3.1. Estimating Virial-Action Lapse Rates in Atmospheres as a Function of Composition
Estimates for virial-action dry lapse rates using Equation (17) for the troposphere on Earth for the mixture of predominant gases in the atmosphere are given in
Table 2. Given that water in a humid equatorial atmosphere can reach 100,000 ppmv, or 10%, the lapse rate will be reduced from 6.894 to 6.562. This is close to the observed values for the US Standard atmosphere by the US Airforce [
15]. The values shown in
Table 2 are taken from Kennedy [
14] for the other two rocky planets, with their atmospheres also shown, consistent with observations. The rate in air is estimated using a program in the
Supplementary Materials for the three predominant gases, N
2, O
2 and argon. Since CO
2 is only 0.0004 atm, one-twenty-fifth of argon, its lapse rate of 9.65 °C per km has little effect on Earth, although on planet Venus, it is decisive.
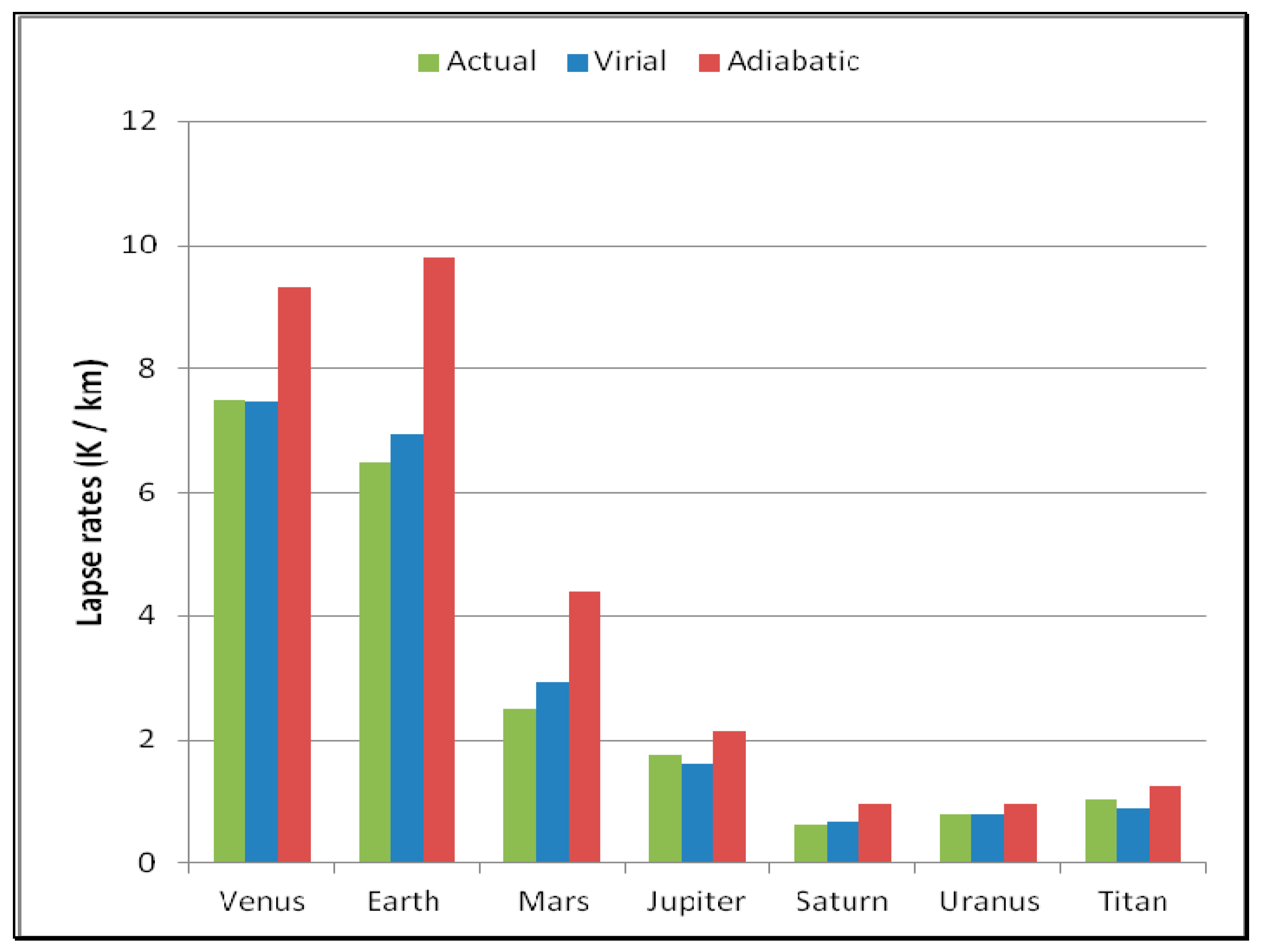
Figure 6 shows comparisons between the actual, virial and adiabatic lapse rates on planets with an atmosphere, with data described in more detail previously [
14]. The observed lapse rates [
15] correspond much more closely to the virial-action lapse rates than to adiabatic expansions into the atmosphere. By comparison, the adiabatic lapse rate favoured by meteorologists plays little role in climate modelling, given its variability locally. Verification that the virial form shown in
Table 1 occurs generally on all planets [
14] should give confidence for the meteorological modelling of tropospheric configurations with altitude on Earth.
3.2. Carnot Heat Engine Cycles Between 288.15 and 208.15 K at Constant Gravity
For relevance to the troposphere, a Carnot cycle in this temperature range is considered appropriate. The Carnot engine cycle starts at the highest pressure and temperature, conditions conducive to driving a work process. In stage 1 of
Figure 3, the volume per molecule increases from the minimum, relieving the high pressure, which tends to cool the gas as work is performed by expansion, but remaining at the same temperature (isothermal), with heat being provided by the hot source (a). At some arbitrary point, the source is insulated from the gas cylinder, and expansion continues with an external pressure exactly matching that in the cylinder until it reaches about 0.1 atm pressure and 208.15 K, like conditions at the tropopause. Inertia from the cycling engine shown in
Figure 1 then recompresses the gas at a constant external temperature with the insulation removed, until the entropy change (δ
s = δq/δT) is equal to that of the original heating. With insulation restored, the engine then recompresses the gas in the cylinder to the same volume, pressure and temperature as at the beginning of the cycle. Then, as Carnot showed, the maximum work possible for each cycle is given by the difference between the heat input in stage 1 and the output (a′) removed at the minimum temperature of stage 3.
The outputs for a Carnot cycle using either argon at 0.01 atm or N
2 at 1 atm as working fluid are shown in
Table 3. Note that the increase in the negative Gibbs energy (−δ
gt = a) between isothermal stages 1 and 2 at high temperature is almost twice as much as the isothermal decrease between stages 3 and 4 (−δ
gt = −a′) at low temperature. These bracketed letters were used by Carnot in 1824 [
3]. As explained by Carnot (on page 31 in French edition), (a + b) must equal (a′ + b′), balancing the two heating stages for the working fluid cycle and the two cooling stages, respectively. Therefore, the difference between the variations (a − a′) or (b′ − b) is the maximum work possible at a given temperature range. Carnot insists that the first two sets be equal so that “after a complete cycle of operations the gas is brought back exactly to its primitive state”. The total heat input from the hot source, that is, stage 1 plus engine inertia in
Figure 1, must equal the maximum external work performed (a − a′) as hot source heat in minus cold heat out plus the external work performed, either as torque sustaining fly wheel momentum or any increased gravitational potential.
Table 3 also shows how other parameters associated with the cycle vary during each stage of the cycle. This includes variations in the pressure, temperature, action, entropy and Gibbs energy and mean values for quanta in the Gibbs energy of molecules in the cycle. This quantum field was considered as caloric by Carnot, drawing attention to its characteristic properties of state for each stage of the cycle. The Gibbs field quanta are regarded as causing the vis viva of the working fluid and its kinetic energy by exerting torques [
2,
4], as indicated in the Lagrangian analysis indicated in Equation (14) as the second derivative of the inertia of independent particles. The heat input at a given temperature indicates the entropy change involved (δq/T), enabling the effective torque maintaining the mean kinetic energy constant for different molecules. Clausius [
4] referred to this field energy performing work on the fluid in the engine as the ergal in his early research but later preferred his entropy or transformation terminology, indicating this was equivalent to the external work. In fact, this action of absorbed heat exerts a field pressure needed to produce a translational configuration of the molecules able to sustain the external work, such as lifting a weight or pumping water upwards by evacuation. In textbook accounts of the Carnot cycle, this expansion work phase is described as the integral of the dV/V differential expressing the external work without reference to the working fluid’s details. In action mechanics, this is described as a differential increase in action with changes in the negative Gibbs energy, as in line 5 in the argon table.
For argon in
Table 3, code a is 0.398, a′ is 0.287, code b′ is 2.387 and b is 2.276. Every cycle of the engine can perform the same amount of work, allowing the rate of work or power to be estimated. It is of interest that the difference in the Gibbs energies for inputs and outputs is the same for argon and N
2, although the adiabatic expansion between stage 2 and 3 extends the cylinder further because of the release of internal rotational quanta in the diatomic gas. This is no advantage in work performed since all the rotational energy consumed during expansion (b′) is needed to reheat the gas on recompression (b).
Only the variations in the translational Gibbs energy participate in the work process because both rotational and vibrational internal energies vary only with temperature and not with volume. It is remarkable that even Carnot’s codes for variation in the caloric at different stages of the engine cycle correspond exactly to variations in the Gibbs energy in a quantum field or as changes in the gravitational potential, shown in
Table 1 for both gases. Of course, the subsequent theoretical work of Clausius, Gibbs, Boltzmann, Planck and Einstein was needed to establish this link.
Schrödinger’s classic text
Statistical Thermodynamics [
16] provides a theoretical basis validating action mechanics estimates of Gibbs energy; this is displayed in
Table 3, where the Gibbs field is also partitioned into translational quanta in line 7 for argon. These apply to monatomic gases like argon but also to all gas molecules for the translational partition, certainly when too cold for rotation and vibration if polyatomic. In a chapter entitled The n-particle problem, Irwin equates the translational quantum field, as shown in Equation (28), with the thermodynamic potential of Gibbs.
Then,
Note that the Gibbs energy will be negative, indicating spontaneity;
ST must exceed (
E +
RT) [
1]. Schrödinger had no hesitation in applying quantum theory to translational action in a zone where mechanics are classical at ambient temperature [
6].
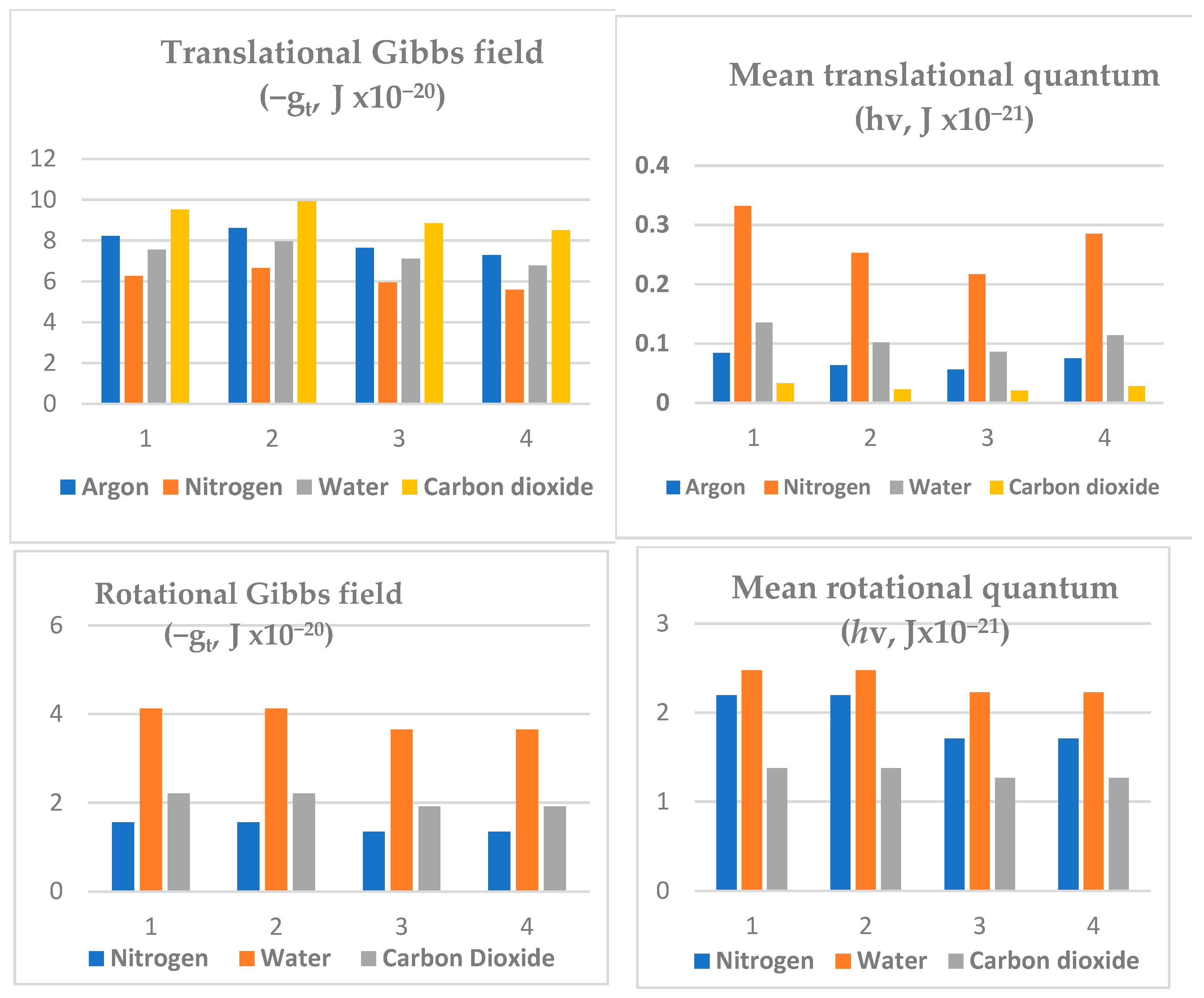
The Carnot cycle simulation shown in
Table 3 for the atmospheric gases argon and nitrogen can be extended to greenhouse gases, water vapour and CO
2, its thermal effect to be examined in later sections. However, the variation in spectral properties is shown in
Figure 7. The mean values of quantum properties in a cycle corresponding to an average global surface temperature of 288.15 K and at 5 km of 255.65 K have been estimated with the same Astrocal program (Carnot8/Cal) described in the
Supplementary Materials. In all cases, the translational energies are in the mm wavelength zone with frequencies near 10
11 around 100 GHz. The rotational properties for N
2, the predominant gas in air, are also given in
Table 3. However, the power of action mechanics to provide access to significant rotational spectra is revealed in the data generated for water vapour and CO
2. The Gibbs energy values can be resolved for the Earth’s surface spectrum for less than 100 μm for water molecules for its three inertial moments rotating as an asymmetric top.
By contrast, the simpler rotational spectrum for the linear symmetrical top CO
2 with a wavelength near 150 μm is greater than the predominant surface emission spectrum. A significant role for the water rotational spectral absorptions is evident in
Figure 7. Earlier, we showed with accuracy [
1] that the vibrational entropy of water vapour is negligible by comparison with CO
2, the latter with its major absorption and emission at 15 μm or 667 cm
−1. The vibrational spectrum for water with much shorter wave numbers of 1595, 3652 and 3756 can retain only 1% of the entropy for CO
2 per molecule, with correspondingly lower pressure of up to two orders of magnitude less. The far greater number density of water molecules in the troposphere at lower altitudes up to 5 km explains its predominant role in establishing the natural greenhouse warming on Earth, by comparison to CO
2.
This unique ability of action mechanics to easily estimate accurate mean values for the rotational Gibbs field energies is impressive, verifying its theoretical basis [
1,
2]. We intend to further investigate this capacity. The contrast in rotational energies between water vapour and CO
2, given that the quantum values for water lie in the Earth’s infrared emission zone, offers the potential for a better understanding of greenhouse processes.
3.3. Virial-Action Atmospheres on Earth
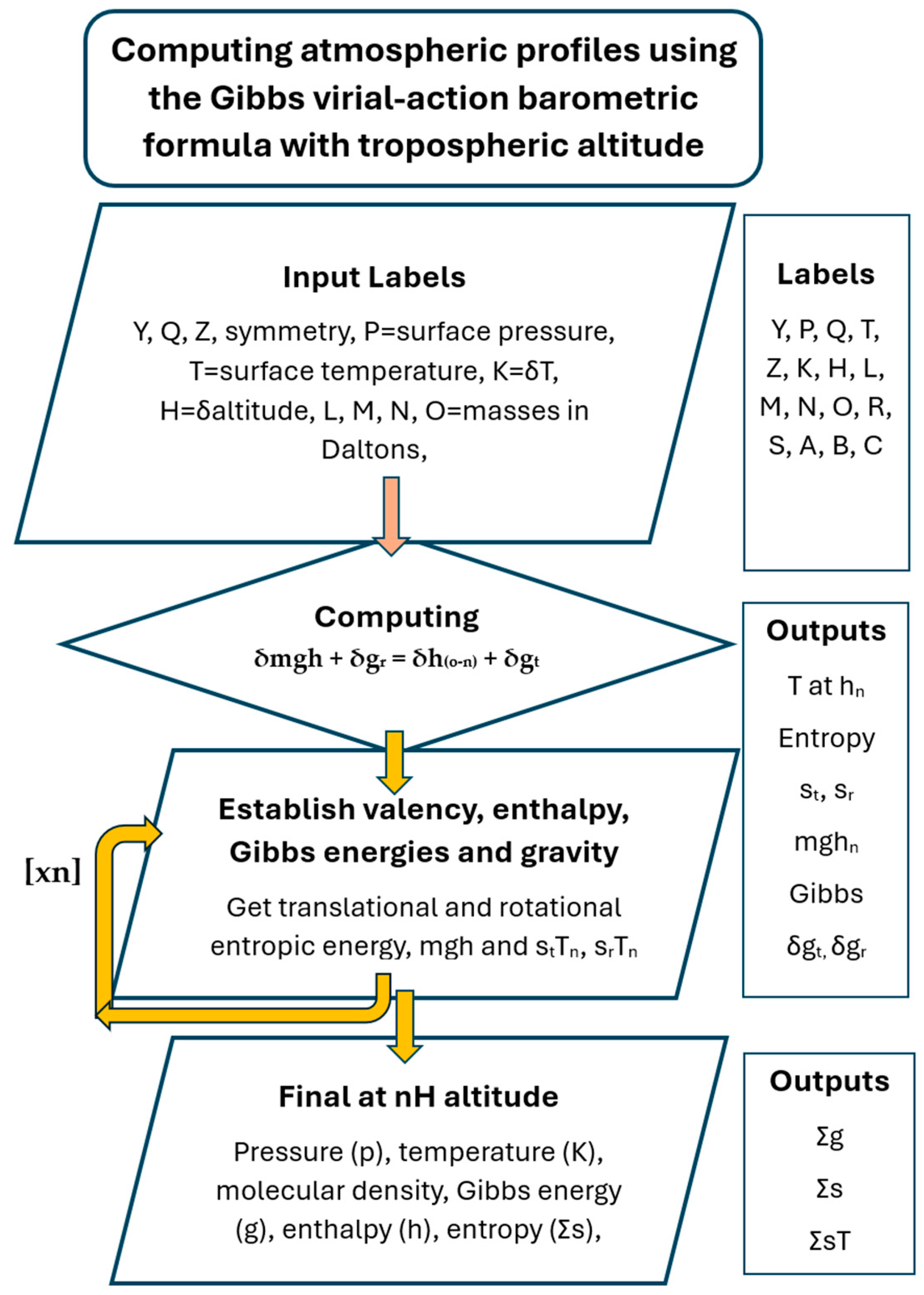
In
Table 4, the thermodynamic properties of air with properties averaged for nitrogen and oxygen with root-mean-square velocities characteristic of a surface temperature of 288.15 K are shown. These data were calculated using numerical computation, as outlined in
Figure 5, for each height. A comparison between data generated by the action barometric model and actual field observations collated by the United States Air Force [
15] is also shown in
Table 4, with close agreement, although the temperature fall is disrupted near the troposphere possibly because of atmospheric dynamics.
The data sets in
Table 4 and
Table 5 were computed using the virial-action gravitation Equation (27), assuming a base temperature of 288.15 K and the Earth’s average pressure at sea level, with gravity (
g) 9.8066, using the program Baromet10/Cal. A standard diatomic molecule of mass 29 with a bond length of 1.13 × 10
−10 cm, a rotational symmetry of 2 and a Q
e value of 1.41 to account for oxygen’s spin multiplicity of 3 was used. However, the same data could have been produced using the respective proportions of nitrogen and oxygen. Minor gases, including argon, were ignored in the calculation. The plot was made by solving Equation (20), as follows:
kTnln[(@
tn/
ħ)
3Q
e/z
t] = 3.5
k(
Tn −
To) +
kToln[(@
to/
ħ)
3Q
e/z
t] −
kToln[(@
ro/
ħ)
2/σ
r] +
kTnln[(@
rn/
ħ)
2/σ
r] −
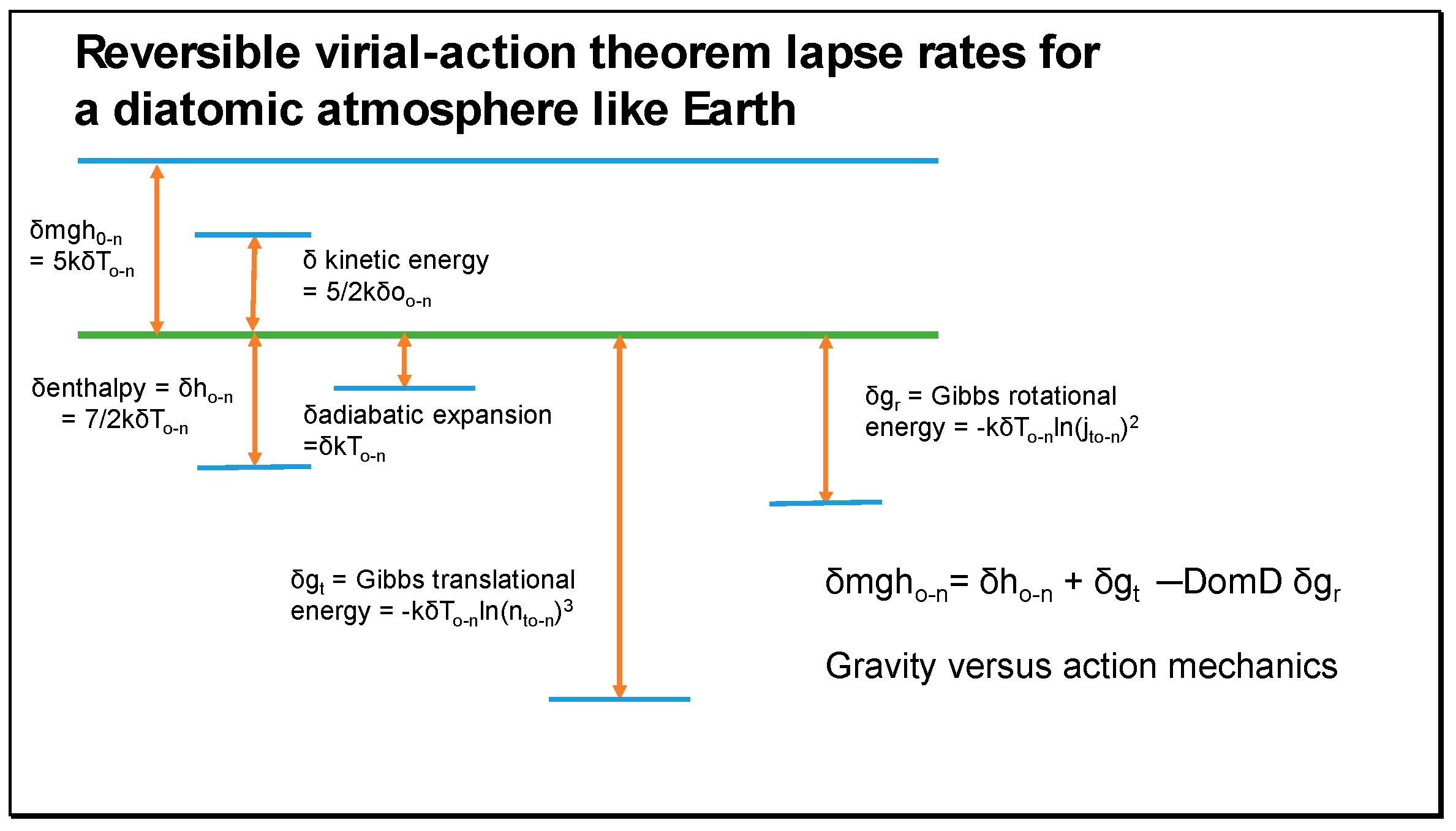
mghn. A data set including the variation in gravitational potential is shown in
Table 5, confirming virial-action Equation (27). In
Table 5, the close correspondence between the increasing gravitational potential and decreasing rotational Gibbs energy is significant. The inertial effect of rotation in diatomic molecules effectively renders the molecules weightless. The rotational Gibbs field reduces with altitude as the atmosphere becomes colder, being replaced with increasing gravitational potential, explaining Equation (27). Its vibrational component expressed as a harmonic oscillator has no such inertial or centrifugal component and cannot compensate mechanically for gravity.
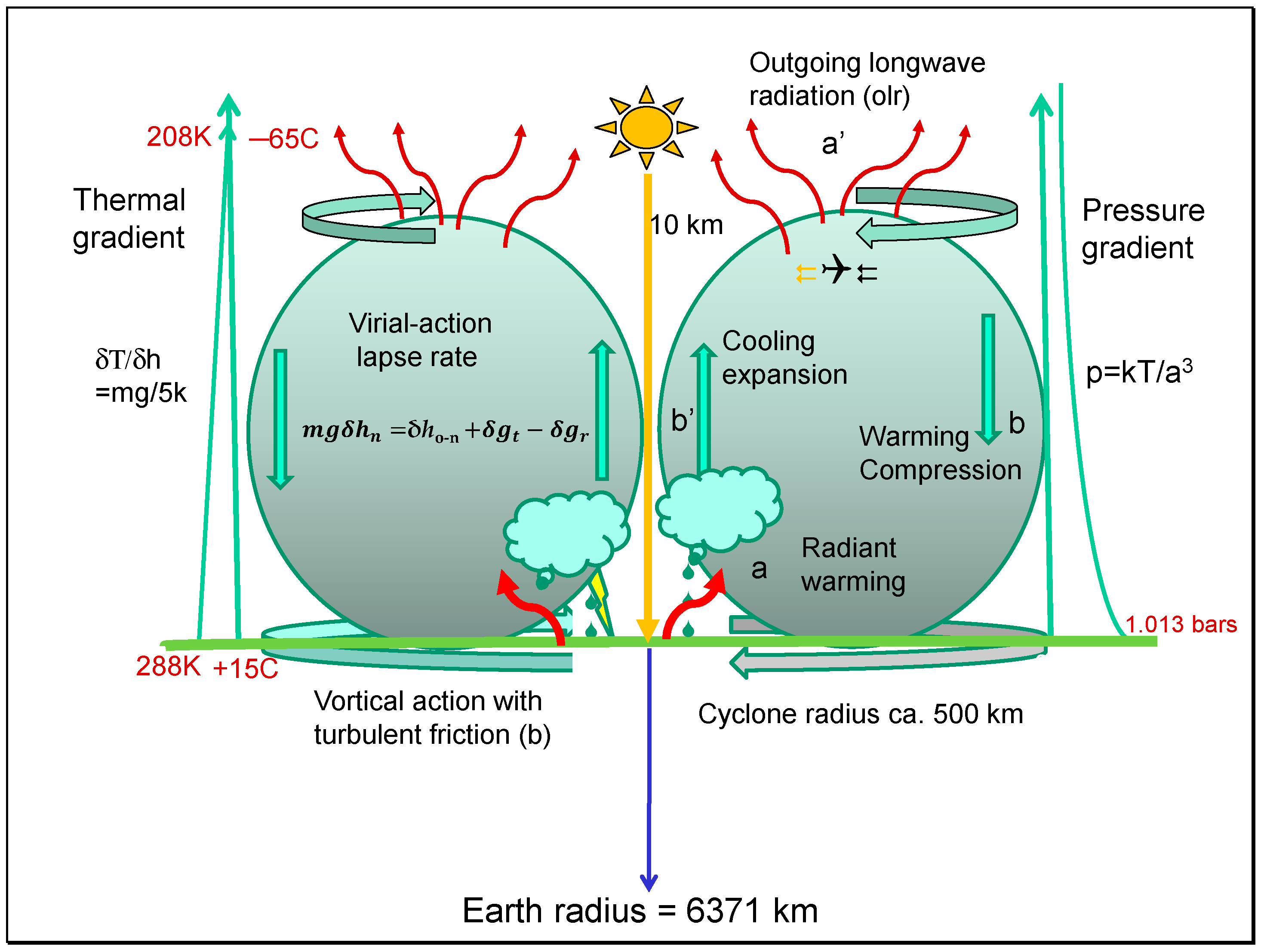
3.5. How Closely Do Anticyclones and Cyclones Function as Carnot Heat Engines?
Just as in the case of real heat engines, because of friction, the maximum power sustaining action in the troposphere must be imperfect. In a perfect cycle, factors like the rates of heat transfer are not limiting; there is no friction generating waste heat loss, nor is there the chaotic loss of heat in the working fluid by turbulence [
6]. The principle of least action regarding the action mechanics of particles in the working fluid applies. By contrast, tropospheric work processes are largely dissipative but continuously replenished with new energy. The internal work of the troposphere is a function of the increase in entropy of the radiant energy emitted from the Sun at almost 6000 K and the outgoing longwave radiation (OLR) emitted above the surface of the tropopause as infrared or microwave radiation at about 255 K. The average surface temperature near 288 K is a result of greenhouse warming.
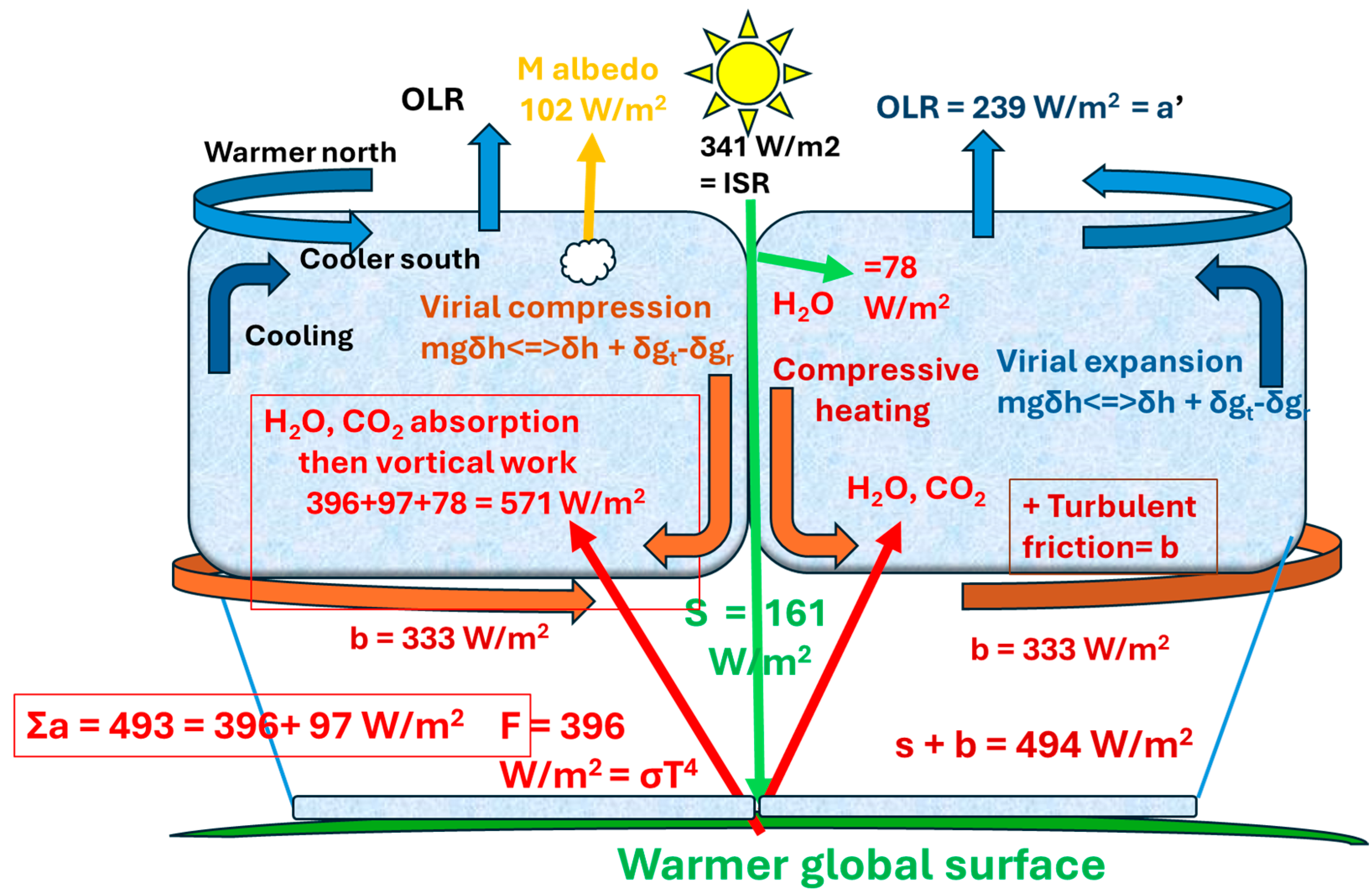
In
Figure 10, the Earth’s surface on land and sea provides the warmer heat source for radiant energy, sustaining the troposphere up to the colder tropopause. The Trenberth model for greenhouse warming [
17] involves the difference between black body radiation from the surface (R =
σT4) and outgoing longwave radiation (OLR ≡ a′). One of Carnot’s insights for reversibility in the engine working fluid was the need for the equality of heating (Σa + A = 396 + 97 + 78 = 571 W/m
2) and cooling processes (b′ + a′ = 333 + 239 = 572 W/m
2), as shown in
Figure 10. For warming, the troposphere is partly heated by solar absorption by water in air (A = 78 W/m
2), with the latent heat of evapotranspiration at the surface (e = 78 W/m
2) with heat release by condensation, under cooling convection. Thermals also conduct from the surface (c = 19 W/m
2). For 239 W/m
2 of absorbed solar radiation, a total of 571 W/m
2 is absorbed in the troposphere, and 239 is emitted through the tropopause to space. About 332 or 333 W/m
2 is available for frictional dissipation at the surface by turbulent heat release rather than by radiation. The greenhouse effect can be considered as (161 + 333) or 494 W/m
2 or a trebling of the total direct heat received at the surface while still allowing 239 W/m
2 of outgoing longwave radiation. Just considering the enhanced radiation from the surface because of a greenhouse effect and subtracting the OLR suggests a lower black body yield of 157 W/m
2 (R-OLR = 157 W/m
2). It is important to understand that, in terms of the work potential, the rating power or Joules per sec (W/m
2) takes no heed of the quality of the work achieved; 239 W/m
2 for the incoming solar radiation (ISR) and the same value for power as OLR cannot be compared for the kinds of work achieved given the large variation in the impulsive momentum between the two kinds of quanta concerned.
By contrast, there is a low likelihood of fully adiabatic expansions or compressions in the troposphere, given that radiative processes that transfer energy occur everywhere there are greenhouse gases with high emissivity. Cooling expansions and warming compressions with changes in altitude exist, but we claim these represent virial-action thermal and gravitational transitions, as already discussed. Heat and work processes in the troposphere also differ from heat engines in the smaller range of pressure variation, particularly in anticyclones. The ranges of pressures shown in
Table 3 for argon and nitrogen gases in the Carnot cycle are 7–10-fold for an 80 K temperature gradient. As a result, winds flowing to balance relatively low pressure gradients are far gentler. Wind droughts are more typical of periods of higher pressure [
18], explaining why wind turbines generating electricity perform weakly. In high-pressure anticyclonic zones with pressure above 1013 hPa (1.0 atm) up to 1030 hPa, descending compressing air still absorbs surface radiation with similar warming rates; the higher pressure is then relieved by radial Coriolis flow from horizontal rarefaction to zones of lower peripheral pressure.
In cyclonic zones with pressure falling as low as 900 hPa, the surface radiation also has intensity with Planck spectrum peaks defined by the varying temperature. Stronger winds flow to the centre of these depressions with vertical expansion rarefying air, cooling it quickly, and the likelihood of exceeding 100% relative humidity with the precipitation of rain is far greater. Black body radiation from the surface may be absorbed as a function of the concentration and absorptivity in the troposphere of greenhouse gases such as water, CO
2, CH
4 and N
2O. Having been excited to a higher quantum state, all these polar gases dissipate their vibrationally excited states almost immediately, warming other air molecules through collision processes [
19]. Near-surface warming can perform work sustaining 10 tonnes per square metre of the atmosphere, like phase 1 in a horizontal piston, increasing action and entropy. In sub-tropical or tropical marine environments, the cyclonic motion is enhanced by the release of infrared radiation from the condensation of water vapor by gravitationally cooling convection near a central eyewall. Surface evaporation by solar heating provides this source of latent energy. Similar though less intense processes can occur on land, provided there exists surface water.
In both cases of air cells of low and high pressure, vortical action provides an additional degree of freedom for the storage of Gibbs energy, sustaining mass action. This work process can be calculated using action mechanics, as described elsewhere [
2]. The conservation of vortical action was used to explain the mechanical intensification of tropical cyclones [
7], using the latent heat of water vapour to sustain vortical rotation, a higher level of Gibbs field energy. For vortical action, quantum levels are partitioned by the reduced Planck’s constant of action (
h/2π = 1.054 × 10
−34) (n
t =
mrv/
ћ), assuming a symmetry factor of 2. The vortical Gibbs energy for the matter per cell (M/
a3) is then estimated using the logarithm of the quantum number multiplied by the appropriate torque factor (Σ
mv
2). The total vortical Gibbs energy is obtained from the product of the number of molecules per cubic metre. This is then extended by altitude.
3.6. Greenhouse Gas Enhancement of Surface Temperature by Vortical Action
This work process requires the significant absorption of heat radiated from the surface, mainly by water and carbon dioxide, without significant rises in temperature. Greenhouse gases like H
2O and CO
2 act like temporary capacitors, rapidly giving up their energised state by absorbing quanta of infrared energy emitted from the surface. This process occurs for CO
2 molecules in collisions with water molecules after only about 105 collisions, dissipating this into translational kinetic energy from a collision rate of 10
10 per sec. Nitrous oxide has the same dissipation rate with water, and methane and CO
2 persist in an excited state for 406 collisions according to the experiments of Leffler and Grunwald [
19]. In the southern hemisphere, this powers anticlockwise anticyclone motion because the absorption of surface emission by GHGs is greater further north (
Figure 9), increasing pressure as the inertial Coriolis effect guides the air flow; this veers in the direction of the Earth’s motion in both hemispheres, moving away from the equator as latitudes increase. The increased translational and rotational energy provide the freedom for low-frequency vortical action and energy (
hv) [
6], providing a mechanism for the turbulent release of heat as radiation (T) near the surface. Given the negative surface gradient in temperature as solar intensity (S) declines, greenhouse gas activity will be greater nearer the equator, providing a pressure gradient from north to south in the southern hemisphere, as indicated in
Figure 10.
The release of radiation by friction nearer the surface, consistent with gravitational potential, does not conflict with the second law of thermodynamics, solving objections to downwelling radiation warming from a colder source. As shown in the Carnot cycle, any increase in the freedom of relative translational motion of molecules increases the heat capacity of the gas phase. For anticyclones, this allows turbulent friction processes nearer the surface to release heat within the boundary layer of the lower atmosphere (Σ
hv), to the extent of about 332 watts per m
2 [
6,
17] as a global average, rather than by direct radiation from a colder atmosphere to the surface, more in accordance with the second law of thermodynamics. The decreasing wind speed near the surface regarded as vorticity represents the loss of power with wind speed as frictional heat is released in turbulence, warming the air and causing spectral radiation proportional to the temperature. If the Earth had no greenhouse gases in its atmosphere, the surface radiation would be emitted directly to space, and the profile with height would require slow heating by conduction.
The vortical translational energy in anticyclones and cyclones must be consistent with the global Kiehl–Trenberth heat flow budget [
17] for black body radiation from the surface into the atmosphere, where some is absorbed as infrared radiation by greenhouse gases. The Kiehl–Trenberth budget proposes that 332 W per m
2 of downwelling radiation is then returned from the heated atmosphere, greater than direct solar radiation alone would sustain. In action mechanics, it is proposed that frictional turbulence near the surface returns absorbed radiation instead of net radiation from higher in a colder atmosphere to the surface, with vortical action in anticyclones generated as work processes in the air, facilitated by Coriolis accelerations in each hemisphere.
While the rates of energy flow can be indicated as a power function of W/m
2, the chemical potential of the Gibbs field is important for function. In the Carnot cycle illustrated in
Figure 1 and the data in
Table 2, the differences in the variation in the Gibbs energy in the isothermal stages are the maximum work potential (a − a′). Direct sunlight falling on chlorophyll provides low-entropy quanta emitted at the temperature of the Sun with a short mean wavelength of 500 nm, generating high action impulses. Radiation emitted from the Earth’s surface has a longer wavelength of about 10 micrometres, and that from the tropopause is even less impulsive at around 15 micrometres, with rotational energy exciting water molecules up to 100 micrometres. While the total energy as Joules per sec must be conserved, the quality of the quanta very much determines the work potential.
A wind speed of 10 m per sec is predicted to contain vortical energy [
6] of 1.47 × 10
3 J per m
3 of air in wind, many times greater than the wind’s kinetic energy, with an additional 2.4 MJ per m
3 of thermal energy required for the air to be heated from absolute 0 K to 298 K [
1]. In a previous paper [
6], we explained how vortical energy as the latent heat of condensation of water and the conservation of momentum can power the intensification of tropical cyclones. It is important to be aware that the Trenberth diagram gives details of mean global energy flows per square metre of the Earth’s surface, irrespective of wavelength. In terms of heat energy performing work in Carnot cycles, as shown in
Figure 4 and
Figure 9, the variation in the Gibbs energy as the kinetic potential must be considered separately. The sunlight within plants decomposing water into oxygen and reducing CO
2 to carbohydrates has wavelengths of less than 1 micrometre. The heat energy radiated from the Earth’s surface near 10 micrometres at a higher temperature than the black body temperature of 255 K, at a mean value of 288 K (a), is able to power anticyclones and cyclones [
6] in vortical motion as winds often in laminar flow without turbulence. Radiation into space occurs (a′) near the black body temperature at the tropopause, identified in
Table 1 at different latitudes and in
Figure 9.
3.7. Estimates of Gibbs Energies for Atmospheric Gases
In
Table 6, Gibbs energies have been determined for the major tropospheric gases, including key greenhouse gases. Of these, only CO
2 has significant vibrational potential energy in ambient conditions. The very stable bonds of hydrogen in water and high zero-point potential energies for unpolarised N
2 and O
2 molecules show there is no infrared absorption by these gases, or by monatomic argon. Only molecules with a significant magnetic moment from asymmetry freely exchange quanta. Water is greenhouse-active in terms of rotational quanta. It is capable of primary excitation by solar energy at its vibrational frequencies or by quanta emitted when water condenses in the atmosphere, as in tropical cyclones, as we have discussed elsewhere [
6,
20,
21]. However, its function as a greenhouse gas has by far the largest effect in the atmosphere. Varying with temperature, this form of entropy does not vary with concentration. When water is at the same pressure as CO
2, its vibrational Gibbs energy (half its total) is more than 100 times less.
The results obtained from the program Entropy8/Cal in the
Supplementary Materials for atmospheric gases are given in
Table 6. Gases without greenhouse properties have little or no vibrational entropy above absolute zero temperature [
20]. CO
2 is outstanding in this degree of freedom, although at tropospheric temperature, it has only minor activation of this property. The major greenhouse gas at lower altitudes only has about 1% as much vibrational excitation at tropospheric temperatures.
This information on atmospheric gases is significant in function. CO2 is distributed evenly in the troposphere, both by altitude and latitude, and it has no significant potential gradients. Therefore, it has little effect on weather, despite its significant role in climate. Methane and nitrous oxide are two more impermanent greenhouse gases. Water is by far the most significant for warming, given that all three of its phases are common on the Earth’s surface. Its rapid condensation when convecting upwards in storms may lead to cavities in the air into which about 10 tonnes of air falls, perhaps generating thunderclaps and lightning from compressive reactions and friction.
Uniquely amongst greenhouse gases, water can undergo all three of its phase transitions at surface conditions, offering prospects for surface temperature management [
22]. Our previous research [
1] showed that of the major greenhouse gases, only CO
2 and nitrous oxide (N
2O) have significant excitation of the temperature-dependent vibrational energy and Gibbs energy. Water and methane, while vibrationally active at shorter infrared wavelengths, also provide capacitance for trapping specific wavelengths of surface radiation, discharging this almost immediately by collisions, generating translational energy, as explained above [
18]. Similar rapid discharges of this heat trapping capacity will occur with all greenhouse gases. By raising the temperature, this will mean subsequent radiation will be a function of the virial-action temperature at any altitude. Radiation from the surface absorbed by molecules with high optical density therefore heats the troposphere and contributes to the gravitational work of sustaining the troposphere at altitude. In a diurnal cycle, the variation in surface temperature as the intensity of sunlight increases with the Earth’s rotation will result in gravitational elevation. The chaotic nature of meteorological conditions for convective and advective flows in the troposphere will mean that these reversible processes for virial-action and heat–work processes will also be erratic in nature.
Like vibration, the rotational energy per molecule is dependent on temperature only, irrespective of the density of the molecules, although the total energy stored does depend on concentration.
Table 6 shows that at 0.016 atm, water is 50 times as dense as CO
2 and therefore stores almost the same total vibrational energy per unit volume of air. Of course, water also stores far more rotational energy and, from its ability to condense, can release far more radiant heat from its latent energy store, mainly by sacrificing its translational entropy [
1,
6,
21]. Of the total translational negative Gibbs energy shown in the table as mixed oxygen, nitrogen and argon, we have estimated there is 1.066503 × 10
10 J of energy stored per square metre up to 10 km, equivalent to 87 days of solar radiation at 161 W per m
2, or 311 days of the mean radiation from the surface of 396 W/m
2 at 288 K, according to the Stefan–Boltzmann equation after greenhouse enhancement.
3.8. What Carnot Meant by Heat and Caloric
It is of interest to discuss whether Carnot distinguished between the terms
chaleur and
calorique he used in describing heat. He seems to suggest a specific nature for caloric, characteristic of a physical state of the working fluid in different conditions of temperature and volume, similar to how we show the forms can be considered as different quantum states in
Table 3. Mendoza, in a footnote in the edition [
4] he edited, equated caloric with entropy. Hermann and Pohlig [
23] have recently discussed which physical quantity deserves the name for the quantity of heat, deciding that Freeman Dyson’s 1954 definition as disordered energy is most accurate. They decided that entropy is the best name. We agree, as long as it is considered as a capacity factor consistent with its physical dimensions of Joules per degree Kelvin. In discussing the thermal capacity of solids, Feldhof [
24] considers entropy capacity as key to thermal processes. This may be true for materials as a second derivative, but it is important to be aware that entropy itself is a capacity factor for energy per degree.
The importance of the virial theorem was accepted by Ludwig Boltzmann [
25], as derived from the Lagrangian calculus of variations. In his lectures on gas theory, he set an important foundation for statistical and action mechanics, pointing out on numerous occasions that entropy (and thus Gibbs energy) was controlled by volume and temperature, the latter to the exponential power 3/2, that is,
f(
VT3/2). This is also obvious in the statistical mechanics Equations (2) and (4) from which the action mechanics equations for estimating entropy and Gibbs energy are developed. His derivation of the virial theorem [
25] owes much to Clausius but with a strong emphasis on the principle of stationary action on which the absolute determination of negative Gibbs energy is based.
Given that we have shown that Carnot’s formula [
4] for caloric in contrast to heat is equal to the variation in Gibbs energy considered as quanta, it seems that the rejection of Carnot’s version of caloric in the 19th century was premature. Clearly, his term meant ordered energy, whereas entropy means energy that is disordered or distributed into many different quantum states, as a function of environmental conditions.