SNP Effects on Yield and Agronomic Traits in an International Winter Wheat Collection Grown in Western Siberia
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Trials and Phenotyping
2.2. DNA Extraction and SNP Genotyping
2.3. Statistical Analysis
3. Results
3.1. Field Experiment and Phenotyping
3.2. Analysis of SNP Effects
3.3. Outperforming Genotypes Revealed on Yield
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MAS | Marker assisted selection |
| SL | Spike length |
| NFT | No. of fertile tillers per unit area |
| NGS | No. of grains per spike |
| GWS | Grain weight per spike |
| GWP | Grain weight per plant |
| TKW | Thousand kernel weight |
References
- Caporaso, L.; Paola, F.D.; Bombelli, A.; Vasenev, I.; Nesterova, O.V.; Castaldi, S.; Valentini, R. The expansion of wheat thermal suitability of Russia in response to climate change. Land Use Policy 2018, 78, 70–77. [Google Scholar]
- Goncharov, A.A.; Safonov, T.A.; Malko, A.M.; Bocharov, G.A.; Goncharov, S.V. Climate change expected to increase yield of spring cereals and reduce yield of winter cereals in the Western Siberian grain belt. Field Crops Res. 2023, 302, 109038. [Google Scholar] [CrossRef]
- Sharma, S.; Schulthess, A.W.; Bassi, F.M.; Badaeva, E.D.; Neumann, K.; Graner, A.; Özkan, H.; Werner, P.; Knüpffer, H.; Kilian, B. Introducing Beneficial Alleles from Plant Genetic Resources into the Wheat Germplasm. Biology 2021, 10, 982. [Google Scholar] [CrossRef]
- Svoboda, P.; Holubec, V.; Reif, J.C.; Berkner, M.O. Curation of historical phenotypic wheat data from the Czech Genebank for research and breeding. Sci. Data 2024, 11, 763. [Google Scholar] [CrossRef] [PubMed]
- Eltaher, S.; Hashem, M.; Ahmed, A.A.M.; Baenziger, P.S.; Börner, A.; Sallam, A. Effectiveness of TaDreb-B1 and 1-FEH w3 KASP Markers in Spring and Winter Wheat Populations for Marker-Assisted Selection to Improve Drought Tolerance. Int. J. Mol. Sci. 2023, 24, 8986. [Google Scholar] [CrossRef]
- Kaur, B.; Mavi, G.S.; Gill, M.S.; Saini, D.K. Utilization of KASP technology for wheat improvement. Cereal Res. Commun. 2020, 48, 409421. [Google Scholar] [CrossRef]
- Shokat, S.; Grobkinsky, D.K.; Singh, S.; Liu, F. The role of genetic diversity and pre-breeding traits to improve drought and heat tolerance of bread wheat at the reproductive stage. Food Energy Secur. 2023, 12, e478. [Google Scholar] [CrossRef]
- Song, L.; Wang, R.; Yang, X.; Zhang, A.; Liu, D. Molecular Markers and Their Applications in Marker Assisted Selection (MAS) in Bread Wheat (Triticum aestivum L.). Agriculture 2023, 13, 642. [Google Scholar] [CrossRef]
- Rasheed, A.; Wen, W.; Gao, F.; Zhai, S.; Jin, H.; Liu, J.; He, Z. Development and validation of KASP assays for genes underpinning key economic traits in bread wheat. Theor. Appl. Genet. 2016, 129, 1843–1860. [Google Scholar] [CrossRef]
- Li, T.; Deng, G.; Su, Y.; Yang, Z.; Tang, Y.; Wang, J.; Qiu, X.; Pu, X.; Li, J.; Liu, Z.; et al. Identification and validation of two major QTLs for spike compactness and length in bread wheat (Triticum aestivum L.) showing pleiotropic effects on yield-related traits. Theor. Appl. Genet. 2021, 134, 3625–3641. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, L.; Hu, M.; Liu, Q.; Xu, J.; Mu, L.; Wang, J.; Yang, J.; Wang, P.; Li, Q.; et al. Pleiotropic Quantitative Trait Loci (QTL) Mining for Regulating Wheat Processing Quality- and Yield-Related Traits. Plants 2024, 13, 2545. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, D.; Meng, Z.; Xu, K.; Yan, J.; Xia, X.; Cao, S.; Tian, Y.; He, Z.; Zhang, Y. QTL mapping for grain yield-related traits in bread wheat via SNP-based selective genotyping. Theor. Appl. Genet. 2020, 133, 857–872. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.-C.; Jia, H.; Powers, C.; Carver, B.F.; Yan, L. Genetic characterization and deployment of a major gene for grain yield on chromosome arm 1BS in winter wheat. Mol. Breed. 2020, 40, 26. [Google Scholar] [CrossRef]
- Shahi, D.; Guo, J.; Babar, M.A.; Pradhan, S.; AVCI, M.; McBreen, J.; Liu, Z.; Bai, G.; Amand, P.S.; Bernardo, A.; et al. Dissecting the genetic basis of fruiting efficiency for genetic enhancement of harvest index, grain number, and yield in wheat. BMC Plant Biol. 2025, 25, 101. [Google Scholar] [CrossRef]
- Singh, D.; Wang, X.; Kumar, U.; Gao, L.; Noor, M.; Imtiaz, M.; Singh, R.P.; Poland, J. High-Throughput Phenotyping Enabled Genetic Dissection of Crop Lodging in Wheat. Front. Plant Sci. 2019, 10, 394. [Google Scholar] [CrossRef]
- Berkner, M.O.; Jiang, Y.; Reif, J.C.; Schulthess, A.W. Trait-customized sampling of core collections from a winter wheat gene bank collection supports association studies. Front. Plant Sci. 2024, 15, 1451749. [Google Scholar] [CrossRef]
- Lozada, D.N.; Ward, B.P.; Carter, A.H. Gains through selection for grain yield in a winter wheat breeding program. PLoS ONE 2020, 15, e0221603. [Google Scholar] [CrossRef]
- Chen, X.; Min, D.; Yasir, T.A.; Hu, Y.-G. Genetic Diversity, Population Structure and Linkage Disequilibrium in Elite Chinese Winter Wheat Investigated with SSR Markers. PLoS ONE 2012, 7, e44510. [Google Scholar] [CrossRef]
- Tadesse, W.; Sanchez-Garcia, M.; Assefa, S.G.; Amri, A.; Bishaw, Z.; Ogbonnaya, F.C.; Baum, M. Genetic Gains in Wheat Breeding and Its Role in Feeding the World. Crop Breed. Genet. Genom. 2019, 1, e190005. [Google Scholar]
- Shamanin, V.P.; Shepelev, S.S.; Pozherukova, V.E.; Pototskaya, I.V.; Kocherina, N.V.; Lohwasser, U.; Börner, A.; Chesnokov, Y.V. QTL mapping in hexaploid soft wheat (Triticum aestivum L.) in the West Siberian Plain. Agric. Biol. 2018, 53, 50–60. [Google Scholar] [CrossRef]
- Yang, C.J.; Ladejobi, O.; Mott, R.; Powell, W.; Mackay, I. Analysis of historical selection in winter wheat. Theor. Appl. Genet. 2022, 135, 30053023. [Google Scholar] [CrossRef] [PubMed]
- Leonova, I.N.; Kiseleva, A.A.; Berezhnaya, A.A.; Stasyuk, A.I.; Likhenko, I.E.; Salina, E.A. Identification of QTLs for Grain Protein Content in Russian Spring Wheat Varieties. Plants 2022, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Morgounov, A.; Li, H.; Shepelev, S.; Ali, M.; Flis, P.; Koksel, H.; Savin, T.; Shamanin, V. GeneticCharacterization of Spring Wheat Germplasm for Macro-, Microelements and Trace Metals. Plants 2022, 11, 2173. [Google Scholar] [CrossRef]
- Dorofeev, V.F. (Ed.) Methodical Instructions. Exploring the Wheat Collection; VIR: Leningrad, Russia, 1985. [Google Scholar]
- Amalova, A.; Yermekbayev, K.; Griffiths, S.; Abugalieva, S.; Babkenov, A.; Fedorenko, E.; Abugalieva, A.; Turuspekov, Y. Identification of quantitative trait loci of agronomic traits in bread wheat using a Pamyati Azieva × Paragon mapping population harvested in three regions of Kazakhstan. PeerJ 2022, 10, e14324. [Google Scholar] [CrossRef]
- Genievskaya, Y.; Turuspekov, Y.; Rsaliyev, A.; Abugalieva, S. Genome-wide association mapping for resistance to leaf, stem, and yellow rusts of common wheat under field conditions of South Kazakhstan. PeerJ 2020, 8, e9820. [Google Scholar] [CrossRef]
- Amalova, A.; Abugalieva, S.; Babkenov, A.; Babkenova, S.; Turuspekov, Y. Genome-wide association study of yield components in spring wheat collection harvested under two water regimes in Northern Kazakhstan. PeerJ 2021, 9, e11857. [Google Scholar] [CrossRef]
- Pototskaya, I.V.; Shepelev, S.S.; Turuspekov, Y.K.; Morgounov, A.I.; Chursin, A.S.; Kovalchuk, A.M.; Savin, T.V.; Shamanin, V.P. Breeding and genetic evaluation of International winter wheat collection under conditions of Western Siberia. Sib. J. Life Sci. Agric. 2024, 6, 313–338. [Google Scholar] [CrossRef]
- Delaporta, S.L.; Wood, J.; Hicks, J.B. A plant DNA minipreparation. Version II. Plant Mol. Biol. Rep. 1983, 4, 19–21. [Google Scholar] [CrossRef]
- Fowler, D.B.; N’Diaye, A.; Laudencia, C.D.; Pozniak, C.J. Quantitative Trait Loci Associated with Phenological Development, Low-Temperature Tolerance, Grain Quality, and Agronomic Characters in Wheat (Triticum aestivum L.). PLoS ONE 2016, 11, e0152185. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Y.; Zhang, D.; Shao, H.; Liu, P.; Hu, J.; Zhang, H.; Zhang, H.; Chang, C.; Lu, J.; et al. Genome-wide association study for grain yield and related traits in elite wheat varieties and advanced lines using SNP markers. PLoS ONE 2017, 12, e0188662. [Google Scholar] [CrossRef]
- Yang, X.; McMaster, G.S.; Yu, Q. Spatial Patterns of Relationship Between Wheat Yield and Yield Components in China. Int. J. Plant Prod. 2018, 12, 61–71. [Google Scholar] [CrossRef]
- Peltonen-Sainio, P.; Kangas, A.; Salo, J.; Jauhiainen, L. Kernel number is more important than grain weight in determining yield of temperate cereal crops: Evidence from 30 years of multi-location trials. Field Crops Res. 2007, 100, 179–188. [Google Scholar] [CrossRef]
- Tsenov, N.; Gubatov, T.; Yanchev, I. Correlations between grain yield and related traits in winter wheat under multi-environmental traits. Agric. Sci. Technol. 2020, 12, 295–300. [Google Scholar] [CrossRef]
- Li, X.; Xu, X.; Liu, W.; Li, X.; Yang, X.; Ru, Z.; Li, L. Dissection of Superior Alleles for Yield-Related Traits and Their Distribution in Important Cultivars of Wheat by Association Mapping. Front. Plant Sci. 2020, 11, 175. [Google Scholar] [CrossRef]
- Gizaw, S.A.; Godoy, J.G.V.; Garland-Campbell, K.; Carter, A.H. Genome-Wide Association Study of Yield and Component Traits in Pacific Northwest Winter Wheat. Crop Sci. 2018, 58, 2315–2330. [Google Scholar] [CrossRef]
- Guan, P.; Lu, L.; Jia, L.; Kabir, M.R.; Zhang, J.; Lan, T.; Zhao, Y.; Xin, M.; Hu, Z.; Yao, Y.; et al. Global QTL Analysis Identifies Genomic Regions on Chromosomes 4A and 4B Harboring Stable Loci for Yield-Related Traits Across Different Environments in Wheat (Triticum aestivum L.). Front. Plant Sci. 2018, 9, 529. [Google Scholar] [CrossRef]
- Zanke, C.D.; Ling, J.; Plieske, J.; Kollers, S.; Ebmeyer, E.; Korzun, V.; Argillier, O.; Stiewe, G.; Hinze, M.; Neumann, F.; et al. Analysis of main effect QTL for thousand grain weight in European winter wheat (Triticum aestivum L.) by genome-wide association mapping. Front. Plant Sci. 2015, 6, 644. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, F.; Yan, X.; Zhang, X.; Dong, Z.; Cui, D.; Chen, F. Genome-wide association study for 13 agronomic traits reveals distribution of superior alleles in bread wheat from the Yellow and Huai Valley of China. Plant Biotechnol. J. 2017, 15, 953–969. [Google Scholar] [CrossRef]
- Würschum, T.; Langer, S.M.; Longin, C.F.H.; Korzun, V.; Akhunov, E.; Ebmeyer, E.; Schachschneider, R.; Schacht, J.; Kazman, E.; Reif, J.C. Population structure, genetic diversity and linkage disequilibrium in elite winter wheat assessed with SNP and SSR markers. Theor Appl. Genet. 2013, 126, 1477–1486. [Google Scholar] [CrossRef]
- Sun, C.; Dong, Z.; Zhao, L.; Ren, Y.; Zhang, N.; Chen, F. The Wheat 660K SNP array demonstrates great potential for marker-assisted selection in polyploid wheat. Plant Biotechnol. J. 2020, 18, 1354–1360. [Google Scholar] [CrossRef]
- Cao, S.; Xu, D.; Hanif, M.; Xia, X.; He, Z. Genetic architecture underpinning yield component traits in wheat. Theor. Appl. Genet. 2020, 133, 1811–1823. [Google Scholar] [CrossRef]
- Xiong, H.; Li, Y.; Guo, H.; Xie, Y.; Zhao, L.; Gu, J.; Zhao, S.; Ding, Y.; Liu, L. Genetic mapping by integration of 55K SNP array and KASP markers reveals candidate genes for important agronomic traits in hexaploid wheat. Front. Plant Sci. 2021, 12, 628478. [Google Scholar] [CrossRef]
- Jung, W.J.; Lee, Y.J.; Kang, C.S.; Seo, Y.W. Identification of genetic loci associated with major agronomic traits of wheat (Triticum aestivum L.) based on genome-wide association analysis. BMC Plant Biol. 2021, 21, 418. [Google Scholar] [CrossRef]
- Nouraei, S.; Mia, M.S.; Liu, H.; Turner, N.C.; Yan, G. Genome-wide association study of drought tolerance in wheat (Triticum aestivum L.) identifies SNP markers and candidate genes. Mol. Genet. Genom. 2024, 299, 22. [Google Scholar] [CrossRef]
- Lozada, D.N.; Mason, R.E.; Babar, M.A.; Carver, B.F.; Guedira, G.-B.; Merrill, K.; Arguello, M.N.; Acuna, A.; Vieiram, L.; Holder, A.; et al. Association mapping reveals loci associated with multiple traits that affect grain yield and adaptation in soft winter wheat. Euphytica 2017, 213, 222. [Google Scholar] [CrossRef]
- Ma, F.; Xu, Y.; Wang, R.; Tong, Y.; Zhang, A.; Liu, D.; An, D. Identification of major QTLs for yield-related traits with improved genetic map in wheat. Front. Plant Sci. 2023, 14, 1138696. [Google Scholar] [CrossRef]
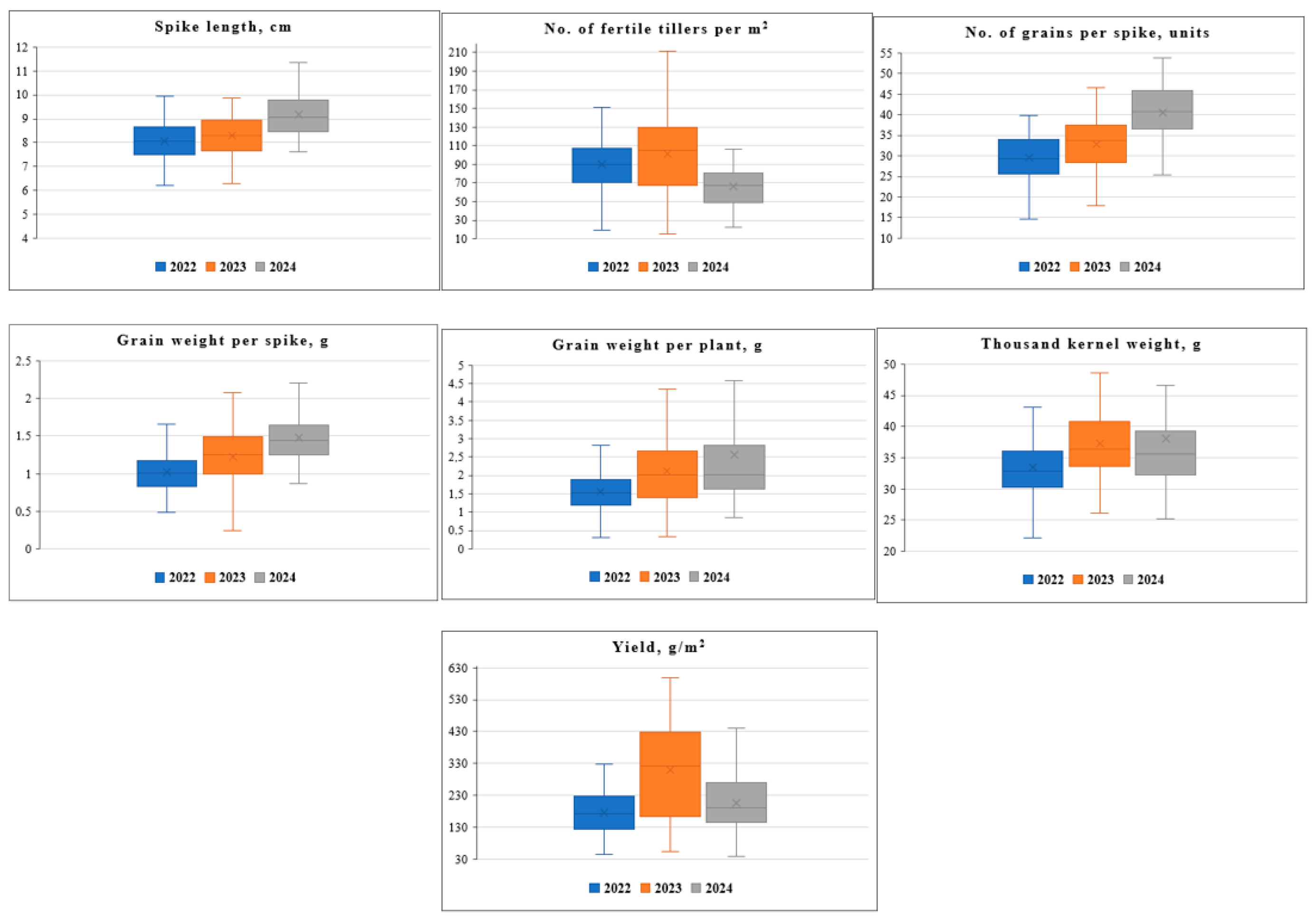
| Trait | 2022 | 2023 | 2024 | ANOVA | ||||
|---|---|---|---|---|---|---|---|---|
| Means * | H2 ** | Means | H2 | Means | H2 | G, % | E, % | |
| Spike length, cm | 8.00 ± 0.82 | 0.56 | 8.30 ± 0.85 | 0.59 | 9.20 ± 0.54 | 0.62 | 72 | 28 |
| No. of fertile tillers per m2 | 89.8 ± 2.90 | 0.58 | 101 ± 4.00 | 0.58 | 66.5 ± 1.80 | 0.61 | 73 | 27 |
| No. of grains per spike, units | 29.5 ± 3.01 | 0.58 | 32.8 ± 3.35 | 0.58 | 40.6 ± 2.39 | 0.64 | 49 | 51 |
| Grain weight per spike, g | 1.00 ± 0.10 | 0.58 | 1.20 ± 0.13 | 0.56 | 1.50 ± 0.87 | 0.63 | 61 | 39 |
| Grain weight per plant, g | 1.70 ± 0.12 | 0.56 | 1.90 ± 0.19 | 0.56 | 2.40 ± 0.24 | 0.66 | 82 | 18 |
| Thousand kernel weight, g | 33.2 ± 3.39 | 0.55 | 37.7 ± 3.84 | 0.56 | 38.1 ± 2.24 | 0.62 | 90 | 10 |
| Yield, g/m2 | 173 ± 17.6 | 0.56 | 315 ± 32.2 | 0.54 | 215 ± 22.2 | 0.60 | 68 | 32 |
| Traits | SL | NFT | NGS | GWS | GWP | TKW | Yield |
|---|---|---|---|---|---|---|---|
| SL | 1.00 | ||||||
| NFT | −0.35 *** | 1.00 | |||||
| NGS | 0.11 * | 0.07 | 1.00 | ||||
| GWS | 0.18 ** | −0.05 | 0.67 *** | 1.00 | |||
| GWP | 0.04 | 0.04 | 0.13 * | 0.42 *** | 1.00 | ||
| TKW | 0.20 *** | −0.06 | −0.12 * | 0.41 *** | 0.65 *** | 1.00 | |
| Yield | −0.16 ** | 0.68 *** | 0.13* | 0.23 *** | 0.18 ** | 0.15 ** | 1.00 |
| KASP-ID | Chr. | Position, cM | Trait | p-Value | MAF |
|---|---|---|---|---|---|
| ipbb_ta_114 | 1A | 50.2 | No. of fertile tillers | 8.85 × 10−5 | 0.116 |
| ipbb_ta_274 | 1B | 99.1 | Vitreousness | 9.32 × 10−6 | 0.031 |
| ipbb_ta_149 | 2B | 76.8 | Seed maturation time | 6.66 × 10−5 | 0.063 |
| ipbb_ta_289 | 2B | 92.0 | Plant height | 7.23 × 10−6 | 0.389 |
| ipbb_ta_259 | 3B | 36.4 | No. of grains per spike | 5.81 × 10−6 | 0.340 |
| ipbb_ta_260 | 3B | 61.2 | No. of grains per spike | 4.62 × 10−6 | 0.348 |
| ipbb_ta_261 | 4A | 40.1 | Grain protein content | 6.02 × 10−6 | 0.063 |
| ipbb_ta_107 | 4A | 161.8 | No. of fertile tillers | 3.80 × 10−6 | 0.021 |
| ipbb_ta_263 | 4B | 85.3 | Spike length | 4.74 × 10−6 | 0.042 |
| ipbb_ta_116 | 5A | 53.5 | Leaf/Stem rust resistance | 3.09 × 10−4 | 0.365 |
| ipbb_ta_239 | 5D | 167.0 | Seed maturation time | 6.62 × 10−6 | 0.106 |
| ipbb_ta_229 | 6A | 0.88 | Heading time | 1.94 × 10−6 | 0.010 |
| ipbb_ta_283 | 6A | 56.3 | Sedimentation value | 6.13 × 10−6 | 0.281 |
| Trait | KASP-ID | Chr. | Allele * | Favourable Season | p-Value | R2, % | SNP Effect |
|---|---|---|---|---|---|---|---|
| SL, cm | ipbb_ta_149 | 2B | C/T | 2022 | 0.01 | 6 | 0.64 |
| 2023 | 0.18 | 2 | 0.35 | ||||
| 2024 | 0.28 | 2 | 0.27 | ||||
| ipbb_ta_261 | 4A | A/G | 2022 | 0.35 | 2 | 0.17 | |
| 2023 | 0.03 | 6 | 0.43 | ||||
| 2024 | 0.25 | 2 | 0.22 | ||||
| NFT, per m2 | ipbb_ta_259 | 3B | C/T | 2022 | 0.24 | 3 | 4.75 |
| 2023 | 0.03 | 8 | 13.2 | ||||
| 2024 | 0.54 | 1 | 1.95 | ||||
| ipbb_ta_107 | 4A | A/G | 2022 | 0.02 | 6 | 24.4 | |
| 2023 | 0.29 | 2 | 15.3 | ||||
| 2024 | 0.47 | 1 | 5.67 | ||||
| ipbb_ta_261 | 4A | A/G | 2022 | 0.40 | 2 | 4.80 | |
| 2023 | 0.01 | 8 | 21.8 | ||||
| 2024 | 0.32 | 2 | 4.51 | ||||
| ipbb_ta_263 | 4B | A/G | 2022 | 0.003 | 8 | 23.0 | |
| 2023 | 0.12 | 2 | 16.8 | ||||
| 2024 | 0.87 | 1 | 0.96 | ||||
| ipbb_ta_239 ** | 5D | A/G | 2022 | 0.05 | 6 | 8.32 | |
| 2023 | 0.45 | 1 | 4.90 | ||||
| 2024 | 0.05 | 6 | 7.09 | ||||
| ipbb_ta_229 | 6A | A/C | 2022 | 0.04 | 5 | 27.5 | |
| 2023 | 0.18 | 2 | 26.2 | ||||
| 2024 | 0.48 | 1 | 7.47 | ||||
| ipbb_ta_283 ** | 6A | C/T | 2022 | 0.05 | 5 | 6.71 | |
| 2023 | 0.64 | 1 | 2.28 | ||||
| 2024 | 0.04 | 5 | 5.55 | ||||
| NGS, units | ipbb_ta_114 ** | 1A | A/G | 2022 | 0.01 | 11 | 2.53 |
| 2023 | 0.04 | 6 | 2.52 | ||||
| 2024 | 0.17 | 4 | 1.48 | ||||
| ipbb_ta_260 | 3B | A/G | 2022 | 0.04 | 7 | 1.82 | |
| 2023 | 0.87 | 1 | 0.19 | ||||
| 2024 | 0.57 | 1 | 0.58 | ||||
| ipbb_ta_229 | 6A | A/C | 2022 | 0.04 | 5 | 6.01 | |
| 2023 | 0.59 | 1 | 2.01 | ||||
| 2024 | 0.63 | 1 | 1.56 | ||||
| GWS, g | ipbb_ta_114 | 1A | A/G | 2022 | 0.88 | 1 | 0.01 |
| 2023 | 0.01 | 11 | 0.16 | ||||
| 2024 | 0.47 | 2 | 0.04 | ||||
| ipbb_ta_289 | 2B | G/T | 2022 | 0.05 | 6 | 0.10 | |
| 2023 | 0.37 | 2 | 0.05 | ||||
| 2024 | 0.64 | 1 | 0.02 | ||||
| ipbb_ta_229 | 6A | A/C | 2022 | 0.13 | 3 | 0.26 | |
| 2023 | 0.04 | 4 | 0.36 | ||||
| 2024 | 0.74 | 1 | 0.05 | ||||
| GWP, g | ipbb_ta_116 | 5A | C/T | 2022 | 0,44 | 1 | 0.17 |
| 2023 | 0,72 | 1 | 0.13 | ||||
| 2024 | 0.01 | 8 | 2.08 | ||||
| ipbb_ta_239 | 5D | A/G | 2022 | 0.81 | 1 | 0.10 | |
| 2023 | 0.02 | 8 | 1.33 | ||||
| 2024 | 0,64 | 1 | 0.23 | ||||
| TKW, g | ipbb_ta_289 | 2B | G/T | 2022 | 0.03 | 8 | 1.73 |
| 2023 | 0.23 | 3 | 1.22 | ||||
| 2024 | 0.18 | 4 | 3.14 | ||||
| ipbb_ta_107 | 4A | A/G | 2022 | 0.01 | 6 | 5.58 | |
| 2023 | 0.23 | 2 | 3.02 | ||||
| 2024 | 0.53 | 1 | 3.58 | ||||
| ipbb_ta_116 | 5A | C/T | 2022 | 0.77 | 1 | 0.26 | |
| 2023 | 0.95 | 1 | 0.07 | ||||
| 2024 | 0.02 | 7 | 5.80 | ||||
| Yield, g/m2 | ipbb_ta_274 | 1B | A/G | 2022 | 0.33 | 1 | 24.0 |
| 2023 | 0.03 | 5 | 102.7 | ||||
| 2024 | 0.50 | 1 | 20.7 | ||||
| ipbb_ta_259 | 3B | C/T | 2022 | 0.40 | 2 | 9.98 | |
| 2023 | 0.01 | 9 | 58.5 | ||||
| 2024 | 0.44 | 2 | 11.2 | ||||
| ipbb_ta_107 ** | 4A | A/G | 2022 | 0.05 | 4 | 57.4 | |
| 2023 | 0.27 | 1 | 30.7 | ||||
| 2024 | 0.05 | 4 | 65.0 |
| Variety, Line | SL, cm | NFT per m2 | NGS, Units | GWS, g | GWP, g | TKW, g | Yield, g/m2 | No. of Alleles |
|---|---|---|---|---|---|---|---|---|
| Russia | ||||||||
| Zhiva | 9.76 * | 91.8 | 37.7 * | 1.43 * | 2.13 * | 38.5 * | 384 * | 7 |
| Donskaya Lira | 8.10 | 115 * | 30.9 * | 1.17 * | 2.33 * | 37.9 * | 367 * | 7 |
| Donna | 8.15 | 94.5 | 31.0 * | 1.18 * | 1.96 * | 37.9 * | 351 * | 8 |
| Zolushka | 9.02 * | 119 * | 34.1 * | 1.30 * | 5.01 * | 37.9 * | 344 * | 8 |
| Doneko | 9.01 * | 105 * | 27.6 | 1.35 * | 1.79 | 49.9 * | 331 * | 8 |
| Vestnitsa | 8.63 * | 121 * | 36.1 * | 1.27 * | 2.23 * | 34.4 | 317 * | 9 |
| Line 2293 K 2-4 | 8.67 * | 103 * | 35.9 * | 1.38 * | 1.96 * | 38.4 * | 303 | 10 |
| Line K 18918 | 7.89 | 139 * | 40.3 * | 1.23 * | 1.96 * | 30.7 | 297 | 12 |
| Bulgaria | ||||||||
| Darunok Podilla | 8.64 * | 97.5 | 38.5 * | 1.59 * | 2.54 * | 40.7 * | 328 * | 7 |
| Türkiye | ||||||||
| Gelibolu | 7.39 | 106 * | 40.3 * | 1.49 * | 1.93 | 36.9 * | 352 * | 7 |
| USA | ||||||||
| CO13D1299 | 8.13 | 139 * | 34.9 * | 1.26 * | 3.06 * | 35.9 * | 495 * | 10 |
| KS13DH0030-32 | 7.78 | 131 * | 41.9 * | 1.44 * | 2.55 * | 34.4 | 362 * | 9 |
| SY Wolf | 7.28 | 133 * | 32.8 * | 1.13 * | 1.65 | 34.0 | 350 * | 8 |
| TCI | ||||||||
| WBLL1*2/Kuruku/5/Chuenmai 18… | 7.62 | 115 * | 34.0 * | 1.09 | 2.02 * | 31.9 | 389 * | 8 |
| OCW03S667T-2/KS020986~1 | 7.98 | 126 * | 31.8 * | 1.16 * | 1.88 | 36.3 * | 369 * | 8 |
| Gondvana//HBK0935-29-15/ KS90W077-2-2 | 8.61 * | 98.2 | 35.5 * | 1.38 * | 2.08 * | 38.6 * | 312 * | 7 |
| Omskaya 4, St | 8.28 | 96.8 | 29.1 | 1.06 | 1.77 | 32.9 | 288 | 8 |
| LCD05 | 0.17 | 4.65 | 0.94 | 0.08 | 0.17 | 1.90 | 16.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamanin, V.; Shepelev, S.; Kovalchuk, A.; Morgounov, A.; Turuspekov, Y.; Pototskaya, I. SNP Effects on Yield and Agronomic Traits in an International Winter Wheat Collection Grown in Western Siberia. Crops 2025, 5, 41. https://doi.org/10.3390/crops5040041
Shamanin V, Shepelev S, Kovalchuk A, Morgounov A, Turuspekov Y, Pototskaya I. SNP Effects on Yield and Agronomic Traits in an International Winter Wheat Collection Grown in Western Siberia. Crops. 2025; 5(4):41. https://doi.org/10.3390/crops5040041
Chicago/Turabian StyleShamanin, Vladimir, Sergey Shepelev, Alexandr Kovalchuk, Alexey Morgounov, Yerlan Turuspekov, and Inna Pototskaya. 2025. "SNP Effects on Yield and Agronomic Traits in an International Winter Wheat Collection Grown in Western Siberia" Crops 5, no. 4: 41. https://doi.org/10.3390/crops5040041
APA StyleShamanin, V., Shepelev, S., Kovalchuk, A., Morgounov, A., Turuspekov, Y., & Pototskaya, I. (2025). SNP Effects on Yield and Agronomic Traits in an International Winter Wheat Collection Grown in Western Siberia. Crops, 5(4), 41. https://doi.org/10.3390/crops5040041






