Scale-Up of Dark Fermentative Biohydrogen Production by Artificial Microbial Co-Cultures
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Set-Up
2.3. Optical Density (OD) Measurements and Cell Counting
2.4. Quantification of Gas Composition
2.5. Quantification of Metabolites
2.6. DNA Extraction and qPCR
2.7. Data Analysis
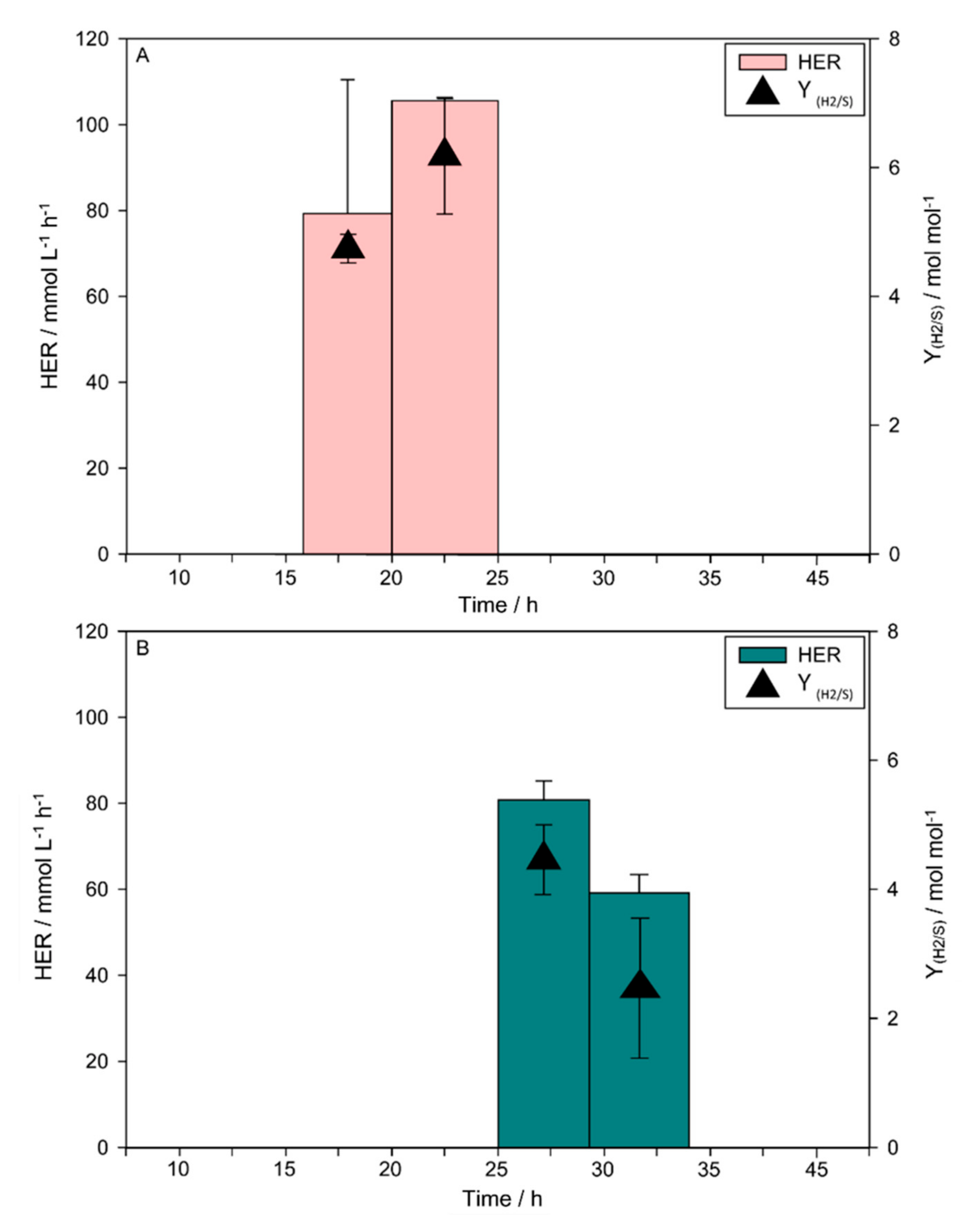
3. Results
3.1. Experimental Set-Up
3.2. H2 Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hay, J.X.W.; Wu, T.Y.; Juan, J.C.; Md. Jahim, J. Biohydrogen Production through Photo Fermentation or Dark Fermentation Using Waste as a Substrate: Overview, Economics, and Future Prospects of Hydrogen Usage. Biofuels Bioprod. Biorefining 2013, 7, 334–352. [Google Scholar] [CrossRef]
- Vinoth Kanna, I.; Paturu, P. A Study of Hydrogen as an Alternative Fuel. Int. J. Ambient Energy 2020, 41, 1433–1436. [Google Scholar] [CrossRef]
- Ergal, İ.; Fuchs, W.; Hasibar, B.; Thallinger, B.; Bochmann, G.; Rittmann, S.K.-M. The Physiology and Biotechnology of Dark Fermentative Biohydrogen Production. Biotechnol. Adv. 2018, 36, 2165–2186. [Google Scholar] [CrossRef]
- Rittmann, S.; Herwig, C. A Comprehensive and Quantitative Review of Dark Fermentative Biohydrogen Production. Microb. Cell Factories 2012, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Thauer, R.K.; Jungermann, K.; Decker, K. Energy Conservation in Chemotrophic Anaerobic Bacteria. Bacteriol. Rev. 1977, 41, 100–180. [Google Scholar] [CrossRef] [PubMed]
- Hallenbeck, P.C. Fermentative Hydrogen Production: Principles, Progress, and Prognosis. Int. J. Hydrogen Energy 2009, 34, 7379–7389. [Google Scholar] [CrossRef]
- Masset, J.; Calusinska, M.; Hamilton, C.; Hiligsmann, S.; Joris, B.; Wilmotte, A.; Thonart, P. Fermentative Hydrogen Production from Glucose and Starch Using Pure Strains and Artificial Co-Cultures Of Clostridium spp. Biotechnol. Biofuels 2012, 5, 35. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Kumar, P.; Mehariya, S.; Purohit, H.J.; Lee, J.-K.; Kalia, V.C. Enhancement in Hydrogen Production by Co-Cultures of Bacillus and Enterobacter. Int. J. Hydrogen Energy 2014, 39, 14663–14668. [Google Scholar] [CrossRef]
- Alshiyab, H.; Kalil, M.S.; Hamid, A.A.; Yusoff, W.M.W. Effect of Salts Addition on Hydrogen Production by C. acetobutylicum. Pak. J. Biol. Sci. 2008, 11, 2193–2200. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yokoi, H.; Ohkawara, T.; Hirose, J.; Hayashi, S.; Takasaki, Y. Characteristics of Hydrogen Production by Aciduric Enterobacter Aerogenes Strain HO-39. J. Ferment. Bioeng. 1995, 80, 571–574. [Google Scholar] [CrossRef]
- Pachapur, V.L.; Kutty, P.; Pachapur, P.; Brar, S.K.; Le Bihan, Y.; Galvez-Cloutier, R.; Buelna, G. Seed Pretreatment for Increased Hydrogen Production Using Mixed-Culture Systems with Advantages over Pure-Culture Systems. Energies 2019, 12, 530. [Google Scholar] [CrossRef]
- Pachapur, V.L.; Sarma, S.J.; Brar, S.K.; Bihan, Y.L.; Buelna, G.; Verma, M. Hydrogen Production from Biodiesel Industry Waste by Using a Co-Culture of Enterobacter aerogenes and Clostridium butyricum. Biofuels 2017, 8, 651–662. [Google Scholar] [CrossRef]
- Ergal, İ.; Gräf, O.; Hasibar, B.; Steiner, M.; Vukotić, S.; Bochmann, G.; Fuchs, W.; Rittmann, S.K.-M.R. Biohydrogen Production beyond the Thauer Limit by Precision Design of Artificial Microbial Consortia. Commun. Biol 2020, 3, 443. [Google Scholar] [CrossRef]
- Ergal, İ.; Bochmann, G.; Fuchs, W.; Rittmann, S.K.-M. Design and Engineering of Artificial Microbial Consortia for Biohydrogen Production. Curr. Opin. Biotechnol. 2022, 73, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.-L.; Sabaratnam, V.; Shirai, Y.; Hassan, M.A. Biohydrogen Production from Biomass and Industrial Wastes by Dark Fermentation. Int. J. Hydrogen Energy 2009, 34, 3277–3287. [Google Scholar] [CrossRef]
- Eriksen, N.T.; Nielsen, T.M.; Iversen, N. Hydrogen Production in Anaerobic and Microaerobic Thermotoga neapolitana. Biotechnol. Lett. 2007, 30, 103–109. [Google Scholar] [CrossRef]
- Nguyen, T.-A.D.; Han, S.J.; Kim, J.P.; Kim, M.S.; Sim, S.J. Hydrogen Production of the Hyperthermophilic Eubacterium, Thermotoga neapolitana under N2 Sparging Condition. Bioresour. Technol. 2010, 101, S38–S41. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.A.; Nguyen, T.H.; Bui, H.T.V. Thermophilic Fermentative Hydrogen Production from Xylose by Thermotoga neapolitana DSM 4359. Renew. Energy 2012, 37, 174–179. [Google Scholar] [CrossRef]
- Rittmann, S.K.-M.R.; Lee, H.S.; Lim, J.K.; Kim, T.W.; Lee, J.-H.; Kang, S.G. One-Carbon Substrate-Based Biohydrogen Production: Microbes, Mechanism, and Productivity. Biotechnol. Adv. 2015, 33, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, C.; Hiligsmann, S.; Beckers, L.; Masset, J.; Wilmotte, A.; Thonart, P. Optimization of Culture Conditions for Biological Hydrogen Production by Citrobacter Freundii CWBI952 in Batch, Sequenced-Batch and Semicontinuous Operating Mode. Int. J. Hydrogen Energy 2010, 35, 1089–1098. [Google Scholar] [CrossRef]
- Vatsala, T.M.; Raj, S.M.; Manimaran, A. A Pilot-Scale Study of Biohydrogen Production from Distillery Effluent Using Defined Bacterial Co-Culture. Int. J. Hydrogen Energy 2008, 33, 5404–5415. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Singh, M.; Kumar, P.; Purohit, H.J.; Kalia, V.C. Exploitation of Defined Bacterial Cultures for Production of Hydrogen and Polyhydroxybutyrate from Pea-Shells. Biomass Bioenergy 2012, 36, 218–225. [Google Scholar] [CrossRef]
- Reischl, B.; Ergal, İ.; Rittmann, S.K.-M.R. Biohydrogen Production Characteristics of Desulfurococcus amylolyticus DSM 16532. Int. J. Hydrogen Energy 2018, 43, 8747–8753. [Google Scholar] [CrossRef]
- Zeidan, A.A.; Van Niel, E.W.J. Developing a Thermophilic Hydrogen-Producing Co-Culture for Efficient Utilization of Mixed Sugars. Int. J. Hydrogen Energy 2009, 34, 4524–4528. [Google Scholar] [CrossRef]
- Wang, A.; Ren, N.; Shi, Y.; Lee, D.-J. Bioaugmented Hydrogen Production from Microcrystalline Cellulose Using Co-Culture—Clostridium acetobutylicum X9 and Ethanoigenens harbinense B49. Int. J. Hydrogen Energy 2008, 33, 912–917. [Google Scholar] [CrossRef]
- Yokoi, H.; Saitsu, A.; Uchida, H.; Hirose, J.; Hayashi, S.; Takasaki, Y. Microbial Hydrogen Production from Sweet Potato Starch Residue. J. Biosci. Bioeng. 2001, 91, 58–63. [Google Scholar] [CrossRef]
- Cappelletti, B.M.; Reginatto, V.; Amante, E.R.; Antônio, R.V. Fermentative Production of Hydrogen from Cassava Processing Wastewater by Clostridium acetobutylicum. Renew. Energy 2011, 36, 3367–3372. [Google Scholar] [CrossRef]
- Pawar, S.S.; Vongkumpeang, T.; Grey, C.; van Niel, E. Biofilm Formation by Designed Co-Cultures of Caldicellulosiruptor Species as a Means to Improve Hydrogen Productivity. Biotechnol. Biofuels 2015, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Roy, S.; Das, D. Comparative Evaluation of the Hydrogen Production by Mixed Consortium, Synthetic Co-Culture and Pure Culture Using Distillery Effluent. Bioresour. Technol. 2015, 198, 593–602. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.L.; Esposito, G. A Review on Dark Fermentative Biohydrogen Production from Organic Biomass: Process Parameters and Use of By-Products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Wu, S.-Y.; Lin, P.-J.; Chang, J.-S.; Hung, C.-H.; Lee, K.-S.; Chang, F.-Y.; Chu, C.-Y.; Cheng, C.-H.; Lay, C.-H.; et al. Pilot-Scale Hydrogen Fermentation System Start-Up Performance. Int. J. Hydrogen Energy 2010, 35, 13452–13457. [Google Scholar] [CrossRef]
- Lee, H.-S.; Salerno, M.B.; Rittmann, B.E. Thermodynamic Evaluation on H2 Production in Glucose Fermentation. Environ. Sci. Technol. 2008, 42, 2401–2407. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, B.; Perego, P. Thermodynamic Study and Optimization of Hydrogen Production by Enterobacter Aerogenes. Int. J. Hydrogen Energy 2002, 27, 149–156. [Google Scholar] [CrossRef]
- Dürre, P. Physiology and Sporulation in Clostridium. Microbiol. Spectr. 2014, 2, 315–329. [Google Scholar] [CrossRef]
- Van Ginkel, S.; Logan, B.E. Inhibition of Biohydrogen Production by Undissociated Acetic and Butyric Acids. Environ. Sci. Technol. 2005, 39, 9351–9356. [Google Scholar] [CrossRef]
- Zeidan, A.A.; Rådström, P.; van Niel, E.W. Stable Coexistence of Two Caldicellulosiruptor Species in a de Novo Constructed Hydrogen-Producing Co-Culture. Microb. Cell Factories 2010, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Bagy, M.M.K.; Abd-Alla, M.H.; Morsy, F.M.; Hassan, E.A. Two Stage Biodiesel and Hydrogen Production from Molasses by Oleaginous Fungi and Clostridium acetobutylicum ATCC 824. Int. J. Hydrogen Energy 2014, 39, 3185–3197. [Google Scholar] [CrossRef]
- Perego, P.; Fabiano, B.; Ponzano, G.P.; Palazzi, E. Experimental Study of Hydrogen Kinetics from Agroindustrial By-Product: Optimal Conditions for Production and Fuel Cell Feeding. Bioprocess Eng. 1998, 19, 205–211. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, K.; Lu, Y.; Zhang, C.; Wang, L.; Xing, X.-H. Cloning and Knockout of Formate Hydrogen Lyase and H2-Uptake Hydrogenase Genes in Enterobacter Aerogenes for Enhanced Hydrogen Production. Int. J. Hydrogen Energy 2009, 34, 186–194. [Google Scholar] [CrossRef]
- Martinez-Porqueras, E.; Rittmann, S.; Herwig, C. Analysis of H2 to CO2 Yield and Physiological Key Parameters of Enterobacter aerogenes and Caldicellulosiruptor saccharolyticus. Int. J. Hydrogen Energy 2013, 38, 10245–10251. [Google Scholar] [CrossRef]

| Microorganism | Feeding Substrate | pH | Temperature/°C | Medium Composition (Complex/Defined) | Y(H2/S)/mol mol−1 | HER/mmol L−1 h−1 | Operating Conditions | Ref. |
|---|---|---|---|---|---|---|---|---|
| Pure cultures | ||||||||
| Clostridium acetobutylicum | glucose | 7 | 30 | complex | 3.14 | NA | 100 mL in 500 mL Schott bottle | [9] |
| Clostridium acetobutylicum | cassava waste-water | 7 | 36 | complex | 2.41 | NA | 300 mL bioreactor | [27] |
| Clostridium acetobutylicum | sugarcane molasses | 6.5 | 30 | complex | 1.3 | NA | 1950 mL in 2 L MultiGen fermenter | [37] |
| Enterobacter aerogenes | maltose | 6.5 | 35 | complex | 2.16 | NA | 52 mL culture in Erlenmeyer flask | [10] |
| Enterobacter aerogenes | corn starch | 5.5 | 40 | complex | 1.8 | 5.2 | 1.5 L in 2 L Gallenkamp FBL-195 bioreactor | [38] |
| Enterobacter aerogenes | glucose | uncontrolled (initial pH 6.9) | 37 | defined | 1.36 | NA | 3 L in 5 L bioreactor | [39] |
| Enterobacter aerogenes | glucose | 6.8 | 30 | defined | 0.55 | 39.92 | 0.8 L in 2 L table-top bioreactor | [40] |
| Enterobacter aerogenes | corn starch | 6.5 | 39 | complex | 1.09 | 17.39 | 1.5 L in 2 L Gallenkamp FBL-195 bioreactor | [33] |
| Co-cultures | ||||||||
| Clostridium acetobutylicum and Desulfovibrio vulgaris | glucose | NA | 37 | complex | 3.46 | NA | cultivated in Hungate tubes | [24] |
| Clostridium butyricum and Enterobacter aerogenes | sweet potato starch | 5.25 | 37 | complex | 2.4 | NA | 200 mL in 250 mL stirred reactor | [26] |
| Clostridium butyricum and Clostridium pasteurianum | starch | 5.3 | 30 | complex | 2.32 | NA | 18 L in 20 L stainless steel tank bioreactor | [7] |
| Clostridium butyricum and Clostridium pasteurianum | glucose | 5.3 | 30 | complex | 2.12 | NA | 18 L in 20 L stainless steel tank bioreactor | [7] |
| Klebsiella pneumoniae and Citrobacter freundii | glucose | 6.5 | 37 | complex | 2.07 | NA | 2 L in a controlled fermenter | [29] |
| Enterobacter aerogenes and Clostridium acetobutylicum | glucose | 6 | 37 | defined | 6.18 | 105.59 | 1.5 L in 2 L stirred tank reactor | This study |
| Enterobacter aerogenes and Clostridium acetobutylicum | glucose | uncontrolled | 37 | defined | 4.45 | 80.76 | 1.5 L in 2 L stirred tank reactor | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ergal, İ.; Zech, E.; Hanišáková, N.; Kushkevych, I.; Fuchs, W.; Vítěz, T.; Vítězová, M.; Bochmann, G.; Rittmann, S.K.-M.R. Scale-Up of Dark Fermentative Biohydrogen Production by Artificial Microbial Co-Cultures. Appl. Microbiol. 2022, 2, 215-226. https://doi.org/10.3390/applmicrobiol2010015
Ergal İ, Zech E, Hanišáková N, Kushkevych I, Fuchs W, Vítěz T, Vítězová M, Bochmann G, Rittmann SK-MR. Scale-Up of Dark Fermentative Biohydrogen Production by Artificial Microbial Co-Cultures. Applied Microbiology. 2022; 2(1):215-226. https://doi.org/10.3390/applmicrobiol2010015
Chicago/Turabian StyleErgal, İpek, Elisa Zech, Nikola Hanišáková, Ivan Kushkevych, Werner Fuchs, Tomáš Vítěz, Monika Vítězová, Günther Bochmann, and Simon K.-M. R. Rittmann. 2022. "Scale-Up of Dark Fermentative Biohydrogen Production by Artificial Microbial Co-Cultures" Applied Microbiology 2, no. 1: 215-226. https://doi.org/10.3390/applmicrobiol2010015
APA StyleErgal, İ., Zech, E., Hanišáková, N., Kushkevych, I., Fuchs, W., Vítěz, T., Vítězová, M., Bochmann, G., & Rittmann, S. K.-M. R. (2022). Scale-Up of Dark Fermentative Biohydrogen Production by Artificial Microbial Co-Cultures. Applied Microbiology, 2(1), 215-226. https://doi.org/10.3390/applmicrobiol2010015






