Tolerance and Adaptability of Tomato Genotypes to Saline Irrigation
Abstract
:1. Introduction
2. Materials and Methods
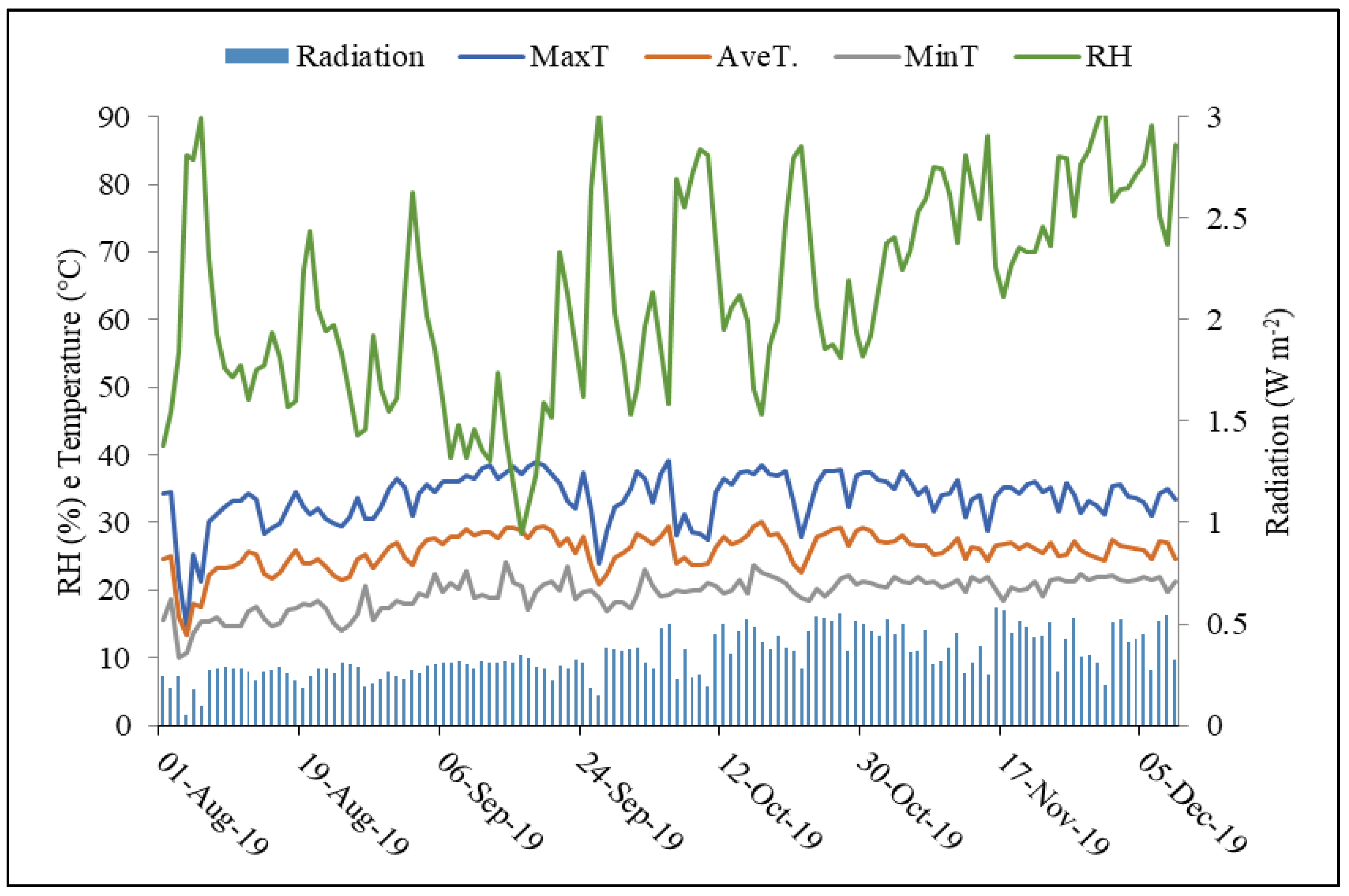
2.1. Experimental Characterization and Conduction
2.2. Experimental Design
2.3. Traits
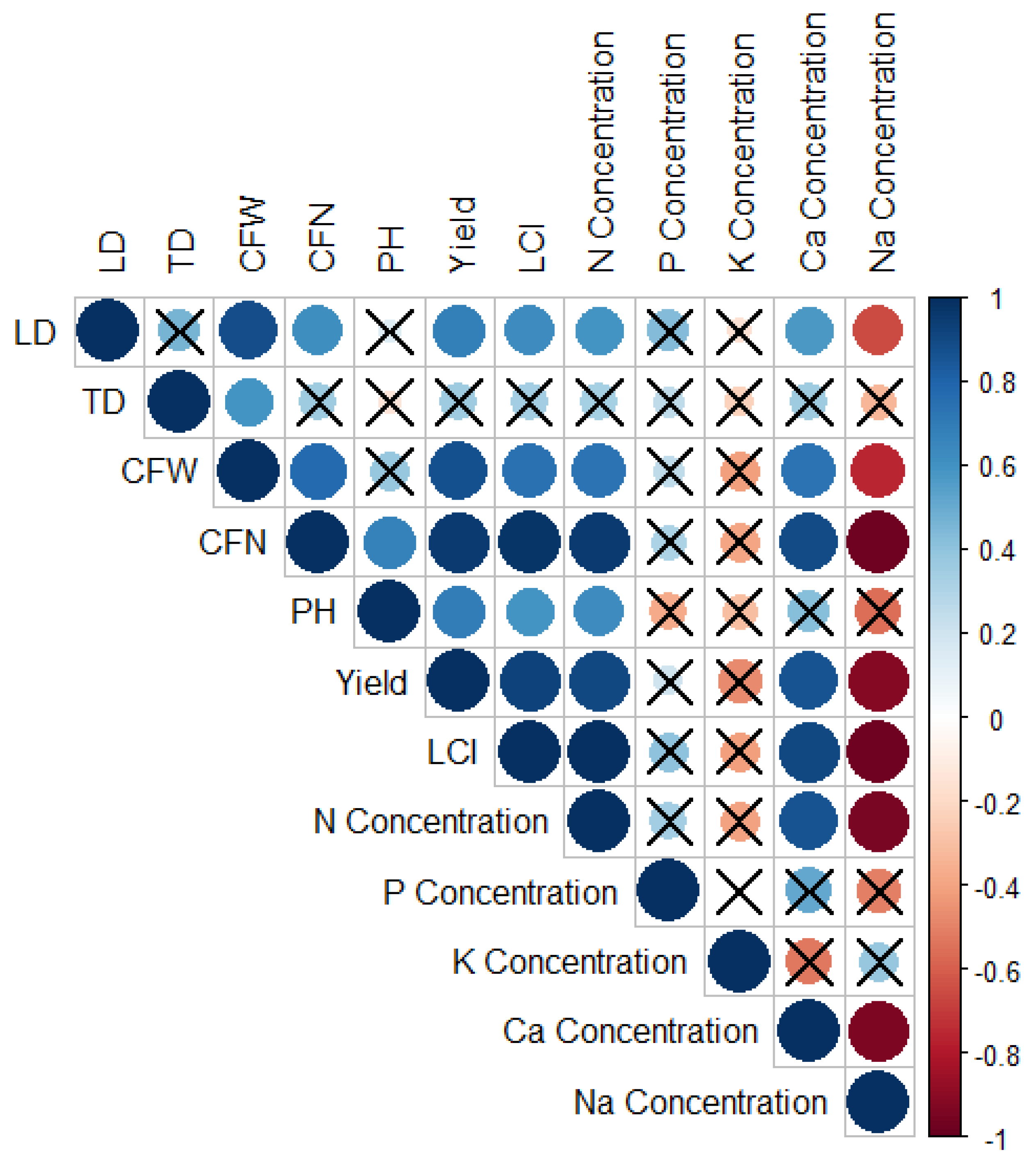
2.4. Statistical and Multivariate Analysis
3. Results
3.1. Tomato Growth and Fruit Yield under Salt Stress
3.2. Effect of Salt Stress on Chlorophyll and Nutrient Concentrations in Tomato Genotypes
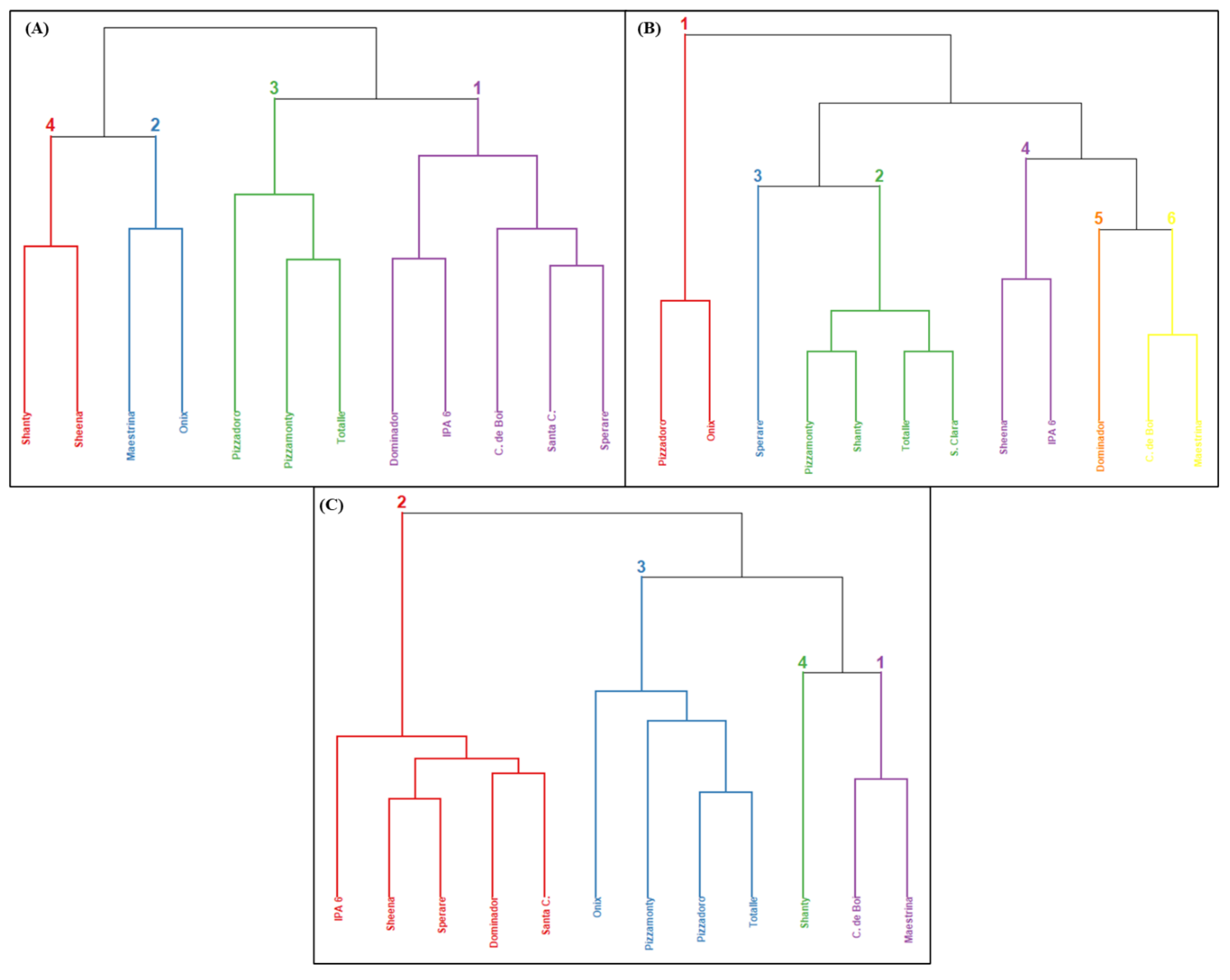
3.3. Cluster Analysis
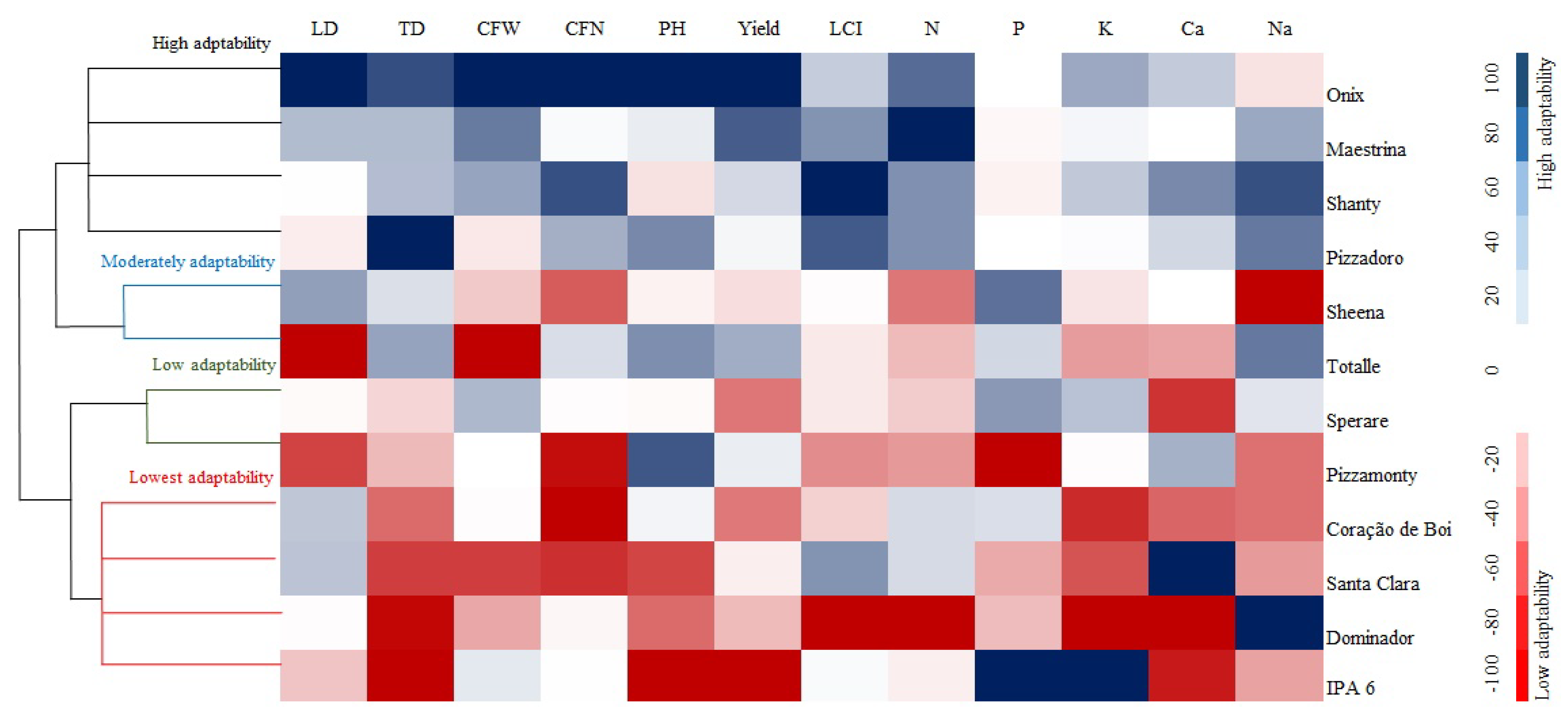
3.4. Analysis of Adaptability and Stability under Stress Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oliveira, C.E.S.; Steiner, F.; Zuffo, A.M.; Zoz, T.; Alves, C.Z.; Aguiar, V.C.B. Seed priming improves the germination and growth rate of melon seedlings under saline stress. Ciênc. Rural 2019, 49, 1–11. [Google Scholar] [CrossRef]
- Pereira, F.A.L.; Medeiros, J.F.; Gheyi, H.R.; Dias, N.S.; Preston, W.; Vasconcelos, C.B.E.L. Tolerance of melon cultivars to irrigation water salinity. Rev. Bras. Eng. Agríc. Ambient. 2017, 21, 846–851. [Google Scholar] [CrossRef]
- Pacheco, J.; Plazas, M.; Pettinari, I.; Landa-Faz, A.; González-Orenga, S.; Boscaiu, S.; Prohens, J.; Vicente, O.; Gramazio, P. Moderate and severe water stress effects on morphological and biochemical traits in a set of pepino (Solanum muricatum) cultivars. Sci. Hortic. 2021, 284, 110143. [Google Scholar] [CrossRef]
- Safdar, H.; Amin, A.; Shafiq, Y.; Ali, A.; Yasin, R.; Shoukat, A.; Hussan, M.U.; Sarwar, M.I. A review: Impact of salinity on plant growth. Nat. Sci. 2019, 17, 34–40. [Google Scholar]
- Mohanavelu, A.; Naganna, S.R.; Al-Ansari, N. Irrigation Induced Salinity and Sodicity Hazards on Soil and Groundwater: An Overview of Its Causes, Impacts and Mitigation Strategies. Agriculture 2021, 11, 983. [Google Scholar] [CrossRef]
- Viana, J.S.; Palaretti, L.F.; Sousa, V.M.; Barbosa, J.A.; Bertino, A.M.P.; Faria, R.T.; Darli, A.B. Saline irrigation water indices affect morphophysiological characteristics of collard. Hortic. Bras. 2021, 39, 79–85. [Google Scholar] [CrossRef]
- Campos, C.A.B.; Fernandes, P.D.; Gheyi, H.R.; Blanco, F.F.; Gonçalves, C.B.; Campos, S.A.F. Yield and fruit quality of industrial tomato under saline irrigation. Sci. Agric. 2006, 63, 146–152. [Google Scholar] [CrossRef]
- Wei, D.; Zhang, W.; Wang, C.; Meng, Q.; Li, G.; Chen, T.H.H.; Yang, X. Genetic engineering of the biosynthesis of glycinebetaine leads toalleviate salt-induced potassium efflux and enhances salt tolerance in tomato plants. Plant Sci. 2017, 257, 74–83. [Google Scholar] [CrossRef]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J.M. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef]
- Munns, R.; James, R.A.; Gilliham, M.; Flowers, T.J.; Colmer, T.D. Tissue tolerance: An essential but elusive trait for salt-tolerant crops. Funct. Plant Biol. 2016, 43, 1103–1113. [Google Scholar] [CrossRef]
- Ali, A.A.M.; Romdhane, W.B.; Tarroum, M.; Al-Dakhil, M.; Al-Doss, A.; Alsadon, A.A.; Hassairi, A. Analysis of Salinity Tolerance in Tomato Introgression Lines Based on Morpho-Physiological and Molecular Traits. Plants 2021, 10, 2594. [Google Scholar] [CrossRef] [PubMed]
- Annicchiarico, P. Cultivar adaptation and recomendation from alfafa trials in Northern Italy. J. Genet. Breed. 1992, 46, 269–278. [Google Scholar]
- Schmildt, E.R.; Nascimento, A.L.; Cruz, C.D.; Oliveira, J.A.R. Evaluation of methodologies of adaptability and stability in corn cultivars. Acta Sci. Agron. 2011, 33, 51–58. [Google Scholar] [CrossRef]
- Chattopadhyay, K.; Marndi, B.C.; Sarkar, R.K.; Singh, O.N. Stability analysis of backcross population for salinity tolerance at reproductive stage in rice. Indian J. Genet. Plant Breed. 2017, 77, 51–58. [Google Scholar] [CrossRef]
- Yan, K.; He, W.; Bian, L.; Zhang, Z.; Tang, X.; An, M.; Li, L.; Han, G. Salt adaptability in a halophytic soybean (Glycine max) involves photosystems coordination. BMC Plant Biol 2020, 20, 155. [Google Scholar] [CrossRef] [PubMed]
- EL Sabagh, A.; Islam, M.S.; Skalicky, M.; Ali Raza, M.; Singh, K.; Anwar Hossain, M.; Hossain, A.; Mahboob, W.; Iqbal, M.A.; Ratnasekera, D.; et al. Salinity Stress in Wheat (Triticum aestivum L.) in the Changing Climate: Adaptation and Management Strategies. Front. Agron. 2021, 3, 661932. [Google Scholar] [CrossRef]
- Bakhshi, B.; Mohammad, S.; Tabatabaei, T.; Reza, M.; Rad, N. Identification of salt tolerant genotypes in wheat using stress tolerance indices. BioRxiv Genet. 2021. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Oliveira, J.B.; Coelho, M.R.; Lumbreras, J.F.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 4th ed.; EMBRAPA: Rio de Janeiro, Brazil, 2018. [Google Scholar]
- Marouelli, W.A.; Oliveira, Á.S.; Coelho, E.F.; Nogueira, L.C.; Sousa, V.F. Irrigation water management. In Irrigation and Fertigation in Fruit and Vegetables; Sousa, V.F., Marouelli, W.A., Coelho, E.F., Pinto, J.M., Coelho Filho, M.A., Eds.; Embrapa Informação Tecnológica: Brasília, Brazil, 2011; pp. 157–232. (In Portuguese) [Google Scholar]
- Silva, D.J.; Vale, F.X.R. Tomato—Production Tecnology; Suprema: Viçosa, Brazil, 2007. (In Portuguese) [Google Scholar]
- Oliveira, C.E.S.; Zoz, T.; Jalal, A.; Seron, C.C.; Silva, R.A.; Teixeira Filho, M.C.M. Tolerance of tomato seedling cultivars to different values of irrigation water salinity. Rev. Bras. Eng. Agríc. Ambient. 2022, 26, 10. [Google Scholar] [CrossRef]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Assessment of the Nutritional Status of Plants: Principles and Applications, 2nd ed.; Brazilian Association for the Research of Potash and Phosphate: Piracicaba, Brazil, 1997. [Google Scholar]
- Rao, C.R. Advanced Statistical Methods in Biometric Research; John Wiley & Sons: New York, NY, USA, 1952. [Google Scholar]
- Cargnelutti-Filho, A.; Ribeiro, N.D.; Reis, R.C.P.; Souza, J.R.; Jost, E. Comparison of cluster methods for the study of genetic diversity in common bean cultivars. Ciênc. Rural 2008, 38, 2138–2145. [Google Scholar] [CrossRef]
- Zuffo, A.M.; Steiner, F.; Sousa, T.O.; Aguilera, J.G.; Teodoro, P.E.; Alcântara Neto, F.; Ratke, R.F. How does water and salt stress affect the germination and initial growth of Brazilian soya bean cultivars? J. Agron. Crop Sci. 2020, 206, 837–850. [Google Scholar] [CrossRef]
- Schmildt, E.R.; Cruz, C.D. Análise da adaptabilidade e estabilidade do milho pelos métodos de Eberhart e Russell (1966) e de Annicchiarico (1992). Rev. Ceres 2005, 52, 45–58. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: https://www.R-project.org/ (accessed on 20 July 2021).
- Oliveira, F.A.; Paiva, F.I.G.; Medeiros, J.F.; Melo, M.R.S.; Oliveira, M.K.T.; Silva, R.C.P. Salinity tolerance of tomato fertigated with different K+/Ca2+ proportions in protected environment. Braz. J. Agric. Environ. Eng. 2021, 25, 620–625. [Google Scholar] [CrossRef]
- Guo, J.; Dong, X.; Han, G.; Wang, B. Salt-Enhanced Reproductive Development of Suaeda salsa L. Coincided with Ion Transporter Gene Upregulation in Flowers and Increased Pollen K+ Content. Front. Plant Sci. 2019, 10, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, H.A.A.; Sahin, N.K.; Akdogan, G.; Yaman, C.; Kom, D.; Uranbey, S. Variability in salinity stress tolerance of potato (Solanum tuberosum L.) varieties using in vitro screening. Ciênc. Agrotecnol. 2020, 44, e004220. [Google Scholar] [CrossRef]
- Wilkinson, L.G.; Bird, D.C.; Tucker, M.R. Exploring the Role of the Ovule in cereal grain development and reproductive stress tolerance. Annu. Plant Rev. 2018, 1, 1–35. [Google Scholar] [CrossRef]
- Siddik, M.A.; Khan, M.S.; Rahman, M.M.; Uddin, M.K. Performance of tomato (Lycopersicon esculentum Mill.) germplasm to salinity stress. Bangladesh J. Bot. 2015, 44, 193–200. [Google Scholar] [CrossRef]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Tariq, A.; Pan, K.; Olatunji, O.A.; Graciano, C.; Li, Z.; Sun, F.; Zhang, L.; Wu, X.; Chen, W.; Song, D.; et al. Phosphorous fertilization alleviates drought effects on Alnus cremastogyne by regulating its antioxidant and osmotic potential. Sci. Rep. 2018, 8, 5644. [Google Scholar] [CrossRef]
- Alves, R.C.; Medeiros, A.S.; Nicolau, M.C.M.; Pizolato Neto, A.; Oliveira, F.A.; Lima, L.W.; Tezotto, T.; Gratão, P.L. The partial root-zone saline irrigation system and antioxidant responses in tomato plants. Plant Physiol. Biochem. 2018, 127, 366–379. [Google Scholar] [CrossRef]
- Meza, S.L.R.; Egea, I.; Massaretto, I.L.; Morales, B.; Purgatto, E.; Egea-Fernández, J.M.; Bolarin, M.C.; Flores, F.B. Traditional tomato varieties improve fruit quality without affecting fruit yield under moderate salt stress. Front. Plant Sci. 2020, 11, 587754. [Google Scholar] [CrossRef]
- Hajlaoui, H.; El Ayeb, N.; Garrec, J.P.; Denden, M. Differential effects of salt stress on osmotic adjustment and solutes allocation on the basis of root and leaf tissue senescence of two silage maize (Zea mays L.) varieties. Ind. Crops Prod. 2010, 31, 122–130. [Google Scholar] [CrossRef]
- Domingues, L.S.; Ribeiro, N.D.; Andriolo, J.L.; Possobom, M.T.D.F.; Zemolin, A.E.M. Growth, grain yield and calcium, potassium and magnesium accumulation in common bean plants as related to calcium nutrition. Acta Sci. Agron. 2016, 38, 207–217. [Google Scholar] [CrossRef]
- Alharby, H.F.; Metwali, E.M.; Fuller, M.P.; Aldhebiani, A.Y. Impact of application of zinc oxide nanoparticles on callus induction, plant regeneration, element content and antioxidant enzyme activity in tomato (Solanum lycopersicum Mill.) under salt stress. Arch. Biol. Sci. 2016, 68, 723–735. [Google Scholar] [CrossRef] [Green Version]
- Cruz, J.L.; Coelho, E.F.; Coelho Filho, M.A.; Santos, A.A. Salinity reduces nutrients absorption and efficiency of their utilization in cassava plants. Cienc. Rural 2018, 48, 1–12. [Google Scholar] [CrossRef]
- Oliveira, C.E.S.; Andrade, A.F.; Zoz, A.; Lustosa Sobrinho, R.; Zoz, T. Genetic divergence and path analysis in wheat cultivars under heat stress. Pesqui. Agropecu. Trop. 2020, 50, 1–9. [Google Scholar] [CrossRef]
- Hanin, M.; Ebel, C.; Ngom, M.; Laplaze, L.; Masmoudi, K. New insights on plant salt tolerance mechanisms and their potential use for breeding. Front. Plant Sci. 2016, 7, 1787. [Google Scholar] [CrossRef]
- Oliveira, C.E.S.; Steiner, F. Potassium nitrate priming to mitigate the salt stress on cucumber seedlings. Sci. Agrar. Parana. 2017, 16, 454–462. [Google Scholar] [CrossRef]
- Szareski, V.J.; Carvalho, I.R.; Kehl, K.; Levien, A.M.; Nardino, M.; Dellagostin, S.M.; Demari, G.H.; Lautenchleger, F.; Villela, F.A.; Pedó, T.; et al. Adaptability and stability of wheat genotypes according to the phenotypic index of seed vigor. Pesqui. Agropecu. Bras. 2018, 53, 727–735. [Google Scholar] [CrossRef]
- Wright, C.K.; Wimberly, M.C. Recent land use change in the Western Corn Belt threatens grasslands and wetlands. Proc. Natl. Acad. Sci. USA 2013, 110, 4134–4139. [Google Scholar] [CrossRef] [Green Version]
| Genotypes | Number of Fruits | Commercial Fruit Weight | ||||
|---|---|---|---|---|---|---|
| Fruits Bunch−1 | g Fruit−1 | |||||
| Control | Moderate | Severe | Control | Moderate | Severe | |
| Coração de Boi | 5.64 bA | 4.93 aB | 4.20 aC | 79.08 bA | 45.02 cB | 39.36 bB |
| Dominador | 5.21 bA | 4.39 bB | 3.77 bC | 53.97 cA | 47.92 cAB | 36.77 bB |
| IPA 6 | 5.27 bA | 4.83 aA | 3.71 bB | 58.94 cA | 49.36 cAB | 38.26 bB |
| Maestrina | 5.57 bA | 4.46 bB | 3.68 bC | 89.17 bA | 54.54 bB | 47.26 aB |
| Onix | 6.75 aA | 5.18 aB | 3.63 bC | 111.58 aA | 68.98 aB | 50.08 aC |
| Pizzadoro | 5.57 bA | 4.25 bB | 3.68 bC | 57.66 cA | 36.18 cB | 30.67 bB |
| Pizzamonty | 5.29 bA | 4.51 bB | 4.46 aB | 46.84 cA | 34.02 cAB | 29.38 bB |
| S. Clara | 5.39 bA | 4.32 bB | 3.55 bC | 51.03 cA | 39.68 cB | 40.55 bB |
| Shanty | 5.28 bA | 4.36 bB | 3.72 bC | 76.63 bA | 47.68 cB | 50.81 aB |
| Sheena | 5.43 bA | 4.39 bB | 3.46 bC | 61.83 cA | 45.90 cB | 52.28 aB |
| Sperare | 5.27 bA | 4.39 bB | 3.49 bC | 56.44 cA | 45.64 cAB | 36.56 bB |
| Totalle | 5.49 bA | 4.83 aB | 3.81 bC | 57.31 cA | 39.60 cB | 37.71 bB |
| Mean | 5.51 | 4.59 | 3.76 | 66.71 | 46.21 | 40.81 |
| CV (%) | 10.15 | 14.28 | ||||
| Standard error (±) | 1.03 | 1.00 | ||||
| Genotypes (G) | 0.00001 ** | 0.00001 ** | ||||
| Salt stress (S) | 0.00001 ** | 0.00001 ** | ||||
| GxS | 0.0026 ** | 0.00001 ** | ||||
| Genotypes | Transversal Diameter | Longitudinal Diameter | ||||
|---|---|---|---|---|---|---|
| cm | cm | |||||
| Control | Moderate | Severe | Control | Moderate | Severe | |
| Coração de Boi | 66.51 aA | 46.99 bB | 46.98 aB | 54.00 bA | 43.84 bB | 39.59 bC |
| Dominador | 51.72 cA | 44.36 bB | 42.37 bB | 44.92 bB | 56.06 aA | 39.00 bC |
| IPA 6 | 55.06 bA | 53.32 aA | 45.92 aB | 41.60 bB | 40.36 bC | 43.90 bA |
| Maestrina | 59.52 bA | 52.18 aB | 51.14 aB | 48.09 bA | 38.39 bC | 41.89 bB |
| Onix | 66.29 aA | 51.77 aB | 49.85 aB | 52.52 bA | 43.84 bB | 42.71 bB |
| Pizzadoro | 40.06 dA | 34.42 cB | 33.92 cB | 54.63 aA | 48.02 aB | 48.12 aB |
| Pizzamonty | 41.65 dA | 32.82 cAB | 29.76 cB | 51.72 bA | 50.70 aA | 45.42 bB |
| S. Clara | 55.98 bA | 44.95 bB | 41.82 bB | 45.75 bA | 39.53 bB | 41.02 bB |
| Shanty | 50.35 cA | 45.76 bAB | 42.65 bB | 64.02 aA | 54.88 aB | 55.08 aB |
| Sheena | 56.81 bA | 42.48 bB | 38.92 bC | 59.57 aA | 48.01 aB | 50.95 aB |
| Sperare | 51.47 cA | 42.56 bB | 41.20 bB | 55.60 aA | 46.20 bB | 44.21 bB |
| Totalle | 43.30 dA | 34.63 cB | 25.66 cC | 65.20 aA | 52.09 aB | 53.37 aB |
| Mean | 53.21 | 43.85 | 41.02 | 53.14 | 47.75 | 45.44 |
| CV (%) | 11.42 | 14.26 | ||||
| Standard error (±) | 2.62 | 3.45 | ||||
| Genotypes (G) | 0.00001 ** | 0.00001 ** | ||||
| Salt stress (S) | 0.00001 ** | 0.00001 ** | ||||
| GxS | 0.009 ** | 0.01 ** | ||||
| Genotypes | Plant Height | Fruit Yield | ||||
|---|---|---|---|---|---|---|
| cm | kg m−2 | |||||
| Control | Moderate | Severe | Control | Moderate | Severe | |
| C. de Boi | 178 aA | 168 aA | 165 aA | 6.08 dA | 2.68 dB | 1.98 aB |
| Dominador | 166 aA | 137 aA | 145 aA | 6.09 dA | 2.52 dB | 1.10 bC |
| IPA 6 | 120 bA | 114 bA | 136 aA | 4.34 fA | 1.70 eB | 1.03 bB |
| Maestrina | 198 aA | 162 aA | 159 aA | 11.84 bA | 4.69 bB | 2.23 aC |
| Onix | 182 aA | 185 aA | 158 aA | 14.66 aA | 6.90 aB | 2.44 aC |
| Pizzadoro | 187 aA | 152 aA | 98 bB | 4.97 eA | 2.45 dB | 1.35 bC |
| Pizzamonty | 185 aA | 171 aA | 148 aA | 5.29 eA | 3.47 cB | 1.97 aC |
| S. Clara | 191 aA | 166 aA | 150 aA | 6.23 dA | 2.94 cB | 1.04 bC |
| Shanty | 130 bA | 100 bA | 103 bA | 6.54 dA | 4.35 bB | 3.05 aC |
| Sheena | 103 bA | 105 bA | 100 bA | 5.37 eA | 3.26 cB | 2.51 aB |
| Sperare | 183 aA | 160 aA | 140 aA | 5.51 eA | 4.24 bB | 1.57 bC |
| Totalle | 168 aA | 148 aAB | 120 bB | 8.36 cA | 4.44 bB | 1.73 bC |
| Mean | 166 | 147 | 135 | 7.11 | 3.64 | 1.83 |
| CV (%) | 14.86 | 11.54 | ||||
| Standard error (±) | 0.13 | 2.71 | ||||
| Genotypes (G) | 0.00001 ** | 0.00001 ** | ||||
| Salt stress (S) | 0.00001 ** | 0.00001 ** | ||||
| GxS | 0.003 ** | 0.00001 ** | ||||
| Genotypes | LCI | N-Concentration | ||||
| SPAD | g kg−1 | |||||
| Control | Moderate | Severe | Control | Moderate | Severe | |
| Coração de Boi | 47.4 dA | 45.8 aA | 32.0 cB | 31.3 bA | 28.6 aB | 22.1 cC |
| Dominador | 46.0 dA | 42.4 bA | 28.7 dB | 25.6 dA | 26.5 bA | 19.8 dB |
| IPA 6 | 49.3 cA | 41.8 cB | 31.8 cC | 27.4 cA | 26.1 cA | 21.9 cB |
| Maestrina | 50.3 cA | 44.3 aB | 35.3 bC | 29.3 cA | 27.7 aA | 24.3 bB |
| Onix | 54.8 bA | 44.4 aB | 40.6 aB | 30.8 bA | 27.7 aB | 28.0 aB |
| Pizzadoro | 59.9 aA | 42.9 bB | 34.9 bC | 33.3 aA | 26.8 bB | 24.0 bC |
| Pizzamonty | 57.9 aA | 39.3 dB | 34.3 bC | 32.2 aA | 24.5 dB | 23.6 bB |
| S. Clara | 51.5 cA | 39.9 dB | 34.7 bC | 28.6 cA | 25.0 dB | 23.9 bB |
| Shanty | 51.4 cA | 45.7 aB | 34.7 bC | 28.6 cA | 28.6 aA | 23.9 bB |
| Sheena | 44.0 eA | 40.4 cB | 29.6 dC | 24.4 eA | 25.3 cA | 20.4 dB |
| Sperare | 50.6 cA | 38.7 dB | 36.2 bB | 28.1 cA | 24.2 dB | 24.9 bB |
| Totalle | 48.2 dA | 44.9 aB | 33.4 bC | 26.8 dA | 28.0 aA | 23.0 bB |
| Mean | 51.0 | 42.5 | 33.8 | 28.9 | 26.6 | 23.3 |
| CV (%) | 8.76 | 3.98 | ||||
| Standard error (±) | 0.67 | 0.49 | ||||
| Genotypes (G) | 0.0001 ** | 0.0002 ** | ||||
| Salt stress (S) | 0.0003 ** | 0.0001 ** | ||||
| GxS | 0.002 ** | 0.0018 ** | ||||
| Genotypes | P-Concentration | K-Concentration | ||||
| g kg−1 | g kg−1 | |||||
| Control | Moderate | Severe | Control | Moderate | Severe | |
| C. de Boi | 4.95 gA | 4.47 dB | 3.33 fC | 48.12 aA | 39.28 bB | 21.94 gC |
| Dominador | 4.22 hA | 3.75 fB | 3.55 eC | 36.48 bB | 26.66 fC | 57.48 aA |
| IPA 6 | 9.76 aA | 8.56 aB | 5.69 aC | 38.41 bA | 37.95 bA | 37.92 bA |
| Maestrina | 4.56 hA | 3.51 gB | 3.86 dB | 34.69 cB | 41.18 aA | 31.39 dC |
| Onix | 4.27 hA | 3.65 gB | 2.83 gC | 27.50 dB | 32.08 cA | 27.75 eB |
| Pizzadoro | 5.81 eA | 4.30 eB | 3.31 fC | 35.24 cB | 30.82 dC | 39.68 bA |
| Pizzamonty | 6.01 dA | 3.15 hB | 2.31 hC | 27.63 dC | 30.97 dB | 32.19 dA |
| S. Clara | 3.48 iB | 4.09 eA | 2.71 gC | 29.59 dA | 30.08 dA | 19.08 gB |
| Shanty | 5.47 eB | 8.60 aA | 4.74 cC | 34.38 cB | 39.13 bA | 28.60 eC |
| Sheena | 6.70 bA | 6.95 cA | 5.17 bB | 28.53 dA | 28.74 eA | 25.01 fB |
| Sperare | 6.38 cB | 7.35 bA | 4.76 cC | 28.23 dA | 29.01 eA | 26.01 fB |
| Totalle | 5.28 fA | 4.62 dB | 3.64 eC | 23.78 eC | 32.89 cB | 35.24 cA |
| Mean | 5.58 | 5.25 | 3.82 | 32.71 | 33.23 | 31.86 |
| CV (%) | 2.05 | 3.78 | ||||
| Standard error (±) | 0.07 | 0.71 | ||||
| Genotypes (G) | 0.00001 ** | 0.00002 ** | ||||
| Salt stress (S) | 0.00002 ** | 0.00001 ** | ||||
| GxS | 0.00001 ** | 0.00002 ** | ||||
| Genotypes | Ca-Concentration | Na-Concentration | ||||
| g kg−1 | g kg−1 | |||||
| Control | Moderate | Severe | Control | Moderate | Severe | |
| Coração de Boi | 32.18 cA | 22.48 dB | 22.14 eB | 4.00 aC | 27.88 bB | 33.20 dA |
| Dominador | 33.51 bA | 26.60 cB | 17.78 fC | 3.75 aC | 17.47 cB | 39.39 cA |
| IPA 6 | 38.08 aA | 26.33 cB | 19.24 fC | 4.11 aC | 11.42 dB | 30.72 eA |
| Maestrina | 35.48 aA | 25.46 cB | 17.50 fC | 3.05 aC | 33.30 aB | 49.50 aA |
| Onix | 36.95 aA | 29.18 bB | 22.57 eC | 2.98 aC | 12.58 dB | 35.67 dA |
| Pizzadoro | 34.43 bA | 26.88 cB | 24.49 dB | 3.58 aC | 15.76 cB | 48.74 aA |
| Pizzamonty | 34.16 bA | 27.89 cB | 27.22 cB | 5.41 aC | 17.70 cB | 24.99 fA |
| S. Clara | 36.39 aA | 30.03 bB | 28.63 bB | 3.28 aC | 16.70 cB | 20.98 gA |
| Shanty | 35.95 aA | 31.95 aB | 30.23 bB | 5.53 aC | 17.91 cB | 41.98 bA |
| Sheena | 30.96 cA | 33.27 aA | 32.44 aA | 3.25 aC | 11.09 dB | 50.48 aA |
| Sperare | 26.08 dB | 31.26 aA | 29.08 bA | 3.24 aC | 13.48 dB | 34.42 dA |
| Totalle | 29.40 cA | 29.82 bA | 26.17 cB | 3.73 aC | 16.10 cB | 38.15 cA |
| Mean | 33.63 | 28.43 | 24.79 | 3.83 | 17.62 | 37.35 |
| CV (%) | 4.57 | 6.76 | ||||
| Standard error (±) | 0.76 | 0.77 | ||||
| Genotypes (G) | 0.00001 ** | 0.00001 ** | ||||
| Salt stress (S) | 0.00001 ** | 0.00001 ** | ||||
| GxS | 0.0002 ** | 0.0001 ** | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Silva Oliveira, C.E.; Zoz, T.; Jalal, A.; Vendruscolo, E.P.; Nogueira, T.A.R.; Jani, A.D.; Teixeira Filho, M.C.M. Tolerance and Adaptability of Tomato Genotypes to Saline Irrigation. Crops 2022, 2, 306-322. https://doi.org/10.3390/crops2030022
Da Silva Oliveira CE, Zoz T, Jalal A, Vendruscolo EP, Nogueira TAR, Jani AD, Teixeira Filho MCM. Tolerance and Adaptability of Tomato Genotypes to Saline Irrigation. Crops. 2022; 2(3):306-322. https://doi.org/10.3390/crops2030022
Chicago/Turabian StyleDa Silva Oliveira, Carlos Eduardo, Tiago Zoz, Arshad Jalal, Eduardo Pradi Vendruscolo, Thiago Assis Rodrigues Nogueira, Arun Dilipkumar Jani, and Marcelo Carvalho Minhoto Teixeira Filho. 2022. "Tolerance and Adaptability of Tomato Genotypes to Saline Irrigation" Crops 2, no. 3: 306-322. https://doi.org/10.3390/crops2030022
APA StyleDa Silva Oliveira, C. E., Zoz, T., Jalal, A., Vendruscolo, E. P., Nogueira, T. A. R., Jani, A. D., & Teixeira Filho, M. C. M. (2022). Tolerance and Adaptability of Tomato Genotypes to Saline Irrigation. Crops, 2(3), 306-322. https://doi.org/10.3390/crops2030022









