Seed Priming and Pericarp Removal Improve Germination in Low-Germinating Seed Lots of Industrial Hemp
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Germination Conditions
2.3. Tetrazolium Testing
2.4. Seed Priming
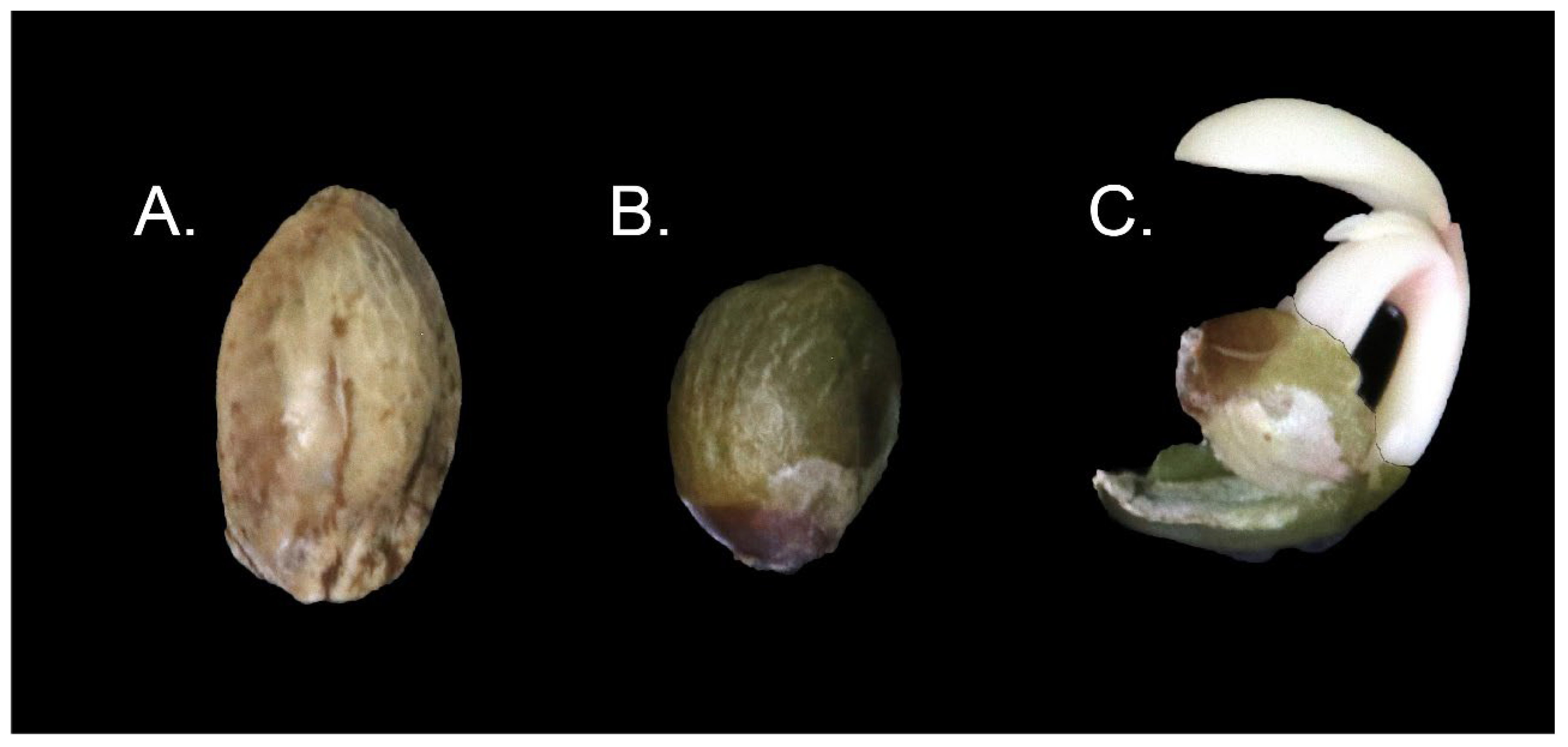
2.5. Pericarp Removal
2.6. Initial Germination Substrate and Normal Seedling Development
2.7. Statistics
3. Results
3.1. Germination and Tetrazolium Staining
3.2. Seed Priming
3.3. Pericarp Removal
4. Discussion
4.1. Germination
4.2. Seed Priming
4.3. Pericarp Removal
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pain, S. A potted history. Nature 2015, 525, S10–S11. [Google Scholar] [CrossRef] [PubMed]
- Schluttenhofer, C.; Yuan, L. Challenges towards revitalizing hemp: A multifaceted crop. Trends Plant Sci. 2017, 22, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Welling, M.T.; Shapter, T.; Rose, T.J.; Liu, L.; Stanger, R.; King, G.J. A belated green revolution for Cannabis: Virtual genetic resources to fast-track cultivar development. Front. Plant Sci. 2016, 7, 1113. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.C.; Merlin, M.D. Cannabis domestication, breeding history, present-day genetic diversity, and future prospects. Crit. Rev. Plant Sci. 2016, 35, 293–327. [Google Scholar] [CrossRef]
- de Meijer, E.P.; Van Soest, L.J.M. The CPRO Cannabis germplasm collection. Euphytica 1992, 62, 201–211. [Google Scholar] [CrossRef]
- Small, E.; Marcus, D. Hemp: A new crop with new uses for North America. In Trends in New Crops and New Uses; Janick, J., Whipkey, A., Eds.; Environmental Science: Washington, DC, USA, 2002; pp. 284–326. [Google Scholar]
- Mankowska, G.; Silska, G. Genetic resources of Cannabis sativa L. in the Collection of the Gene Bank at INF&MP in Poznan. J. Nat. Fibers 2015, 12, 332–340. [Google Scholar]
- Salentijn, E.M.J.; Zhang, Q.; Amaducci, S.; Yang, M.; Trindade, L.M. New developments in fiber hemp (Cannabis sativa L.) breeding. Ind. Crops Prod. 2004, 68, 32–41. [Google Scholar] [CrossRef]
- Welling, M.T.; Liu, L.; Shapter, T.; Raymond, C.A.; King, G.J. Characterization of cannabinoid composition in a diverse Cannabis sativa L. germplasm collection. Euphytica 2016, 208, 463–475. [Google Scholar] [CrossRef]
- Elias, S.G.; Wu, Y.; Stimpson, D. Seed quality and dormancy of hemp (Cannabis staiva L.). J. Agric. Hemp. Res. 2020, 2, 1–15. [Google Scholar]
- Parihar, S.S.; Dadlani, M.; Lal, S.K.; Tonapi, V.A.; Nautiyal, P.C.; Basu, S. Effect of seed moisture content and storage temperature on seed longevity of hemp (Cannabis sativa). Indian J. Agric. Sci. 2014, 84, 1303–1309. [Google Scholar]
- Malik, C.P. Seed deterioration: A review. Int. J. Life Sci. Biotechnol. Pharma Res. 2013, 2, 374–385. [Google Scholar]
- Anderson, J.D.; Baker, J.E. Deterioration of seeds during aging. Phytopathology 1983, 73, 321–325. [Google Scholar] [CrossRef]
- Ellis, R.; Roberts, E. The quantification of ageing and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Perry, D. Seed vigour and seedling establishment. Adv. Res. Technol. Seeds 1980, 5, 25–40. [Google Scholar]
- Zheng, Y.-L.; Ma, H.-C. Effects of seed aging on seed germination and seed reserve utilization in mumian. HortTechnology 2014, 24, 471–474. [Google Scholar] [CrossRef]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- Rudrapal, D.; Nakamura, S. Use of halogens in controlling eggplant and radish seed deterioration. Seed Sci. Techol. 1988, 16, 115–121. [Google Scholar]
- Dearman, J.; Koornneef, M.; Bentsink, L.; Hilhorst, H. Effects of osmotic priming and ageing on onion seed germination. Ann. Appl. Biol. 1986, 108, 639–648. [Google Scholar] [CrossRef]
- Dell’Aquila, A.; Tritto, V. Ageing and osmotic priming in wheat seeds: Effects upon certain components of seed quality. Ann. Bot. 1990, 65, 21–26. [Google Scholar] [CrossRef]
- Varier, A.; Vari, A.K.; Dadlani, M. The subcellular basis of seed priming. Curr. Sci. 2010, 99, 450–456. [Google Scholar]
- Nirmala, K.; Umarani, R. Evaluation of seed priming methods to improve seed vigour of okra (Abelmoschus esculentus) and beetroot (Beta vulgaris). Seed Sci. Res. 2008, 36, 56–65. [Google Scholar] [CrossRef]
- Ueno, K.; Miyoshi, K. Difference of optimum germination temperature of seeds of intact and dehusked japonica rice during seed development. Euphytica 2005, 143, 271–275. [Google Scholar] [CrossRef]
- Griffith, L.W.; Booth, D.T. Indian ricegrass seed damage and germination responses to mechanical treatments. J. Range Manag. 1988, 41, 335–337. [Google Scholar] [CrossRef]
- McWilliam, J.; Phlllips, P. Effect of osmotic and matric potentials on the availability of water for seed germination. Aust. J. Biol. Sci. 1971, 24, 423–432. [Google Scholar] [CrossRef][Green Version]
- Roohi, R.; Jameson, D.A. The Effect of hormone, dehulling and seedbed treatments on germinaton and adventititious root formation in blue grama. J. Range Manag. 1991, 44, 237–241. [Google Scholar] [CrossRef]
- Miyajima, D. Germination of zinnia seed with and without pericarp. Seed Sci. Technol. 1996, 24, 465–473. [Google Scholar]
- Vigliocco, A.E.; Andrade, A.M.; Lindström, L.I.; Alemano, S.G. Dormancy in sunflower line A-3: The role of the pericarp. Botany 2017, 95, 853–858. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing, Edition; International Seed Testing Association (ISTA): Basserdorf, Switzerland, 2008. [Google Scholar]
- AOSA. Rules for Seed Testing; Association of Official Seed Analysts: Witchita, KS, USA, 2021. [Google Scholar]
- Schopfer, P.; Plachy, C. Control of seed germination by abscisic acid: II. Effect on embryo water uptake in Brassica napus L. Plant Physiol. 1984, 76, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Manz, B.; Müller, K.; Kucera, B.; Volke, F.; Leubner-Metzger, G. Water uptake and distribution in germinating tobacco seeds investigated in vivo by nuclear magnetic resonance imaging. Plant Physiol. 2005, 138, 1538–1551. [Google Scholar] [CrossRef]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef]
- Kucera, B.; Cohn, M.A.; Leubner-Metzger, G. Plant hormone interactions during seed dormancy release and germination. Seed Sci. Res. 2005, 15, 281–307. [Google Scholar] [CrossRef]
- Hilhorst, H.W. A critical update on seed dormancy. I. Primary dormancy. Seed Sci. Res. 1995, 5, 61–73. [Google Scholar] [CrossRef]
- Bewley, J.D. Breaking down the walls—A role for endo-β-mannanase in release from seed dormancy? Trends Plant Sci. 1997, 2, 464–469. [Google Scholar] [CrossRef]
- Leubner-Metzger, G. Functions and regulation of β-1, 3-glucanases during seed germination, dormancy release and after-ripening. Seed Sci. Res. 2003, 13, 17–34. [Google Scholar] [CrossRef]
- Koornneef, M.; Bentsink, L.; Hilhorst, H. Seed dormancy and germination. Curr. Opin. Plant Biol. 2002, 5, 33–36. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phtol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Bradford, K.J. Manipulation of seed water relations via osmotic priming to improve germination under stress conditions. HortScience 1986, 21, 1105–1112. [Google Scholar] [CrossRef]
- Jett, L.W.; Welbaum, G.E.; Morse, R.D. Effects of matric and osmotic priming treatments on broccoli seed germination. J. Am. Soc. Hort. Sci. 1996, 121, 423–429. [Google Scholar] [CrossRef]
- Türkmen, Ö.; Dursun, A.; Turan, M.; Erdinç, Ç. Calcium and humic acid affect seed germination, growth, and nutrient content of tomato (Lycopersicon esculentum L.) seedlings under saline soil conditions. Acta Agric. Scand. Sect. B Soil Plant Sci. 2004, 54, 168–174. [Google Scholar] [CrossRef]
- Zehra, A.; Gul, B.; Ansari, R.; Khan, M.A. Role of calcium in alleviating effect of salinity on germination of Phragmites karka seeds. S. Afr. J. Bot. 2012, 78, 122–128. [Google Scholar] [CrossRef]
- Katzman, L.S.; Taylor, A.G.; Langhans, R.W. Seed enhancements to improve spinach germination. HortScience 2001, 36, 979–981. [Google Scholar] [CrossRef]
| Hemp Seed Line | ||||
|---|---|---|---|---|
| TZ Staining (%) | Victoria | Finola | Canda 2014 | Canda 2015 |
| Fully stained z | 76 | 16 | 8 | 15 |
| Variable | 24 | 72 | 60 | 56 |
| Unstained | 0 | 12 | 32 | 29 |
| Victoria | Finola | Canda 2014 | Canda 2015 | |||||
|---|---|---|---|---|---|---|---|---|
| Time (h) | Untreated | Primed | Untreated | Primed | Untreated | Primed | Untreated | Primed |
| 16 | 0 | 49 * | 0 | 18 * | 0 | 7 * | 0 | 15 * |
| 40 | 71 | 82 * | 8 | 20 * | 5 | 11 * | 18 | 26 * |
| 56 | 76 | 90 * | 9 | 22 * | 9 | 12 | 19 | 26 * |
| 72 | 86 | 92 | 13 | 24 | 10 | 12 | 24 | 26 |
| Victoria | Finola | Canda 2014 | Canda 2015 | |||||
|---|---|---|---|---|---|---|---|---|
| Time (h) | Untreated | Primed | Untreated | Primed | Untreated | Primed | Untreated | Primed |
| 16 | 1 | 26 * | 1 | 6 * | 1 | 6 * | 1 | 5 * |
| 40 | 64 | 85 * | 16 | 23 | 4 | 23 * | 9 | 25 * |
| 56 | 79 | 90 * | 17 | 25 | 6 | 25 * | 11 | 31 * |
| 72 | 87 | 90 | 17 | 25 | 6 | 25 * | 12 | 31 * |
| Victoria | Finola | Canda 2014 | Canda 2015 | |||||
|---|---|---|---|---|---|---|---|---|
| Time (h) | Untreated | No Pericarp | Untreated | No Pericarp | Untreated | No Pericarp | Untreated | No Pericarp |
| 16 | 0.5 | 76 * | 2 | 9 | 0.5 | 6 * | 2 | 29 * |
| 40 | 77 | 90 * | 14 | 21 * | 8 | 16 | 23 | 40 * |
| 56 | 87 | 93 | 16 | 26 * | 11 | 20 * | 27 | 47 * |
| 72 | 93 | 96 | 18 | 28 * | 13 | 21 * | 29 | 49 * |
| Cultivar | Treatment | Substrate | Seedling Length (cm) | Seedling Emergence (%) | Abnormal (%) |
|---|---|---|---|---|---|
| Victoria | Intact | Towel | 2.37 ab,* | 86.7 ab | 0 |
| No pericarp | Towel | 2.88 a | 96.7 a | 0 | |
| Intact | Dish 3 mL | 2.00 b | 96.7 a | 0 | |
| No pericarp | Dish 3 mL | 1.51 b | 86.7 ab | 10.3 | |
| Intact | Dish 6 mL | 1.76 b | 80.0 b | 15.4 | |
| No pericarp | Dish 6 mL | 1.62 b | 66.7 c | 35.0 | |
| Canda | Intact | Towel | 1.86 b | 33.3 ab | 10.0 |
| No pericarp | Towel | 2.56 a | 43.3 a | 20.0 | |
| Intact | Dish 3 mL | 1.12 b | 33.3 ab | 25.0 | |
| No pericarp | Dish 3 mL | 1.65 b | 36.7 ab | 23.1 | |
| Intact | Dish 6 mL | 1.56 b | 26.7 b | 45.5 | |
| No pericarp | Dish 6 mL | 1.02 b | 20.0 b | 83.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, J.W.; Kester, S.T.; Su, K.; Hildebrand, D.F.; Geneve, R.L. Seed Priming and Pericarp Removal Improve Germination in Low-Germinating Seed Lots of Industrial Hemp. Crops 2022, 2, 407-414. https://doi.org/10.3390/crops2040028
Tan JW, Kester ST, Su K, Hildebrand DF, Geneve RL. Seed Priming and Pericarp Removal Improve Germination in Low-Germinating Seed Lots of Industrial Hemp. Crops. 2022; 2(4):407-414. https://doi.org/10.3390/crops2040028
Chicago/Turabian StyleTan, Jia W., Sharon T. Kester, Kai Su, David F. Hildebrand, and Robert L. Geneve. 2022. "Seed Priming and Pericarp Removal Improve Germination in Low-Germinating Seed Lots of Industrial Hemp" Crops 2, no. 4: 407-414. https://doi.org/10.3390/crops2040028
APA StyleTan, J. W., Kester, S. T., Su, K., Hildebrand, D. F., & Geneve, R. L. (2022). Seed Priming and Pericarp Removal Improve Germination in Low-Germinating Seed Lots of Industrial Hemp. Crops, 2(4), 407-414. https://doi.org/10.3390/crops2040028








