Corda) Komárková-Legnerová 1969 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | P-B | - | st-str | - | 2.3 | b | - | - | - | e |
| Oedogonium sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | B | - | - | - | - | - | - | alb | - | - |
| Pseudagloë polychloris (Pascher) K.I.Meyer 1952 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | P | - | - | - | - | - | - | - | - | e |
| Tetraëdron minimum (A.Braun) Hansgirg 1889 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 2 | P-B | - | st-str | - | 2.1 | b | i | alf | - | e |
| Tetraëdron trigonum (Nägeli) Hansgirg 1888 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | P | - | - | - | 2.0 | b | - | - | - | e |
| Cyanobacteria | ||||||||||||||||||
| Calothrix elenkinii Kossinskaja 1924 | 0 | 0 | 0 | 0 | 2 | 0 | 5 | 0 | - | - | - | - | - | - | - | - | - | - |
| Chroococcus minutus (Kützing) Nägeli 1849 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | P-B | - | - | - | 1.8 | o-a | i | ind | - | e |
| Leptolyngbya tenuis (Gomont) Anagnostidis & Komárek 1988 | 0 | 0 | 0 | 3 | 2 | 0 | 6 | 2 | B,S | - | st-str | - | 2.4 | b-a | i | alf | - | - |
| Merismopedia minima G.Beck 1897 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | B,S | - | aer | - | - | - | - | - | - | ot |
| Tychonema decoloratum (G.S.West) Anagnostidis & Komárek 1988 | 0 | 6 | 0 | 3 | 0 | 0 | 6 | 0 | - | - | - | - | - | - | - | - | - | - |
| Euglanozoa | ||||||||||||||||||
| Trachelomonas pusilla Playfair 1915 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | - | - | - | - | 2.3 | b | - | - | - | - |
| Euglenaformis proxima (P.A.Dangeard) M.S.Bennett & Triemer 2014 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | P-B | eterm | st-str | - | 3.5 | p-a | mh | ind | - | e |
| Phacus pusillus Lemmermann 1910 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | P-B | - | st-str | - | 2.5 | b-a | - | acf | - | - |
| Miozoa | ||||||||||||||||||
| Gymnodinium inversum Nygaard 1929 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 1 | P | - | - | - | 1.6 | b-o | - | - | - | - |
References
- Hula-Agmon Natural Park Authority. Available online: http://agamon-hula.co.il/ (accessed on 24 December 2021).
- Rayss, T.; Katchalsky, E. On the plankton in Lake Hula. Hatevah Ve’Haaretz 1938, 5, 483–490. (In Hebrew) [Google Scholar]
- Hambright, K.D.; Zohary, T. Lakes Hula and Agmon: Destruction and creation of wetland ecosystems in northern Israel. Wetl. Ecol. Manag. 1998, 6, 83–89. [Google Scholar] [CrossRef]
- Hambright, K.D.; Zohary, T. The Hula Valley (Northern Israel) Wetlands Rehabilitation Project. In An International Perspective on Wetland Rehabilitation; Streever, W., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 73–180. [Google Scholar]
- Pollingher, U.; Zohary, T.; Fishbein, T. Algal flora in the Hula valley—Past and present. Isr. J. Plant Sci. 1998, 46, 155–168. [Google Scholar] [CrossRef]
- Barinova, S.; Alster, A. Algae and Cyanobacteria Diversity and Bioindication of Long-Term Changes in the Hula Nature Reserve, Israel. Diversity 2021, 13, 583. [Google Scholar] [CrossRef]
- Freshwater Ecoregions of the World, (FEOW). Available online: https://www.feow.org/ (accessed on 21 September 2021).
- Payne, R.J. A longer-term perspective on human exploitation and management of peat wetlands: The Hula Valley, Israel. Mires Peat. 2012, 4, 1–9. [Google Scholar]
- Barinova, S. How to Align and Unify the Cell Counting of Organisms for Bioindication. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 555–585. [Google Scholar] [CrossRef]
- Desikachary, T. Cyanophyta; Pyarelal Sah at the Times of India Press: Bombay, India, 1959; 686p. [Google Scholar]
- Dillard, G. Freshwater algae of the southeastern United States, part 1, Chlorophyceae: Volvocales, Tetrasporales and Chlorococcales. In Bibliotheca Phycologica Band 81; J. Cramer: Stuttgart, Germany, 1989. [Google Scholar]
- Forest, H. Handbook of Algae; Univ. Tenn. Press: Knoxville, TN, USA, 1954; 467p. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota, 1. Teil, Chroococcales. In Süsswasserflora von mitteleuropa, Band 19/1; Ettl, H., Gärtner, G., Heynig, H., Mollenhauer, E., Eds.; Gustav Fisher: Jena, Germany, 1999; 548p. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota, 2. Teil, Oscillatoriales. In Süßwasserflora von mitteleuropa, Band 19/2; Büdel, B., Krienitz, L., Gärtner, G., Schagerl, M., Eds.; Spektrum Akademischer Verlag, Elsevier GmbH: München, Germany, 2005; 759p. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae, Teil 1, Naviculaceae. In Süsswasserflora von Mitteleuropa, Band 2/1; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fisher: Jena, Germany, 1986; 876p. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae, Teil 2, Bacillariaceae, Epithemiaceae, Surirellaceae. In Süsswasserflora von Mitteleuropa, Band 2/2; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fisher: Jena, Germany, 1988; 596p. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae, Teil 3, Centrales, Fragilariaceae, Eunotiaceae. In Süsswasserflora von Mitteleuropa, Band 2/3; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fisher: Jena, Germany, 1991; 598p. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae, Teil 4, Achnantaceae, Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema. In Süsswasserflora von Mitteleuropa, Band 2/4; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fisher: Jena, Germany, 1991; 468p. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae, Teil 5, English and French translation of the keys. In Süsswasserflora von Mitteleuropa, Band 2/5; Büdel, B., Gärtner, G., Krienitz, L., Lokhorst, G., Eds.; Spektrum Akademischer Verlag: Heidelberg-Berlin, Germany, 2000; 311p. [Google Scholar]
- Prescott, G. Algae of the Western Great Lakes Area; WM. C. Brown Co. Publishers: Dubuque, IA, USA, 1962; 977p. [Google Scholar]
- Whitford, L.; Schumacher, G. A Manual of Freshwater Algae; Sparks Pr Inc.: San Francisco, CA, USA, 1984; 324p. [Google Scholar]
- John, D.M.; Whitton, B.A.; Brook, A.J. (Eds.) The Freshwater Algal Flora of the British Isles: An Identification Guide to Freshwater and Terrestrial Alga; Cambridge University Press: Cambridge, UK, 2002; 702p. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase World-Wide Electronic Publication. National University of Ireland, Galway. Available online: http://www.algaebase.org (accessed on 24 June 2019).
- Sládeček, V. Diatoms as indicators of organic pollution. Acta Hydroch. Hydrobiol. 1986, 14, 555–566. [Google Scholar] [CrossRef]
- Love, J.; Selker, R.; Marsman, M.; Jamil, T.; Dropmann, D.; Verhagen, J.A.; Ly, A.; Gronau, F.Q.; Smira, M.; Epskamp, S.; et al. JASP: Graphical statistical software for common statistical designs. J. Stat. Softw. 2019, 88, 1–17. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power Press: Ithaca, NY, USA, 2002; 500p. [Google Scholar]
- Novakovsky, A.B. Abilities and base principles of program module “GRAPHS”. Sci. Rep. Komi Sci. Cent. Ural. Div. Russ. Acad. Sci. 2004, 27, 1–28. [Google Scholar]
- Barinova, S.S.; Medvedeva, L.A.; Anissimova, O.V. Diversity of Algal Indicators in Environmental Assessment; Pilies Studio Publisher: Tel Aviv, Israel, 2006; 498p. (In Russian) [Google Scholar]
- Barinova, S.S.; Bilous, O.P.; Tsarenko, P.M. Algal Indication of Water Bodies in Ukraine: Methods and Prospects; Publishing House of Haifa University: Haifa, Kyiv, Israel, 2019; 367p. (In Russian) [Google Scholar]
- Barinova, S. Essential and practical bioindication methods and systems for the water quality assessment. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 1–11. [Google Scholar] [CrossRef]
- Barinova, S. Ecological mapping in application to aquatic ecosystems bioindication: Problems and methods. Int. J. Environ. Sci. Nat. Resour. 2017, 3, 1–7. [Google Scholar] [CrossRef]
- Barinova, S. On the Classification of Water Quality from an Ecological Point of View. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 1–8. [Google Scholar] [CrossRef]
- Barinova, S.; Krassilov, V.A. Algal diversity and bio-indication of water resources in Israel. Int. J. Environ. Resour. 2012, 1, 62–72. [Google Scholar]
- Dokulil, M.T. Algae as ecological bio-indicators. In Bioindicators and Biomonitors. Principles, Concepts and Applications, Chapter 9; Market, B.A., Breure, A.M., Zechmeistter, H.G., Eds.; Elsevier: Oxford, UK, 2003; pp. 285–327. [Google Scholar] [CrossRef]
- Barinova, S.; Bilous, O.; Ivanova, N. New Statistical Approach to Spatial Analysis of Ecosystem of the Sasyk Reservoir, Ukraine. Int. J. Ecotoxicol. Ecobiol. 2016, 1, 118–126. [Google Scholar] [CrossRef]
- Barinova, S.; Romanov, R. How a new locality of algal community in the Negev Desert, Israel was formed. Expert Opin. Environ. Biol. 2015, 4, 1–7. [Google Scholar] [CrossRef]
- Barinova, S.; Romanov, R. Unique charophytes locality in the Borot Loz Natural Reserve, Negev Desert, Israel. Discov. Nat. 2015, 9, 33–41. [Google Scholar]
- Barinova, S.; Romanov, R. The Ein El Balad Charophyte Locality in the Mount Carmel Biosphere Reserve, Israel. Int. J. Adv. Res. Bot. 2015, 1, 1–12. [Google Scholar]
- Barinova, S.; Romanov, R. Charophyte community in the lowermost locality in the world near the Dead Sea, Israel. Int. J. Plant Soil Sci. 2015, 6, 229–243. [Google Scholar] [CrossRef]
- Barinova, S.; Romanov, R. Charophyte Communities in the Ein Afeq Natural Reserve, Israel. Nat. Resour. Conserv. 2015, 3, 31–44. [Google Scholar] [CrossRef]
- Barinova, S.; Romanov, R. The ancient locality Syndianna with charophytes in the northern Israel. Nat. Resour. Conserv. 2016, 4, 1–14. [Google Scholar] [CrossRef][Green Version]
- Krupa, E.; Barinova, S.; Aubakirova, M. Tracking pollution and its sources in the catchment-lake system of major waterbodies in Kazakhstan. Lakes Reserv. Res. Manag. 2020, 25, 18–30. [Google Scholar] [CrossRef]
- Lutzu, G.A.; Dunford, N.T. Interactions of microalgae and other microorganisms for enhanced production of high-value compounds. Front. Biosci. 2018, 23, 1487–1504. [Google Scholar] [CrossRef] [PubMed]
- Bănăduc, D.; Joy, M.; Olosutean, H.; Afanasyev, S.; Curtean-Bănăduc, A. Natural and anthropogenic driving forces as key elements in the Lower Danube Basin–South-Eastern Carpathians–North-Western Black Sea coast area lakes: A broken stepping stones for fish in a climatic change scenario? Environ. Sci. Eur. 2020, 32, 73. [Google Scholar] [CrossRef]
- Bănăduc, D.; Sas, A.; Cianfaglione, K.; Barinova, S.; Curtean-Bănăduc, A. The Role of Aquatic Refuge Habitats for Fish, and Threats in the Context of Climate Change and Human Impact, during Seasonal Hydrological Drought in the Saxon Villages Area (Transylvania, Romania). Atmosphere 2021, 12, 1209. [Google Scholar] [CrossRef]
- Qin, B.Q.; Gao, G.; Zhu, G.W.; Zhang, Y.L.; Song, Y.Z.; Tang, X.M.G.; Xu, H.; Deng, J.M. Lake eutrophication and its ecosystem response. Chin. Sci. Bull. 2013, 58, 961–970. [Google Scholar] [CrossRef]
- Muylaert, K.; Pérez-Martínez, C.; Sánchez-Castillo, P.; Lauridsen, T.L.; Vanderstukken, M.; Declerck, S.A.J.; Van der Gucht, K.; Conde-Porcuna, J.M.; Jeppesen, E.; De Meester, L.; et al. Influence of nutrients, submerged macrophytes and zooplankton grazing on phytoplankton biomass and diversity along a latitudinal gradient in Europe. Hydrobiologia 2010, 653, 79–90. [Google Scholar] [CrossRef]



| Score | Visual Estimate | Cell Numbers of Plankton per L | Cell Numbers of Periphyton per Slide (20 × 20 mm) | Cell Number of Each Species, % |
|---|---|---|---|---|
| 1 | Occasional | 1–103 cell L−1 | 1–5 cells per slide | <1 |
| 2 | Rare | 103–104 cell L−1 | 10–15 cells per slide | 2–10 |
| 3 | Common | 104–105 cell L−1 | 25–30 cells per slide | 10–40 |
| 4 | Frequent | 105–107 cell L−1 | 1 cell over a slide transect | 40–60 |
| 5 | Very frequent | 106–107 cell L−1 | Several cells over a slide transect | 60–80 |
| 6 | Abundant | More than 107 cell L−1 | One or more cells in each field of view | 80–100 |
| Date | Season | pH | EC, ms cm−1 | TDS, mg L−1 | N-NO3, mg L−1 | Temperature °C |
|---|---|---|---|---|---|---|
| 30 March 2011 | Wet | 7.61 | 1.01 | 734.75 | 5.67 | 22.50 |
| 21 May 2012 | Wet | 7.74 | 0.71 | 510.40 | 1.47 | 24.88 |
| 31 August 2012 | Dry | 7.99 | 0.46 | 331.95 | 1.83 | 28.89 |
| 5 August 2018 | Dry | 7.84 | 0.87 | 632.38 | 2.28 | 19.20 |
| Station | North | East | Index S | No of Species | pH | Electrical Conductivity ms cm−1 | TDS, mg L−1 | Temperature, °C | N-NO3 mg L−1 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 33.06.36 | 35.35.46 | 1.77 | 5 | 7.7 | 0.92 | 662 | 17.0 | 3.8 |
| 2 | 33.06.33 | 35.35.58 | 2.06 | 8 | 8.0 | 0.45 | 327 | 19.9 | 0.7 |
| 3 | 33.06.29 | 35.36.47 | 1.77 | 12 | 7.8 | 0.36 | 265 | 18.3 | 1.6 |
| 4 | 33.06.26 | 35.36.56 | 1.75 | 18 | 7.8 | 0.36 | 246 | 17.6 | 1.6 |
| 5 | 33.06.24 | 35.37.00 | 1.55 | 11 | 7.7 | 0.40 | 286 | 19.3 | 1.2 |
| 6 | 33.06.22 | 35.37.03 | 2.17 | 20 | 7.9 | 1.74 | 1285 | 21.8 | 4.5 |
| 7 | 33.06.06 | 35.37.17 | 1.94 | 18 | 7.5 | 1.31 | 955 | 18.0 | 3.8 |
| 8 | 33.05.49 | 35.36.44 | 1.99 | 19 | 8.3 | 1.42 | 1033 | 21.7 | 1.0 |
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Phyla | ||||||||
| Bacillariophyta | 4 | 6 | 8 | 15 | 9 | 11 | 11 | 10 |
| Charophyta | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Euglenozoa | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 1 |
| Cyanobacteria | 0 | 1 | 0 | 3 | 2 | 1 | 3 | 1 |
| Chlorophyta | 0 | 0 | 3 | 0 | 0 | 6 | 3 | 6 |
| Miozoa | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Habitat | ||||||||
| B | 2 | 2 | 6 | 8 | 7 | 6 | 6 | 8 |
| P-B | 2 | 4 | 5 | 7 | 3 | 10 | 10 | 8 |
| P | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 2 |
| Temperature | ||||||||
| cool | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| temp | 3 | 4 | 4 | 10 | 6 | 9 | 9 | 8 |
| eterm | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Oxygen | ||||||||
| aer | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| st-str | 3 | 5 | 0 | 13 | 9 | 11 | 11 | 12 |
| st | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 2 |
| Watanabe | ||||||||
| sx | 1 | 0 | 2 | 4 | 5 | 2 | 2 | 3 |
| es | 3 | 4 | 4 | 8 | 4 | 6 | 6 | 5 |
| sp | 0 | 1 | 0 | 0 | 0 | 2 | 2 | 1 |
| Salinity | ||||||||
| hb | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| i | 3 | 6 | 8 | 10 | 9 | 10 | 10 | 11 |
| hl | 1 | 0 | 1 | 3 | 0 | 2 | 2 | 1 |
| mh | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| pH | ||||||||
| acf | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 |
| ind | 2 | 4 | 5 | 6 | 7 | 4 | 4 | 5 |
| alf | 2 | 2 | 4 | 8 | 3 | 6 | 6 | 7 |
| alb | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| Autotrophy-Heterotrophy | ||||||||
| ats | 0 | 0 | 2 | 2 | 2 | 0 | 0 | 3 |
| ate | 3 | 3 | 4 | 9 | 6 | 5 | 5 | 3 |
| hne | 1 | 1 | 0 | 2 | 0 | 4 | 4 | 2 |
| hce | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| Trophy | ||||||||
| ot | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| om | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| m | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 |
| me | 0 | 1 | 4 | 5 | 2 | 2 | 2 | 1 |
| e | 2 | 1 | 1 | 5 | 1 | 8 | 8 | 8 |
| o-e | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 2 |
| he | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barinova, S.; Smith, T.; Tsarenko, P. Microalgae, in Spatial Assessment of the Drainage Basin, Influences on the Ecosystem of Lake Agmon, Israel. Appl. Microbiol. 2022, 2, 197-214. https://doi.org/10.3390/applmicrobiol2010014
Barinova S, Smith T, Tsarenko P. Microalgae, in Spatial Assessment of the Drainage Basin, Influences on the Ecosystem of Lake Agmon, Israel. Applied Microbiology. 2022; 2(1):197-214. https://doi.org/10.3390/applmicrobiol2010014
Chicago/Turabian StyleBarinova, Sophia, Thomas Smith, and Petro Tsarenko. 2022. "Microalgae, in Spatial Assessment of the Drainage Basin, Influences on the Ecosystem of Lake Agmon, Israel" Applied Microbiology 2, no. 1: 197-214. https://doi.org/10.3390/applmicrobiol2010014
APA StyleBarinova, S., Smith, T., & Tsarenko, P. (2022). Microalgae, in Spatial Assessment of the Drainage Basin, Influences on the Ecosystem of Lake Agmon, Israel. Applied Microbiology, 2(1), 197-214. https://doi.org/10.3390/applmicrobiol2010014








