Experience of Using Antifungal Rocima GT for Protection of Paper from Biological Damage Caused by Fungi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strain Isolation and Identification
2.2. Fungal Cultivation and Paper Inoculation
- B-5—82 g/m2, 100% sulfite cellulose, ash content—5% (filler-kaolin);
- B-19—82 g/m2, 100% sulfate cellulose, ash content—5% (filler-kaolin);
- B-25—85g/m2, 100% bleached cotton half-mass, ash content—9% (filler-kaolin).
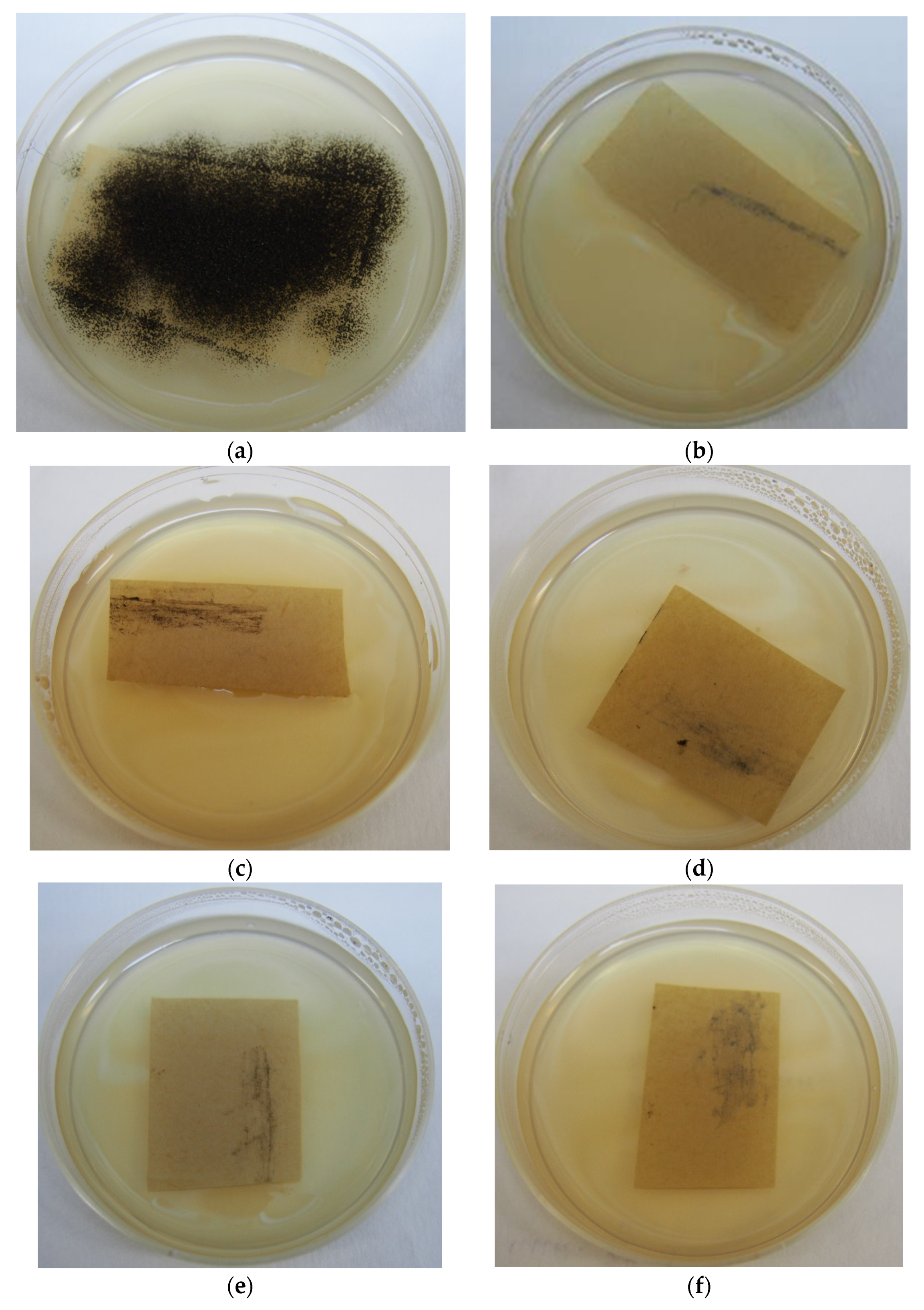
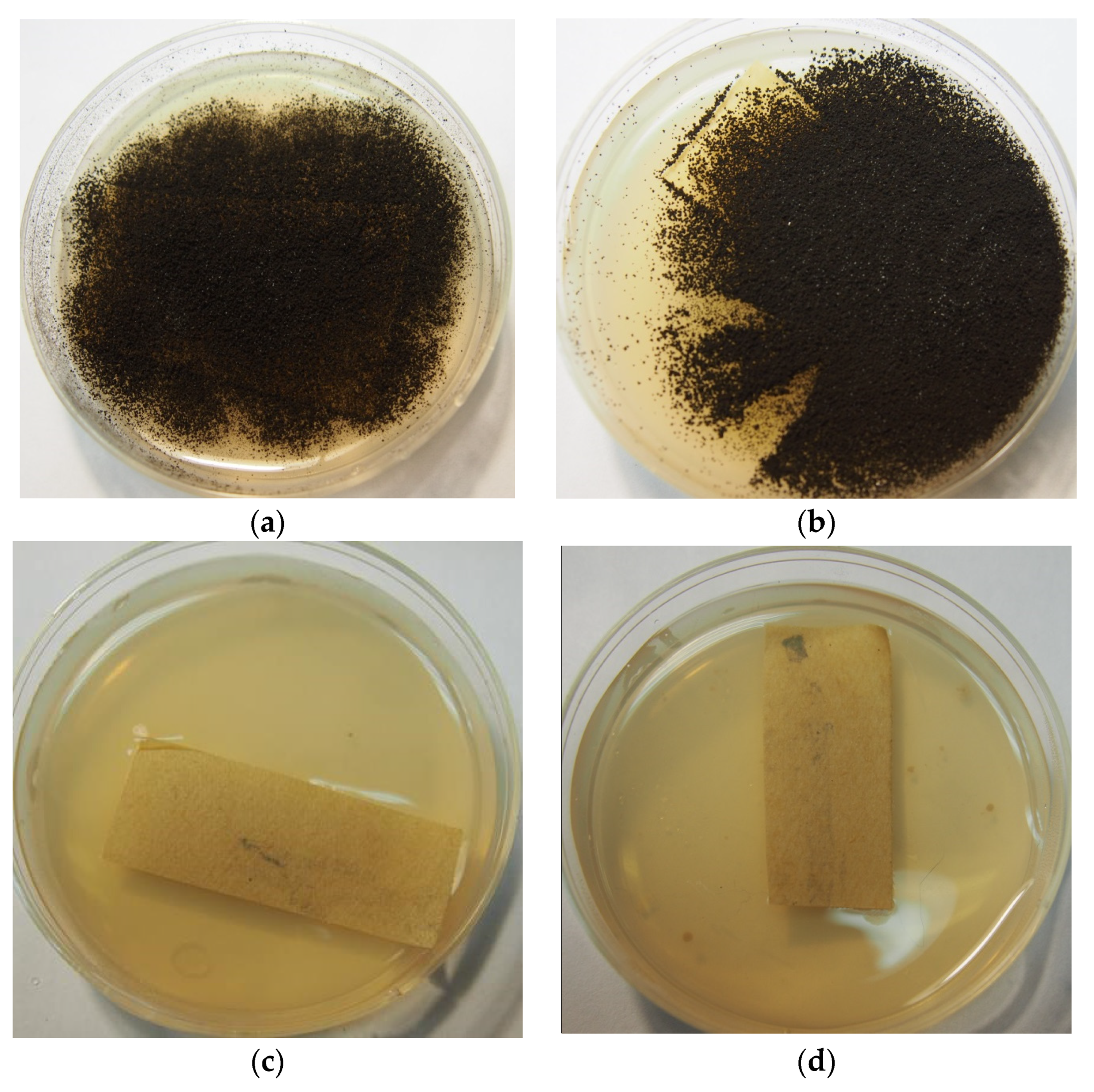
2.3. Rocima GT Treatment
2.3.1. The Treatment of the Inoculated Paper Samples with Rocima GT
Swab Treatment
Treatment in Chamber
2.3.2. The Cultivation of Aspergillus niger, Medium Enriched with Rocima GT
2.4. Organic Acids Analysis
2.4.1. Sample Preparation
2.4.2. Gas Chromatography–Mass Spectrometry
2.5. Estimating the Physical Characteristic of Paper
2.6. Statistical Analysis
3. Results
3.1. Fungal Species Identification
3.2. Evaluation of the Viability of A. niger after Treatment by Rocima GT
3.3. The Influence of Rocima GT on the Physical Properties of Paper
3.4. Effect of Rocima GT on Organic Acids Produced by A. niger
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oetari, A.; Natalius, A.; Komalasari, D.; Susetyo-Salim, T.; Sjamsuridzal, W. Fungal deterioration of old manuscripts of European paper origin. AIP Conf. Proc. 2018, 2023, 020156. [Google Scholar] [CrossRef]
- Pinzari, F.; Pasquariello, G.; De Mico, A. Biodeterioration of Paper: A SEM Study of Fungal Spoilage Reproduced Under Controlled Conditions. Macromol. Symp. 2006, 238, 57–66. [Google Scholar] [CrossRef]
- Sequeira, S.; Cabrita, E.J.; Macedo, M.F. Antifungals on paper conservation: An overview. Int. Biodeterior. Biodegrad. 2012, 74, 67–86. [Google Scholar] [CrossRef]
- Sequeira, S.O.; Cabrita, E.J.; Macedo, M.F. Fungal Biodeterioration of Paper: How are Paper and Book Conservators Dealing with it? An International Survey. Restaurator 2014, 35, 181–199. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Sequeira, S.O.; Macedo, M.F. Fungi in archives, libraries, and museums: A review on paper conservation and human health. Crit. Rev. Microbiol. 2019, 45, 686–700. [Google Scholar] [CrossRef]
- Sterflinger, K. Fungi: Their role in deterioration of cultural heritage. Fungal Biol. Rev. 2010, 24, 47–55. [Google Scholar] [CrossRef]
- Borrego, S.; Patricia, G.S.; Vivar, I.; Battistoni, P. Fungi involved in biodeterioration of documents in paper and effect on substrate. Acta Microsc. 2018, 27, 37–44. [Google Scholar]
- Lugauskas, A.; Krikŝtaponis, A. Microscopic Fungi Found in the Libraries of Vilnius and Factors Affecting their Development. Indoor Built Environ. 2004, 13, 169–182. [Google Scholar] [CrossRef]
- Nitiu, D.S.; Mallo, A.C.; Saparrat, M.C.N. Fungal melanins that deteriorate paper cultural heritage: An overview. Mycologia 2020, 112, 859–870. [Google Scholar] [CrossRef]
- Magnuson, J.K.; Lasure, L.L. Organic Acid Production by Filamentous Fungi; Plenum Publishers: New York, NY, USA, 2004; pp. 307–340. [Google Scholar]
- Grbic, M.V.L.; Vukojevic, J.B. Role of fungi in biodeterioration process of stone in historic buildings. Proc. Natl. Acad. Sci. USA 2009, 116, 245–251. [Google Scholar]
- Sequeda-Castañeda, L.G.; Ortiz-Ardila, A.E.; Correa-Cuadros, J.P.; López-Pérez, C. Chemical and microbiological comparison of biodeterioration in Colombian heritage constructions. Univ. Sci. 2013, 18, 51–63. [Google Scholar] [CrossRef]
- Sazanova, K.V.; Frank-Kamenetskaya, O.V.; Vlasov, D.Y.; Zelenskaya, M.S.; Vlasov, A.D.; Rusakov, A.V.; Petrova, M.A. Carbonate and Oxalate Crystallization by Interaction of Calcite Marble with Bacillus subtilis and Bacillus subtilis–Aspergillus niger Association. Crystals 2020, 10, 756. [Google Scholar] [CrossRef]
- Sturm, E.V.; Frank-Kamenetskaya, O.V.; Vlasov, D.Y.; Zelenskaya, M.S.; Sazanova, K.V.; Rusakov, A.V.; Kniep, R. Crystallization of calcium oxalate hydrates by interaction of calcite marble with fungus Aspergillus niger. Am. Miner. 2015, 100, 2559–2565. [Google Scholar] [CrossRef]
- Sequeira, S.O.; Phillips, A.J.L.; Cabrita, E.J.; Macedo, M.F. Antifungal treatment of paper with calcium propionate and parabens: Short-term and long-term effects. Int. Biodeterior. Biodegrad. 2017, 120, 203–215. [Google Scholar] [CrossRef]
- Baughan, E. (Ed.) Aspergillus niger. Pathogenicity, Cultivation and Uses; Nova Science Pub. Inc.: New York, NY, USA, 2020; 197p. [Google Scholar]
- Sazanova, K.V.; Vlasov, D.Y.; Osmolovskay, N.G.; Schiparev, S.M.; Rusakov, A.V. Significance and regulation of acids production by rock-inhabited fungi. In Biogenic–Abiogenic Interactions in Natural and Anthropogenic Systems. Lecture Notes in Earth System Sciences; Springer Nature: Berlin/Heidelberg, Germany, 2016; pp. 379–392. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. In Encyclopedia of Microbiology, 3rd ed.; Moselio, S., Ed.; Academic Press: Oxford, UK, 2009; pp. 559–565. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Macedo, M.F.; Jurado, V.; Saiz-Jimenez, C.; Viegas, C.; Brandao, J.; Rosado, L. Mold and yeast identification in archival settings: Preliminary results on the use of traditional methods and molecular biology options in Portuguese archives. Int. Biodeterior. Biodegrad. 2011, 65, 619–627. [Google Scholar] [CrossRef]
- Wiszniewska, M.J.; Walusiak-Skorupa, I.; Pannenko, M.; Draniak, M.; Paczyski, C. Occupational exposure and sensitization to fungi among museum workers. Occup. Med. 2009, 59, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.; Yu, C.W.F.; Kim, J.T. Building Pathology, Investigation of Sick Buildings Toxic Molds. Indoor Built. Environ. 2010, 19, 40–47. [Google Scholar] [CrossRef]
- Micalli, O.; Montacutelli, R.; Tarsitani, G. Pathogenic microorganisms and situations of risk to man. In Cultural Heritage and Aerobiology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 31–43. [Google Scholar] [CrossRef]
- Khan, A.A.H.; Karuppayil, S.M.; Manoharachary, C.; Kunwar, I.K.; Waghray, S. Isolation, identification and testing for allergenicity of fungi from air-conditioned indoor environments. Aerobiologia 2009, 25, 119–123. [Google Scholar] [CrossRef]
- Nyuksha, Y.P. Biological Damage to Paper and Books; Publishing house of the Russian Academy of Sciences: Saint-Petersburg, Russia, 1994; 210p. (In Russ) [Google Scholar]
- Nitte´rus, M. Ethanol as fungal sanitizer in paper conservation. Restaurator 2000, 21, 101–115. [Google Scholar] [CrossRef]
- Gutarowska, B.; Pietrzak, K.; Skóra, J. Disinfection as a factor reducing microbial threat at workposts in museum and library—A comparison of the effectiveness of photocatalytic ionization, UV irradiation and chemical misting. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 945–959. [Google Scholar]
- Xu, J.; Bai, Y.; Wan, M.; Liu, Y.; Tao, L.; Wang, X. Antifungal Paper Based on a Polyborneolacrylate Coating. Polymers 2018, 10, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakotonirainy, M.; Fohrer, F.; Flieder, F. Research on fungicides for aerial disinfection by thermal fogging in libraries and archives. Int. Biodeterior. Biodegrad. 1999, 44, 133e139. [Google Scholar] [CrossRef]
- Gutarowska, B.; Rembisz, D.; Zduniak, K.; Skóra, J.; Szynkowska, M.; Gliscinska, E.; Koziróg, A. Optimization and application of the misting method with silver nanoparticles for disinfection of the historical objects. Int. Biodeterior. Biodegrad. 2012, 75, 167–175. [Google Scholar] [CrossRef]
- Special Chem. The Material Selection Platform. Available online: https://coatings.specialchem.com/product/a-dow-rocima-gt. (accessed on 25 January 2022).
- Velikova, T.; Trepova, E.; Rosen, T. The use of biocides for the protection of library documents: Before and now. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Microbiology Series, Formatex; Formatex Research Center: Badajoz, Spain, 2011; Volume 1, pp. 152–159. [Google Scholar]
- Ellis, M.B. More Dematiaceous Hyphomycetes; Commonwealth Mycological Institute: London, UK, 1976; p. 507. [Google Scholar]
- De Hoog, G.S.; Guarro, J. Atlas of Clinical Fungi; CBS: Baarn, The Netherlands, 1995; p. 1160. [Google Scholar]
- Stielow, J.B.; Lévesque, C.A.; Seifert, K.A. One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia 2015, 35, 242–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AMDIS. Available online: http://www.amdis.net/index.html (accessed on 15 November 2021).
- UniChrom. Available online: http://www.unichrom.com/unichrome.shtml (accessed on 15 November 2021).
- ISO (International Organization for Standardization). Paper—Determination of Folding Endurance; ISO 5626:1993; ISO: Geneva, Switzerland, 1993. [Google Scholar]
- ISO (International Organization for Standardization). Paper and Board—Accelerated Ageing; ISO 5630-5:2008; ISO: Geneva, Switzerland, 2008. [Google Scholar]
- Franke, W. Internationale Normung fur Papier und. Pappe. Wochenbl. Papierfabr 1974, 102, 175–177. [Google Scholar]
- ISO (International Organization for Standardization). Paper, Board and Pulps—Measurement of Diffuse Blue Reflectance Factor—Part 1: Indoor Daylight Conditions (ISO Brightness); ISO 2470:1999ISO 2470-1:2016; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- Perrone, G.; Stea, G.; Epifani, F.; Varga, J.; Frisvad, J.C.; Samson, R.A. Aspergillus niger contains the cryptic phylogenetic species A. awamori. Fungal Biol. 2011, 115, 1138–1150. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.B.; Lee, M.; Kim, D.H.; Varga, J.; Frisvad, J.C.; Perrone, G.; Gomi, K.; Yamada, O.; Machida, M.; Houbraken, J.; et al. Aspergillus luchuensis, an industrially important black Aspergillus in East Asia. PLoS ONE 2013, 28, e63769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinzari, F.; Zotti, M.; De Mico, A.; Calvini, P. Biodegradation of inorganic components in paper documents: Formation of calcium oxalate crystals as a consequence of Aspergillus terreus Thom growth. Int. Biodeterior. Biodegr. 2010, 64, 499–505. [Google Scholar] [CrossRef]
- Zheng, X.; Tian, S.; Meng, X.; Li, B. Physiological and biochemical responses in peach fruit to oxalic acid treatment during storage at room temperature. Food Chem. 2007, 104, 156–162. [Google Scholar] [CrossRef]
| Type of Paper | Treatment | Break Resistance (h.p.) | Whiteness, % | Thermo-Aging | |||
|---|---|---|---|---|---|---|---|
| Longitudinal | Transverse | Break Resistance (h.p.) | Whiteess, % | ||||
| Longitudinal | Transverse | ||||||
| B-5 | without treatment | 11.6 ± 2.4 | 32.6 ± 3.9 | 71.8 ± 0.2 | 8.9 ± 1.7 | 19.0 ± 4.8 | 69.0 ± 0.0 |
| B-19 | 165.5 ± 36.3 | 92.3 ± 18.4 | 72.2 ± 0.3 | 187.8 ± 33.0 | 88.8 ± 13.5 | 70.0 ± 0.0 | |
| B-25 | 10.1 ± 1.1 | 7.2 ± 0.4 | 85.0 ± 0.0 | 11 ± 1.0 | 8.1 ± 1.3 | 84.0 ± 0.0 | |
| B-5 | distilled water | 21.4 ± 3.4 | 30.1 ± 5.0 | 71.2 ± 0.3 | 16.8 ± 6.2 | 33.6 ± 4.7 | 69.0 ± 0.0 |
| B-19 | 161.5 ± 29.3 | 86.0 ± 12.5 | 70.0 ± 0.0 | 189.5 ± 39.5 | 135.5 ± 30.1 | 70.0 ± 0.0 | |
| B-25 | 8.9 ± 0.6 | 6.5 ± 0.5 | 83.0 ± 0.0 | 10.3 ± 0.9 | 8.2 ± 0.8 | 84.0 ± 0.0 | |
| B-5 | 0.5% aqueous solution of Rocima GT | 16.3 ± 3.2 | 24.4 ± 4.7 | 69.0 ± 0.6 | 15.1 ± 3.5 | 22.1 ± 4.7 | 69.0 ± 0.0 |
| B-19 | 86.1 ± 17.9 | 48.3 ± 4.5 | 69.0 ± 0.6 | 110.3 ± 18.8 | 48.1 ± 7.7 | 70.0 ± 0.0 | |
| B-25 | 6.2 ± 0.4 | 5.2 ± 0.4 | 82.7 ± 0.3 | 7.4 ± 0.7 | 6.4 ± 0.5 | 84.0 ± 0.0 | |
| B-5 | 2% aqueous solution of Rocima GT | 12.5 ± 1.5 | 19.7 ± 2.8 | 70.3 ± 0.3 | 9.9 ± 1.5 | 15.5 ± 1.6 | 70.0 ± 0.2 |
| B-19 | 46.4 ± 7.3 | 24.7 ± 2.9 | 69.5 ± 0.0 | 62.5 ± 17.3 | 33.6 ± 6.6 | 69.0 ± 0.0 | |
| B-25 | 4.9 ± 0.3 | 4.0 ± 0.0 | 84.0 ± 0.0 | 5.8 ± 0.4 | 4.9 ± 0.3 | 84.0 ± 0.0 | |
| B-5 | water mist | 18.7 ± 2.6 | 34.3 ± 7.3 | 69.0 ± 0.0 | 10.6 ± 2.7 | 22.8 ± 2.9 | 70.0 ± 0.1 |
| B-19 | 211.1 ± 43.5 | 110.9 ± 26.9 | 70.0 ± 0.0 | 189.6 ± 51.4 | 125.4 ± 22.4 | 68.8 ± 0.7 | |
| B-25 | 10.6 ± 1.0 | 6.2 ± 0.4 | 84.0 ± 0.0 | 11.0 ± 1.9 | 8.1 ± 0.6 | 84.0 ± 0.0 | |
| B-5 | mist of 5% aqueous solution of Rocima GT | 17.6 ± 5.1 | 23.0 ± 2.8 | 70.5 ± 0.0 | 7.0 ± 1.0 | 22.2 ± 4.8 | 70.0 ± 0.0 |
| B-19 | 168.5 ± 54.0 | 30.2 ± 7.0 | 70.0 ± 0.0 | 152.5 ± 49.1 | 41.5 ± 6.9 | 68.5 ± 0.0 | |
| B-25 | 7.4 ± 0.6 | 5.2 ± 0.4 | 84.0 ± 0.0 | 10.3 ± 1.2 | 8.1 ± 0.9 | 84.0 ± 0.0 | |
| Rocima GT Concentration, % | Growth Suppression, % | The Amount of Organic Acids, mg/g Mycelium | ||||
|---|---|---|---|---|---|---|
| Oxalic Acid | Citric Acid | Fumaric Acid | Succinic Acid | Malic Acid | ||
| 0%(control) | 0 | 125.0 ± 34.8 | 6.8 ± 2.5 | 0.8 ± 0.3 | 1.5 ± 0.7 | 1.7 ± 0.6 |
| 0.01% | 10 | 20.0 ± 6.2 | 8.0 ± 2.8 | 1.0 ± 0.3 | 1.0 ± 0.4 | 1.1 ± 0.3 |
| 0.05% | 50 | 194.8 ± 28.7 | 8.1 ± 2.3 | 0.3 ± 0.1 | 0.4 ± 0.2 | 0.8 ± 0.3 |
| 0.1% | 80 | - * | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlasov, A.D.; Sazanova, K.V.; Hosid, E.G.; Tkachenko, T.S.; Alekseev, A.I.; Pchelin, I.M.; Galushkin, A.A. Experience of Using Antifungal Rocima GT for Protection of Paper from Biological Damage Caused by Fungi. Appl. Microbiol. 2022, 2, 185-196. https://doi.org/10.3390/applmicrobiol2010013
Vlasov AD, Sazanova KV, Hosid EG, Tkachenko TS, Alekseev AI, Pchelin IM, Galushkin AA. Experience of Using Antifungal Rocima GT for Protection of Paper from Biological Damage Caused by Fungi. Applied Microbiology. 2022; 2(1):185-196. https://doi.org/10.3390/applmicrobiol2010013
Chicago/Turabian StyleVlasov, Alexey D., Katerina V. Sazanova, Elena G. Hosid, Tat’yana S. Tkachenko, Andrey I. Alekseev, Ivan M. Pchelin, and Alexandr A. Galushkin. 2022. "Experience of Using Antifungal Rocima GT for Protection of Paper from Biological Damage Caused by Fungi" Applied Microbiology 2, no. 1: 185-196. https://doi.org/10.3390/applmicrobiol2010013
APA StyleVlasov, A. D., Sazanova, K. V., Hosid, E. G., Tkachenko, T. S., Alekseev, A. I., Pchelin, I. M., & Galushkin, A. A. (2022). Experience of Using Antifungal Rocima GT for Protection of Paper from Biological Damage Caused by Fungi. Applied Microbiology, 2(1), 185-196. https://doi.org/10.3390/applmicrobiol2010013






