Flow Analysis of Mastectomy Patients Using Length of Stay: A Single-Center Study
Abstract
1. Introduction
2. Materials and Methods
- Gender (Male/Female);
- Age;
- Discharge ward:
- ○
- 0911: General Surgery;
- ○
- 0912: General Surgery;
- ○
- 0914: General Surgery;
- ○
- 0921: General Surgery;
- ○
- 0941: General Surgery;
- ○
- 0915: Breast Unit;
- ○
- 1211: Plastic Surgery;
- ○
- 2411: Infectious Disease;
- ○
- 2911: Nephrology;
- ○
- 6411: Oncology.
- Type of procedure:
- ○
- 8512: Open Biopsy of the Breast;
- ○
- 8520: Removal or Demolition of Breast Tissue;
- ○
- 8521: Local Removal of Breast Injury;
- ○
- 8522: Breast Quadrectomy;
- ○
- 8523: Subtotal Mastectomy;
- ○
- 8532: Bilateral Reductive Mammoplasty;
- ○
- 8533: Unilateral Subcutaneous Mammectomy with Simultaneous Implantation of Prosthesis;
- ○
- 8534: Other Unilateral Subcutaneous Mammectomy;
- ○
- 8535: Bilateral Subcutaneous Mammectomy with Simultaneous Implantation of Prosthesis;
- ○
- 8541: Unilateral Simple Mastectomy;
- ○
- 8542: Bilateral Simple Mastectomy;
- ○
- 8543: Unilateral Enlarged Simple Mastectomy;
- ○
- 8544: Bilateral Enlarged Simple Mastectomy;
- ○
- 8545: Unilateral Radical Mastectomy;
- ○
- 8546: Bilateral Radical Mastectomy;
- ○
- 8547: Unilateral Enlarged Radical Mastectomy;
- ○
- 8548: Bilateral Enlarged Radical Mastectomy.
- ○
- 8553: Unilateral Prosthesis Implantation;
- ○
- 8554: Bilateral Prosthesis Implantation;
- ○
- 8599: Other Breast Surgeries;
- Hypertension (Yes/No);
- Diabetes (Yes/No);
- Cardiovascular disease (Yes/No);
- Respiratory disease (Yes/No);
- Secondary tumors (Yes/No);
- Surgery with complications (Yes/No);
- Pre-operative LOS.
- Class 0: LOS ≤ 3;
- Class 1: 4 ≤ LOS ≤ 7;
- Class 2: LOS ≥ 8.
2.1. Regression Algorithms
- (1)
- The linear relationship between the independent and dependent variable;
- (2)
- Absence of multicollinearity;
- (3)
- The independence of the residuals;
- (4)
- The residuals have constant variance;
- (5)
- The residuals are normally distributed;
- (6)
- Absence of outliers.
2.2. Classification Algorithms
2.3. Statistical Analysis
- Group 0: all patients discharged in the two-year period 2018–2019 (pre-COVID);
- Group 1: all patients discharged in the biennium 2020–2021 (COVID era).
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| LOS | length of stay |
| MLR | multiple linear regression |
| DT | decision tree |
| RF | random forest |
| GBT | gradient boosted tree |
| SVM | support vector machine |
| NB | naïve Bayes |
| VC | voting classifier |
| RMSE | root mean squared error |
References
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Associazione Italiana Oncologia Medica (AIOM). I Numeri Del Cancro in Italia 2018 I Numeri Del Cancro in Italia; Associazione Italiana Oncologia Medica: Milano, Italy, 2018. [Google Scholar]
- Vanni, G.; Pellicciaro, M.; Materazzo, M.; Palombi, L.; Buonomo, O.C. Breast cancer diagnosis in coronavirus-era: Alert from italy. Front. Oncol. 2020, 10, 938. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. Jama 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Parker, S. Clinical Guidelines for the Management of Breast Cancer; NHS: London, UK, 2019. [Google Scholar]
- The American Society of Breast Surgeons. Performance and Practice Guidelines for Mastectomy; The American Society of Breast Surgeons: Columbia, MD, USA, 2015. [Google Scholar]
- American Cancer Society, “Mastectomy”. Available online: https://www.cancer.org/cancer/breast-cancer/treatment/surgery-for-breast-cancer/mastectomy.html (accessed on 1 March 2024).
- Breast Cancer. National Comprehensive Cancer Network: National Clinical Practice Guidelines in Oncology. Version 3. 2018. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 1 March 2024).
- Morrow, M.; Jagsi, R.; Alderman, A.K.; Griggs, J.J.; Hawley, S.T.; Hamilton, A.S.; Graff, J.J.; Katz, S.J. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. Jama 2009, 302, 1551–1556. [Google Scholar] [CrossRef]
- Tuttle, T.M.; Habermann, E.B.; Grund, E.H.; Morris, T.J.; Virnig, B.A. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: A trend toward more aggressive surgical treatment. J. Clin. Oncol. 2007, 25, 5203–5209. [Google Scholar] [CrossRef]
- Lostumbo, L.; Carbine, N.E.; Wallace, J. Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database Syst. Rev. 2010, 11, CD002748. [Google Scholar] [CrossRef]
- Capri, S.; Russo, A. Cost of breast cancer based on real-world data: A cancer registry study in Italy. BMC Health Serv. Res. 2017, 17, 84. [Google Scholar] [CrossRef]
- Ponsiglione, A.M.; Marino, M.R.; Raiola, E.; Russo, G.; Borrelli, A.; Improta, G. Predictive Models for Studying Emergency Department Abandonment Rates: A Bicentric Study. In Proceedings of the Biomedical and Computational Biology: Second International Symposium, BECB 2022, Virtual Event, 13–15 August 2022; Revised Selected Papers. Springer International Publishing: Cham, Switzerland, 2023. [Google Scholar]
- Improta, G.; Bottino, V.; Sciambra, A.; Russo, M.A.; Stingone, M.A.; Triassi, M. Data Analysis to Study the Prolonged ED-LOS: The Case of Evangelical Hospital “Betania”. In Proceedings of the Biomedical and Computational Biology: Second International Symposium, BECB 2022, Virtual Event, 13–15 August 2022; Revised Selected Papers. Springer International Publishing: Cham, Switzerland, 2023. [Google Scholar]
- Scala, A.; Borrelli, A.; Improta, G. Predictive analysis of lower limb fractures in the orthopedic complex operative unit using artificial intelligence: The case study of AOU Ruggi. Sci. Rep. 2022, 12, 22153. [Google Scholar] [CrossRef]
- Improta, G.; Donisi, L.; Bossone, E.; Vallefuoco, E.; Ponsiglione, A.M.; Amato, F. Discrete Event Simulation to Improve Clinical Consultations in a Rehabilitation Cardiology Unit. In Proceedings of the 2022 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 17–18 November 2022; IEEE: Piscataway, NJ, USA, 2022. [Google Scholar]
- Montella, E.; Ferraro, A.; Sperlì, G.; Triassi, M.; Santini, S.; Improta, G. Predictive analysis of healthcare-associated blood stream infections in the neonatal intensive care unit using artificial intelligence: A single center study. Int. J. Environ. Res. Public Health 2022, 19, 2498. [Google Scholar] [CrossRef] [PubMed]
- Improta, G.; Mazzella, V.; Vecchione, D.; Santini, S.; Triassi, M. Fuzzy logic–based clinical decision support system for the evaluation of renal function in post-Transplant Patients. J. Eval. Clin. Pract. 2020, 26, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Santini, S.; Pescapé, A.; Valente, A.S.; Abate, V.; Improta, G.; Triassi, M.; Ricchi, P.; Filosa, A. Using fuzzy logic for improving clinical daily-care of β-thalassemia patients. In Fuzzy Systems (FUZZ-IEEE), Proceedings of the 2017 IEEE International Conference, Naples, Italy, 9–12 July 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1–6. [Google Scholar]
- Scala, A.; Loperto, I.; Triassi, M.; Improta, G. Risk Factors Analysis of Surgical Infection Using Artificial Intelligence: A Single Center Study. Int. J. Environ. Res. Public Health 2022, 19, 10021. [Google Scholar] [CrossRef]
- Improta, G.; Luciano, M.A.; Vecchione, D.; Cesarelli, G.; Rossano, L.; Santalucia, I.; Triassi, M. Management of the Diabetic Patient in the Diagnostic Care Pathway. In Proceedings of the 8th European Medical and Biological Engineering Conference, EMBEC 2020, IFMBE Proceedings, Portorož, Slovenia, 29 November–3 December 2020; Jarm, T., Cvetkoska, A., Mahnič-Kalamiza, S., Miklavcic, D., Eds.; Springer: Cham, Switzerland, 2021; Volume 80. [Google Scholar] [CrossRef]
- Improta, G.; Scala, A.; Trunfio, T.A.; Guizzi, G. Application of Supply Chain Management at Drugs Flow in an Italian Hospital District. J. Phys. Conf. Ser. 2021, 1828, 012081. [Google Scholar] [CrossRef]
- Colella, Y.; Di Laura, D.; Borrelli, A.; Triassi, M.; Amato, F.; Improta, G. Overcrowding analysis in emergency department through indexes: A single center study. BMC Emerg. Med. 2022, 22, 181. [Google Scholar] [CrossRef]
- Scala, A.; Trunfio, T.A.; De Coppi, L.; Rossi, G.; Borrelli, A.; Triassi, M.; Improta, G. Regression Models to Study the Total LOS Related to Valvuloplasty. Int. J. Environ. Res. Public Health 2022, 19, 3117. [Google Scholar] [CrossRef]
- Rosa, A.; Trunfio, T.A.; Marolla, G.; Costantino, A.; Nardella, D.; McDermott, O. Lean Six Sigma to reduce the acute myocardial infarction mortality rate: A single center study. TQM J. 2023, 35, 25–41. [Google Scholar] [CrossRef]
- Ferraro, A.; Centobelli, P.; Cerchione, R.; Cicco, M.V.; Montella, E.; Raiola, E.; Triassi, M.; Improta, G. Implementation of lean practices to reduce healthcare associated infections. Int. J. Healthc. Technol. Manag. 2020, 18, 51. [Google Scholar] [CrossRef]
- Cesarelli, G.; Montella, E.; Scala, A.; Raiola, E.; Triassi, M.; Improta, G. DMAIC Approach for the Reduction of Healthcare-Associated Infections in the Neonatal Intensive Care Unit of the University Hospital of Naples ‘Federico II’. In Proceedings of the European Medical and Biological Engineering Conference, Portorož, Slovenia, 29 November–3 December 2020; Springer: Cham, Switzerland, 2020; pp. 414–423. [Google Scholar]
- Converso, G.; Improta, G.; Mignano, M.; Santillo, L.C. A Simulation Approach for Agile Production Logic Implementation in a Hospital Emergency Unit. In Proceedings of the Intelligent Software Methodologies, Tools and Techniques, Naples, Italy, 15–17 September 2015; Springer International Publishing: Cham, Switzerland, 2015; Volume 532, pp. 623–634. [Google Scholar]
- Brasel, K.J.; Lim, H.J.; Nirula, R.; Weigelt, J.A. Length of stay: An appropriate quality measure? Arch. Surg. 2007, 142, 461–466. [Google Scholar] [CrossRef]
- Buttigieg, S.C.; Abela, L.; Pace, A. Variables affecting hospital length of stay: A scoping review. J. Health Organ. Manag. 2018, 32, 463–493. [Google Scholar] [CrossRef]
- Armel, W.S.; Samaha, S.; Starks, D.W. The use of simulation to reduce the length of stay in an emergency department. In Proceedings of the 2003 Winter Simulation Conference, New Orleans, LA, USA, 7–10 December 2003. [Google Scholar]
- Combes, C.; Kadri, F.; Chaabane, S. Predicting hospital length of stay using regression models: Application to emergency department. In Proceedings of the 10ème Conférence Francophone de Modélisation, Optimisation et Simulation-MOSIM’14, Nancy, France, November 2014. [Google Scholar]
- Sun, S.X.; Leung, A.N.; Dillon, P.W.; Hollenbeak, C.S. Length of stay and readmissions in mastectomy patients. Breast J. 2015, 21, 526–532. [Google Scholar] [CrossRef]
- Mandelbaum, A.; Nakhla, M.; Seo, Y.J.; Dobaria, V.; Attai, D.J.; Baker, J.L.; Thompson, C.K.; DiNome, M.L.; Benharash, P.; Lee, M.K. National trends and predictors of mastectomy with immediate breast reconstruction. Am. J. Surg. 2021, 222, 773–779. [Google Scholar] [CrossRef]
- Butz, D.R.; Shenaq, D.S.; Rundell, V.L.M.; Kepler, B.; Liederbach, E.B.; Thiel, J.; Pesce, C.; Murphy, G.S.; Sisco, M.; Howard, M.A. Postoperative pain and length of stay lowered by use of exparel in immediate, implant-based breast reconstruction. Plast. Reconstr. Surg. Glob. Open 2015, 3, e391. [Google Scholar] [CrossRef]
- Tartter, P.I.; Beck, G.; Fuchs, K. Determinants of hospital stay after modified radical mastectomy. Am. J. Surg. 1994, 168, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Manum, J.; Veith, J.; Wei, G.; Kwok, A.; Agarwal, J. Variables associated with length of stay in patients undergoing mastectomy and delayed-immediate breast reconstruction with tissue expander. Breast J. 2019, 25, 927–931. [Google Scholar] [CrossRef]
- Loperto, I.; Scala, A.; Borrelli, A.; Rossi, G.; Triassi, M. Analysis of the adequacy of admissions in a Complex Operative Unit of General Surgery and Day Surgery and Breast Unit. In Proceedings of the 2021 International Symposium on Biomedical Engineering and Computational Biology, Nanchang, China, 13–15 August 2021. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, version 27.0; IBM Corp: Armonk, NY, USA, 2020. [Google Scholar]
- Rodriguez-Galiano, V.; Sanchez-Castillo, M.; Chica-Olmo, M.; Chica-Rivas, M. Machine learning predictive models for mineral prospectivity: An evaluation of neural networks, random forest, regression trees and support vector machines. Ore Geol. Rev. 2015, 71, 804–818. [Google Scholar] [CrossRef]
- Friedman, J.H. Greedy function approximation: A gradient boosting machine. Ann. Stat. 2001, 29, 1189–1232. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. Xgboost: A scalable tree boosting system. In Proceedings of the 22nd Acm Sigkdd International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016. [Google Scholar]
- Ostertagová, E. Modelling using polynomial regression. Procedia Eng. 2012, 48, 500–506. [Google Scholar] [CrossRef]
- Google. Google Colaboratory. 2024. Available online: https://colab.research.google.com/ (accessed on 28 April 2024).
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Priyanka; Kumar, D. Decision tree classifier: A detailed survey. Int. J. Inf. Decis. Sci. 2020, 12, 246–269. [Google Scholar] [CrossRef]
- Suthaharan, S. Support vector machine. In Machine Learning Models and Algorithms for Big Data Classification. Integrated Series in Information Systems; Springer: Boston, MA, USA, 2016; Volume 36. [Google Scholar]
- Alkan, A. Analysis of knee osteoarthritis by using fuzzy c-means clustering and SVM classification. Sci. Res. Essays 2011, 6, 4213–4219. [Google Scholar]
- Leung, K.M. Naive bayesian classifier. Polytech. Univ. Dep. Comput. Sci./Financ. Risk Eng. 2007, 2007, 123–156. [Google Scholar]
- Ruta, D.; Gabrys, B. Classifier selection for majority voting. Inf. Fusion 2005, 6, 63–81. [Google Scholar] [CrossRef]
- Cross Validation, Scikit-Learn Python Library. Available online: https://scikit-learn.org/stable/modules/cross_validation.html (accessed on 28 April 2024).
- GridSearchCV, Scikit-Learn Python Library. Available online: https://scikit-learn.org/stable/modules/generated/sklearn.model_selection.GridSearchCV.html (accessed on 28 April 2024).
- Senaviratna, N.A.M.R.; Cooray, T.M.J.A. Diagnosing multicollinearity of logistic regression model. Asian J. Probab. Stat. 2019, 5, 1–9. [Google Scholar] [CrossRef]
- Rutledge, D.N.; Barros, A.S. Durbin–Watson statistic as a morphological estimator of information content. Anal. Chim. Acta 2002, 454, 277–295. [Google Scholar] [CrossRef]
- Hamilton, D.F.; Ghert, M.; Simpson, A.H.R.W. Interpreting regression models in clinical outcome studies. Bone Jt. Res. 2015, 4, 152–153. [Google Scholar] [CrossRef] [PubMed]
- Tatachar, A.V. Comparative assessment of regression models based on model evaluation metrics. Int. Res. J. Eng. Technol. (IRJET) 2021, 8, 853–860. [Google Scholar]
- Seliya, N.; Khoshgoftaar, T.M.; Van Hulse, J. A study on the relationships of classifier performance metrics. In Proceedings of the 2009 21st IEEE International Conference on Tools with Artificial Intelligence, Newark, NJ, USA, 2–4 November 2009; IEEE: Piscataway, NJ, USA, 2009; pp. 59–66. [Google Scholar]
- Lee, A.H.; Gracey, M.; Wang, K.; Yau, K.K. A robustified modeling approach to analyze pediatric length of stay. Ann. Epidemiol. 2005, 15, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C.; Rothwell, D.M.; Tu, J.V. A comparison of statistical modeling strategies for analyzing length of stay after CABG surgery. Health Serv. Outcomes Res. Methodol. 2002, 3, 107–133. [Google Scholar] [CrossRef]
- Li, H.; Jiao, J.; Zhang, S.; Tang, H.; Qu, X.; Yue, B. Construction and comparison of predictive models for length of stay after total knee arthroplasty: Regression model and machine learning analysis based on 1,826 cases in a single Singapore center. J. Knee Surg. 2022, 35, 007–014. [Google Scholar] [CrossRef]
- Bhandari, M.; Wilson, C.; Rifkind, K.; DiMaggio, C.; Ayoung-Chee, P. Prolonged length of stay in delayed cholecystectomy is not due to intraoperative or postoperative contributors. J. Surg. Res. 2017, 219, 253–258. [Google Scholar] [CrossRef]
- Trunfio, T.A.; Scala, A.; Giglio, C.; Rossi, G.; Borrelli, A.; Romano, M.; Improta, G. Multiple regression model to analyze the total LOS for patients undergoing laparoscopic appendectomy. BMC Med. Inform. Decis. Mak. 2022, 22, 141. [Google Scholar] [CrossRef] [PubMed]
- Santoso, U.; Iau, P.T.; Lim, J.; Koh, C.S.; Pang, Y.T. The mastectomy clinical pathway: What has it achieved? Ann. Acad. Med. Singap. 2002, 31, 440–445. [Google Scholar] [PubMed]
- Nwaogu, I.Y.; Bommarito, K.; Olsen, M.A.; Margenthaler, J.A. Economic impact of bleeding complications after mastectomy. J. Surg. Res. 2015, 199, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, J.; Gonzalez, E.; Pal, N.; Roetzheim, R. The use and outcomes of outpatient mastectomy in Florida. Am. J. Surg. 2000, 179, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.P.; Barry, M.; Junqueira, M.; Lee, S.; Mazzella, A.; Sclafani, L. Initial experiences with a multidisciplinary approach to decreasing the length of hospital stay for patients undergoing unilateral mastectomy. Eur. J. Surg. Oncol. (EJSO) 2011, 37, 944–949. [Google Scholar] [CrossRef]
- Wagman, L.D.; Terz, J.J.; Hill, L.R.; Beatty, J.D.; Kokal, W.A.; Riihimaki, D.U.; Hyams, D.M.; Grant, M.; Hart, A.; Kirshner, T.; et al. Evaluation of a short-stay program for patients undergoing mastectomy. J. Surg. Oncol. 1989, 41, 98–102. [Google Scholar] [CrossRef]

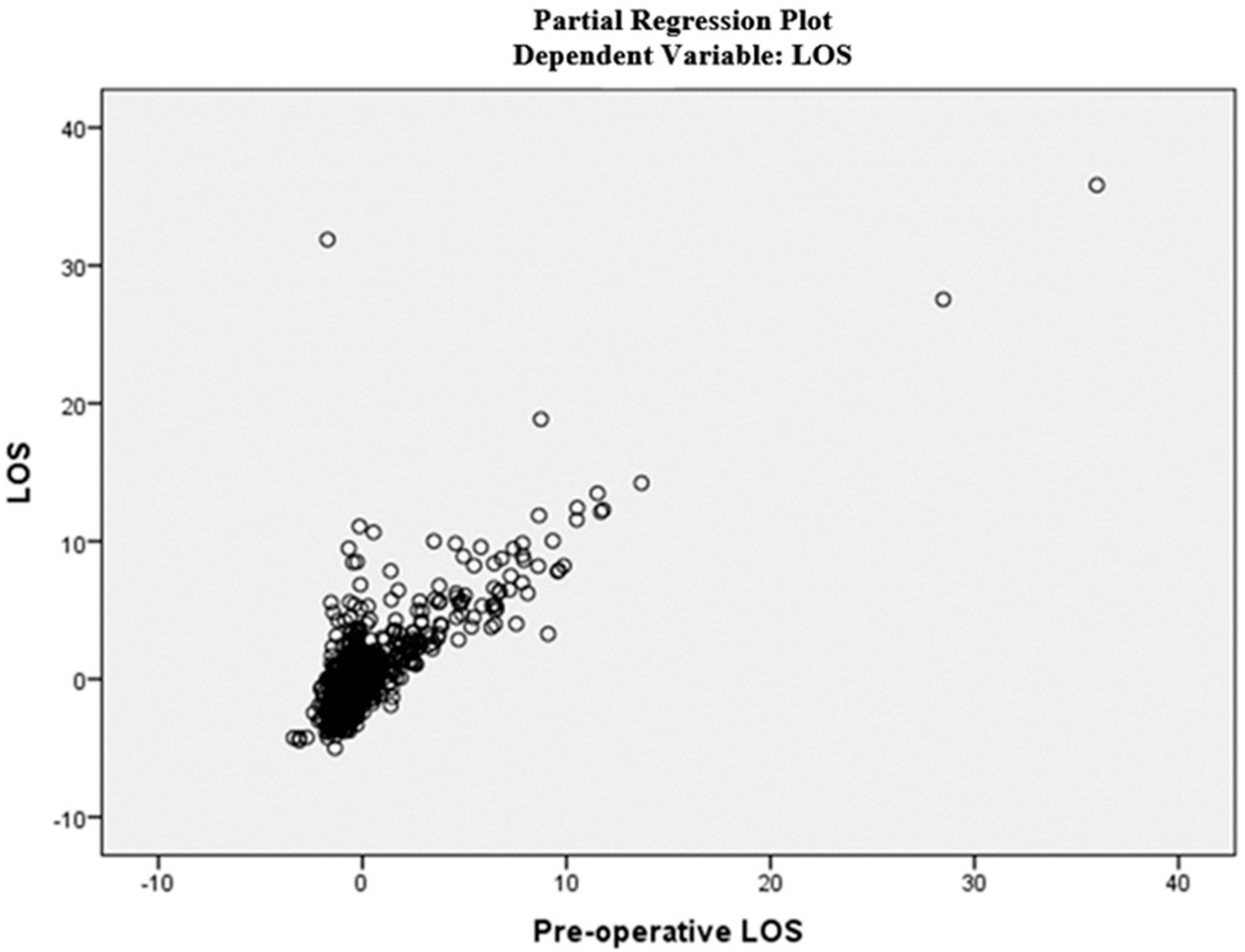
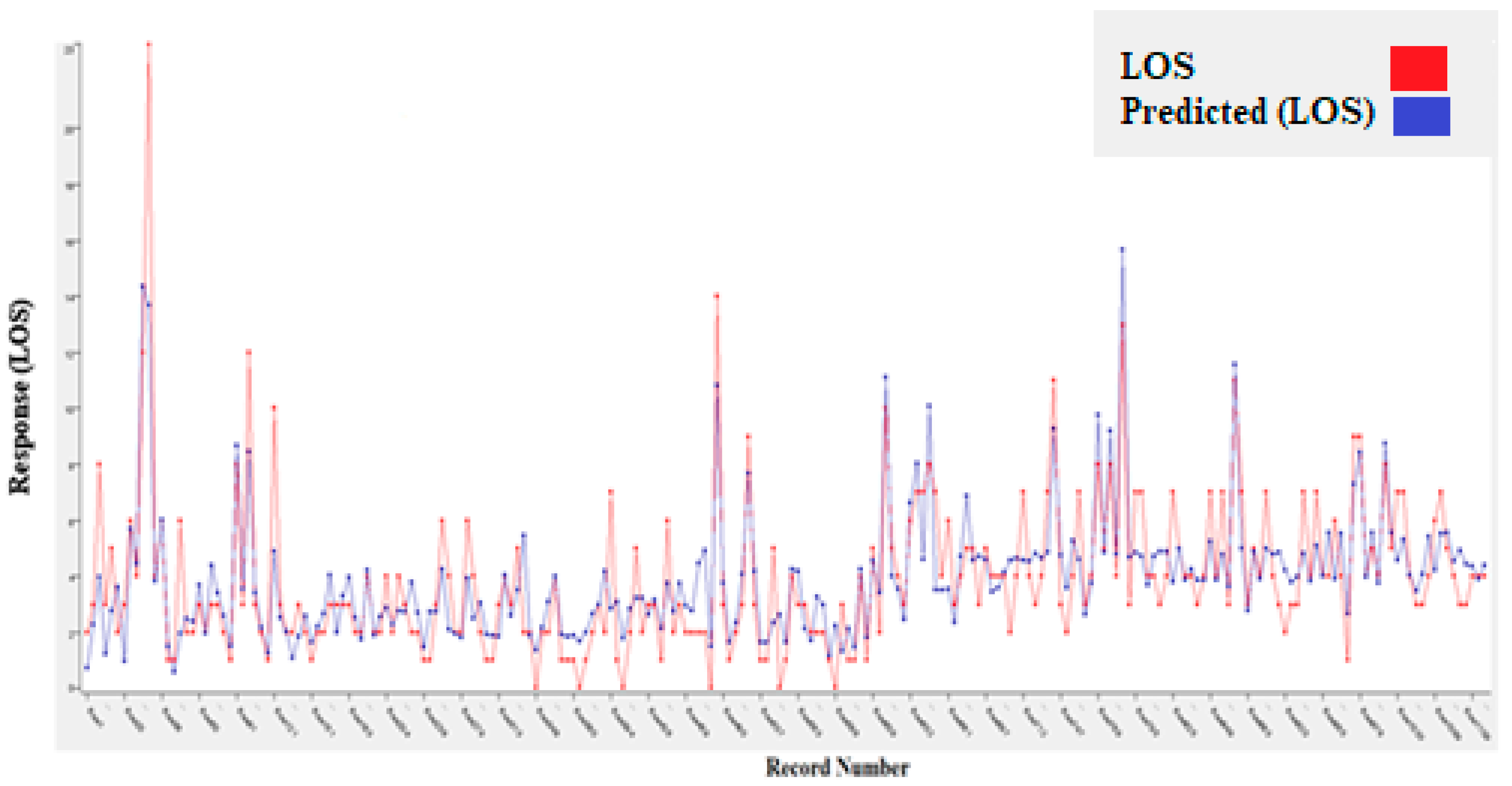

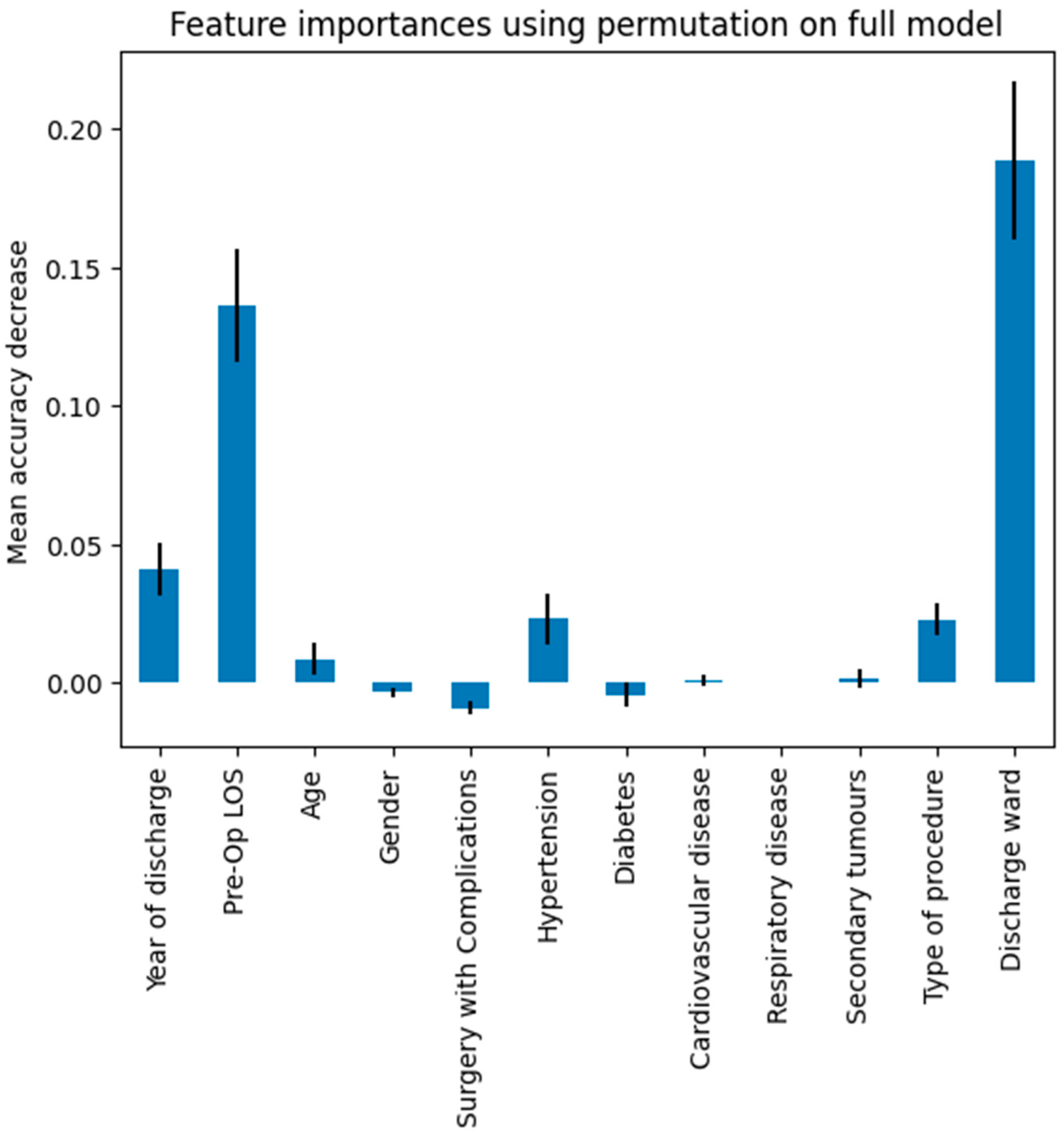
| Features | Dataset (N = 1123) |
|---|---|
| Gender | |
| M | 12 |
| F | 1111 |
| Discharge ward | |
| 0911 | 94 |
| 0912 | 3 |
| 0914 | 8 |
| 0915 | 459 |
| 0921 | 7 |
| 0941 | 547 |
| 1211 | 1 |
| 2411 | 1 |
| 2911 | 2 |
| 6411 | 1 |
| Type of procedure | |
| 8512 | 29 |
| 8520 | 14 |
| 8521 | 33 |
| 8522 | 439 |
| 8523 | 4 |
| 8532 | 1 |
| 8533 | 9 |
| 8534 | 9 |
| 8535 | 2 |
| 8541 | 116 |
| 8542 | 2 |
| 8543 | 175 |
| 8544 | 2 |
| 8545 | 270 |
| 8546 | 4 |
| 8547 | 7 |
| 8548 | 3 |
| 8553 | 1 |
| 8554 | 1 |
| 8599 | 2 |
| Hypertension | |
| Yes | 216 |
| No | 907 |
| Diabetes | |
| Yes | 74 |
| No | 1049 |
| Cardiovascular disease | |
| Yes | 71 |
| No | 1052 |
| Respiratory disease | |
| Yes | 19 |
| No | 1104 |
| Secondary tumors | |
| Yes | 60 |
| No | 1063 |
| Surgery with complications | |
| Yes | 278 |
| No | 845 |
| Algorithms | Hyperparameters |
|---|---|
| SVM | ‘kernel’:(‘linear’, ‘rbf’), ‘C’:[1, 10, 100], cv = 10 |
| RF | ‘n_estimators’: [5, 10, 15, 20], ‘max_depth’: [2, 5, 7, 9], cv = 10 |
| DT | ‘max_depth’: range(3,20), cv = 10 |
| NB | ‘var_smoothing’: np.logspace(0, −9, num = 100), cv = 10 |
| VC | ‘voting’: [hard, soft] |
| Pearson Correlation | Variable/Variable | LOS | Pre-Operative LOS | Age | Gender | Hypertension | Diabetes | Cardiovascular Disease | Respiratory Disease | Type of Procedure | Secondary Tumors | Discharge Ward | Year of Discharge | Surgery with Complications |
| LOS | 1.000 | 0.770 | 0.025 | −0.021 | −0.205 | −0.048 | 0.031 | −0.002 | 0.227 | −0.046 | 0.001 | 0.246 | 0.107 | |
| Pre-operative LOS | 0.770 | 1.000 | 0.042 | −0.034 | −0.141 | −0.051 | −0.012 | 0.063 | 0.036 | −0.021 | 0.015 | 0.070 | 0.016 | |
| Age | 0.025 | 0.042 | 1.000 | −0.055 | 0.320 | 0.211 | 0.241 | 0.129 | −0.059 | 0.025 | 0.051 | −0.071 | 0.109 | |
| Gender | −0.021 | −0.034 | −0.055 | 1.000 | −0.015 | 0.028 | −0.009 | 0.014 | −0.097 | 0.025 | 0.008 | −0.028 | 0.019 | |
| Hypertension | −0.205 | −0.141 | 0.320 | −0.015 | 1.000 | 0.344 | 0.310 | 0.129 | −0.104 | 0.045 | 0.005 | −0.357 | 0.039 | |
| Diabetes | −0.048 | −0.051 | 0.211 | 0.028 | 0.344 | 1.000 | 0.196 | −0.035 | −0.053 | 0.081 | 0.030 | −0.217 | 0.081 | |
| Cardiovascular disease | 0.031 | −0.012 | 0.241 | −0.009 | 0.310 | 0.196 | 1.000 | 0.108 | −0.058 | 0.068 | 0.001 | −0.224 | 0.207 | |
| Respiratory disease | −0.002 | 0.063 | 0.129 | 0.014 | 0.129 | −0.035 | 0.108 | 1.000 | −0.018 | 0.000 | 0.000 | −0.116 | 0.197 | |
| Type of procedure | 0.227 | 0.036 | −0.059 | −0.097 | −0.104 | −0.053 | −0.058 | −0.018 | 1.000 | −0.064 | −0.086 | 0.238 | 0.072 | |
| Secondary tumors | −0.046 | −0.021 | 0.025 | 0.025 | 0.045 | 0.081 | 0.068 | 0.000 | −0.064 | 1.000 | −0.001 | −0.202 | 0.378 | |
| Discharge ward | 0.001 | 0.015 | 0.051 | 0.008 | 0.005 | 0.030 | 0.001 | 0.000 | −0.086 | −0.001 | 1.000 | −0.074 | 0.086 | |
| Year of discharge | 0.246 | 0.070 | −0.071 | −0.028 | −0.357 | −0.217 | −0.224 | −0.116 | 0.238 | −0.202 | −0.074 | 1.000 | 0.152 | |
| Surgery with Complications | 0.107 | 0.016 | 0.109 | 0.019 | 0.039 | 0.081 | 0.207 | 0.197 | 0.072 | 0.378 | 0.086 | 0.152 | 1.000 | |
| Sign. (1-tailed) | LOS | Pre-operative LOS | Age | Gender | Hypertension | Diabetes | Cardiovascular disease | Respiratory disease | Type of procedure | Secondary tumors | Discharge ward | Year of discharge | Surgery with Complications | |
| LOS | . | 0.000 | 0.201 | 0.237 | 0.000 | 0.054 | 0.146 | 0.480 | 0.000 | 0.063 | 0.485 | 0.000 | 0.000 | |
| Pre-operative LOS | 0.000 | . | 0.082 | 0.126 | 0.000 | 0.042 | 0.345 | 0.017 | 0.112 | 0.245 | 0.303 | 0.009 | 0.292 | |
| Age | 0.201 | 0.082 | . | 0.033 | 0.000 | 0.000 | 0.000 | 0.000 | 0.025 | 0.198 | 0.044 | 0.008 | 0.000 | |
| Gender | 0.237 | 0.126 | 0.033 | . | 0.305 | 0.178 | 0.387 | 0.324 | 0.001 | 0.204 | 0.398 | 0.175 | 0.257 | |
| Hypertension | 0.000 | 0.000 | 0.000 | 0.305 | . | 0.000 | 0.000 | 0.000 | 0.000 | 0.067 | 0.437 | 0.000 | 0.093 | |
| Diabetes | 0.054 | 0.042 | 0.000 | 0.178 | 0.000 | . | 0.000 | 0.122 | 0.037 | 0.003 | 0.158 | 0.000 | 0.003 | |
| Cardiovascular disease | 0.146 | 0.345 | 0.000 | 0.387 | 0.000 | 0.000 | . | 0.000 | 0.027 | 0.011 | 0.493 | 0.000 | 0.000 | |
| Respiratory disease | 0.480 | 0.017 | 0.000 | 0.324 | 0.000 | 0.122 | 0.000 | . | 0.276 | 0.494 | 0.495 | 0.000 | 0.000 | |
| Type of procedure | 0.000 | 0.112 | 0.025 | 0.001 | 0.000 | 0.037 | 0.027 | 0.276 | . | 0.016 | 0.002 | 0.000 | 0.008 | |
| Secondary tumors | 0.063 | 0.245 | 0.198 | 0.204 | 0.067 | 0.003 | 0.011 | 0.494 | 0.016 | . | 0.486 | 0.000 | 0.000 | |
| Discharge ward | 0.485 | 0.303 | 0.044 | 0.398 | 0.437 | 0.158 | 0.493 | 0.495 | 0.002 | 0.486 | . | 0.007 | 0.002 | |
| Year of discharge | 0.000 | 0.009 | 0.008 | 0.175 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.007 | . | 0.000 | |
| Surgery with Complications | 0.000 | 0.292 | 0.000 | 0.257 | 0.093 | 0.003 | 0.000 | 0.000 | 0.008 | 0.000 | 0.002 | 0.000 | . |
| Independent Variables | Tolerance | Variance Inflation Factor |
|---|---|---|
| Pre-operative LOS | 0.964 | 1.038 |
| Age | 0.840 | 1.191 |
| Gender | 0.983 | 1.017 |
| Hypertension | 0.693 | 1.443 |
| Diabetes | 0.836 | 1.196 |
| Cardiovascular disease | 0.813 | 1.230 |
| Respiratory disease | 0.892 | 1.121 |
| Type of procedure | 0.923 | 1.083 |
| Secondary tumors | 0.761 | 1.314 |
| Discharge ward | 0.967 | 1.034 |
| Year of discharge | 0.685 | 1.460 |
| Surgery with Complications | 0.682 | 1.466 |
| R | R2 | Adjusted R2 | Std. Error of the Estimate | |
|---|---|---|---|---|
| MLR Model | 0.819 | 0.671 | 0.677 | 1.931 |
| Variable | Unstandardized Coefficients | Standardized Coefficients Beta | t | p-Value * | |
|---|---|---|---|---|---|
| B | Std. Error | ||||
| Intercept | −725.249 | 59.353 | - | −12.219 | 0.000 |
| Pre-operative LOS | 1.043 | 0.024 | 0.749 | 42.686 | 0.000 |
| Age | 0.002 | 0.004 | 0.008 | 0.448 | 0.654 |
| Gender | 0.757 | 0.565 | 0.023 | 1.339 | 0.181 |
| Hypertension | −0.578 | 0.176 | −0.068 | −3.290 | 0.001 |
| Diabetes | 0.386 | 0.254 | 0.029 | 1.519 | 0.129 |
| Cardiovascular disease | 1.226 | 0.263 | 0.089 | 4.669 | 0.000 |
| Respiratory disease | −1.089 | 0.473 | −0.042 | −2.303 | 0.021 |
| Type of procedure | 0.047 | 0.005 | 0.163 | 9.112 | 0.000 |
| Secondary tumors | −0.279 | 0.294 | −0.019 | −0.949 | 0.343 |
| Discharge ward | 0.000 | 0.000 | 0.008 | 0.462 | 0.644 |
| Year of discharge | 0.160 | 0.024 | 0.141 | 6.786 | 0.000 |
| Surgery with Complications | 0.436 | 0.162 | 0.056 | 2.699 | 0.007 |
| GBT | RF | XGBoost | Polynomial Regression | |
|---|---|---|---|---|
| R2 | 0.649 | 0.501 | 0.601 | 0.689 |
| Root Mean Squared Error (RMSE) | 1.660 | 2.448 | 1.769 | 1.562 |
| Algorithm | Best Parameters |
|---|---|
| DT | {‘max_depth’: 3} |
| RF | {‘max_depth’: 7, ‘n_estimators’: 10} |
| SVM | {‘C’: 10, ‘kernel’: ‘linear’} |
| NB | {‘var_smoothing’: 1E-6} |
| Voter | {‘voting’: ‘hard’} |
| Performance Metrics | Class | DT | RF | SVM | NB |
|---|---|---|---|---|---|
| Accuracy | Overall | 0.76 | 0.78 | 0.73 | 0.72 |
| Precision | 0 | 0.77 | 0.79 | 0.75 | 0.74 |
| 1 | 0.73 | 0.73 | 0.68 | 0.71 | |
| 2 | 0.76 | 1.00 | 0.82 | 0.67 | |
| Recall | 0 | 0.86 | 0.83 | 0.82 | 0.86 |
| 1 | 0.60 | 0.71 | 0.62 | 0.52 | |
| 2 | 0.80 | 0.80 | 0.70 | 0.80 | |
| F-measure | 0 | 0.81 | 0.81 | 0.79 | 0.79 |
| 1 | 0.66 | 0.72 | 0.65 | 0.60 | |
| 2 | 0.78 | 0.89 | 0.76 | 0.73 |
| Features | Years 2018–2019 Pre-Pandemic (N = 208) | Years 2020–2021 COVID-19 Era (N = 194) | p-Value |
|---|---|---|---|
| Gender | 0.461 | ||
| M | 4 | 2 | |
| F | 204 | 192 | |
| Discharge ward | 0.541 | ||
| 0911 | 1 | 1 | |
| 0914 | 2 | 0 | |
| 0915 | 202 | 192 | |
| 0941 | 2 | 1 | |
| 1211 | 1 | 0 | |
| Type of procedure | 0.548 | ||
| 8512 | 1 | 1 | |
| 8520 | 0 | 2 | |
| 8521 | 1 | 0 | |
| 8522 | 65 | 60 | |
| 8533 | 3 | 1 | |
| 8535 | 1 | 1 | |
| 8541 | 26 | 32 | |
| 8542 | 1 | 0 | |
| 8543 | 2 | 0 | |
| 8545 | 104 | 95 | |
| 8546 | 1 | 1 | |
| 8548 | 2 | 0 | |
| 8554 | 1 | 0 | |
| 8599 | 0 | 1 | |
| Hypertension | 0.011 | ||
| Yes | 0 | 6 | |
| No | 208 | 188 | |
| Diabetes | 0.300 | ||
| Yes | 0 | 1 | |
| No | 208 | 193 | |
| Cardiovascular disease | 0.334 | ||
| Yes | 1 | 0 | |
| No | 207 | 194 | |
| Respiratory disease | - | ||
| Yes | 0 | 0 | |
| No | 208 | 194 | |
| Secondary tumors | - | ||
| Yes | 0 | 0 | |
| No | 208 | 194 | |
| Surgery with complications | 0.000 | ||
| Yes | 18 | 113 | |
| No | 190 | 81 | |
| Age | 0.831 | ||
| Mean ± STD Deviation | 59.50 ± 13.08 | 59.19 ± 13.41 | |
| Pre-Operative LOS | 0.168 | ||
| Mean ± STD Deviation | 1.32 ± 2.46 | 1.64 ± 2.16 | |
| LOS | 0.083 | ||
| Mean ± STD Deviation | 5.25 ± 3.84 | 5.39 ± 2.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trunfio, T.A.; Improta, G. Flow Analysis of Mastectomy Patients Using Length of Stay: A Single-Center Study. BioMedInformatics 2024, 4, 1725-1744. https://doi.org/10.3390/biomedinformatics4030094
Trunfio TA, Improta G. Flow Analysis of Mastectomy Patients Using Length of Stay: A Single-Center Study. BioMedInformatics. 2024; 4(3):1725-1744. https://doi.org/10.3390/biomedinformatics4030094
Chicago/Turabian StyleTrunfio, Teresa Angela, and Giovanni Improta. 2024. "Flow Analysis of Mastectomy Patients Using Length of Stay: A Single-Center Study" BioMedInformatics 4, no. 3: 1725-1744. https://doi.org/10.3390/biomedinformatics4030094
APA StyleTrunfio, T. A., & Improta, G. (2024). Flow Analysis of Mastectomy Patients Using Length of Stay: A Single-Center Study. BioMedInformatics, 4(3), 1725-1744. https://doi.org/10.3390/biomedinformatics4030094








