Nutritional Content, Phytochemical Profiling, and Physical Properties of Buckwheat (Fagopyrum esculentum) Seeds for Promotion of Dietary and Food Ingredient Biodiversity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Proximate Analysis
2.3. Trace Element Analysis
2.4. Amino Acid Analysis
2.5. Phytochemical Analysis
2.6. Physical Properties
2.7. Statistical Analysis
3. Results
3.1. Macronutrient, Micronutrient, and Phytochemical Composition of the Buckwheat Samples
3.1.1. Macronutrient Composition
3.1.2. Microelement Composition
3.1.3. Amino Acid Composition
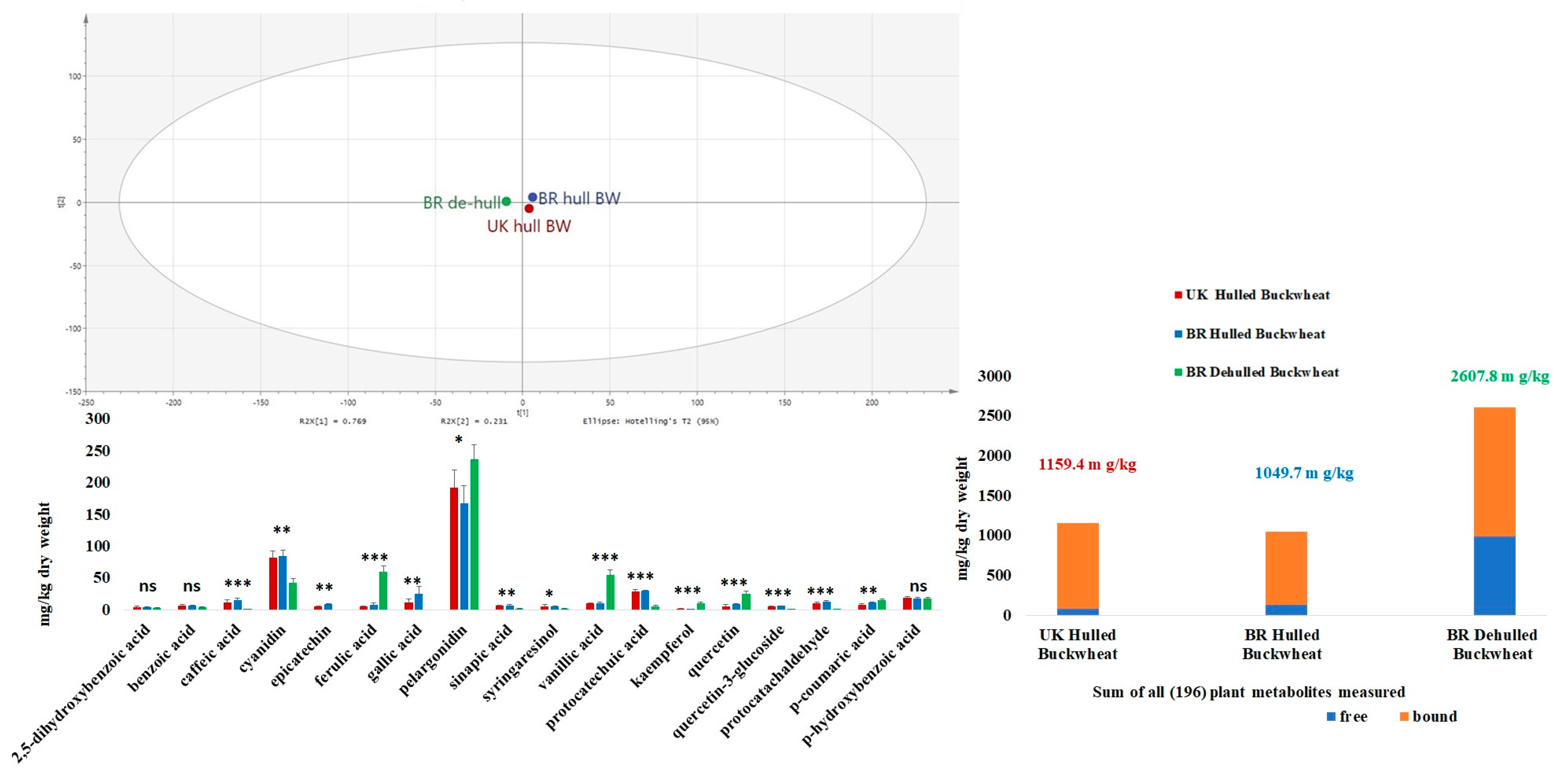
3.1.4. Phytochemical Composition
3.2. Physical Properties of Buckwheat Samples
4. Discussion
4.1. Macronutrient and Micronutrient Composition
4.2. Phytochemical Composition
4.3. Physical Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffman, J.R.; Falvo, M.J. Protein—Which is best? J. Sport Sci. Med. 2004, 3, 118–130. [Google Scholar]
- Odermatt, A. The Western-style diet: A major risk factor for impaired kidney function and chronic kidney disease. Am. J. Physiol. Renal Physiol. 2011, 301, F919–F931. [Google Scholar] [CrossRef] [PubMed]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future protein supply and demand: Strategies and factors influencing a sustainable equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Neacsu, M.; Fyfe, C.; Horgan, G.; Johnstone, A.M. Appetite control and biomarkers of satiety with vegetarian (soy) and meat-based high-protein diets for weight loss in obese men: A randomized crossover trial. Am. J. Clin. Nutr. 2014, 100, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.; Schuster, T.M.; Burke, J.M.; Kron, K.A. Taxonomy of Polygonoideae (Polygonaceae): A new tribal classification. Taxon 2011, 60, 151–160. [Google Scholar] [CrossRef]
- FAOSTAT. Data on Production Quantity of Crops All over the World in 2014; Corporate Statistical Database; UN Food and Agriculture Organization: Rome, Italy, 2014. [Google Scholar]
- FAOSTAT. Buckwheat Production in 2019; Corporate Statistical Database; UN Food and Agriculture Organization: Rome, Italy, 2019. [Google Scholar]
- Habtemariam, S. Antioxidant and Rutin Content Analysis of Leaves of the Common Buckwheat (Fagopyrum esculentum Moench) Grown in the United Kingdom: A Case Study. Antioxidants 2019, 3, 160. [Google Scholar] [CrossRef]
- Mota, C.; Santos, M.; Mauro, R.; Samman, N.; Matos, A.S.; Torres, D.; Castanheira, I. Protein content and amino acids profile of pseudocereals. Food Chem. 2016, 193, 55–61. [Google Scholar] [CrossRef]
- Multari, S.; Neacsu, M.; Scobbie, L.; Cantlay, L.; Duncan, G.; Vaughan, N.; Stewart, D.; Russell, W.R. Nutritional and Phytochemical Content of High-Protein Crops. J. Agric. Food Chem. 2016, 64, 7800–7811. [Google Scholar] [CrossRef]
- Ahmed, A.; Khalid, N.; Ahmad, A.; Abbasi, N.; Latif, M.; Randhawa, M. Phytochemicals and biofunctional properties of buckwheat: A review. J. Agric. Sci. 2014, 152, 349–369. [Google Scholar] [CrossRef]
- Alonso-Miravalles, L.; O’Mahony, J.A. Composition, protein profile and rheological properties of pseudocereal-based protein-rich ingredients. Foods 2018, 7, 73. [Google Scholar] [CrossRef]
- Huda, M.N.; Lu, S.; Jahan, T.; Ding, M.; Jha, R.; Zhang, K.; Zhang, W.; Georgiev, M.I.; Park, S.U.; Zhou, M. Treasure from garden: Bioactive compounds of buckwheat. Food Chem. 2021, 335, 127653. [Google Scholar] [CrossRef] [PubMed]
- Neacsu, M.; Vaughan, N.J.; Multari, S.; Haljas, E.; Scobbie, L.; Duncan, G.J.; Cantlay, L.; Fyfe, C.; Anderson, S.; Horgan, G.; et al. Hemp and buckwheat are valuable sources of dietary amino acids, beneficially modulating gastrointestinal hormones and promoting satiety in healthy volunteers. Eur. J. Nutr. 2022, 61, 1057–1072. [Google Scholar] [CrossRef]
- Zhu, F. Buckwheat proteins and peptides: Biological functions and food applications. Trends Food Sci. Technol. 2021, 110, 155–167. [Google Scholar] [CrossRef]
- Chen, X.D.; Patel, K.C. Manufacturing Better Quality Food Powders from Spray Drying and Subsequent Treatments. Dry. Technol. 2005, 26, 1313–1318. [Google Scholar] [CrossRef]
- Russell, W.R.; Burkitt, M.J.; Forrester, A.R.; Chesson, A. Oxidative coupling during lignin polymerization is determined by unpaired electron delocalization within parent phenylpropanoid radicals. Arch. Biochem. Biophys. 1996, 332, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.R.; Burkitt, M.J.; Scobbie, L.; Chesson, A. Radical formation and coupling of hydroxycinnamic acids containing 1,2-dihydroxy substituents. Biorg. Chem. 2003, 31, 206–215. [Google Scholar] [CrossRef]
- Food and Agriculture Organisation of the United Nations Methods of Food Analysis. Food Energy Methods of Analysis and Conversion Factors; Food and Agriculture Organisation of the United Nations: Rome, Italy, 2003; pp. 7–17. [Google Scholar]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar]
- Anderson, S. Soxtec: Its principles and applications. In Oil Extraction and Analysis: Critical Issues and Competitive Studies; Luthria, D.L., Ed.; American Oil Chemists’ Society: Urbana, IL, USA, 2004; pp. 11–24. [Google Scholar]
- AOAC Official Method 985.28 Amino Acids, Sulphur Amino Acids in Food, Feed Ingredients, and Processed Foods, a Official Method of Analysis, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2003.
- Waters Application for Acquity UPLC Amino Acid Analysis Solution Using Waters AccQ Tag Chemistry Derivatisation Amino Acid Analysis Standards & Kits: Waters. In UPLC Amino Acid Analysis Solution; Thomas, E.W., Eric, S.G., Jeffrey, R.M., Eds.; Waters Corporation: Milford, MA, USA, 2021; Available online: https://www.waters.com/webassets/cms/library/docs/720001683en.pdf (accessed on 14 July 2022).
- Neacsu, M.; Vaughan, N.J.; Perri, V.; Duncan, G.J.; Walker, R.; Coleman, M.; Russell, W.R. Nutritional and chemical profiling of UK-grown potato bean (Apios americana Medik) reveal its potential for diet biodiversification and revalorization. J. Food Comp. Anal. 2021, 98, 103821. [Google Scholar] [CrossRef]
- Zhang, Z.; Kou, X.; Fugal, K.; Mclaughlin, J. Comparison of HPLC methods for determination of anthocyanins and anthocyanidins in bilberry extracts. J. Agric. Food Chem. 2004, 52, 688–691. [Google Scholar] [CrossRef]
- Neacsu, M.; McMonagle, J.; Fletcher, R.J.; Scobbie, L.; Duncan, G.J.; Cantlay, L.; De Roos, B.; Duthie, G.G.; Russell, W.R. Bound phytophenols from ready-to-eat cereals; comparison with other plant-based foods. Food Chem. 2013, 141, 2880–2886. [Google Scholar] [CrossRef]
- Neacsu, M.; Vaughan, N.J.; Raikos, V.; Multari, S.; Duncan, G.J.; Duthie, G.G.; Russell, W.R. Phytochemical profile of food plant powders: Their potential role in healthier food reformulations. Food Chem. 2015, 179, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Freudig, B.; Hogekamp, S.; Schubert, H. Dispersion of powders in liquids in a stirred vessel. Chem. Eng. Proc. Process. Intensif. 1999, 38, 525–532. [Google Scholar] [CrossRef]
- Carr, R.L. Evaluating flow properties of solids. Chem. Eng. 1965, 72, 163–168. [Google Scholar]
- Hausner, H.H. Friction conditions in a mass of metal powder. Int. J. Powder Metall. 1967, 3, 7–13. [Google Scholar]
- Santana, A.A.; Martin, L.G.P.; de Oliveira, R.A.; Kurozawa, L.E.; Park, K.J. Spray drying of babassu coconut milk using different carrier agents. Dry. Technol. 2017, 35, 76–87. [Google Scholar] [CrossRef]
- Doğan, M.; Aslan, D.; Gürmeriç, V.; Özgür, A.; Saraç, M.G. Powder caking and cohesion behaviours of coffee powders as affected by roasting and particle sizes: Principal component analyses (PCA) for flow and bioactive properties. Powder Technol. 2019, 344, 222–232. [Google Scholar] [CrossRef]
- Mitchell, W.R.; Forny, L.; Althaus, T.O.; Niederreiter, G.; Palzer, S.; Hounslow, M.J.; Salman, A.D. Mapping the rate-limiting regimes of food powder reconstitution in a standard mixing vessel. Powder Technol. 2015, 270, 520–527. [Google Scholar] [CrossRef]
- Forny, L.; Marabi, A.; Palzer, S. Wetting, disintegration and dissolution of agglomerated water soluble powders. Powder Technol. 2011, 206, 72–78. [Google Scholar] [CrossRef]
- Tien, N.N.T.; Trinh, L.N.D.; Inoue, N.; Morita, N.; Hung, P.V. Nutritional composition, bioactive compounds, and diabetic enzyme inhibition capacity of three varieties of buckwheat in Japan. Cereal Chem. 2018, 95, 615–624. [Google Scholar] [CrossRef]
- Vrancheva, R.; Krystev, L.; Popova, A.; Mihaylova, D. Proximate nutritional composition and heat-induced changes of starch in selected grains and seeds. Emir. J. Food Agric. 2019, 31, 718–724. [Google Scholar]
- Scientific Advisory Committee on Nutrition (SACN). Carbohydrates and Health Report. 2015. Available online: https://www.gov.uk/government/publications/sacn-carbohydrates-and-health-report (accessed on 14 July 2022).
- Neacsu, M.; Anderson, S.E.; Verschoor, P.; Vaughan, N.J.; Horgan, G.W.; Hulshof, T.; Duncan, S.H.; Duthie, S.; Russell, W.R. Consumption of a Recommended Serving of Wheat Bran Cereals Significantly Increases Human Faecal Butyrate Levels in Healthy Volunteers and Reduces Markers of Inflammation Ex Vivo. Rec. Progr. Nutr. 2021, 1, 4. [Google Scholar] [CrossRef]
- Duncan, S.H.; Russell, W.R.; Quartieri, A.; Rossi, M.; Parkhill, J.; Walker, A.W.; Flint, H.J. Wheat bran promotes enrichment within the human colonic microbiota of butyrate-producing bacteria that release ferulic acid. Environ. Microbiol. 2016, 18, 2214–2225. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Brestic, M.; Zivcak, M.; Tran, L.S. The Contribution of Buckwheat Genetic Resources to Health and Dietary Diversity. Curr. Genom. 2016, 3, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Ina, S.; Hamada, A.; Yamaguchi, Y.; Akao, M.; Shinmachi, F.; Hitoshi Kumagai, H.; Kumagai, H. Suppressive Effect of the α-Amylase Inhibitor Albumin from Buckwheat (Fagopyrum esculentum Moench) on Postprandial Hyperglycaemia. Nutrients 2018, 10, 1503. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Li, Z.; Qin, Y.; Yue, Y.; Liu, Y. Protective effect of tartary buckwheat on renal function in type 2 diabetics: A randomized controlled trial. Ther. Clin. Risk Manag. 2016, 12, 1721–1727. [Google Scholar] [CrossRef]
- Tomotake, H.; Yamamoto, N.; Kitabayashi, H.; Kawakami, A.; Kayashita, J.; Ohinata, H.; Karasawa, H.; Kato, N. Preparation of tartary buckwheat protein product and its improving effect on cholesterol metabolism in rats and mice fed cholesterol-enriched diet. J. Food Sci. 2007, 72, S528–S533. [Google Scholar] [CrossRef]
- Tang, C.H.; Peng, J.; Zhen, D.-W.; Chen, Z. Physicochemical and antioxidant properties of buckwheat (Fagopyrum esculentum Moench) protein hydrolysates. Food Chem. 2009, 115, 672–678. [Google Scholar] [CrossRef]
- Pomeranz, Y.; Marshall, H.G.; Robbins, G.S.; Gilbertson, J.T. Protein content and amino acid composition of maturing buckwheat (Fagopyrum esculentum Moench). Cereal Chem. 1975, 52, 479–484. [Google Scholar]
- Neacsu, M.; Christie, J.S.; Duncan, G.J.; Vaughan, N.J.; Russell, W.R. Buckwheat, Fava Bean and Hemp Flours Fortified with Anthocyanins and Other Bioactive Phytochemicals as Sustainable Ingredients for Functional Food Development. Nutraceuticals 2022, 2, 150–161. [Google Scholar] [CrossRef]
- Roy, M.; Sen, S.; Chakraborti, A.S. Action of pelargonidin on hyperglycemia and oxidative damage in diabetic rats: Implication for glycation-induced hemoglobin modification. Life Sci. 2008, 82, 1102–1110. [Google Scholar] [CrossRef]
- Vaiyapuri, M.; Thimmarayan, S.; Dhupal, M.; Rallabandi, H.R.; Mekapogu, M.; Vasamsetti, B.M.K.; Swamy, M.K.; Natesan, K. Pelargonidin, a Dietary Anthocyanidin in the Prevention of Colorectal Cancer and Its Chemoprotective Mechanisms. In Plant-Derived Bioactives; Swamy, M., Ed.; Springer: Singapore, 2020. [Google Scholar]
- Kozłowska, A.; Szostak-Węgierek, D. Flavonoids—Food sources, health benefits, and mechanisms involved. In Bioactive Molecules in Food. Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, E47. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, E.; Dong, Y.J. Buckwheat allelopathy: Use in weed management. Allelopath. J. 2003, 12, 1–11. [Google Scholar]
- Kalinova, J.; Vrchotova, N.; Triska, J. Exudation of allelopathic substances in buckwheat (Fagopyrum esculentum Moench). J. Agric. Food Chem. 2007, 55, 6453–6459. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Tran, D.X.; Tsuzuki, E.; Terao, H.; Matsuo, M.; Tran, D.K. Screening for allelopathic potential of higher plants from Southeast Asia. Crop Prot. 2003, 22, 829–836. [Google Scholar]
- Iqbal, Z.; Hiradate, S.; Noda, A.; Isojima, S.; Fujii, Y. Allelopathic activity of buckwheat: Isolation and characterization of phenolics. Weed Sci. 2003, 51, 657–662. [Google Scholar] [CrossRef]
- Falquet, B.; Gfeller, A.; Pourcelot, M.; Tschuy, F.; Wirth, J. Weed Suppression by Common Buckwheat: A Review. Environ. Control Biol. 2015, 53, 1–6. [Google Scholar] [CrossRef]
- Szwed, M.; Wiczkowski, W.; Szawara-Nowak, D.; Obendorf, R.L.; Horbowicz, M. Allelopathic infuence of common buckwheat root residues on selected weed species. Acta Physiol. Plant. 2019, 41, 92. [Google Scholar] [CrossRef]
- Suzihaque, M.U.H.; Hashib, S.A.; Ibrahim, U.K. Effect of Inlet Temperature on Pineapple Powder and Banana Milk Powder. Procedia Soc. Behav. Sci. 2015, 195, 2829–2838. [Google Scholar] [CrossRef][Green Version]
- Tontul, I.; Topuz, A. Spray-drying of fruit and vegetable juices: Effect of drying conditions on the product yield and physical properties. Trends Food Sci. Technol. 2017, 63, 91–102. [Google Scholar] [CrossRef]
- Chegini, G.R.; Ghobadian, B. Effect of spray-drying conditions on physical properties of orange juice powder. Drying Technol. 2005, 23, 657–668. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Pallet, D.; Brat, P.; Hubinger, M.D. Physicochemical and morphological characterisation of açai (Euterpe oleraceae Mart.) powder produced with different carrier agents. Int. J. Food Sci. Technol. 2009, 44, 1950–1958. [Google Scholar] [CrossRef]
- Bicudo, M.O.P.; Jó, J.; de Oliveira, G.A.; Chaimsohn, F.P.; Sierakowski, M.R.; de Freitas, R.-A.; Ribani, R.H. Microencapsulation of Juçara (Euterpe edulis M.) Pulp by Spray Drying Using Different Carriers and Drying Temperatures. Drying Technol. 2015, 33, 153–161. [Google Scholar] [CrossRef]
- Jinapong, N.; Jamnong, P.; Suphantharika, M. Production of instant soymilk powders by ultrafiltration, spray drying and fluidized bed agglomeration. J. Food Eng. 2009, 84, 194–205. [Google Scholar] [CrossRef]
- Fitzpatrick, J.J.; van Lauwe, A.; Coursol, M.; O’Brian, A.; Fitzpatrick, K.L.; Ji, J.; Miao, S. Investigation of the rehydration behaviour of food powders by comparing the behaviour of twelve powders with different properties. Powder Technol. 2016, 297, 340–348. [Google Scholar] [CrossRef]
- Fazaeli, H.; Emam-Djomeh, Z.; Kalbasi-Ashtari, A.; Omid, M. Effect of process conditions and carrier concentration for improving drying yield and other quality attributes of spray dried black mulberry (Morus nigra) juice. Int. J. Food Eng. 2012, 8, 1–20. [Google Scholar] [CrossRef]
| Flowability | Carr Index (%) | Cohesiveness | Hausner Ratio |
|---|---|---|---|
| Very good | <15 | Low | <1.2 |
| Good | 15–20 | Intermediate | 1.2–1.4 |
| Fair | 20–35 | High | >1.4 |
| Bad | 35–45 | ||
| Very bad | >45 | ||
| Buckwheat Sample | Dry Matter | Ash | Protein | Fat | Carbohydrate Total | Resistant Starch | Soluble NSP | Insoluble NSP |
|---|---|---|---|---|---|---|---|---|
| % Dry Weight | ||||||||
| BR Dehulled | 86.43 ± 0.1 | 1.89 ± 0.01 | 14.13 ± 0.50 | 2.30 ± 0.05 | 69.30 ± 2.55 | 0.64 ± 0.00 | 0.06 ± 0.00 | 1.96 ± 0.07 |
| BR Hulled | 86.42 ± 0.02 | 1.88 ± 0.03 | 11.71 ± 0.40 | 1.71 ± 0.07 | 56.52 ± 1.85 | 0.56 ± 0.03 | 0.06 ± 0.03 | 7.80 ± 0.14 |
| UK Hulled | 83.38 ± 0.07 | 1.75 ± 0.03 | 12.24 ± 0.42 | 1.44 ± 0.02 | 46.70 ± 4.43 | 0.38 ± 0.02 | 0.03 ± 0.00 | 11.05 ± 0.25 |
| Overall ANOVA (p-values) | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | ns | <0.001 |
| LSD | 0.1406 | 0.05099 | 0.879 | 0.1001 | 6.27 | 0.04680 | 0.04007 | 0.3212 |
| Buckwheat Samples | Monosaccharides (% Dry Weight) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rhamnose | Fucose | Arabinose | Xylose | Mannose | Galactose | Glucose | Uronic Acid | ||
| BR Dehulled | soluble NSP | 0 ± 0 | 0 ± 0 | 0.001 ± 0.0016 | 0.003 ± 0.0044 | 0 ± 0 | 0 ± 0 | 0.004 ± 0.0032 | 0.052 ± 0.0054 |
| insoluble NSP | 0.068 ± 0.0138 | 0.028 ± 0.0049 | 0.39 ± 0.005 | 0.133 ± 0.0093 | 0.026 ± 0.0018 | 0.107 ± 0.0041 | 0.656 ± 0.0051 | 0.549 ± 0.0406 | |
| BR Hulled | soluble NSP | 0 ± 0 | 0.002 ± 0.0043 | 0.002 ± 0.0032 | 0 ± 0.0005 | 0.002 ± 0.0037 | 0.002 ± 0.0029 | 0.002 ± 0.0043 | 0.048 ± 0.0154 |
| insoluble NSP | 0.118 ± 0.0003 | 0.042 ± 0.0159 | 0.448 ± 0.0243 | 1.807 ± 0.0515 | 0.056 ± 0.001 | 0.199 ± 0.0037 | 3.939 ± 0.0677 | 1.192 ± 0.0211 | |
| UK Hulled | soluble NSP | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.034 ± 0.0037 |
| insoluble NSP | 0.139 ± 0.0039 | 0.04 ± 0.0018 | 0.701 ± 0.0101 | 3.499 ± 0.064 | 0.091 ± 0.0021 | 0.251 ± 0.0025 | 4.704 ± 0.0623 | 1.629 ± 0.1312 | |
| mg/kg Dry Weight | BR Dehulled Buckwheat | BR Hulled Buckwheat | UK Hulled Buckwheat | Overall ANOVA (p-Values) | LSD |
|---|---|---|---|---|---|
| Na | 14.36 ± 8.46 | 8.91 ± 14.4 | 24.02 ± 2.93 | ns | 19.56 |
| Mg | 2573.5 ± 55.38 | 2079.8 ± 158.58 | 2203.22 ± 89.8 | <0.01 | 219.7 |
| P | 4384.1 ± 155.58 | 3181.76 ± 235.42 | 3768.07 ± 237.99 | 0.001 | 425.8 |
| K | 4978.26 ± 155.71 | 5076.9 ± 389.68 | 5276.4 ± 319.08 | ns | 608.1 |
| Ca | 117.52 ± 7.64 | 403.52 ± 30.36 | 491.33 ± 24.81 | <0.001 | 46.08 |
| Mn | 13.69 ± 0.55 | 16.42 ± 1.18 | 43.32 ± 1.62 | <0.001 | 2.400 |
| Fe | 24.81 ± 0.66 | 35.63 ± 3.38 | 35.97 ± 1.55 | 0.001 | 4.361 |
| Cu | 5.6 ± 0.14 | 4.52 ± 0.27 | 6.05 ± 0.27 | <0.001 | 0.4723 |
| Zn | 25.32 ± 1.34 | 20.78 ± 1.41 | 34.44 ± 1.53 | <0.001 | 2.851 |
| Mo | 0.41 ± 0.02 | 0.41 ± 0.01 | 0.23 ± 0.01 | <0.001 | 0.03712 |
| µmoles/g Sample | BR Dehulled Buckwheat | BR Hulled Buckwheat | UK Hulled Buckwheat | Overall ANOVA (p-Values) | LSD |
|---|---|---|---|---|---|
| His | 34.36 ± 1.77 | 31.7 ± 1.38 | 35.66 ± 3.11 | 0.01 | 2.593 |
| Ser | 101.53 ± 3.58 | 92.99 ± 2.83 | 101.97 ± 5.2 | ns | 7.31 |
| Arg | 129.34 ± 6.27 | 113.93 ± 4.04 | 131.42 ± 9.31 | <0.01 | 8.62 |
| Gly | 168.16 ± 7.6 | 154.01 ± 5.12 | 169.65 ± 7.02 | <0.05 | 11.76 |
| Asp | 94.43 ± 4.89 | 85.09 ± 2.42 | 88.14 ± 6.89 | ns | 7.65 |
| Glu + Gln | 156.9 ± 9.37 | 142.32 ± 7.96 | 146.98 ± 9.91 | ns | 14.46 |
| Thr | 64.74 ± 2.07 | 59.84 ± 1.34 | 64.7 ± 6.3 | 0.01 | 3.463 |
| Ala | 72.32 ± 3.12 | 67.27 ± 3.28 | 69.41 ± 4.2 | ns | 5.799 |
| Pro | 58.58 ± 2.38 | 54.87 ± 2.18 | 57.93 ± 3.37 | ns | 4.031 |
| Lys | 51.13 ± 2.05 | 47.86 ± 3.87 | 46.99 ± 4.19 | ns | 5.317 |
| Tyr | 32.39 ± 1.68 | 27.5 ± 1.49 | 32.76 ± 1.76 | <0.01 | 2.936 |
| Val | 68.07 ± 2.43 | 63.66 ± 3.91 | 63.6 ± 12.64 | ns | 5.666 |
| Ileu | 45.1 ± 1.71 | 42.12 ± 2.6 | 42.47 ± 8.6 | ns | 3.827 |
| Leu | 89.42 ± 2.96 | 82.03 ± 2.98 | 87.15 ± 8.14 | <0.05 | 5.522 |
| Phe | 64.37 ± 3.74 | 58.08 ± 2.95 | 67.29 ± 6.3 | <0.01 | 5.631 |
| Cys | 59.7 ± 3.3 | 51.85 ± 4.61 | 50.84 ± 4.68 | ns | 8.48 |
| Met | 21.54 ± 1.4 | 18.36 ± 1.66 | 17.9 ± 1.62 | ns | 3.132 |
| Plant Metabolite | BR Dehulled Buckwheat | BR Hulled Buckwheat | UK Hulled Buckwheat | Overall ANOVA p-Values When <0.05 (with LSD Value) |
|---|---|---|---|---|
| mg/kg Dry Weight | ||||
| Benzoic acid | 4.281 ± 0.798 | 5.832 ± 1.338 | 6.467 ± 1.504 | <0.001 (1.649) |
| Salicylic acid | 0.622 ± 0.197 | 3.381 ± 0.574 | 4.082 ± 0.744 | |
| m-Hydroxybenzoic acid | nd | nd | nd | |
| p-Hydroxybenzoic acid | 17.194 ± 2.509 | 17.291 ± 1.786 | 18.661 ± 1.93 | |
| 2,3-Dihydroxybenzoic acid | 0.029 ± 0.003 | 2.682 ± 0.465 | 3.488 ± 0.8 | <0.001 (1.089) |
| 2,4-Dihydroxybenzoic acid | 0.189 ± 0.037 | 0.13 ± 0.008 | 0.133 ± 0.063 | |
| 2,5-Dihydroxybenzoic acid | 2.728 ± 0.356 | 4.311 ± 0.604 | 4.296 ± 0.996 | |
| 2,6-Dihydroxybenzoic acid | 0.18 ± 0.018 | 0.074 ± 0.01 | 0.088 ± 0.029 | |
| Protocatechuic acid | 5.3 ± 1.344 | 29.545 ± 1.453 | 28.381 ± 3.349 | <0.001 (4.142) |
| 3,5-Dihydroxybenzoic acid | nd | nd | nd | |
| o-Anisic acid | nd | nd | nd | |
| m-Anisic acid | nd | 0.109 ± 0.007 | 0.113 ± 0.012 | |
| p-Anisic acid | 0.522 ± 0.106 | 0.833 ± 0.124 | 0.967 ± 0.158 | |
| Gallic acid | nd | 24.877 ± 11.394 | 11.55 ± 5.095 | 0.01 (12.90) |
| Vanillic acid | 54.471 ± 8.46 | 10.68 ± 1.664 | 9.924 ± 1.1 | <0.001 (3.075) |
| Syringic acid | 0.934 ± 0.152 | 4.003 ± 3.221 | 2.405 ± 0.309 | |
| 3,4-Dimethoxybenzoic acid | 0.299 ± 0.043 | 0.401 ± 0.046 | 0.37 ± 0.044 | |
| p-Hydroxybenzaldehyde | 1.686 ± 0.37 | 3.246 ± 0.364 | 3.794 ± 0.604 | |
| Protocatechualdehyde | 0.18 ± 0.048 | 12.446 ± 2.119 | 10.47 ± 1.494 | <0.001 (2.067) |
| 3,4,5-Trihydroxybenzaldehyde | nd | nd | nd | |
| Vanillin | 0.977 ± 0.297 | 3.603 ± 0.747 | 3.906 ± 1.026 | <0.01 (1.425) |
| Isovanillin | nd | nd | nd | |
| Syringin | 0.286 ± 0.054 | 1.225 ± 0.157 | 1.523 ± 0.253 | |
| 3-Methoxybenzaldehyde | nd | nd | nd | |
| 3,4-Dimethoxybenzaldehyde | nd | 0.01 ± 0.002 | nd | |
| 3,4,5-Trimethoxybenzaldehyde | nd | nd | nd | |
| Cinnamic acid | 1.311 ± 0.246 | 1.038 ± 0.131 | 0.649 ± 0.112 | |
| o-Coumaric acid | nd | nd | nd | |
| m-Coumaric acid | nd | nd | nd | |
| p-Coumaric acid | 14.846 ± 2.573 | 11.03 ± 1.037 | 8.033 ± 0.906 | <0.01 (2.525) |
| Caffeic acid | 0.562 ± 0.12 | 15.223 ± 2.451 | 11.48 ± 3.624 | <0.001 (4.290) |
| Ferulic acid | 59.91 ± 9.101 | 7.496 ± 3.042 | 5.649 ± 0.618 | <0.001 (8.65) |
| Sinapic acid | 1.974 ± 0.49 | 6.57 ± 1.758 | 6.088 ± 0.98 | <0.01 (2.290) |
| 3-Methoxycinnamic acid | nd | nd | nd | |
| 4-Methoxycinnamic acid | nd | nd | nd | |
| 3,4-Dimethoxycinnamic acid | nd | 0.096 ± 0.004 | 0.11 ± 0.014 | |
| 3,4,5-Trimethoxycinnamic acid | nd | 0.942 ± 0.035 | 0.949 ± 0.134 | |
| Phenyl propionic acid | nd | nd | nd | |
| 2-Hydroxyphenylpropionic acid | nd | nd | nd | |
| 3-Hydroxyphenylpropionic acid | nd | nd | nd | |
| 4-Hydroxyphenylpropionic acid | nd | nd | nd | |
| 3,4-Dihydroxyphenylpropionic acid | nd | nd | nd | |
| 4-Hydroxy-3-methoxyphenylpropionic acid | 0.292 ± 0.071 | 0.187 ± 0.051 | 0.269 ± 0.083 | |
| 3-Methoxyphenylpropionic acid | nd | nd | nd | |
| Phenol | nd | 32.366 ± 6.472 | 31.267 ± 3.407 | |
| 1,2-Hydroxybenzene | nd | 0.066 ± 0.059 | nd | |
| 1,3-Hydroxybenzene | nd | nd | nd | |
| 1,2,3-Trihydroxybenzene | nd | nd | nd | |
| 4-Hydroxyacetophenone | 0.307 ± 0.038 | 0.158 ± 0.019 | 0.173 ± 0.015 | |
| 4-Hydroxy-3-methoxyacetophenone | 0.28 ± 0.042 | 0.259 ± 0.056 | 0.246 ± 0.035 | |
| 4-Hydroxy-3,5-dimethoxyacetophenone | nd | 0.087 ± 0.01 | nd | |
| 3,4-Dimethoxyacetophenone | nd | nd | nd | |
| 3,4,5-Trimethoxyacetophenone | nd | nd | nd | |
| Phenylacetic acid | 0.472 ± 0.224 | 1.809 ± 0.505 | 1.113 ± 0.216 | |
| 3-Hydroxyphenylacetic acid | nd | nd | nd | |
| 4-Hydroxyphenylacetic acid | 0.914 ± 0.36 | 38.692 ± 5.791 | 28.872 ± 9.651 | |
| 3,4-Dihydroxyphenylacetic acid | nd | 0.262 ± 0.228 | 0.221 ± 0.214 | |
| 4-Hydroxy-3-methoxyphenylacetic acid | nd | nd | nd | |
| 4-Methoxyphenylacetic acid | nd | nd | nd | |
| Mandelic acid | nd | nd | nd | |
| 3-Hydroxymandelic acid | nd | nd | nd | |
| 4-Hydroxymandelic acid | nd | nd | nd | |
| 3,4-Dihydroxymandelic acid | 0.118 ± 0.055 | 0.49 ± 0.117 | 0.4 ± 0.082 | |
| 4-Hydroxy-3-methoxymandelic acid | nd | nd | nd | |
| Phenyl pyruvic acid | 0.415 ± 0.119 | 0.126 ± 0.02 | 0.118 ± 0.082 | |
| 4-Hydroxyphenylpyruvic acid | 25.02 ± 4.931 | 24.769 ± 2.597 | 23.232 ± 4.753 | |
| Phenyl lactic acid | 0.433 ± 0.047 | 1.474 ± 0.195 | 1.088 ± 0.215 | |
| 4-hydroxyphenyllactic acid | 0.479 ± 0.144 | 1.758 ± 0.23 | 1.473 ± 0.278 | |
| Anthranilic acid | 0.296 ± 0.036 | 0.276 ± 0.028 | 0.265 ± 0.038 | |
| Quinaldinic acid | nd | nd | nd | |
| Chlorogenic acid | 0.137 ± 0.038 | 0.744 ± 0.642 | 0.137 ± 0.031 | |
| o-Hydroxyhippuric acid | nd | nd | nd | |
| Ethylferulate | nd | nd | nd | |
| Coniferyl alcohol | 0.46 ± 0.104 | nd | 0.218 ± 0.068 | |
| p-Cresol | nd | nd | nd | |
| 4-Ethylphenol | nd | nd | nd | |
| 4-Methylcatechol | nd | 0.075 ± 0.01 | 0.075 ± 0.015 | |
| Ellagic acid | nd | nd | nd | |
| Ferulic dimer (5-5 linked) | 1.375 ± 0.237 | 0.065 ± 0.045 | 0.043 ± 0.011 | |
| Ferulic dimer (8-8 linked) | nd | nd | nd | |
| Ferulic dimer (8-5 linked) | 3.297 ± 0.372 | 0.079 ± 0.065 | 0.051 ± 0.004 | |
| Ferulic dimer (5-5 hydrogenated) | nd | nd | nd | |
| Resveratrol | nd | nd | nd | |
| Indole | 0.187 ± 0.026 | 0.145 ± 0.013 | 0.168 ± 0.016 | |
| Indole-3-acetic acid | 0.097 ± 0.004 | nd | nd | |
| Indole-3-acrylic acid | nd | nd | nd | |
| Indole-3-propionic acid | nd | nd | nd | |
| Indole-3-carbinol | nd | nd | nd | |
| Indole-3-carboxylic acid | 0.283 ± 0.052 | 0.172 ± 0.01 | 0.2 ± 0.031 | |
| Indole-3-pyruvic acid | 2049.461 ± 149.169 | 474.242 ± 121.003 | 611.974 ± 112.205 | |
| Indole-3-methyl | nd | nd | nd | |
| Indoe-3-lactic acid | 0.025 ± 0.001 | 0.024 ± 0.005 | nd | |
| Coumarin | 0.007 ± 0.006 | 0.018 ± 0 | 0.003 ± 0.006 | |
| Psoralen | nd | nd | nd | |
| 8-Methylpsoralen | 0.002 ± 0.002 | 0.001 ± 0.001 | nd | |
| Bergapten | 0.006 ± 0.002 | 0.01 ± 0.008 | 0.001 ± 0.001 | |
| Tangeretin | 0.041 ± 0.016 | 0.074 ± 0.07 | 0.007 ± 0.001 | |
| Coumesterol | nd | nd | nd | |
| Catechin | nd | 2.8 ± 0.233 | 1.254 ± 0.301 | <0.001 (0.877) |
| Epicatechin | nd | 9.389 ± 0.347 | 5.698 ± 0.674 | <0.01 (3.848) |
| Gallocatechin | nd | nd | nd | |
| Epigallocatechin | nd | nd | nd | |
| Epigallocatechin gallate | nd | nd | nd | |
| Imperatorin | 0.006 ± 0.002 | 0.013 ± 0.015 | nd | |
| 4-Methylumbelliferone | nd | nd | nd | |
| 7-Hydroxy-4-methyl coumarin | nd | nd | nd | |
| 4-Hydroxy-6-methyl coumarin | nd | nd | nd | |
| Luteolinidin | nd | 0.361 ± 0.099 | 0.244 ± 0.074 | |
| Glycitein | 0.004 ± 0 | 0.002 ± 0.004 | nd | |
| 2-Hydroxybenzyl alcohol | nd | nd | nd | |
| 4-Hydroxy-3-methoxycinnamyl alcohol | nd | nd | nd | |
| Secoisolariciresinol | nd | 0.147 ± 0.02 | nd | |
| Matairesinol | nd | nd | nd | |
| Enterodiol | nd | nd | nd | |
| Enterolactone | nd | nd | nd | |
| Syringaresinol | 1.678 ± 0.449 | 4.881 ± 0.774 | 5.657 ± 3.102 | <0.05 |
| Pinoresinol | nd | 0.211 ± 0.043 | 0.224 ± 0.031 | |
| Lariciresinol | nd | nd | nd | |
| Hydroxymatairesinol | nd | nd | nd | |
| 3-Indoleacetonitrile | nd | nd | nd | |
| Indole-3-carboxaldehyde | 0.282 ± 0.039 | 0.217 ± 0.015 | 0.252 ± 0.037 | |
| Kaempferol | 10.069 ± 1.782 | 0.944 ± 0.155 | 1.107 ± 0.451 | <0.001 (1.982) |
| Quercetin | 25.574 ± 4.009 | 9.206 ± 0.8 | 5.586 ± 2.495 | <0.001 (5.353) |
| Isoliquiritigenin | nd | 0.01 ± 0 | 0.01 ± 0.001 | |
| Phloretin | nd | 0.049 ± 0.002 | 0.025 ± 0.023 | |
| Eriocitrin | 0.06 ± 0.021 | nd | nd | |
| Naringenin | 0.094 ± 0.013 | 0.158 ± 0.016 | 0.092 ± 0.067 | |
| Naringin | nd | nd | nd | |
| Hesperitin | nd | nd | nd | |
| Morin | nd | nd | nd | |
| Myricetin | nd | nd | nd | |
| Quercetin-3-glucoside | 0.537 ± 0.068 | 5.807 ± 0.503 | 4.732 ± 0.527 | <0.001 (0.344) |
| Taxifolin | 0.052 ± 0.005 | 0.208 ± 0.037 | 0.156 ± 0.062 | |
| Genstein | nd | 0.136 ± 0.086 | nd | |
| Scopoletin | 0.012 ± 0.002 | 0.047 ± 0.006 | 0.047 ± 0.013 | |
| Umbelliferone | nd | 0.053 ± 0.007 | 0.046 ± 0.014 | |
| 7,8-Dihydroxy-6-methyl coumarin | nd | nd | nd | |
| Neohesperidin | nd | nd | nd | |
| Hesperidin | nd | 0.004 ± 0.007 | 0.023 ± 0.004 | |
| Quercitrin | nd | 0.11 ± 0.022 | 0.512 ± 0.05 | |
| Biochanin A | 0.182 ± 0.021 | nd | nd | |
| Poncirin | nd | nd | nd | |
| Didymin | nd | nd | nd | |
| Phloridzin | nd | 0.098 ± 0.007 | 0.101 ± 0.012 | |
| Daidzein | nd | 0.136 ± 0.091 | nd | |
| Galangin | nd | nd | nd | |
| Luteolin | 0.016 ± 0.005 | 0.33 ± 0.017 | 0.202 ± 0.107 | |
| Equol | nd | nd | nd | |
| Fisetin | nd | nd | nd | |
| Neoeriocitrin | nd | nd | nd | |
| Formononetin | 0.094 ± 0.097 | 0.176 ± 0.087 | 0.043 ± 0.058 | |
| Apigenin | 0.004 ± 0.001 | 0.038 ± 0.007 | 0.058 ± 0.028 | |
| Gossypin | nd | nd | nd | |
| Tyrosol | nd | nd | nd | |
| Hydroxytyrosol | 0.006 ± 0.01 | nd | nd | |
| Isorhamnetin | 3.194 ± 0.358 | 0.23 ± 0.088 | 0.115 ± 0.086 | |
| Cyanidin | 42.884 ± 5.89 | 84.015 ± 9.672 | 81.463 ± 10.418 | <0.01 (17.75) |
| Pelargonidin | 236.219 ± 23.463 | 167.659 ± 27.457 | 191.592 ± 28.467 | 0.05 (53.05) |
| Buckwheat Sample | Moisture (%) | Loose Bulk Density (g/mL) | Tapped Bulk Density (g/mL) | Carr Index | Hausner Ratio | Wettability (sec) | Dispersibility (%) | Solubility (%) | Color Values | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | |||||||||
| BR Dehulled | 8.66 ± 0.07 b | 0.65 ± 0.00 c | 0.77 ± 0.00 b | 16.13 ± 0.00 c | 1.19 ± 0.00 c | 1344 ± 21 c | 11.32 ± 0.84 a | 4.52 ± 0.87 b | 47.4 ± 0.5 b | 2.0 ± 0.1 b | 11.2 ± 0.0 c |
| BR Hulled | 8.59 ± 0.03 b | 0.61 ± 0.0 b | 0.77 ± 0.00 b | 21.21 ± 0.00 b | 1.27 ± 0.00 b | 620 ± 12 b | 16.74 ± 2.21 a | 9.09 ± 1.73 ab | 38.2 ± 0.1 a | 1.9 ± 0.0 b | 9.4 ± 0.0 b |
| UK Hulled | 7.99 ± 0.06 a | 0.58 ± 0.01 a | 0.73 ± 0.01 a | 20.20 ± 0.20 a | 1.25 ± 0.00 a | 1514 ± 9 a | 12.92 ± 1.69 a | 11.53 ± 1.54 a | 37.9 ± 0.1 a | 2.3 ± 0.0 a | 9.7 ± 0.0 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neacsu, M.; De Lima Sampaio, S.; Hayes, H.E.; Duncan, G.J.; Vaughan, N.J.; Russell, W.R.; Raikos, V. Nutritional Content, Phytochemical Profiling, and Physical Properties of Buckwheat (Fagopyrum esculentum) Seeds for Promotion of Dietary and Food Ingredient Biodiversity. Crops 2022, 2, 287-305. https://doi.org/10.3390/crops2030021
Neacsu M, De Lima Sampaio S, Hayes HE, Duncan GJ, Vaughan NJ, Russell WR, Raikos V. Nutritional Content, Phytochemical Profiling, and Physical Properties of Buckwheat (Fagopyrum esculentum) Seeds for Promotion of Dietary and Food Ingredient Biodiversity. Crops. 2022; 2(3):287-305. https://doi.org/10.3390/crops2030021
Chicago/Turabian StyleNeacsu, Madalina, Shirley De Lima Sampaio, Helen E. Hayes, Gary J. Duncan, Nicholas J. Vaughan, Wendy R. Russell, and Vassilios Raikos. 2022. "Nutritional Content, Phytochemical Profiling, and Physical Properties of Buckwheat (Fagopyrum esculentum) Seeds for Promotion of Dietary and Food Ingredient Biodiversity" Crops 2, no. 3: 287-305. https://doi.org/10.3390/crops2030021
APA StyleNeacsu, M., De Lima Sampaio, S., Hayes, H. E., Duncan, G. J., Vaughan, N. J., Russell, W. R., & Raikos, V. (2022). Nutritional Content, Phytochemical Profiling, and Physical Properties of Buckwheat (Fagopyrum esculentum) Seeds for Promotion of Dietary and Food Ingredient Biodiversity. Crops, 2(3), 287-305. https://doi.org/10.3390/crops2030021






