Efficacy and Differential Physiological–Biochemical Response of Biostimulants in Green Beans Subjected to Moderate and Severe Water Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Crop Management
2.2. Experimental Design
2.3. Plant Sampling
2.4. Plant Analysis
2.4.1. Aerial, Foliar, Root and Total Biomass
2.4.2. Biomass and Yield
2.4.3. Nitrate Reductase Activity In Vivo
2.4.4. Photosynthetic Pigments
2.4.5. Chlorophyll Index
2.4.6. Amino Acids and Soluble Proteins
2.4.7. Proline, Sucrose, Glucose and Fructose Assay
2.4.8. Photosynthetic Activity, Stomatal Conductance, Maximum Fluorescence and Transpiration
2.5. Statistical Analysis
3. Results and Discussion
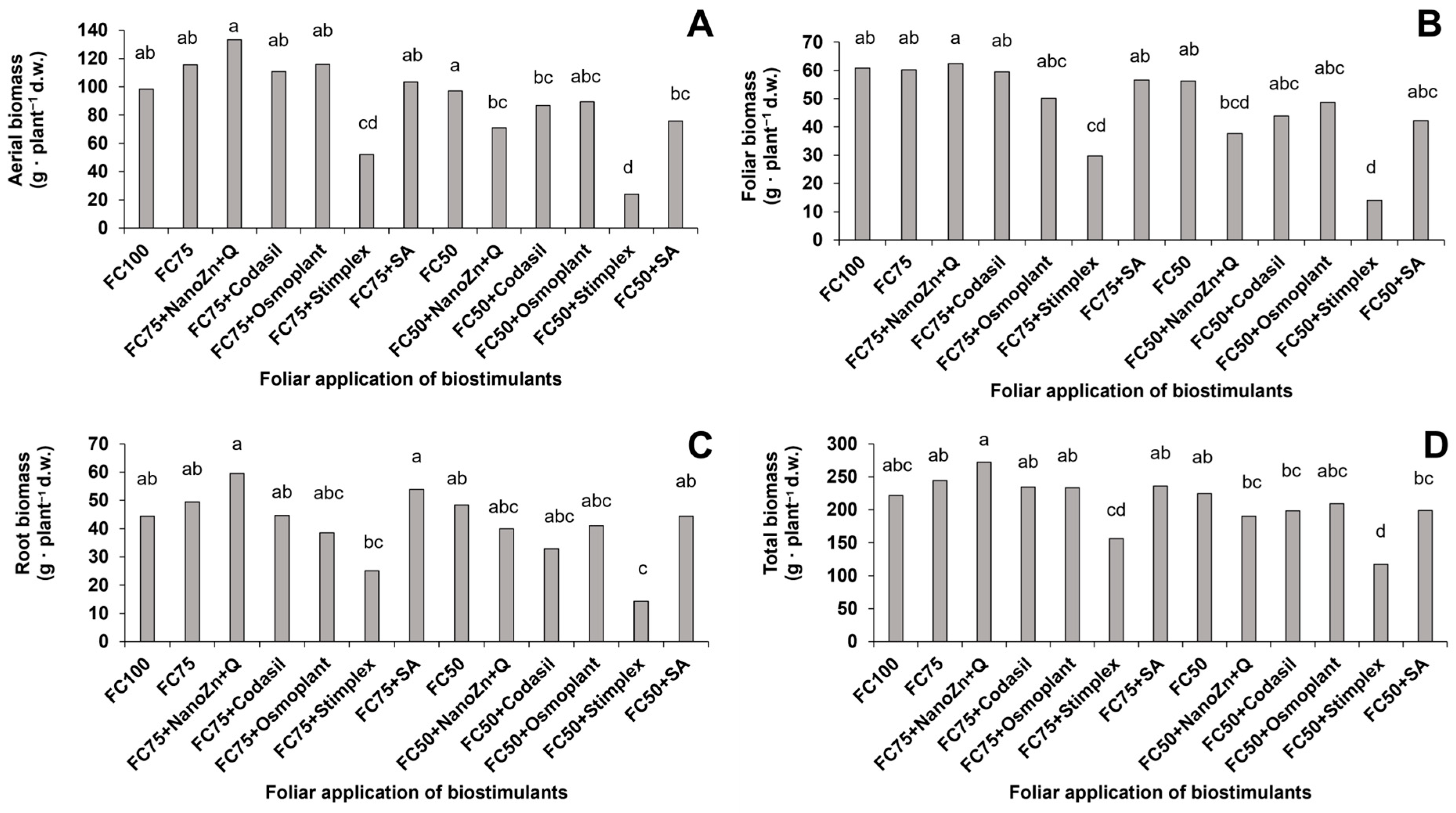
3.1. Aerial, Foliar, Root and Total Biomass
3.2. Yield
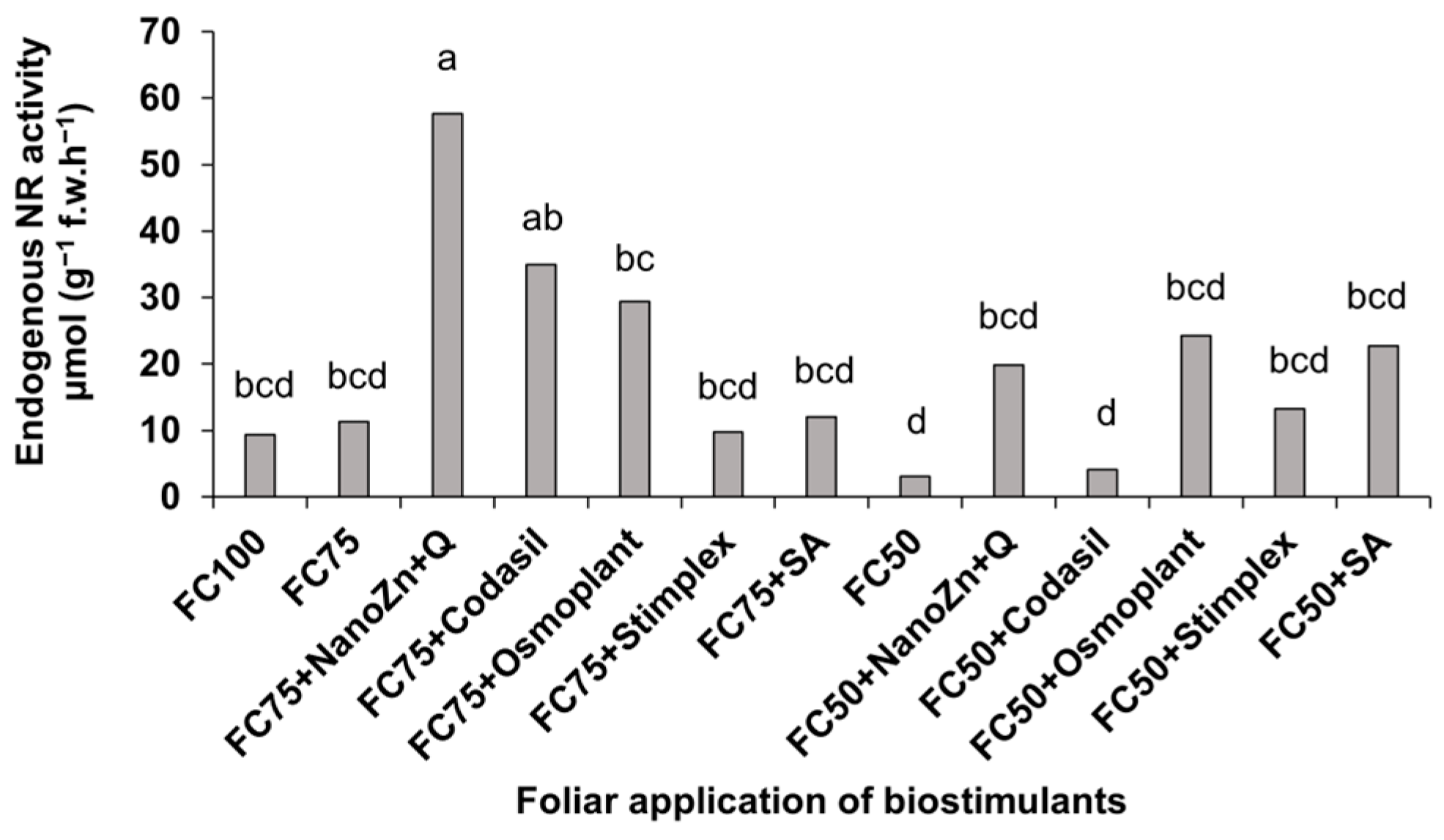
3.3. Nitrate Reductase Activity In Vivo
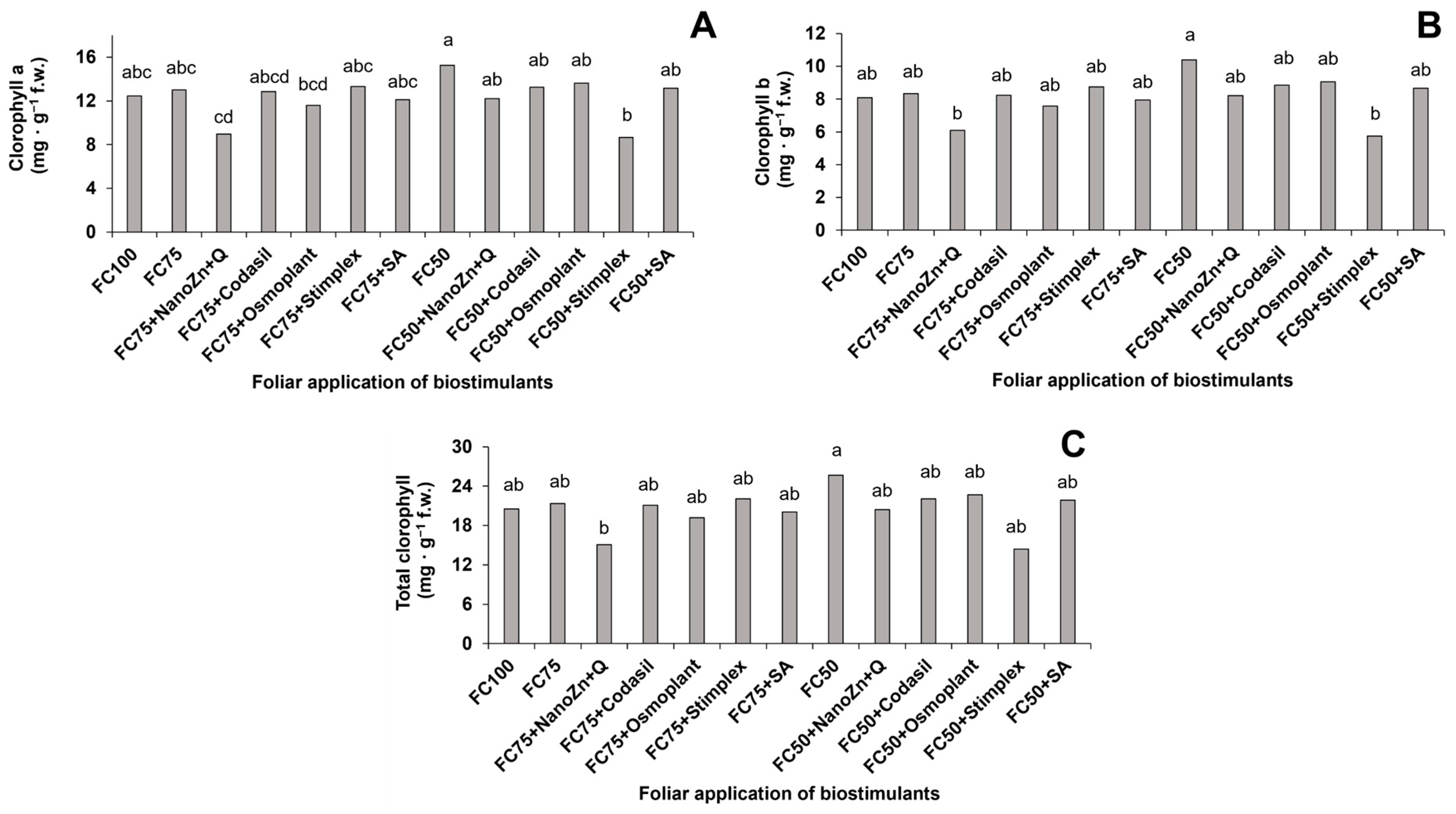
3.4. Photosynthetic Pigments
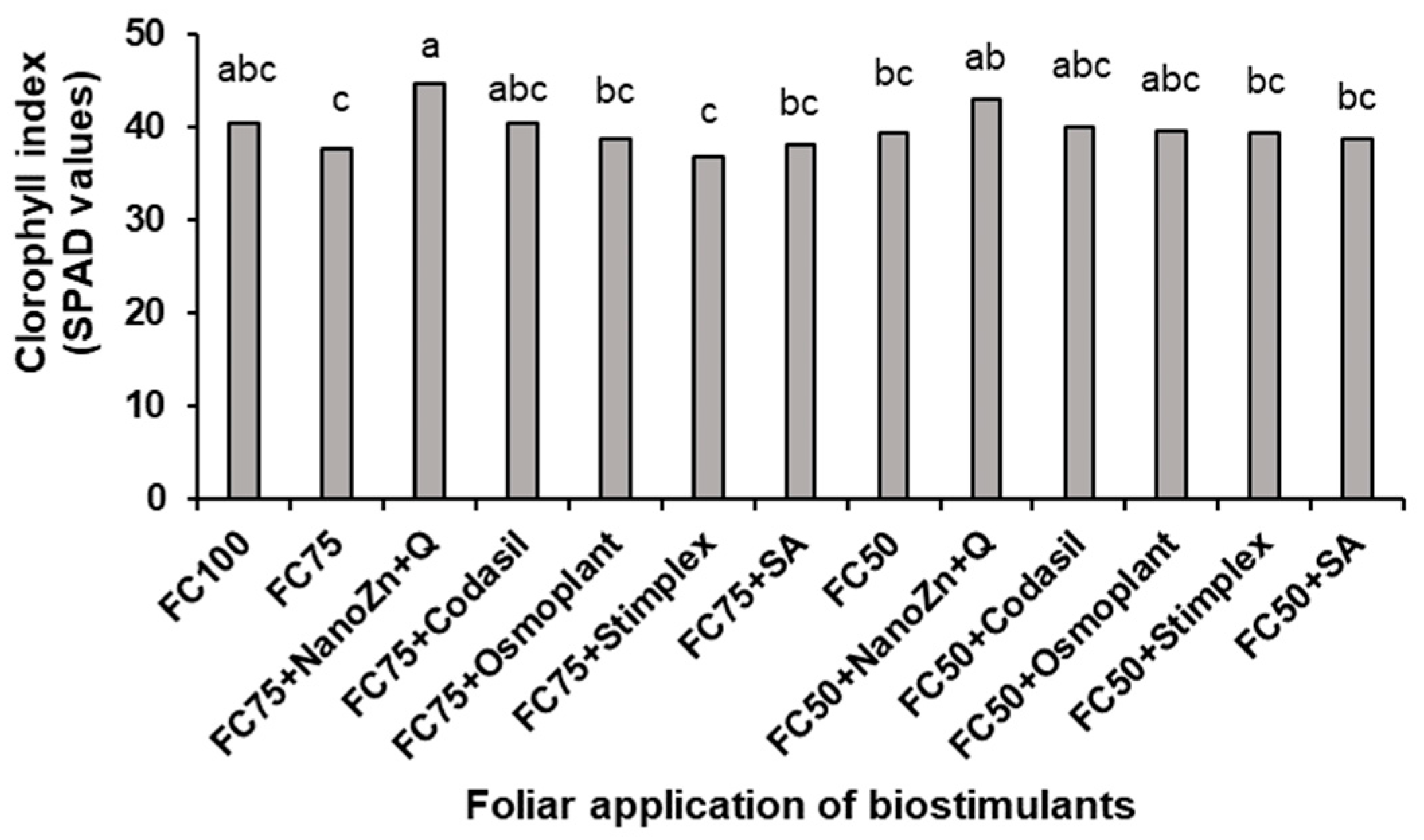
3.5. Chlorophyll Index
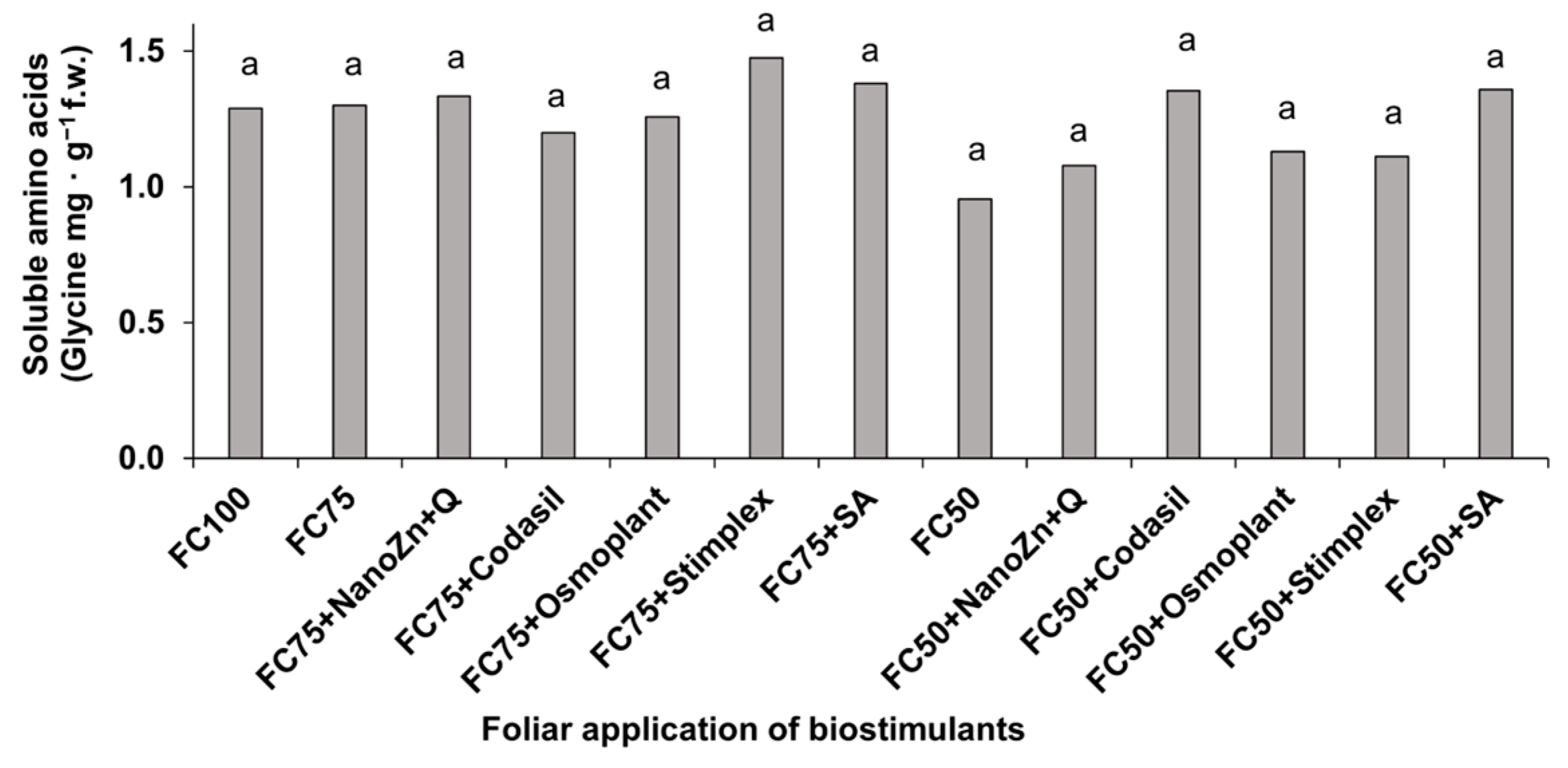
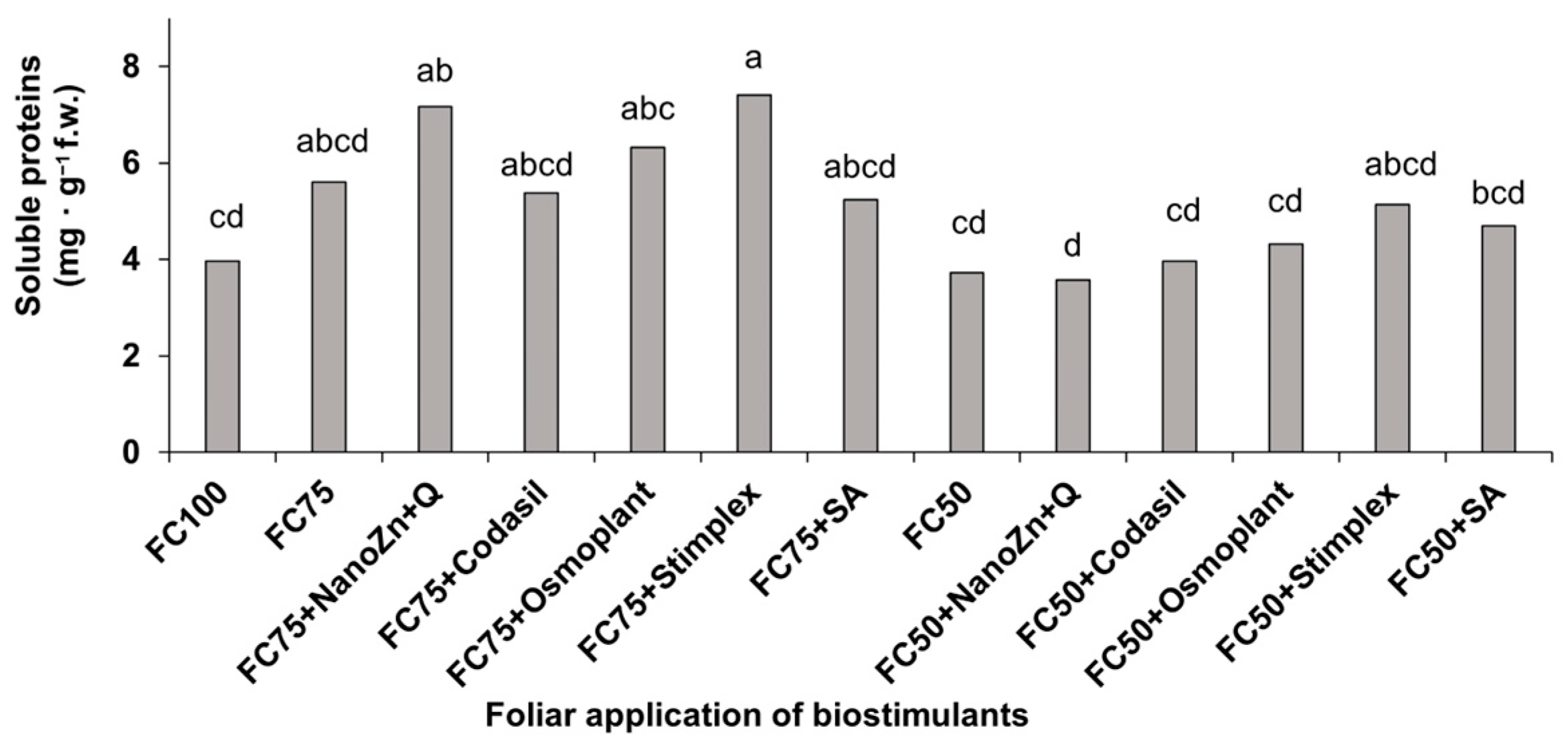
3.6. Amino Acids and Soluble Proteins
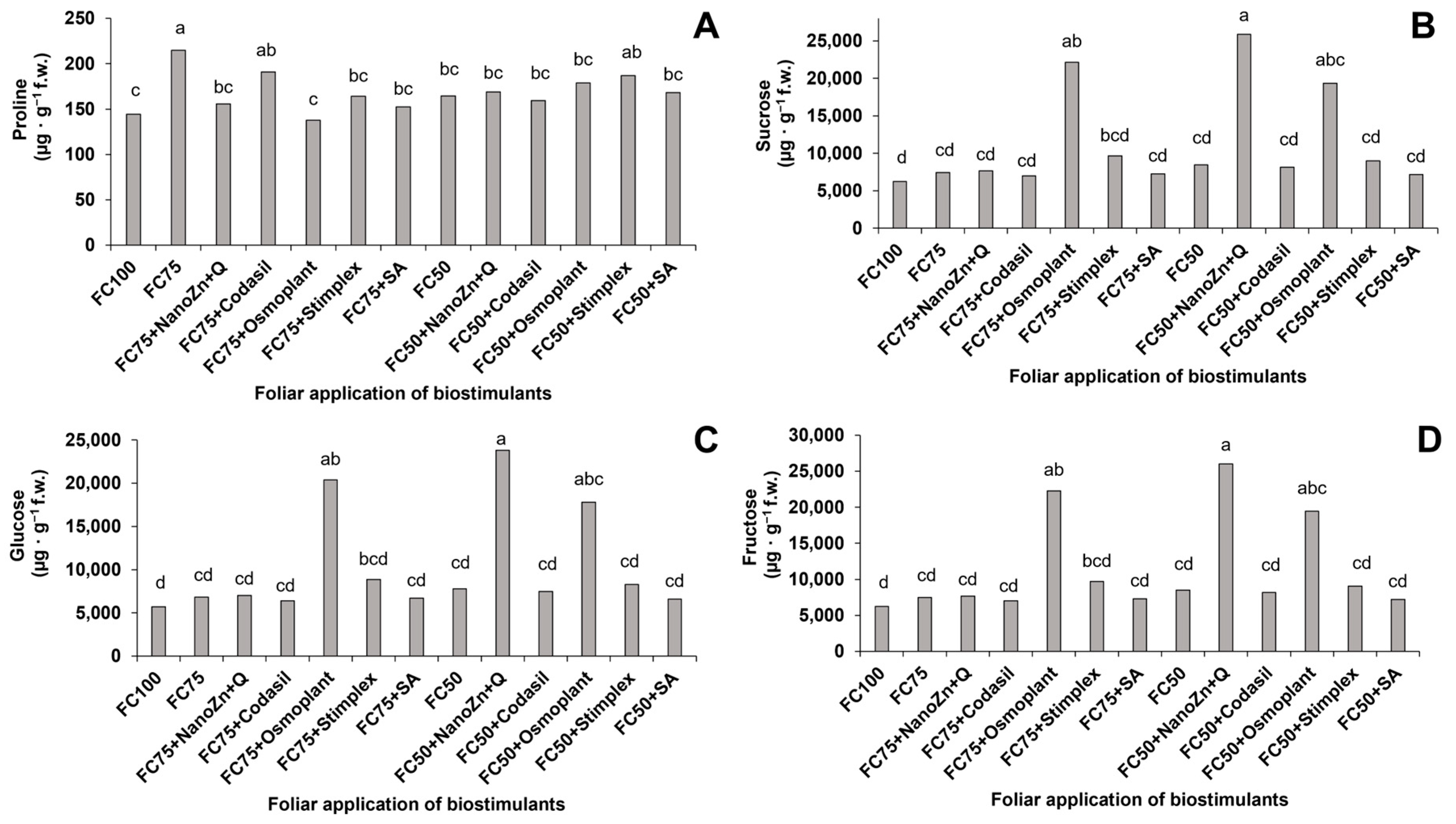
3.7. Content of Proline, Sucrose, Glucose and Fructose
3.8. Photosynthetic Activity, Stomatal Conductance, Fluorescence and Transpiration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Haghighi, M.; Saadat, S.; Abbey, L. Effect of exogenous amino acids application on growth and nutritional value of cabbage under drought stress. Sci. Hortic. 2020, 272, 109561. [Google Scholar] [CrossRef]
- Kissel, E.; Van Asten, P.; Swennen, R.; Lorenzen, J.; Carpentier, S.C. Transpiration efficiency versus growth: Exploring the banana biodiversity for drought tolerance. Sci. Hortic. 2015, 185, 175–182. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Molecular responses to drought stress in plants. Biol. Plant. 2017, 61, 201–209. [Google Scholar] [CrossRef]
- Mathobo, R.; Marais, D.; Steyn, J.M. The effect of drought stress on yield, leaf gaseous exchange and chlorophyll fluorescence of dry beans (Phaseolus vulgaris L.). Agric. Water Manag. 2017, 180, 118–125. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Santiago, L.; Navarro-León, E.; López-Moreno, F.J.; Arjó, G.; González, L.M.; Ruiz, J.M.; Blasco, B. The application of the silicon-based biostimulant Codasil® offset water deficit of lettuce plants. Sci. Hortic. 2021, 285, 110177. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustaka, J.; Ouzounidou, G.; Moustakas, M. Leaf age-dependent photosystem II photochemistry and oxidative stress responses to drought stress in Arabidopsis thaliana are modulated by flavonoid accumulation. Molecules 2021, 26, 4157. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Sun, L.; Song, F.; Zhu, X.; Liu, S.; Liu, F.; Wang, Y.; Li, X. Nano-ZnO alleviates drought stress via modulating the plant water use and carbohydrate metabolism in maize. Arch. Agron. Soil Sci. 2021, 67, 245–259. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response mechanism of plants to drought stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Palacio-Márquez, A.; Ramírez-Estrada, C.A.; Gutiérrez-Ruelas, N.J.; Sánchez, E.; Ojeda-Barrios, D.L.; Chávez-Mendoza, C.; Sida-Arreola, J.P. Efficiency of foliar application of zinc oxide nanoparticles versus zinc nitrate complexed with chitosan on nitrogen assimilation, photosynthetic activity, and production of green beans (Phaseolus vulgaris L.). Sci. Hortic. 2021, 288, 110297. [Google Scholar] [CrossRef]
- Mondal, M.M.A.; Malek, M.A.; Puteh, A.B.; Ismail, M.R. Foliar application of chitosan on growth and yield attributes of mungbean (Vigna radiata (L.) Wilczek). Bangladesh J. Bot. 2013, 42, 179–183. [Google Scholar] [CrossRef]
- Sánchez-Chávez, E.; Barrera-Tovar, R.; Muñoz-Márquez, E.; Ojeda-Barrios, D.L.; Anchondo-Nájera, Á. Efecto del ácido salicílico sobre biomasa, actividad fotosintética, contenido nutricional y productividad del chile jalapeño. Rev. Chapingo Ser. Hortic. 2011, 17, 63–68. [Google Scholar] [CrossRef]
- Sánchez, E.; Rivero, R.M.; Ruiz, J.M.; Romero, L. Changes in biomass, enzymatic activity and protein concentration in roots and leaves of green bean plants (Phaseolus vulgaris L. cv. Strike) under high NH4NO3 application rates. Sci. Hortic. 2004, 99, 237–248. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Shrestha, S.; Brueck, H.; Asch, F. Chlorophyll index, photochemical reflectance index and chlorophyll fluorescence measurements of rice leaves supplied with different N levels. J. Photochem. Photobiol. B Biol. 2012, 113, 7–13. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford Method For Protein Quantitation. In The Protein Protocols Handbook; Walker, J.M., Ed.; Springer Protocols Handbooks; Humana Press: Totowa, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Einerich, D.W.; Sánchez-Díaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Sánchez, E.; Soto, J.M.; Núñez, A.; Ruiz, J.M.; Romero, L. Biosynthesis of non-structural carbohydrates and their distribution in greenbean plants (Phaseolus vulgaris L. Cv. Strike): Deficiency vs toxicity of nitrogen. Rev. Fit. Mex. 2005, 28, 55–61. [Google Scholar] [CrossRef]
- Patel, K.V.; Nath, M.; Bhatt, M.D.; Dobriyal, A.K.; Bhatt, D. Nanofomulation of zinc oxide and chitosan zinc sustain oxidative stress and alter secondary metabolite profile in tobacco. Chem. Eng. J. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Semida, W.M.; Abdelkhalik, A.; Mohamed, G.F.; Abd El-Mageed, T.A.; Abd El-Mageed, S.A.; Rady, M.M.; Ali, E.F. Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.). Plants. 2021, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- De Micco, V.; Aronne, G. Morpho-anatomical traits for plant adaptation to drought. In Plant Responses to Drought Stress, 1st ed.; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 37–61. [Google Scholar] [CrossRef]
- Salcido-Martinez, A.; Sanchez, E.; Licon-Trillo, L.P.; Perez-Alvarez, S.; Palacio-Marquez, A.; Amaya-Olivas, N.I.; Preciado-Rangel, P. Impact of the foliar application of magnesium nanofertilizer on physiological and biochemical parameters and yield in green beans. Not. Bot. Horti Agrobot. 2020, 48, 2167–2181. [Google Scholar] [CrossRef]
- Etienne, P.; Diquelou, S.; Prudent, M.; Salon, C.; Maillard, A.; Ourry, A. Macro and micronutrient storage in plants and their remobilization when facing scarcity: The case of drought. Agriculture 2018, 8, 14. [Google Scholar] [CrossRef]
- Xu, J.; Luo, X.; Wang, Y.; Feng, Y. Evaluation of zinc oxide nanoparticles on lettuce (Lactuca sativa L.) growth and soil bacterial community. Environ. Sci. Pollut. Res. 2018, 25, 6026–6035. [Google Scholar] [CrossRef]
- Lang, C.P.; Merkt, N.; Zörb, C. Different nitrogen (N) forms affect responses to N form and N supply of rootstocks and grafted grapevines. Plant Sci. 2018, 277, 311–321. [Google Scholar] [CrossRef]
- Pejam, F.; Ardebili, Z.O.; Ladan-Moghadam, A.; Danaee, E. Zinc oxide nanoparticles mediated substantial physiological and molecular changes in tomato. PLoS ONE 2021, 16, e0248778. [Google Scholar] [CrossRef]
- Ghani, M.I.; Saleem, S.; Rather, S.A.; Rehmani, M.S.; Alamri, S.; Rajput, V.D.; Kalaji, H.M.; Saleem, N.; Sail, T.A.; Liu, M. Foliar application of zinc oxide nanoparticles: An effective strategy to mitigate drought stress in cucumber seedling by modulating antioxidant defense system and osmolytes accumulation. Chemosphere 2022, 289, 133202. [Google Scholar] [CrossRef]
- Bautista-Diaz, J.; Cruz-Alvarez, O.; Hernández-Rodríguez, O.A.; Sánchez-Chávez, E.; Jacobo-Cuellar, J.L.; Preciado-Rangel, P.; Ávila-Quezada, G.D.; Ojeda-Barrios, D.L. Zinc sulphate or zinc nanoparticle applications to leaves of green beans. Folia Hortic. 2021, 33, 365–375. [Google Scholar] [CrossRef]
- Xu, C.; Leskovar, D.I. Effects of A. nodosum seaweed extracts on spinach growth, physiology and nutrition value under drought stress. Sci. Hortic. 2015, 183, 39–47. [Google Scholar] [CrossRef]
- El-Kaoaua, M.; Chernane, H.; Benaliat, A.; Neamallah, L. Seaweed liquid extracts effect on Salvia officinalis growth, biochemical compounds and water deficit tolerance. Afr. J. Biotechnol. 2013, 12, 4481–4489. [Google Scholar]
- Heidari, M.; Golpayegani, A. Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). J. Saudi Soc. Agric. Sci. 2012, 11, 57–61. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Ahmad, A.; Battaglia, M.L.; Bilal, H.M.; Alhammad, B.A.; Khan, N. Zinc oxide nanoparticles: A unique saline stress mitigator with the potential to increase future crop production. S. Afr. J. Bot. 2023, 159, 208–218. [Google Scholar] [CrossRef]
- Tegeder, M. Transporters for amino acids in plant cells: Some functions and many unknowns. Curr. Opin. Plant Biol. 2021, 15, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Francioso, O.; Tinti, A.; Schiavon, M.; Pizzeghello, D.; Nardi, S. Evaluation of seaweed extracts from Laminaria and Ascophyllum nodosum spp. as biostimulants in Zea mays L. using a combination of chemical, biochemical and morphological approaches. Front. Plant Sci. 2018, 9, 428. [Google Scholar] [CrossRef] [PubMed]
- Kocira, S.; Szparaga, A.; Findura, P.; Treder, K. Modification of yield and fiber fractions biosynthesis in Phaseolus vulgaris L. by treatment with biostimulants containing amino acids and seaweed extract. Agronomy 2020, 10, 1338. [Google Scholar] [CrossRef]
- Latique, S.; Chernane, H.; Mansori, M.; El Kaoua, M. Seaweed liquid fertilizer effect on physiological and biochemical parameters of bean plant (Phaesolus vulgaris variety Paulista) under hydroponic system. Eur. Sci. J. 2013, 9, 174–191. [Google Scholar]
- Hidangmayum, A.; Sharma, R. Effect of different concentration of commercial seaweed liquid extract of Ascophylum nodosum on germination of onion (Allium cepa L.). Int. J. Sci. Res. 2017, 6, 1488–1491. [Google Scholar]
- Dar, M.I.; Naikoo, M.I.; Rehman, F.; Naushin, F.; Khan, F.A. Proline accumulation in plants: Roles in stress tolerance and plant development. In Osmolytes and Plants Acclimation to Changing Environment, 1st ed.; Iqbal, N., Nazar, R., Khan, N.A., Eds.; Springer: New Delhi, India, 2016; pp. 155–166. [Google Scholar] [CrossRef]
- Jungklang, J.; Saengnil, K.; Uthaibutra, J. Effects of water-deficit stress and paclobutrazol on growth, relative water content, electrolyte leakage, proline content and some antioxidant changes in Curcuma alismatifolia Gagnep. cv. Chiang Mai Pink. Saudi J. Biol. Sci. 2017, 24, 1505–1512. [Google Scholar] [CrossRef]
- Reddy, P.S.; Jogeswar, G.; Rasineni, G.K.; Maheswari, M.; Reddy, A.R.; Varshney, R.K.; Kishor, P.K. Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor (L.) Moench]. Plant Physiol. Biochem. 2015, 94, 104–113. [Google Scholar] [CrossRef]
- Piper, F.I. Drought induces opposite changes in the concentration of non-structural carbohydrates of two evergreen Nothofagus species of differential drought resistance. Ann. For. Sci. 2011, 68, 415–424. [Google Scholar] [CrossRef]
- Abdel-Latef, A.A.H.; Abu-Alhmad, M.F.; Abdelfattah, K.E. The possible roles of priming with ZnO nanoparticles in mitigation of salinity stress in lupine (Lupinus termis) plants. J. Plant Growth Regul. 2017, 36, 60–70. [Google Scholar] [CrossRef]
- Zhao, T.; Deng, X.; Xiao, Q.; Han, Y.; Zhu, S.; Chen, J. IAA priming improves the germination and seedling growth in cotton (Gossypium hirsutum L.) via regulating the endogenous phytohormones and enhancing the sucrose metabolism. Ind. Crops Prod. 2020, 155, 112788. [Google Scholar] [CrossRef]
- Matthews, J.S.; Vialet-Chabrand, S.R.; Lawson, T. Diurnal variation in gas exchange: The balance between carbon fixation and water loss. Plant Physiol. 2017, 174, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Sher, A.; Hussain, S.; Cai, L.J.; Ahmad, M.I.; Jamro, S.A.; Rashid, A. Significance of chemical priming on yield and yield components of wheat under drought stress. Am. J. Plant Sci. 2017, 8, 1339. [Google Scholar] [CrossRef]
- Blum, A.; Tuberosa, R. Dehydration survival of crop plants and its measurement. J. Exp. Bot. 2018, 69, 975–981. [Google Scholar] [CrossRef]
- Zhuang, J.; Wang, Y.; Chi, Y.; Zhou, L.; Chen, J.; Zhou, W.; Song, J.; Zhao, N.; Ding, J. Drought stress strengthens the link between chlorophyll fluorescence parameters and photosynthetic traits. PeerJ 2020, 8, e10046. [Google Scholar] [CrossRef]
- Nazar, R.; Umar, S.; Khan, N.A.; Sareer, O. Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. S. Afr. J. Bot. 2015, 98, 84–94. [Google Scholar] [CrossRef]
- Martel, A.B.; Qaderi, M.M. Does salicylic acid mitigate the adverse effects of temperature and ultraviolet-B radiation on pea (Pisum sativum) plants? Environ. Exp. Bot. 2016, 122, 39–48. [Google Scholar] [CrossRef]


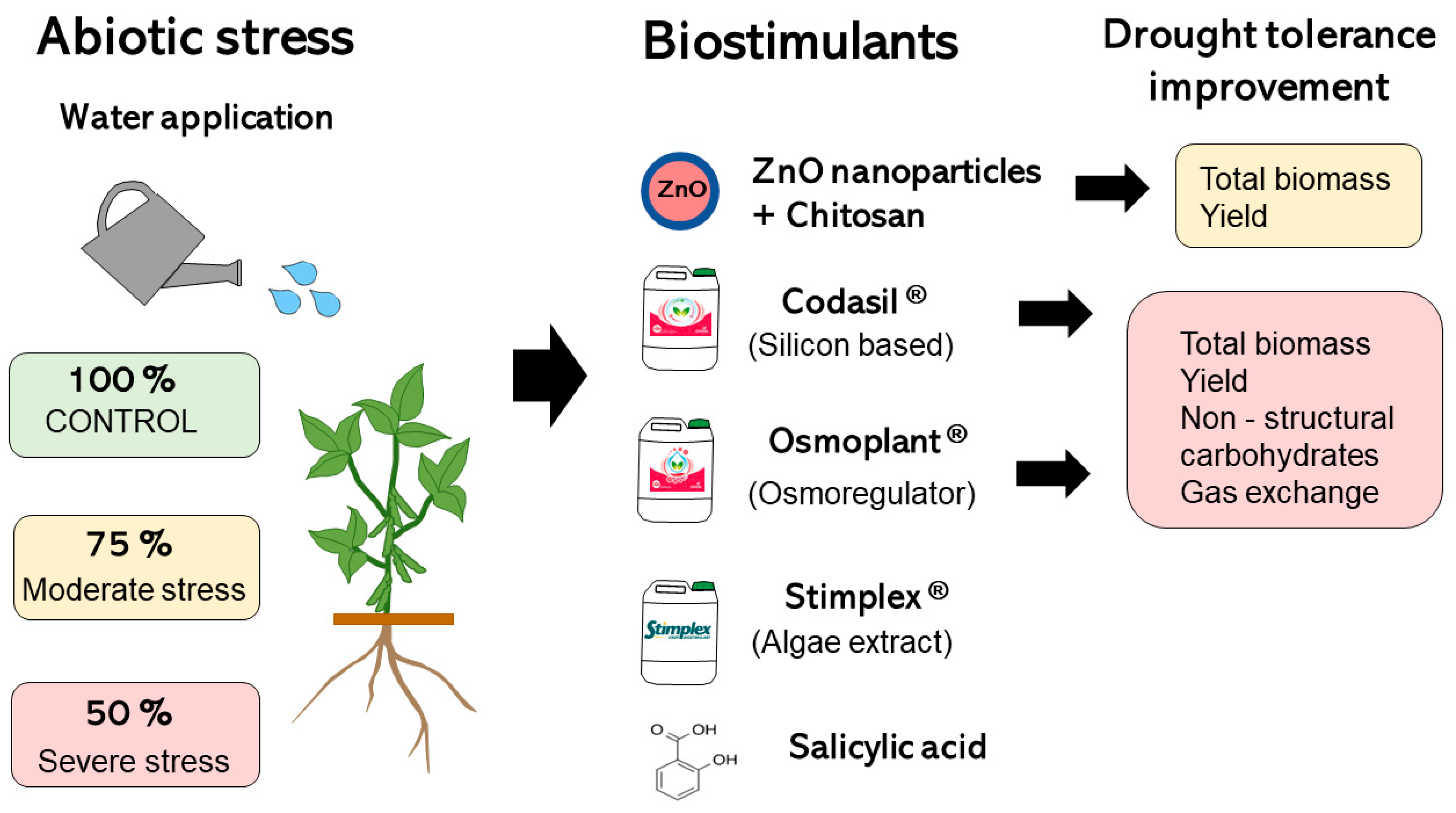
| Percentage of Water Applied (FC) | Added Biostimulant | Doses of Added Biostimulant | Code |
|---|---|---|---|
| 100 | - | - | FC100 |
| 75 | - | - | FC75 |
| 75 | Nano ZnO + Chitosan | 100 ppm | FC75 + NanoZn + Q |
| The75 | Codasil® | 200 ppm | FC75 + Codasil |
| 75 | Osmoplant® | 200 ppm | FC75 + Osmoplant |
| 75 | Stimplex® | 200 ppm | FC75 + Stimplex |
| 75 | Salicylic Acid | 0.1 mM | FC75 + SA |
| 50 | - | - | FC50 |
| 50 | Nano ZnO + Chitosan | 100 ppm | FC50 + NanoZn + Q |
| 50 | Codasil® | 200 ppm | FC50 + Codasil |
| 50 | Osmoplant® | 200 ppm | FC50 + Osmoplant |
| 50 | Stimplex® | 200 ppm | FC50 + Stimlex |
| 50 | Salicylic Acid | 0.1 mM | FC50 + SA |
| Biostimulant | Chemical Composition | Doses |
|---|---|---|
| Codasil® | Liquid solution with a high concentration of soluble silicon composed of 20% silicon, 4% free amino acids and 11.20% potassium. | 2 mL/L (manufacturer’s recommendation). |
| Osmoplant® | Liquid solution composed of 6% free amino acids, 2.4% nitrogen and 3.3% potassium. | 2 mL/L (manufacturer’s recommendation). |
| Stimplex® | Liquid solution composed of Ascophyllum nodosum algae extract as its active ingredient at 0.34%, with a formulation of total nitrogen 0.1% and soluble potassium (K2O) 4.0%. | 2 mL/L (Manufacturer’s recommendation). |
| Zinc Oxide Nanoparticles | <50 nm, 99.9% | 0.1246 g/L (100 ppm) [13]. |
| Chitosan (Poli-D-glucosamine) | 2 mL/L [14]. | |
| Salicylic acid | C7H6O3 | 0.0138 g/1 L. (0.1 mM) [15]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Figueroa, K.I.; Sánchez, E.; Ramírez-Estrada, C.A.; Anchondo-Páez, J.C.; Ojeda-Barrios, D.L.; Pérez-Álvarez, S. Efficacy and Differential Physiological–Biochemical Response of Biostimulants in Green Beans Subjected to Moderate and Severe Water Stress. Crops 2024, 4, 27-42. https://doi.org/10.3390/crops4010003
Hernández-Figueroa KI, Sánchez E, Ramírez-Estrada CA, Anchondo-Páez JC, Ojeda-Barrios DL, Pérez-Álvarez S. Efficacy and Differential Physiological–Biochemical Response of Biostimulants in Green Beans Subjected to Moderate and Severe Water Stress. Crops. 2024; 4(1):27-42. https://doi.org/10.3390/crops4010003
Chicago/Turabian StyleHernández-Figueroa, Karla I., Esteban Sánchez, Carlos A. Ramírez-Estrada, Julio C. Anchondo-Páez, Damaris L. Ojeda-Barrios, and Sandra Pérez-Álvarez. 2024. "Efficacy and Differential Physiological–Biochemical Response of Biostimulants in Green Beans Subjected to Moderate and Severe Water Stress" Crops 4, no. 1: 27-42. https://doi.org/10.3390/crops4010003
APA StyleHernández-Figueroa, K. I., Sánchez, E., Ramírez-Estrada, C. A., Anchondo-Páez, J. C., Ojeda-Barrios, D. L., & Pérez-Álvarez, S. (2024). Efficacy and Differential Physiological–Biochemical Response of Biostimulants in Green Beans Subjected to Moderate and Severe Water Stress. Crops, 4(1), 27-42. https://doi.org/10.3390/crops4010003






