Temperature Limits for Seed Germination in Industrial Hemp (Cannabis sativa L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Germination Conditions
2.3. Germination and Temperature
2.4. Seed Priming
2.5. Data Analysis
3. Results
3.1. Germination and Vigor
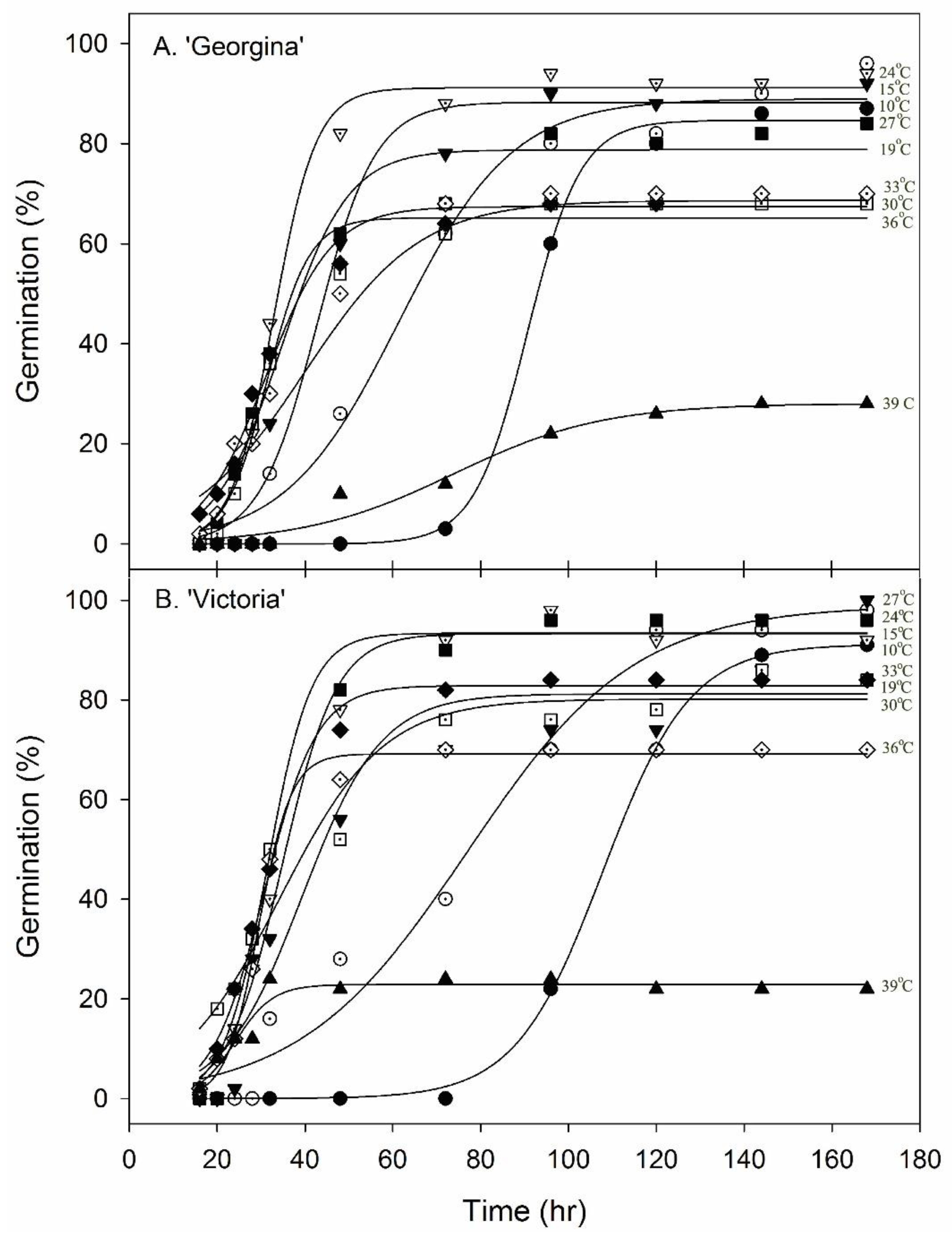
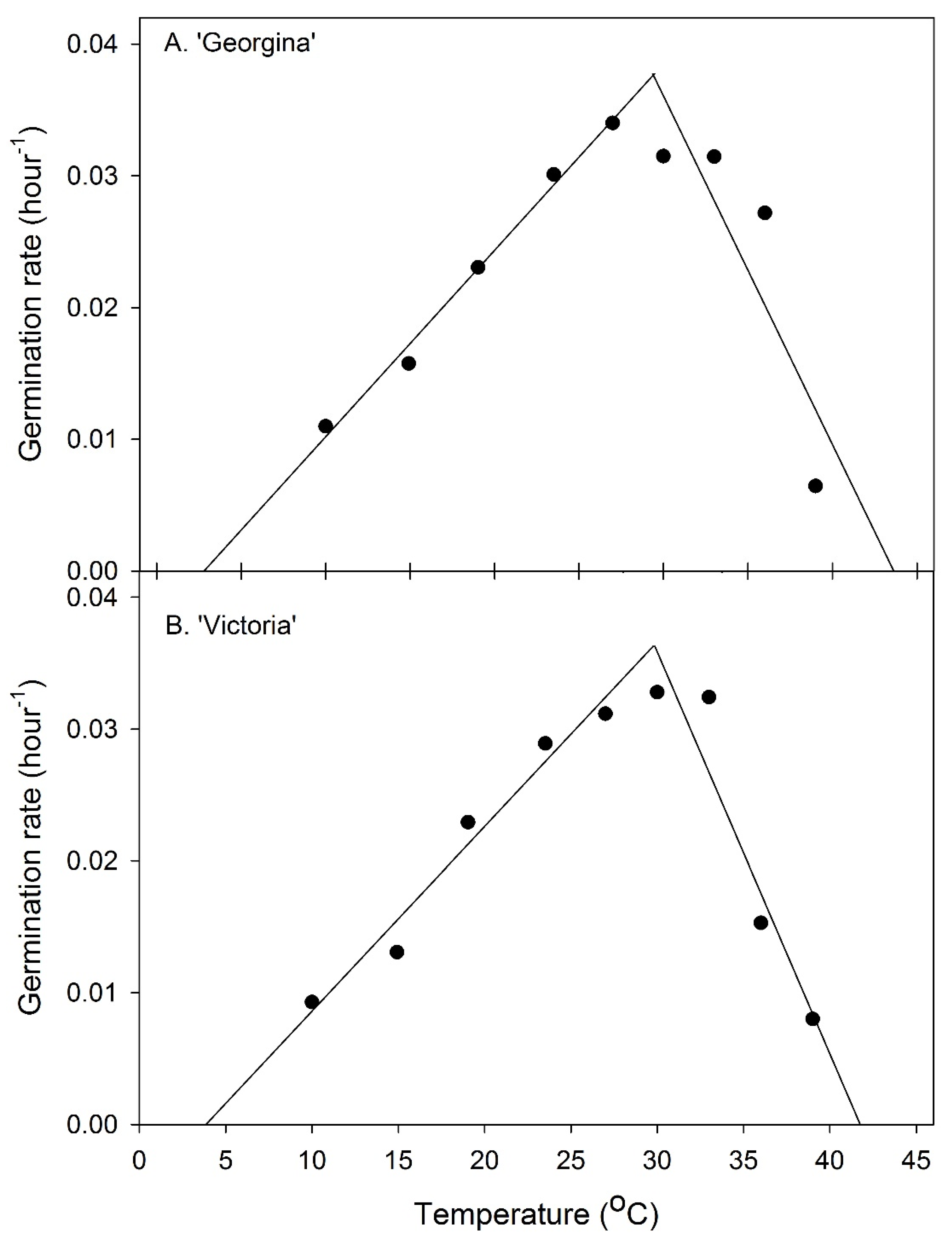
3.2. Germination and Temperature
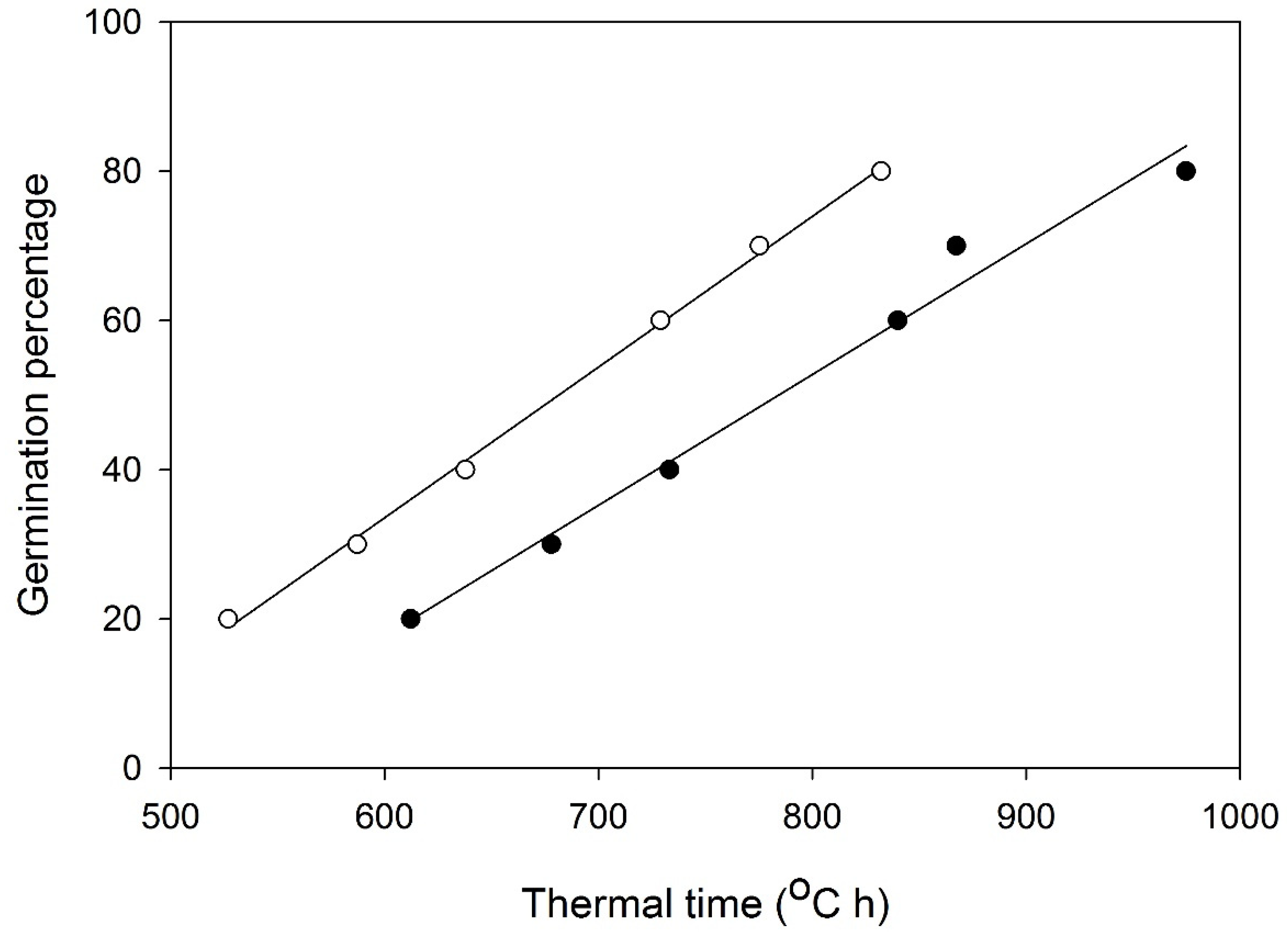
3.3. Thermal Time
3.4. Seed Priming
4. Discussion
4.1. Germination and Vigor
4.2. Germination and Temperature
4.3. Thermal Time
4.4. Seed Priming
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Courtwright, D.T. Forces of Habit: Drugs and the Making of Modern World; Harvard University Press: Cambridge, MA, USA, 2002; ISBN 9780674010031. [Google Scholar]
- Johnson, R. Hemp as an Agricultural Commodity; Congressional Research Service Report 7-5700. 2015. Available online: http://www.fas.org/sgp/crs/misc/RL32725.pdf (accessed on 21 June 2022).
- Karus, M.; Vogt, D. European hemp industry: Cultivation, processing and product lines. Euphytica 2004, 140, 7–12. [Google Scholar] [CrossRef]
- Amaducci, S.; Scordia, D.; Liu, F.H.; Zhang, Q.; Guo, H.; Testa, G.; Cosentino, S.L. Key cultivation techniques for hemp in Europe and China. Industrial Crops Prod. 2015, 68, 2–16. [Google Scholar] [CrossRef]
- Amaducci, S.; Colauzzi, M.; Bellocchi, G.; Venturi, G. Modelling post-emergent hemp phenology (Cannabis sativa L.): Theory and evaluation. Eur. J. Agron. 2008, 28, 90–102. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Testa, G.; Scordia, D.; Copani, V. Sowing time and prediction of flowering of different hemp (Cannabis sativa L.) genotypes in southern Europe. Ind. Crops Prod. 2012, 37, 20–33. [Google Scholar] [CrossRef]
- Lisson, S.N.; Mendham, N.J.; Carberry, P.S. 2000. Development of a hemp (Cannabis sativa L.) simulation model 1. General introduction and the effect of temperature on the pre-emergent development of hemp. Aust. J. Exp. Agric. 2000, 40, 405–411. [Google Scholar] [CrossRef]
- Qin, C.X.; Wang, F.Y.; Wen, D.Q.; Qin, W. The effect of different temperatures treatment on fire hemp seeds germination. Med. Plant 2014, 5, 70–72. [Google Scholar]
- Varga, I.; Iljkić, D.; Tkalec Kojić, M.; Dobreva, T.; Markulj Kulundžić, A.; Antunović, M. Germination of industrial hemp (Cannabis sativa L.) at different level of sodium chloride and temperatures. Agric. Conspec. Sci. 2022, 87, 11–15. [Google Scholar]
- Byrd, J.A. Industrial Hemp (Cannabis sativa L.) Germination Temperatures and Herbicide Tolerance Screening. Master’s Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2019. Available online: http://hdl.handle.net/10919/91431 (accessed on 21 June 2022).
- Akter, N.; Rafiqul Islam, M. Heat stress effects and management in wheat. A review. Agron. Sustain. Dev. 2017, 37, 37. [Google Scholar] [CrossRef]
- Silva-Correia, J.; Freitas, S.; Tavares, R.M.; Lino-Neto, T.; Azevedo, H. Phenotypic analysis of the Arabidopsis heat stress response during germination and early seedling development. Plant Methods 2014, 10, 1. [Google Scholar] [CrossRef]
- Essemine, J.; Ammar, S.; Bouzid, S. Impact of heat stress on germination and growth in higher plants: Physiological, biochemical and molecular repercussions and mechanisms of defence. J. Biosci. 2010, 10, 565–572. [Google Scholar] [CrossRef]
- Howarth, C.J. Genetic improvements of tolerance to high temperature. In Abiotic Stresses: Plant Resistance through Breeding and Molecular Approaches; Howarth Press Inc.: New York, NY, USA, 2005; pp. 277–300. [Google Scholar]
- Pawar, V.A.; Laware, S.L. Seed priming a critical review. Int. J. Sci. Res. Biol. Sci. 2018, 5, 94–101. [Google Scholar] [CrossRef]
- Bruggink, G.T.; Ooms, J.; van der Toorn, P. Induction of longevity in primed seeds. Seed Sci. Res. 1999, 9, 49–53. [Google Scholar] [CrossRef]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant. 2013, 35, 1381–1396. [Google Scholar] [CrossRef]
- Fujita, M.; Fujita, Y.; Noutoshi, Y.; Takahashi, F.; Narusaka, Y.; Yamaguchi-Shinozaki, K.; Shinozak, K. Crosstalk between abiotic and biotic stress responses: A current view from the points of convergence in the stress signaling networks. Curr. Opin. Plant Biol. 2006, 9, 436–442. [Google Scholar] [CrossRef]
- Beckers, G.J.M.; Conrath, U. Priming for stress resistance: From the lab to the field. Curr. Opin. Plant Biol. 2007, 10, 425–431. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing; International Seed Testing Association (ISTA): Basserdorf, Switzerland, 2008. [Google Scholar]
- Gummerson, R.J. The effect of constant temperatures and osmotic potential on the germination of sugar beet. J. Exp. Bot. 1986, 37, 729–741. [Google Scholar] [CrossRef]
- Elias, S.G.; Wu, Y.; Stimpson, D. Seed quality and dormancy of hemp (Cannabis staiva L.). J. Agric. Hemp Res. 2020, 2, 1–15. [Google Scholar]
- Parihar, S.; Dadlani, M.; Lal, S.; Tonapi, V.; Nautiyal, P.; Basu, S.A. Effect of seed moisture content and storage temperature on seed longevity of hemp (Cannabis sativa). Indian J. Agri. Sci. 2014, 84, 1303–1309. [Google Scholar]
- Amaducci, S.; Colauzzi, M.; Zatta, A.; Venturi, G. Flowering dynamics in monoecious and dioecious hemp genotypes. J. Ind. Hemp 2008, 13, 5–19. [Google Scholar] [CrossRef]
- Bradford, K.J. Applications of hydrothermal time to quantifying and modeling seed germination and dormancy. Weed Sci. 2002, 50, 248–260. [Google Scholar] [CrossRef]
- Covell, S.; Ellis, R.H.; Roberts, E.H.; Summerfield, R.J. The influence of temperature on seed germination rate in grain legumes: I. A comparison of chickpea, lentil, soyabean and cowpea at constant temperatures. J. Exp. Bot. 1986, 37, 705–715. [Google Scholar] [CrossRef]
- Hardegree, S.P. Predicting germination response to temperature. I. Cardinal-temperature models and subpopulation-specific regression. Ann. Bot. 2006, 97, 1115–1125. [Google Scholar] [CrossRef]
- Watt, M.S.; Bloomberg, M. Key features of the seed germination response to high temperatures. New Phytol. 2012, 196, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Barney, J.; Ponder, M.A.; Welbaum, G.E. Germination response of six sweet basil (Ocimum basilicum) cultivars to temperature. Seed Technol. 2016, 37, 43–51. [Google Scholar]
- Orozco-Segovia, A.; González-Zertuche, L.; Mendoza, A.; Orozco, S. A mathematical model that uses Gaussian distribution to analyze the germination of Manfreda brachystachya (Agavaceae) in a thermogradient. Physiol. Plant. 1996, 98, 431–438. [Google Scholar] [CrossRef]
- Zhang, H.; McGill, C.R.; Irving, L.J.; Kemp, P.D.; Zhou, D. A modified thermal time model to predict germination rate of ryegrass and tall fescue at constant temperatures. Crop Sci. 2013, 53, 240–249. [Google Scholar] [CrossRef]
- Jett, L.W.; Welbaum, G.E.; Morse, R.D. Effects of matric and osmotic priming treatments on broccoli seed germination. J. Am. Soc. Hortic. Sci. 1996, 121, 423–429. [Google Scholar] [CrossRef]
- Kang, J.; Choi, Y.; Son, B.; Lee, Y.; Ahn, C.; Choi, I.; Park, H. Effect of osmotic priming and solid matrix priming to improved seed vigor and early growth of pepper and tomato seeds. Korean J. Life Sci. 2003, 13, 433–440. [Google Scholar]
- Parera, C.A.; Qiao, P.; Cantliffe, D.J. Enhanced celery germination at stress temperature via solid matrix priming. HortScience 1993, 28, 20–22. [Google Scholar] [CrossRef]
- Tamm, E. Weitere Untersuchungen fiber die Keiming und das Auflaufen landwirt- schaftlicher Kulturpfianzen. Pflanzenbau 1933, 10, 297–313. [Google Scholar]
- Werf, H.V.D.; Brouwer, K.; Wijlhuizen, M.; Withagen, J.M. The effect of temperature on leaf appearance and canopy establishment in fibre hemp (Cannabis sativa L.). Ann. Appl. Biol. 1995, 126, 551–561. [Google Scholar] [CrossRef]
| Standard Germination (%) | Accelerated Aging Germination (%) | |||||
|---|---|---|---|---|---|---|
| Cultivar | 3 days | 7 days | Moisture (%) | 3 days | 7 days | Moisture (%) |
| ‘Georgina’ | 76.5 bx | 89.5 a | 8.3 | 57.5 b | 74.5 b | 24.9 |
| ‘Victoria’ | 84.0 a | 91.5 a | 8.4 | 81.0 a | 88.0 a | 23.5 |
| Cultivar | Temperature (°C) | Germination Percentage | Time to 50% Germination (h) | (T80)–(T20) |
|---|---|---|---|---|
| ‘Georgina’ | 3 10 15 19 24 27 30 33 36 39 42 | 0 87 96 92 94 84 68 70 70 29 0 | - 91.0 63.5 43.4 33.2 32.5 31.7 31.8 36.8 - - | - 17.5 40.5 19.6 14.0 13.8 14.0 21.8 27.0 - - |
| ‘Victoria’ | 3 10 15 19 24 27 30 33 36 39 42 | 0 91 98 100 92 96 84 84 70 22 0 | - 107.5 76.5 43.7 34.6 32.1 30.5 30.9 65.4 - - | - 23.0 51.2 29.2 17.3 12.3 14.8 13.9 39.5 - - |
| Tetrazolium Staining in Non-Germinated Seeds | |||||
|---|---|---|---|---|---|
| Initial Temperature | Cultivar | Duration (Days) | Germination (%) | Viable (%) | Non-Viable (%) |
| 3 °C | ‘Georgina’ | 0 | 87 ax | - | - |
| 7 | 82 a | - | - | ||
| ‘Victoria’ | 0 | 89 a | - | - | |
| 7 | 86 a | - | - | ||
| 42 °C | ‘Georgina’ | 0 | 84 a | 11 | 5 |
| 1 | 70 b | 5 | 25 | ||
| 2 | 64 bc | 3 | 33 | ||
| 3 | 25 c | 0 | 75 | ||
| 4 | 0 | 1 | 99 | ||
| ‘Victoria’ | 0 1 2 3 4 | 86 a 73 b 44 c 8 d 0 | 11 13 10 0 0 | 3 13 45 92 100 | |
| Time (Days after Sowing) | ||||||
|---|---|---|---|---|---|---|
| Temperature (°C) | 2 | 3 | 4 | 5 | 6 | 7 |
| 20/30 constant | 0 | 27 | 72 | 88 | 88 | 91 a |
| 35 constant | 7 | 36 | 55 | 63 | 71 | 71 b |
| 20/30 moved to 35 | 1 | 51 | 76 | 83 | 90 | 90 a |
| 35 moved to 20/30 | 1 | 12 | 51 | 70 | 71 | 71 b |
| ‘Georgina’ | ‘Victoria’ | |||||
|---|---|---|---|---|---|---|
| Germination Percentage Subpopulation | Germination Rate | R2 | Thermal Time (°C h) | Germination Rate | R2 | Thermal Time (°C h) |
| 20 | 0.00163 | 0.99 | 612.11 | 0.00190 | 0.99 | 526.87 |
| 30 | 0.00148 | 0.96 | 677.97 | 0.00170 | 0.96 | 587.20 |
| 40 | 0.00136 | 0.99 | 733.20 | 0.00157 | 0.99 | 637.76 |
| 50 | 0.00144 | 0.98 | 693.96 | 0.00140 | 0.98 | 714.08 |
| 60 | 0.00119 | 0.99 | 839.98 | 0.00137 | 0.99 | 728.92 |
| 70 | 0.00115 | 0.99 | 867.30 | 0.00129 | 0.99 | 775.31 |
| 80 | 0.00103 | 0.96 | 974.75 | 0.00120 | 0.96 | 832.22 |
| Time (h after Imbibition) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Days | 16 | 24 | 40 | 48 | 64 | 72 | Final |
| No priming | 0 | 7 | 56 | 71 | 82 | 83 | 90 | |
| Priming at 24 °C | 2 3 4 | 0 4 * 4 * | 17 * 17 * 12 * | 63 54 45 | 74 61 50 | 83 77 67 | 83 82 68 | 88 84 74 |
| Priming at 10 °C | 2 3 4 | 1 14 * 16 * | 18 * 29 * 36 * | 76 * 75 * 77 * | 80 * 78 * 80 * | 88 * 85 88 * | 88 * 87 88 * | 90 87 90 |
| Time (h after Imbibition) | |||||||
|---|---|---|---|---|---|---|---|
| Days of Priming | 16 | 24 | 40 | 48 | 64 | 72 | Final |
| 0 2 3 4 | 0 25 * 37 * 51 * | 11 39 * 46 * 66 * | 64 64 62 82 * | 78 66 63 85 * | 79 76 76 87 * | 79 78 71 87 * | 88 80 73 87 |
| Time (Days after Planting) | ||||||
|---|---|---|---|---|---|---|
| Temperature (°C) | Treatment | 16 | 24 | 40 | 48 | 64 |
| 33 | No priming Priming | 1 17 * | 17 54 * | 61 72 | 80 82 | 85 84 |
| 35 | No priming Priming | 1 11 * | 14 35 * | 53 63 | 64 71 | 73 77 |
| 37 | No priming Priming | 0 15 * | 9 39 * | 53 54 | 55 54 | 73 61 |
| 39 | No priming Priming | 0 9 * | 3 16 * | 29 43 * | 31 43 | 41 50 |
| 41 | No priming Priming | 0 2 * | 1 7* | 3 8 | 3 6 | 5 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geneve, R.L.; Janes, E.W.; Kester, S.T.; Hildebrand, D.F.; Davis, D. Temperature Limits for Seed Germination in Industrial Hemp (Cannabis sativa L.). Crops 2022, 2, 415-427. https://doi.org/10.3390/crops2040029
Geneve RL, Janes EW, Kester ST, Hildebrand DF, Davis D. Temperature Limits for Seed Germination in Industrial Hemp (Cannabis sativa L.). Crops. 2022; 2(4):415-427. https://doi.org/10.3390/crops2040029
Chicago/Turabian StyleGeneve, Robert L., Evan W. Janes, Sharon T. Kester, David F. Hildebrand, and Derrick Davis. 2022. "Temperature Limits for Seed Germination in Industrial Hemp (Cannabis sativa L.)" Crops 2, no. 4: 415-427. https://doi.org/10.3390/crops2040029
APA StyleGeneve, R. L., Janes, E. W., Kester, S. T., Hildebrand, D. F., & Davis, D. (2022). Temperature Limits for Seed Germination in Industrial Hemp (Cannabis sativa L.). Crops, 2(4), 415-427. https://doi.org/10.3390/crops2040029







