Aedes Larva Detection Using Ensemble Learning to Prevent Dengue Endemic
Abstract
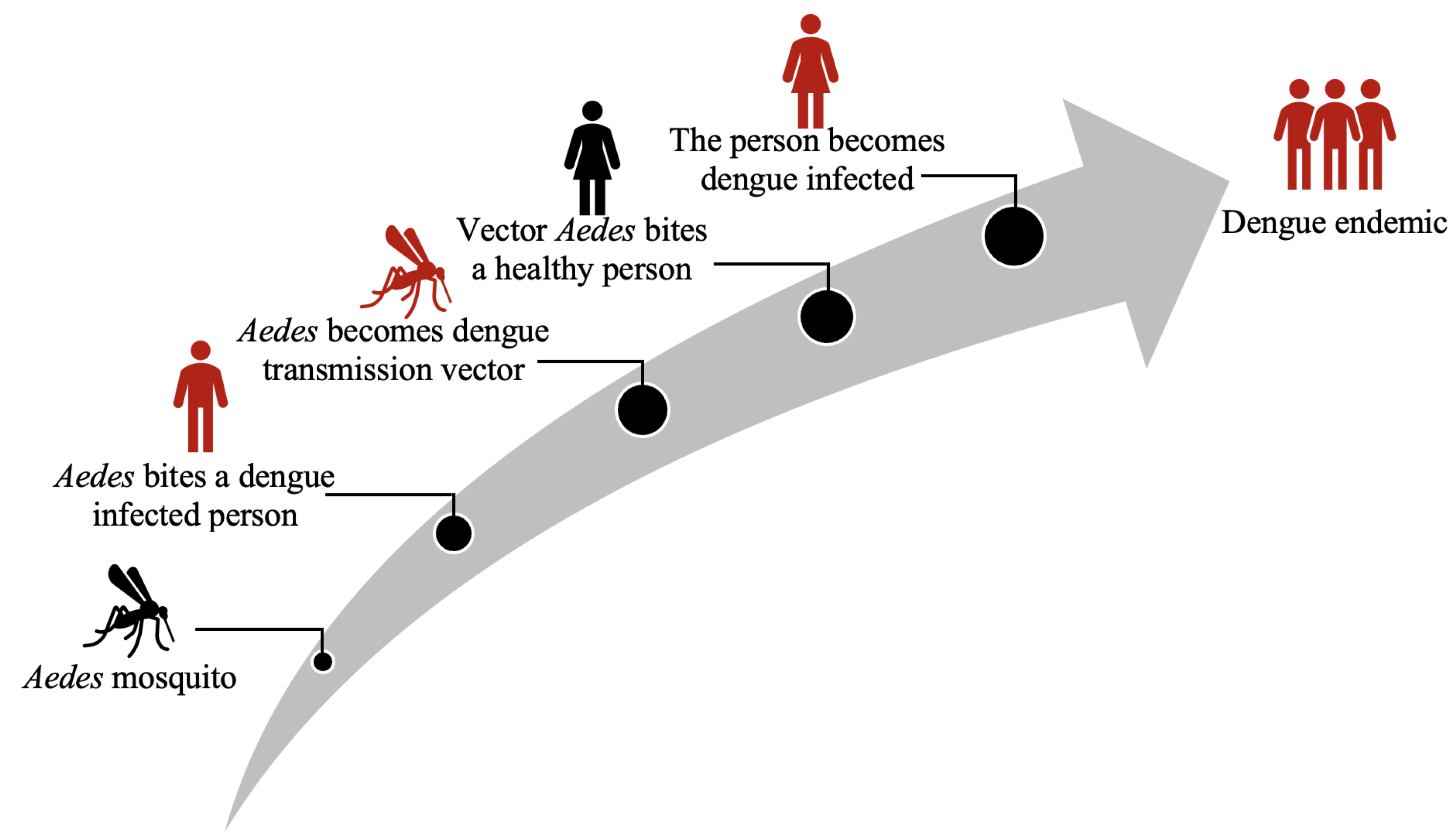
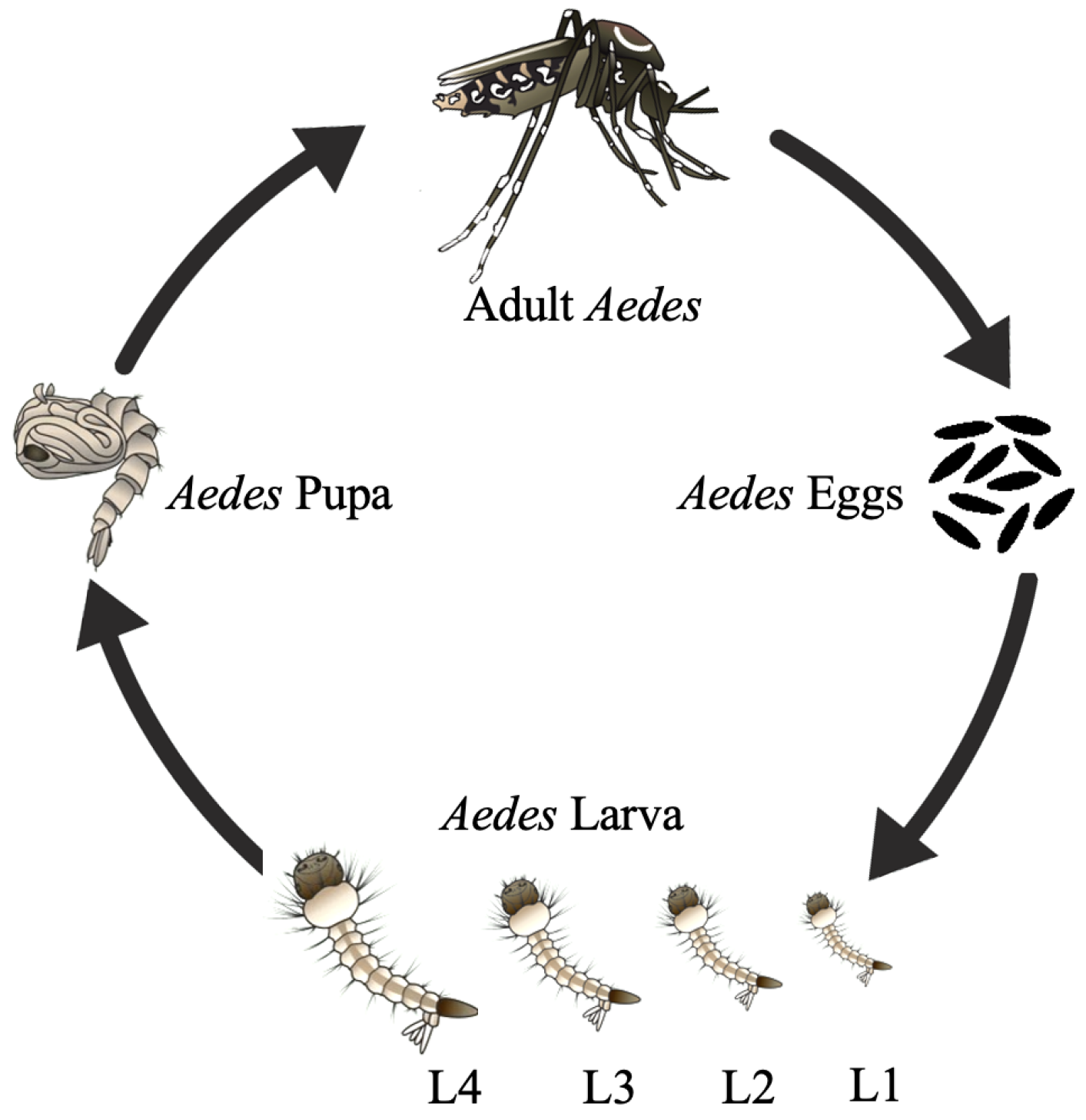
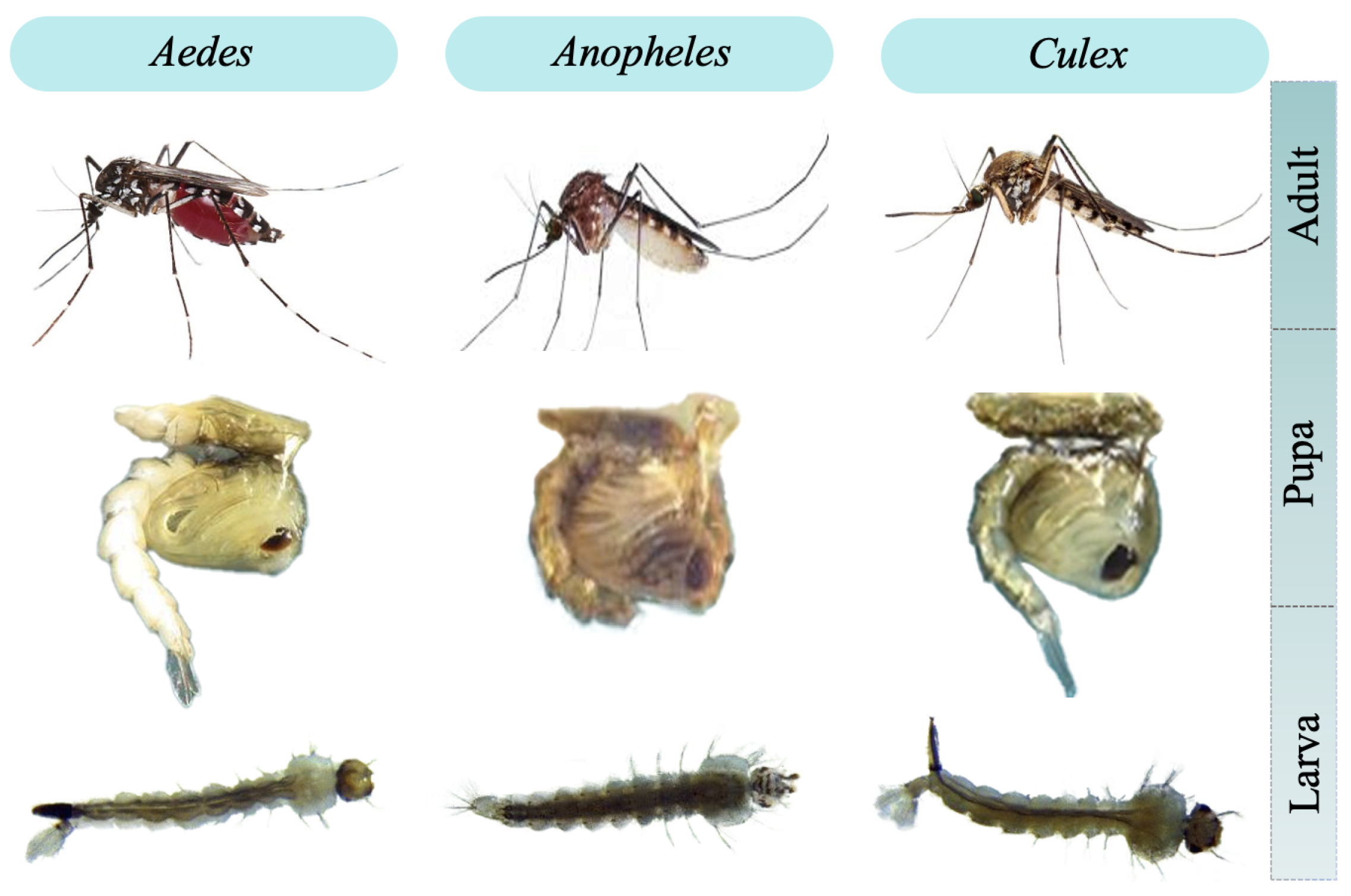
:1. Introduction
2. Related Works
3. Materials and Methods
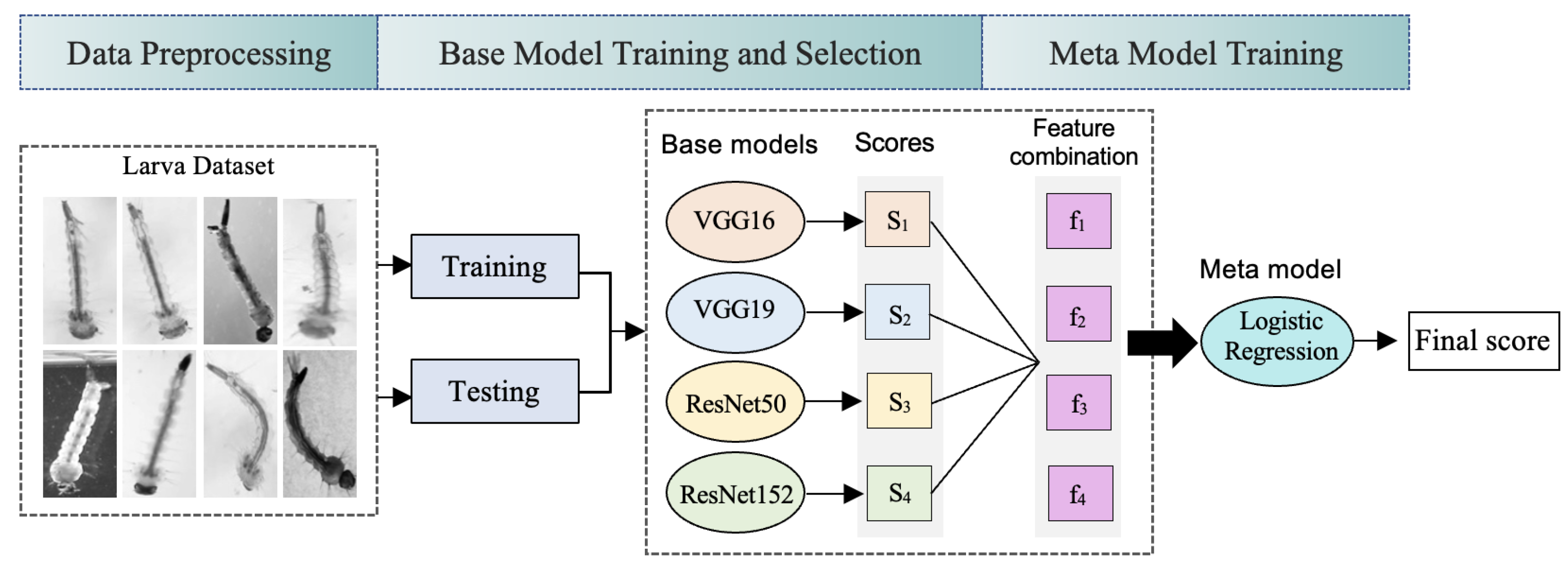
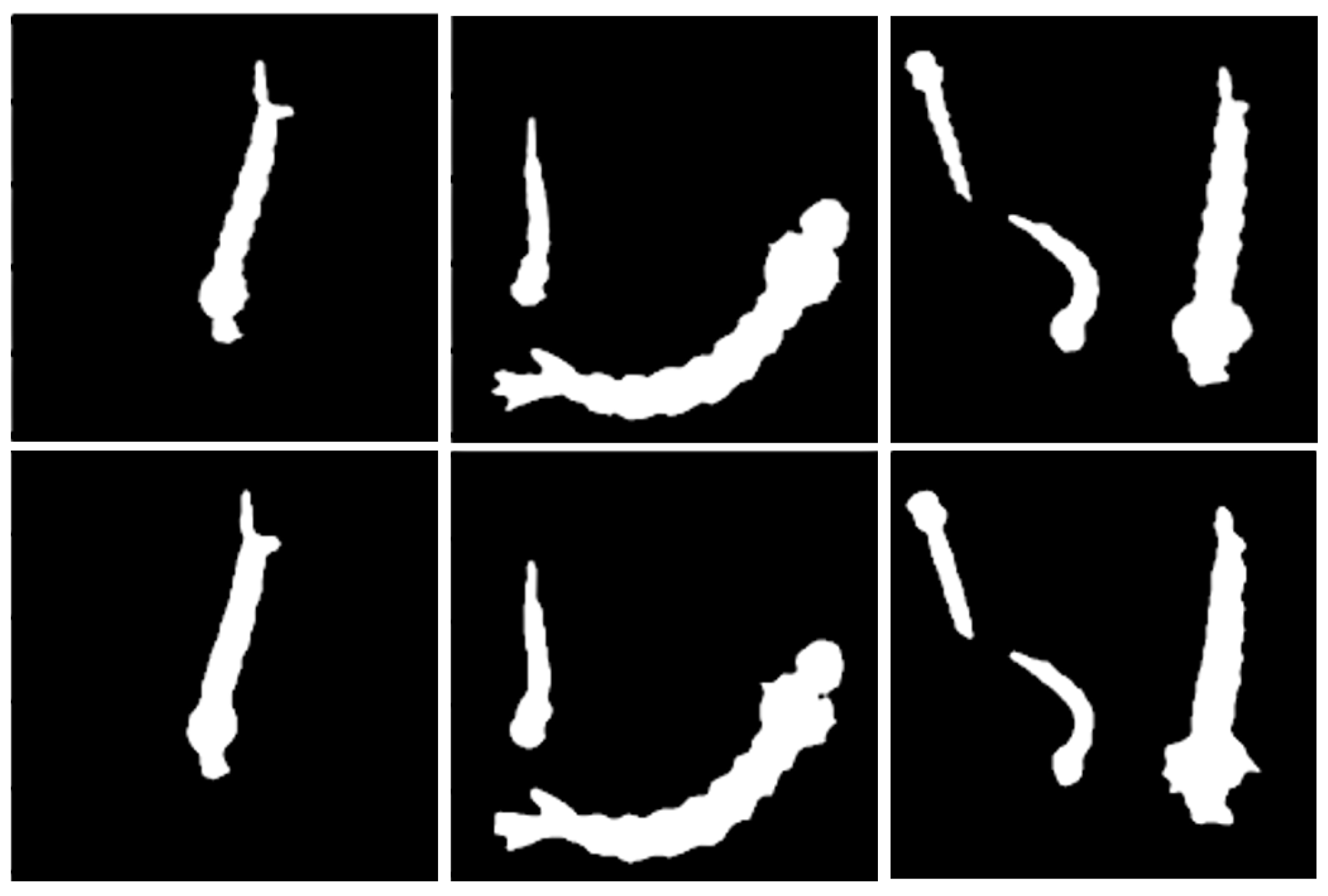
3.1. Image Dataset
3.2. System Overview
| Algorithm 1:Aedes larva identification method |
|
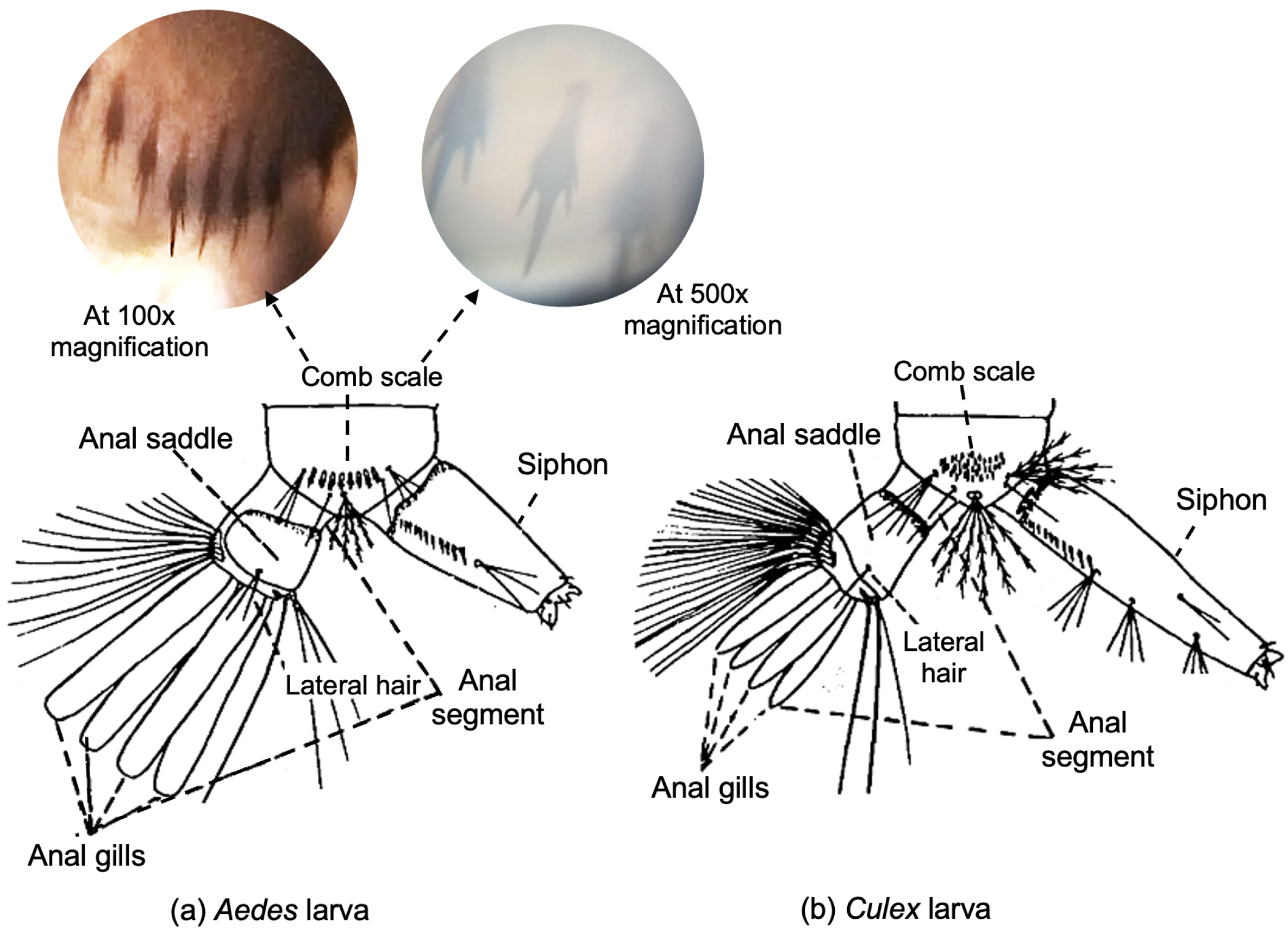
3.2.1. Larva ROI Segmentation
3.2.2. Ensemble Learning for Larva Classification
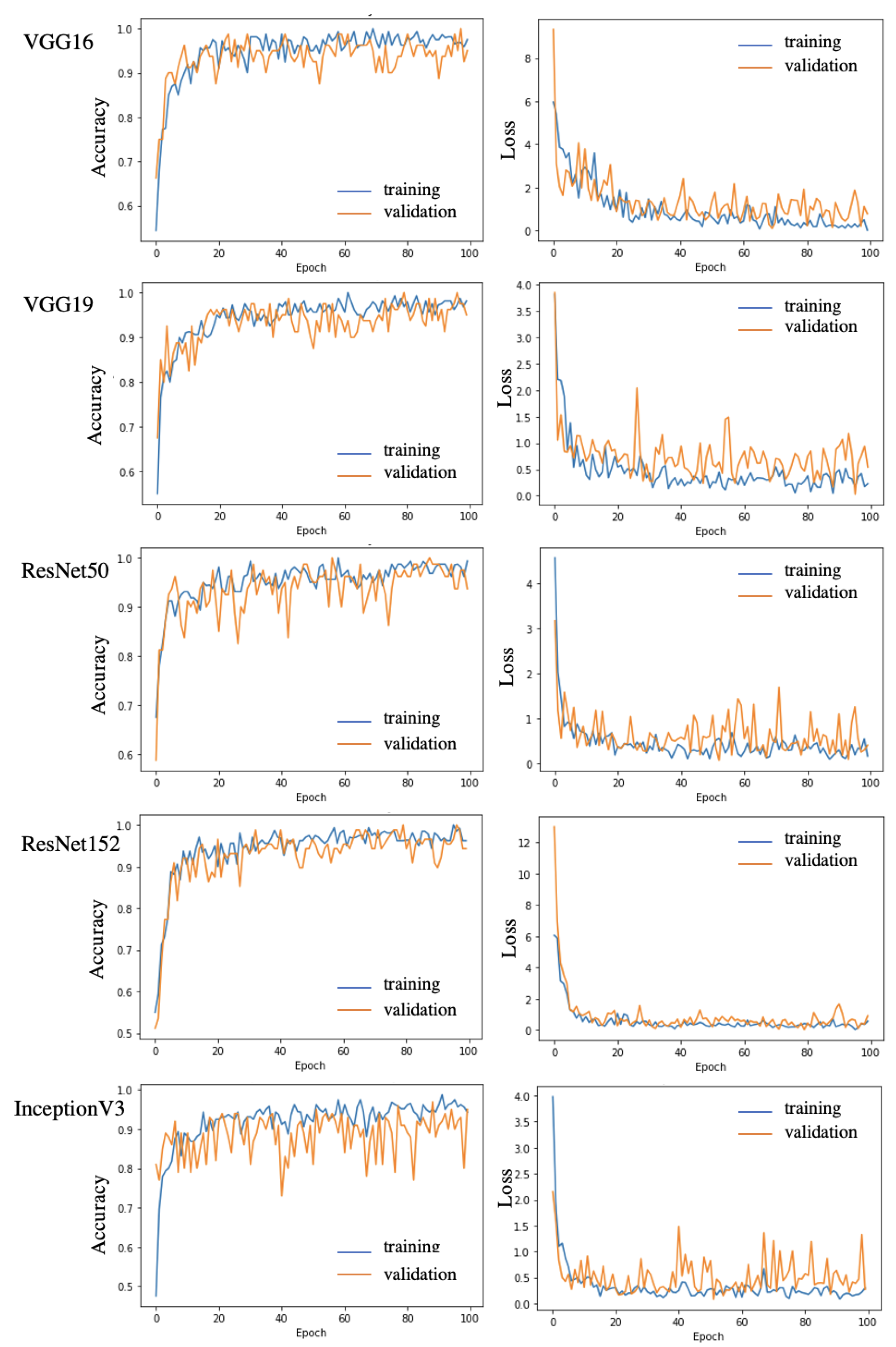
3.2.3. Base Model and Meta Model Selection
4. Results
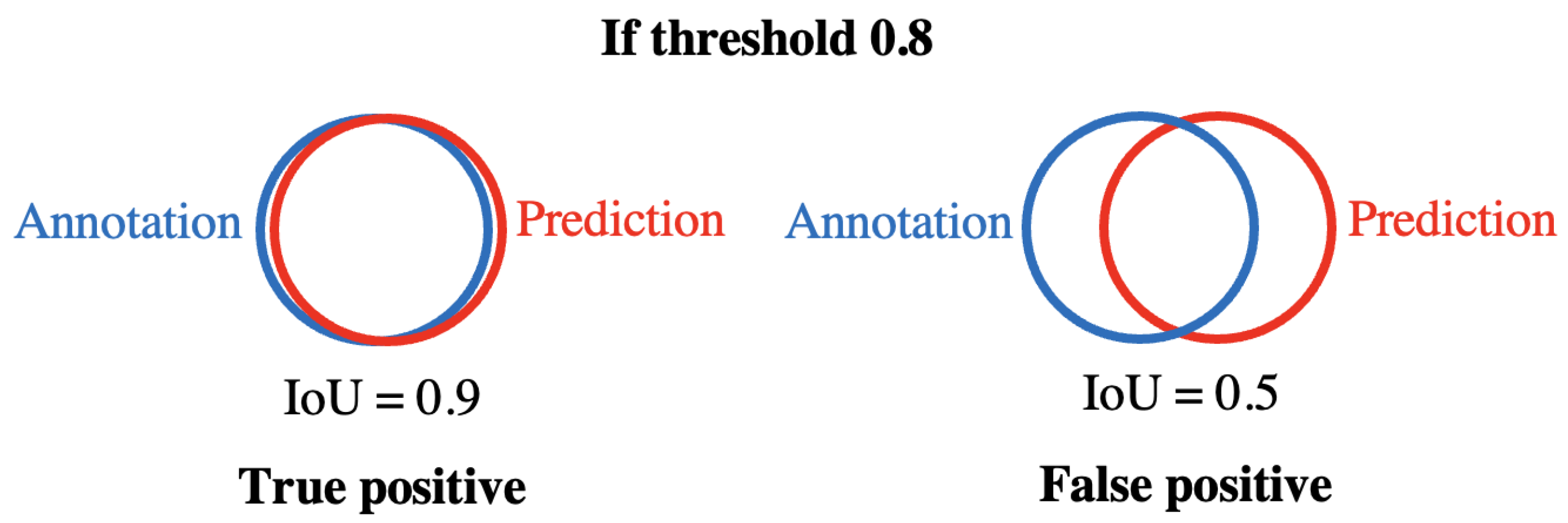
4.1. Evaluation of Larva Segmentation
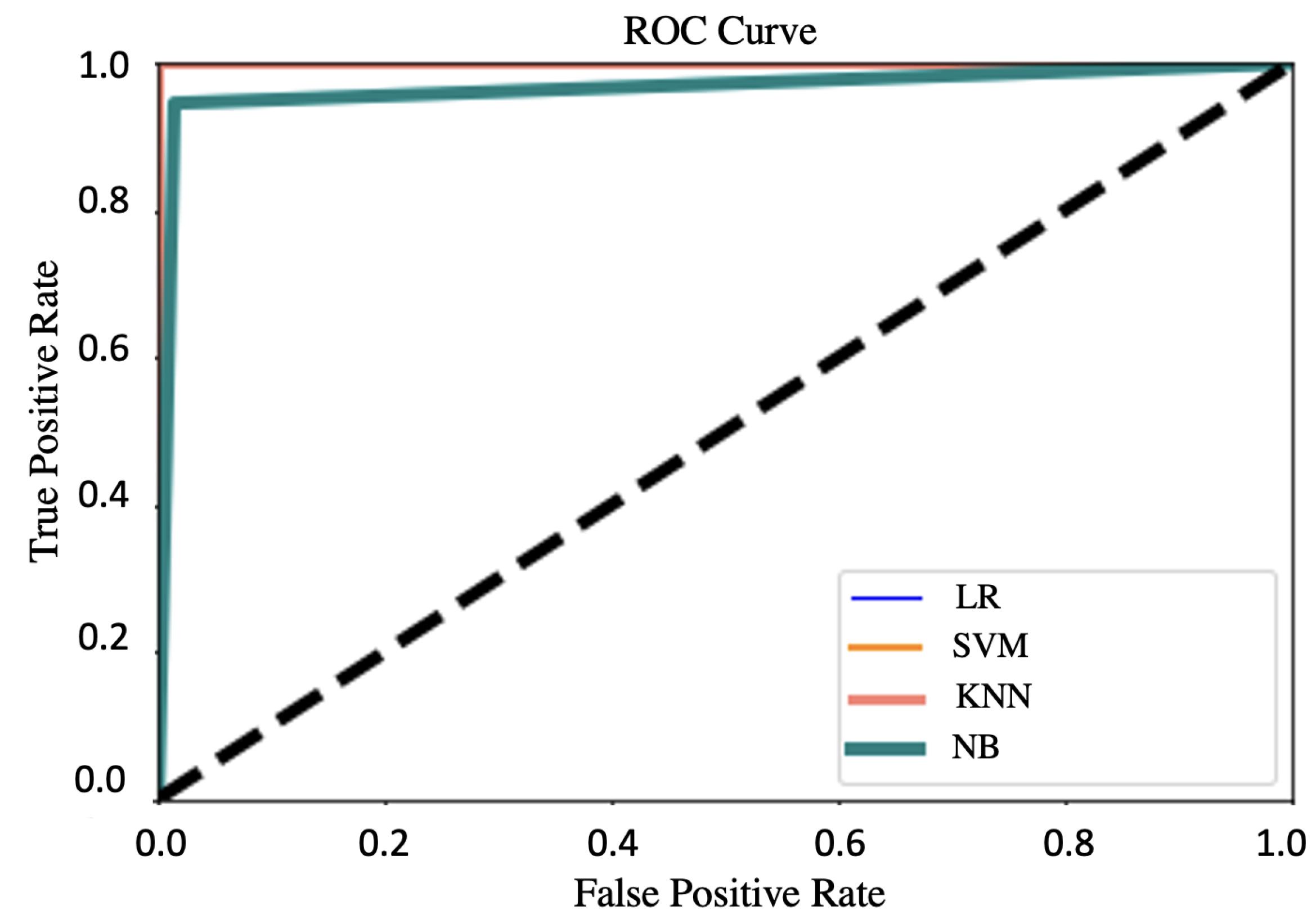
4.2. Evaluation of Larva Classification
4.3. Evaluation of Proposed System
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Vector-Borne Disease: Key Facts; World Health Organization: Geneva, Switzerland, 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 30 July 2022).
- Russell, P.K.; Buescher, E.L.; McCown, J.M.; Ordoñez, J. Recovery of dengue viruses from patients during epidemics in Puerto Rico and East Pakistan. Am. J. Trop. Med. Hyg. 1966, 15, 573–579. [Google Scholar] [CrossRef]
- Dhar-Chowdhury, P.; Paul, K.K.; Haque, C.E.; Hossain, S.; Lindsay, L.R.; Dibernardo, A.; Brooks, W.A.; Drebot, M.A. Dengue seroprevalence, seroconversion and risk factors in Dhaka, Bangladesh. PLoS Negl. Trop. Dis. 2017, 11, e0005475. [Google Scholar] [CrossRef]
- Rahman, M.S.; Faruk, M.O.; Tanjila, S.; Sabbir, N.M.; Haider, N.; Chowdhury, S. Entomological survey for identification of Aedes larval breeding sites and their distribution in Chattogram, Bangladesh. Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 1–11. [Google Scholar] [CrossRef]
- Dash, A.; Bhatia, R.; Kalra, N. Dengue in South-East Asia: An appraisal of case management and vector control. Dengue Bull. 2012, 36, 1–13. [Google Scholar]
- Ahsan, A.; Haider, N.; Kock, R.; Benfield, C. Possible drivers of the 2019 dengue outbreak in Bangladesh: The need for a robust community-level surveillance system. J. Med. Entomol. 2021, 58, 37–39. [Google Scholar] [CrossRef]
- WHO. Comprehensive Guideline for Prevention and Control of Dengue and Dengue Haemorrhagic Fever; World Health Organization: Geneva, Switzerland, 2011.
- Sharmin, S.; Glass, K.; Viennet, E.; Harley, D. Geostatistical mapping of the seasonal spread of under-reported dengue cases in Bangladesh. PLoS Negl. Trop. Dis. 2018, 12, e0006947. [Google Scholar] [CrossRef]
- Wahid, B.; Ali, A.; Rafique, S.; Idrees, M. Global expansion of chikungunya virus: Mapping the 64-year history. Int. J. Infect. Dis. 2017, 58, 69–76. [Google Scholar] [CrossRef]
- Hossain, M.G.; Nazir, K.N.H.; Saha, S.; Rahman, M.T. Zika virus: A possible emerging threat for Bangladesh! J. Adv. Vet. Anim. Res. 2019, 6, 575. [Google Scholar] [CrossRef]
- Anwar, S.; Taslem Mourosi, J.; Khan, M.F.; Ullah, M.O.; Vanakker, O.M.; Hosen, M.J. Chikungunya outbreak in Bangladesh (2017): Clinical and hematological findings. PLoS Negl. Trop. Dis. 2020, 14, e0007466. [Google Scholar] [CrossRef]
- Wagatsuma, Y.; Breiman, R.F.; Hossain, A.; Rahman, M. Dengue fever outbreak in a recreation club, Dhaka, Bangladesh. Emerg. Infect. Dis. 2004, 10, 747. [Google Scholar] [CrossRef]
- Chowdhury, R.; Chowdhury, V.; Faria, S.; Huda, M.M.; Laila, R.; Dhar, I.; Maheswary, N.P.; Dash, A.P. How dengue vector Aedes albopictus (Diptera: Culicidae) survive during the dry season in Dhaka City, Bangladesh? J. Vector Borne Dis. 2014, 51, 179. [Google Scholar]
- Abir, T.; Ekwudu, O.; Kalimullah, N.A.; Nur-A Yazdani, D.M.; Al Mamun, A.; Basak, P.; Osuagwu, U.L.; Permarupan, P.Y.; Milton, A.H.; Talukder, S.H.; et al. Dengue in Dhaka, Bangladesh: Hospital-based cross-sectional KAP assessment at Dhaka north and Dhaka south city corporation area. PLoS ONE 2021, 16, e0249135. [Google Scholar] [CrossRef]
- Barraud, P.J. The Fauna of British India, including Ceylon and Burma. Diptera. Vol. 5. Family Culieldae. Tribes Megarhinini and Culicini; CABI: Wallingford, UK, 1934. [Google Scholar]
- Snodgrass, R.E. The anatomical life of the mosquito. In Smithsonian Miscellaneous Collections; Smithsonian Institution: Auburn, KY, USA, 1959. [Google Scholar]
- Clements, A.N. The Physiology of Mosquitoes: International Series of Monographs on Pure and Applied Biology: Zoology; Elsevier: Amsterdam, The Netherlands, 1963; Volume 17. [Google Scholar]
- Christophers, S. The Yellow Fever Mosquito. Its Life History, Bionomics and Structure; Cambridge University Press: Cambridge, UK, 1960. [Google Scholar]
- Bar, A.; Andrew, J. Morphology and morphometry of Aedes aegypti larvae. Annu. Res. Rev. Biol. 2013, 3, 1–21. [Google Scholar]
- Silva, D.F.; De Souza, V.M.; Batista, G.E.; Keogh, E.; Ellis, D.P. Applying machine learning and audio analysis techniques to insect recognition in intelligent traps. In Proceedings of the 2013 12th International Conference on Machine Learning and Applications, Miami, FL, USA, 4–7 December 2013; IEEE: Piscataway, NJ, USA, 2013; Volume 1, pp. 99–104. [Google Scholar]
- De Los Reyes, A.M.M.; Reyes, A.C.A.; Torres, J.L.; Padilla, D.A.; Villaverde, J. Detection of Aedes Aegypti mosquito by digital image processing techniques and support vector machine. In Proceedings of the 2016 IEEE Region 10 Conference (TENCON), Singapore, 22–25 November 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 2342–2345. [Google Scholar]
- Mulchandani, P.; Siddiqui, M.U.; Kanani, P. Real-time mosquito species identification using deep learning techniques. Int. J. Eng. Adv. Technol. 2019, 9, 2249–8958. [Google Scholar] [CrossRef]
- Ong, S.Q.; Ahmad, H.; Nair, G.; Isawasan, P.; Majid, A.H.A. Implementation of a deep learning model for automated classification of Aedes aegypti (Linnaeus) and Aedes albopictus (Skuse) in real time. Sci. Rep. 2021, 11, 9908. [Google Scholar] [CrossRef]
- Fernandes, M.S.; Cordeiro, W.; Recamonde-Mendoza, M. Detecting Aedes aegypti mosquitoes through audio classification with convolutional neural networks. Comput. Biol. Med. 2021, 129, 104152. [Google Scholar] [CrossRef]
- Sanchez-Ortiz, A.; Fierro-Radilla, A.; Arista-Jalife, A.; Cedillo-Hernandez, M.; Nakano-Miyatake, M.; Robles-Camarillo, D.; Cuatepotzo-Jiménez, V. Mosquito larva classification method based on convolutional neural networks. In Proceedings of the 2017 International Conference on Electronics, Communications and Computers (CONIELECOMP), Cholula, Mexico, 22–24 February 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1–6. [Google Scholar]
- Garcia-Nonoal, Z.; Sanchez-Ortiz, A.; Arista-Jalife, A.; Nakano, M. Comparison of image descriptors to classify mosquito larvae. In Proceedings of the CAIP, Ystad, Sweden, 22–24 August 2017; pp. 271–278. [Google Scholar]
- Asmai, S.; Zukhairin, M.; Jaya, A.; Rahman, A.; Abas, Z. Mosquito larvae detection using deep learning. Int. J. Innov. Technol. Explor. Eng. IJITEE 2019, 8, 804–809. [Google Scholar] [CrossRef]
- Arista-Jalife, A.; Nakano, M.; Garcia-Nonoal, Z.; Robles-Camarillo, D.; Perez-Meana, H.; Arista-Viveros, H.A. Aedes mosquito detection in its larval stage using deep neural networks. Knowl.-Based Syst. 2020, 189, 104841. [Google Scholar] [CrossRef]
- Azman, M.I.A.B.Z.; Sarlan, A.B. Aedes larvae classification and detection (ALCD) system by using deep learning. In Proceedings of the 2020 International Conference on Computational Intelligence (ICCI), Bandar Seri Iskandar, Malaysia, 8–9 October 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 179–184. [Google Scholar]
- De Silva, W.; Jayalal, S. Dengue mosquito larvae identification using digital images. In Proceedings of the 2020 International Research Conference on Smart Computing and Systems Engineering (SCSE), Colombo, Sri Lanka, 24 September 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 31–36. [Google Scholar]
- Hossain, M.S.; Nakamura, T.; Kimura, F.; Yagi, Y.; Yamaguchi, M. Practical image quality evaluation for whole slide imaging scanner. In Proceedings of the Biomedical Imaging and Sensing Conference, Yokohama, Japan, 25–27 April 2018; SPIE: Bellingham, WA, USA, 2018; Volume 10711, pp. 203–206. [Google Scholar]
- Shakhawat, H.M.; Nakamura, T.; Kimura, F.; Yagi, Y.; Yamaguchi, M. Automatic Quality Evaluation of Whole Slide Images for the Practical Use of Whole Slide Imaging Scanner. ITE Trans. Media Technol. Appl. 2020, 8, 252–268. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-net: Convolutional networks for biomedical image segmentation. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Munich, Germany, 5–9 October 2015; Springer: Berlin/Heidelberg, Germany, 2015; pp. 234–241. [Google Scholar]
- Wei, L.; Zhou, C.; Chen, H.; Song, J.; Su, R. ACPred-FL: A sequence-based predictor using effective feature representation to improve the prediction of anti-cancer peptides. Bioinformatics 2018, 34, 4007–4016. [Google Scholar] [CrossRef]
- Sun, W.; Trevor, B. Combining k-nearest-neighbor models for annual peak breakup flow forecasting. Cold Reg. Sci. Technol. 2017, 143, 59–69. [Google Scholar] [CrossRef]
- Xue, D.; Zhou, X.; Li, C.; Yao, Y.; Rahaman, M.M.; Zhang, J.; Chen, H.; Zhang, J.; Qi, S.; Sun, H. An application of transfer learning and ensemble learning techniques for cervical histopathology image classification. IEEE Access 2020, 8, 104603–104618. [Google Scholar] [CrossRef]
- Li, C.; Xue, D.; Kong, F.; Hu, Z.; Chen, H.; Yao, Y.; Sun, H.; Zhang, L.; Zhang, J.; Jiang, T.; et al. Cervical histopathology image classification using ensembled transfer learning. In Proceedings of the International Conference on Information Technologies in Biomedicine, Kamień Śląski, Poland, 18–20 June 2019; Springer: Berlin/Heidelberg, Germany, 2019; pp. 26–37. [Google Scholar]
- Aboneh, T.; Rorissa, A.; Srinivasagan, R. Stacking-Based Ensemble Learning Method for Multi-Spectral Image Classification. Technologies 2022, 10, 17. [Google Scholar] [CrossRef]
- Mohammed, M.; Mwambi, H.; Mboya, I.B.; Elbashir, M.K.; Omolo, B. A stacking ensemble deep learning approach to cancer type classification based on TCGA data. Sci. Rep. 2021, 11, 15626. [Google Scholar] [CrossRef]
- Manavalan, B.; Basith, S.; Shin, T.H.; Wei, L.; Lee, G. Meta-4mCpred: A sequence-based meta-predictor for accurate DNA 4mC site prediction using effective feature representation. Mol. Ther.-Nucleic Acids 2019, 16, 733–744. [Google Scholar] [CrossRef]
- Su, R.; Liu, X.; Xiao, G.; Wei, L. Meta-GDBP: A high-level stacked regression model to improve anticancer drug response prediction. Briefings Bioinform. 2020, 21, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jin, Y.; Zhong, J.; Zhao, R. A Fruit Tree Disease Diagnosis Model Based on Stacking Ensemble Learning. Complexity 2021, 2021, 6868592. [Google Scholar] [CrossRef]
- Breiman, L. Stacked regressions. Mach. Learn. 1996, 24, 49–64. [Google Scholar] [CrossRef]
- Wolpert, D.H. Stacked generalization. Neural Netw. 1992, 5, 241–259. [Google Scholar] [CrossRef]
- Pan, S.J.; Yang, Q. A survey on transfer learning. IEEE Trans. Knowl. Data Eng. 2009, 22, 1345–1359. [Google Scholar] [CrossRef]
- Zhao, W. Research on the deep learning of the small sample data based on transfer learning. AIP Conf. Proc. 2017, 1864, 020018. [Google Scholar]




| Hyperparameters | Optimization Space |
|---|---|
| Epochs | [10, 30, 50, 70, 100] |
| Batch sizes | [5, 10, 20, 30] |
| Learning rates | [0.001, 0.01, 0.03, 0.05] |
| Dropouts | [0.5, 0.6, 0.7, 0.8] |
| Model | Training Accuracy | Training Loss | Validation Accuracy | Validation Loss | AUC |
|---|---|---|---|---|---|
| VGG16 | 0.987 | 0.393 | 0.973 | 0.830 | 0.992 |
| VGG19 | 0.972 | 0.121 | 0.953 | 0.505 | 0.990 |
| ResNet50 | 0.975 | 0.172 | 0.920 | 0.160 | 0.978 |
| ResNet152 | 0.987 | 0.240 | 0.940 | 0.600 | 0.980 |
| InceptionV3 | 0.943 | 0.280 | 0.890 | 0.805 | 0.940 |
| VGG16 | VGG19 | ResNet50 | ResNet152 | Inceptionv3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPR | SPC | ACC | TPR | SPC | ACC | TPR | SPC | ACC | TPR | SPC | ACC | TPR | SPC | ACC | |
| F1 | 0.94 | 0.95 | 0.97 | 0.95 | 0.95 | 0.97 | 0.94 | 1.00 | 0.95 | 0.91 | 1.00 | 0.92 | 0.92 | 0.95 | 0.88 |
| F2 | 0.93 | 1.00 | 0.98 | 0.95 | 1.00 | 0.97 | 0.97 | 1.00 | 0.98 | 0.91 | 1.00 | 0.92 | 0.82 | 0.94 | 0.88 |
| F3 | 0.90 | 0.93 | 0.94 | 1.00 | 0.97 | 0.96 | 1.00 | 0.97 | 0.97 | 0.97 | 0.91 | 0.97 | 0.80 | 1.00 | 0.95 |
| F4 | 0.98 | 0.94 | 0.92 | 0.98 | 0.91 | 0.93 | 0.86 | 0.97 | 0.96 | 0.92 | 0.94 | 0.94 | 0.92 | 0.90 | 0.84 |
| Avg. | 0.93 | 0.95 | 0.95 | 0.97 | 0.95 | 0.95 | 0.94 | 0.98 | 0.96 | 0.93 | 0.96 | 0.94 | 0.86 | 0.94 | 0.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.S.; Raihan, M.E.; Hossain, M.S.; Syeed, M.M.M.; Rashid, H.; Reza, M.S. Aedes Larva Detection Using Ensemble Learning to Prevent Dengue Endemic. BioMedInformatics 2022, 2, 405-423. https://doi.org/10.3390/biomedinformatics2030026
Hossain MS, Raihan ME, Hossain MS, Syeed MMM, Rashid H, Reza MS. Aedes Larva Detection Using Ensemble Learning to Prevent Dengue Endemic. BioMedInformatics. 2022; 2(3):405-423. https://doi.org/10.3390/biomedinformatics2030026
Chicago/Turabian StyleHossain, Md Shakhawat, Md Ezaz Raihan, Md Sakir Hossain, M. M. Mahbubul Syeed, Harunur Rashid, and Md Shaheed Reza. 2022. "Aedes Larva Detection Using Ensemble Learning to Prevent Dengue Endemic" BioMedInformatics 2, no. 3: 405-423. https://doi.org/10.3390/biomedinformatics2030026
APA StyleHossain, M. S., Raihan, M. E., Hossain, M. S., Syeed, M. M. M., Rashid, H., & Reza, M. S. (2022). Aedes Larva Detection Using Ensemble Learning to Prevent Dengue Endemic. BioMedInformatics, 2(3), 405-423. https://doi.org/10.3390/biomedinformatics2030026









