Industrial Technologies for CO2 Reduction Applicable to Glass Furnaces
Abstract
:1. Introduction
2. Sectors of the Glass Industry
- Hollow glass, also called “container glass”.
- Flat glass.
- Glass fibers.
- Tableware.
- Special glass.
- Mineral wool: glass and rock wool.
- High-temperature insulation wool.
- Glass frits.
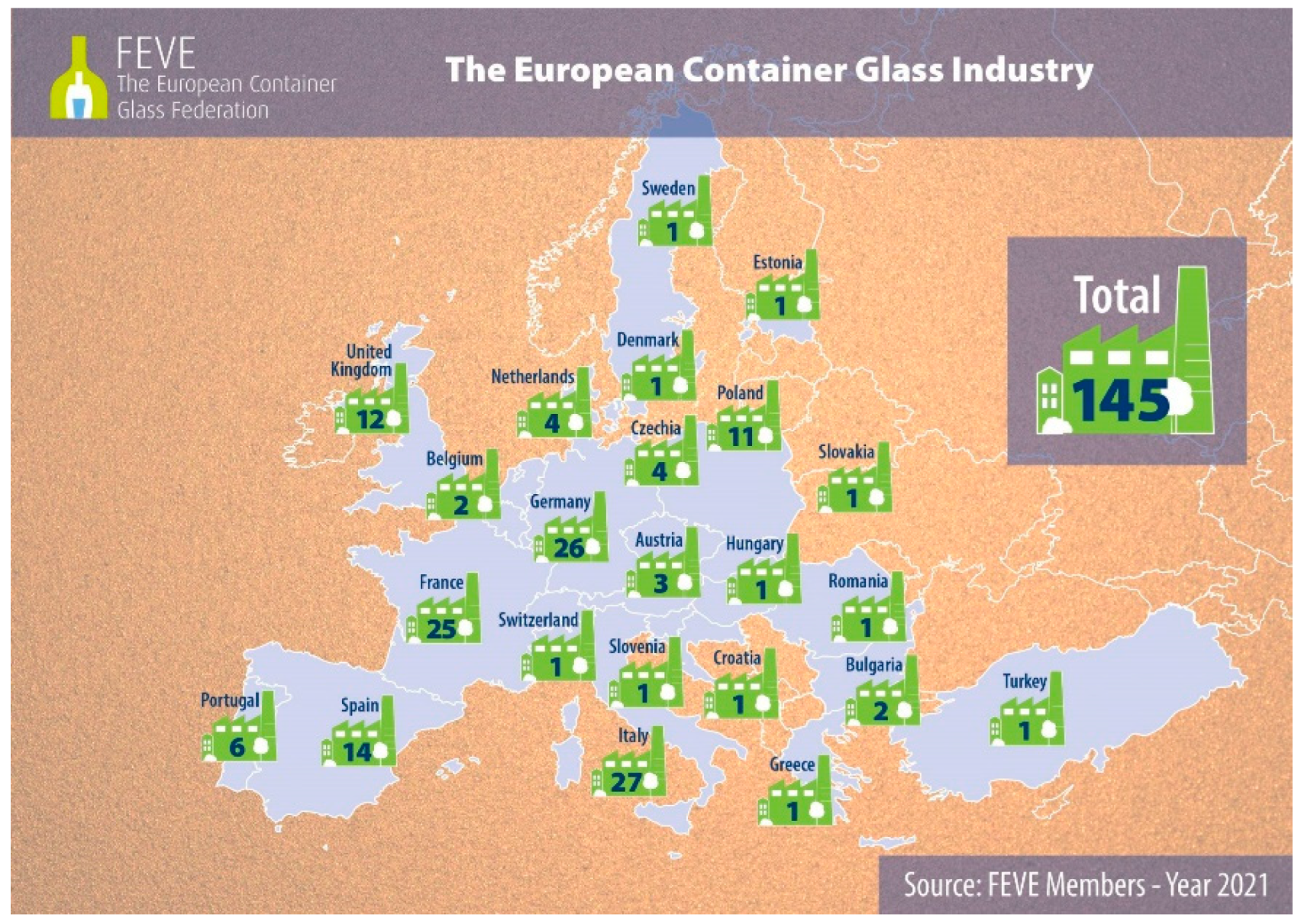
2.1. The Container Glass Sector
- a mixture of virgin raw materials consisting of quarry minerals (silica sand, feldspar sand, dolomite, marble) and inorganic chemical synthesis products (soda Solvay, sodium sulfate);
- glass cullet, partly from the plant’s quality control-rejected production (internal cullet), partly purchased from external suppliers, that acquire raw glass waste coming from the separate collection of municipal solid waste (which cannot be recycled directly in glass plants) and then the main contaminants are removed, such as fragments of ceramics, plastics, metals, lead glass, glass ceramics, etc., in dedicated treatment plants, thus producing a secondary raw material called “furnace ready” cullet.
- The gob is cut from the shears and falls into the gob distributor, the lubro-refrigerated system that directs the drops to almost zero friction towards the various sections of the forming machine;
- The drop falls by gravity to the bottom of the mold;
- During settling time, the container mouth/finish is formed;
- A punch opens a first small cavity inside the mouth, then retracts, and compressed air is blown inside, thus forming the blank by blowing;
- The blank mold opens and the blank is transferred by overturning to the finishing mold;
- The finishing mold closes, the blank undergoes temperature homogenization and the blowing head is positioned;
- The blank is blown with compressed air until the final shape is obtained;
- The mold opens and the container is placed by push-outs onto the exit conveyor.
- Preparation of the batch mixture: <5%.
- Melting: >60%.
- Fabrication (working end, conditioning channels, forming, hot end treatment, annealing and cold end treatment): >10%.
- Final phases: <5%
- Auxiliary services (compressors, fans, pumps, chillers, pollution control system, etc.): >10%
- General services (lighting, winter heating, summer cooling, etc.) <5%.
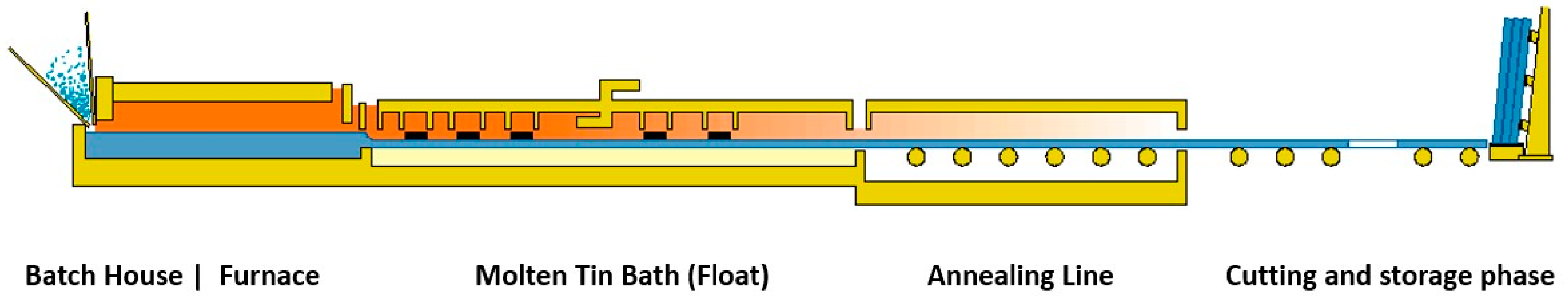
2.2. The Flat Glass Sector
2.3. Industrial Production
3. State of Energy Consumption and Pollutant Emissions
3.1. Energy Consumption
Energy Consumption in the Melting Phase
- (1)
- Enthalpy required to heat the mixture to the process temperatures;
- (2)
- Latent heat of reaction: i.e., the heat necessary to carry out the chemical reactions between minerals that transform the mixture of crystalline compounds into a homogenous “molten” amorphous material;
- (3)
- Enthalpy required to heat the CO2 released by the thermal calcination of limestone, dolomite and soda ash, up to the process temperatures.
- 5.56 MJ/ton (production between 40 and 149.99 tons/day);
- 4.83 MJ/ton (production between 150 and 209.99 tons/day);
- 4.42 MJ/ton (production between 210 and 289.99 tons/day);
- 3.95 MJ/ton (production between 290 and 500 tons/day).
3.2. Polluting Emissions
CO2 and Other Pollutant Emissions
4. Current and Developing Technologies/Solutions
4.1. Increased Combustion Efficiency and Reduced Residence Time of the Glass in the Furnace
4.2. Waste Heat Recovery from Fumes
4.2.1. Cullet and/or Batch Preheating
4.2.2. District Heating System
4.2.3. Electricity Generation through SRC or ORC Turbines
4.2.4. Steam Methane Reforming: The SUGAR Project
- Finding the best catalyst for the process conditions.
- Defining the optimal layout for the steam methane reformer reactor.
- Analyzing the behavior of flames and emissions from the combustion of produced syngas.
4.3. Electrical Boosting State of the Art
4.4. Process Electrification and Super-Boosting
The Furnace for the Future Project and Other Hybrid Furnaces
4.5. Pre-Calcination of Raw Materials
4.6. Hydrogen
4.6.1. The DIVINA Project
4.6.2. The HyGlass Project
4.7. Biogas/Biomass
4.8. Oxygen Combustion
4.9. Carbon Capture Storage and Carbon Capture Utilization on Site
4.10. Other Solutions
- •
- Installation of high-efficiency transformers, replacing the old ones, that are air-cooled (less efficient) and oversized for current needs.
- •
- Compressors: optimization of the compressor fleet and search for leaks within the compressed air distribution system; if compressed air is used for cooling, stirring or mixing of glass, compressors can be replaced with blowers or fans; if they are used to create a vacuum, with vacuum pumps. In particular, interventions related to compressed air are typically very effective:, compressed air, used mostly in the hollow glass industry, is one of the most expensive forms of energy vector used within the industrial plant because of its low efficiency [43].
- •
- Installation of high-efficiency HVAC systems.
- •
- Optimization of cooling systems, with implementation of controls, speed and flow rate balance.
- •
- Installation of heat recovery systems on the thermal strengthening, toughening or annealing furnaces.
- •
- Motors: it is possible to install variable speed motors, re-establish belt tensioning, rewire them, install inverters and/or replace them with hydraulic motors or gearboxes.
- •
- Lighting: installation of LED and lighting control systems in time. Also, the interventions related to the saving of electricity used for lighting, which usually approaches 5% of the total consumption of electricity in the plant, are profitable, both for the ease of construction and for the low cost of intervention.
5. Subdivision into Technologies/Solutions Implementable in Short and Medium Term
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Un Green Deal Europeo. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_it (accessed on 11 June 2023).
- European Climate Law. Available online: https://climate.ec.europa.eu/eu-action/european-green-deal/european-climate-law_en (accessed on 25 October 2022).
- Emission Trading Europeo. Available online: https://www.isprambiente.gov.it/it/servizi/registro-italiano-emission-trading/contesto/emission-trading-europeo (accessed on 11 June 2023).
- Carbon Border Adjustment Mechanism. Available online: https://taxation-customs.ec.europa.eu/carbon-border-adjustment-mechanism_en (accessed on 11 June 2023).
- Accordo di Parigi. Available online: https://climate.ec.europa.eu/eu-action/international-action-climate-change/climate-negotiations/paris-agreement_it (accessed on 21 September 2022).
- National Long-Term Strategies. Available online: https://commission.europa.eu/energy-climate-change-environment/implementation-eu-countries/energy-and-climate-governance-and-reporting/national-long-term-strategies_en (accessed on 11 June 2023).
- European Commission, Joint Research Centre, Institute for Prospective Technological Studies. Best Available Techniques (BAT) Reference Document for the Manufacture of Glass: Industrial Emissions Directive 2010/75/EU: Integrated Pollution Prevention and Control; Publications Office: Luxembourg, 2013. [Google Scholar]
- Griffin, P.W.; Hammond, G.P.; McKenna, R.C. Industrial Energy Use and Decarbonisation in the Glass Sector: A UK Perspective. Adv. Appl. Energy 2021, 3, 100037. [Google Scholar] [CrossRef]
- International Energy Agency. Tracking Industrial Energy Efficiency and CO2 Emissions; OECD: Paris, France, 2007; ISBN 978-92-64-03016-9. [Google Scholar]
- Sengupta, P. Refractories for Glass Manufacturing. In Refractories for the Chemical Industries; Springer International Publishing: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Cattaneo, E. LIFE SUGAR PROJECT—The Idea of Total Recovery Glass Furnace. Hydrogen in Glass Making. 15 June 2021. Available online: https://www.glass-international.com/hydrogen-in-glass-manufacturing/view-the-presentations (accessed on 8 January 2022).
- Assovetro. Relazione Annuale Assovetro 2023; Assovetro: Rome, Italy, 2023. [Google Scholar]
- Ojovan, M.I.; Yudintsev, S.V. Glass, Ceramic, and Glass-Crystalline Matrices for HLW Immobilisation. Open Ceram. 2023, 14, 100355. [Google Scholar] [CrossRef]
- Siddika, A.; Hajimohammadi, A.; Mamun, M.A.A.; Alyousef, R.; Ferdous, W. Waste Glass in Cement and Geopolymer Concretes: A Review on Durability and Challenges. Polymers 2021, 13, 2071. [Google Scholar] [CrossRef]
- FEVE Plants. Available online: https://feve.org/feve-plants/ (accessed on 8 January 2022).
- Ceola Introductory Training Course for Glass Producers—SSV—1° Day. Venice. Available online: https://www.spevetro.it/corsi-formazione/ (accessed on 24 November 2021).
- CEN/TC 129; EN 572 Glass in Building—Basic Soda-Lime Silicate Glass Products. Glass for Europe: Brussels, Belgium, 2012.
- Pellegrino, J.; Greenman, M.; Ross, C.P. Energy and Environmental Profile of the U.S. Glass Industry; Office of Industrial Technologies Energy Efficiency and Renewable Energy, U.S Department of Energy: Washington, DC, USA, 2002. [Google Scholar]
- ENEA. Quaderni Dell’efficienza Energetica—VETRO; Martini, C., Martini, F., Salvio, M., Toro, C., Eds.; ENEA: Rome, Italy, 2021; p. 170. ISBN 978-88-8286-413-2. [Google Scholar]
- Hubert, M. IMI-NFG Course on Processing in Glass: Lecture 3: Basics of Industrial Glass Melting Furnaces; Celsian Glass & Solar: Eindhoven, The Netherland, 2015. [Google Scholar]
- Shelby, J.E. Introduction to Glass Science and Technology; Royal Society of Chemistry: London, UK, 2005; ISBN 978-0-85404-639-3. [Google Scholar]
- Beerkens, R.G.; van der Schaaf, J. Gas Release and Foam Formation During Melting and Fining of Glass. J. Am. Ceram. Soc. 2006, 89, 24–35. [Google Scholar] [CrossRef]
- Key Data—Flat Glass Market. Glass Eur. Available online: https://glassforeurope.com/the-sector/key-data/ (accessed on 10 June 2023).
- Assovetro. L’industria Del Vetro in Italia—Sfide Ed Opportunità per Un Materiale al Centro Della Transizione Ecologica; Assovetro: Rome, Italy, 2021. [Google Scholar]
- Assovetro. Relazione Annuale Assovetro 2014; Assovetro: Rome, Italy, 2014. [Google Scholar]
- Assovetro. Relazione Annuale Assovetro 2015; Assovetro: Rome, Italy, 2015. [Google Scholar]
- Assovetro. Relazione Annuale Assovetro 2016; Assovetro: Rome, Italy, 2016. [Google Scholar]
- Assovetro. Relazione Annuale Assovetro 2017; Assovetro: Rome, Italy, 2017. [Google Scholar]
- Assovetro. Relazione Annuale Assovetro 2018; Assovetro: Rome, Italy, 2018. [Google Scholar]
- Assovetro. Relazione Annuale Assovetro 2019; Assovetro: Rome, Italy, 2019. [Google Scholar]
- Assovetro. Relazione Annuale Assovetro 2020; Assovetro: Rome, Italy, 2020. [Google Scholar]
- Assovetro. Relazione Annuale Assovetro 2021; Assovetro: Rome, Italy, 2021. [Google Scholar]
- Assovetro. Relazione Annuale Assovetro 2022; Assovetro: Rome, Italy, 2022. [Google Scholar]
- Assovetro. Rapporto Di Sostenibilità; Assovetro: Rome, Italy, 2022. [Google Scholar]
- Boston Consulting Group. Industrial Decarbonization Pact: Un’alleanza per la piena decarbonizzazione dei settori Hard to Abate; Boston Consulting Group: Boston, MA, USA, 2022; Available online: https://web-assets.bcg.com/2b/79/89d157b44d9090a549bec316bcf4/working-paper-industrial-decarbonization-pact.pdf (accessed on 11 November 2023).
- Sinton, C.W. Glass and Energy. In Encyclopedia of Energy; Cleveland, C.J., Ed.; Elsevier: New York, NY, USA, 2004; pp. 1–10. ISBN 978-0-12-176480-7. [Google Scholar]
- Rademaekers, K.; Zaki, S.S.; Smith, M. Sustainable Industry: Going for Growth & Resource Efficiency; ECORYS: Rotterdam, The Netherlands, 2011. [Google Scholar]
- Klaassen, G.; Berglund, C.; Wagner, F.; Amann, M. The GAINS Model for Greenhouse Gases—Version 1.0: Carbon Dioxide (CO2); International Institute for Applied Systems Analysis, IIASA: Laxenburg, Austria, 2005. [Google Scholar]
- Glass Alliance Europe. The European Glass Sector Contribution to a Climate Neutral Economy; Glass Alliance Europe: Brussels, Belgium, 2021. [Google Scholar]
- Industrial Decarbonisation & Energy Efficiency Roadmaps to 2050, Glass; Department of Energy & Climate Change and Department for Business, Innovation & Skills: London, UK, 2015.
- Auchet, O.; Riedinger, P.; Malasse, O.; Iung, C. First-Principles Simplified Modelling of Glass Furnaces Combustion Chambers. Control Eng. Pract. 2008, 16, 1443–1456. [Google Scholar] [CrossRef]
- D’Antonio, M.; Hildt, N.; Patil, Y.; Moray, S.; Shields, T. Energy Efficiency Opportunities in the Glass Manufacturing Industry. In Proceedings of the ACEEE Summer Study on Energy Efficiency in Industry, Rye Brook, NY, USA, 29 July–1 August 2003. [Google Scholar]
- Galitsky, C.; Worrell, E.; Galitsky, C.; Masanet, E.; Graus, W. Energy Efficiency Improvement and Cost Saving Opportunities for the Glass Industry. An ENERGY STAR Guide for Energy and Plant Managers; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2008. [Google Scholar]
- Laroia, M. Oxy-Fuel Combustion Systems (OCS); LexInnova: Gurgaon, India, 2013. [Google Scholar]
- Karellas, S.; Giannakopoulos, D.; Hatzilau, C.-S.; Dolianitis, I.; Skarpetis, G.; Zitounis, T. The Potential of WHR/Batch and Cullet Preheating for Energy Efficiency in the EU ETS Glass Industry and the Related Energy Incentives. Energy Effic. 2018, 11, 1161–1175. [Google Scholar] [CrossRef]
- Trier, W. Glass Furnaces: Design, Construction and Operation; Society of Glass Technology: Sheffield, UK, 1987. [Google Scholar]
- Levine, E.; Greenman, M.; Jamison, K. The Development of a Next Generation Melting System for Glass Production: Opportunities and Progress. In ACEEE Summer Study on Energy Efficiency in Industry; ACEEE: Washington, DC, USA, 2003. [Google Scholar]
- Beerkens, R. Energy Saving Options for Glass Furnaces & Recovery of Heat from Their Flue Gases and Experiences with Batch & Cullet Pre-Heaters Applied in the Glass Industry. Ceram. Eng. Sci. Proc. 2009, 30, 143–162. [Google Scholar] [CrossRef]
- Papadogeorgos, I.; Schure, K.M. Decarbonisation Options for the Dutch Container and Tableware Glass Industry; PBL Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2019. [Google Scholar]
- O-I and Dalkia Heat Homes in Reims, France. Available online: https://feve.org/case_study/oi-france-heat-recovery-system-to-heat-homes/ (accessed on 9 June 2023).
- City of Cuneo Taps Factory Waste Heat to Warm Residences. Available online: https://www.automatedbuildings.com/news/jan22/articles/tridium/211229122901triduim.html (accessed on 9 June 2023).
- Hubert, M. Industrial Glass Processing and Fabrication. In Springer Handbook of Glass; Musgraves, J.D., Hu, J., Calvez, L., Eds.; Springer Handbooks; Springer International Publishing: Cham, Switzerland, 2019; pp. 1195–1231. ISBN 978-3-319-93728-1. [Google Scholar]
- Quoilin, S.; Lemort, V. Technological and Economical Survey of Organic Rankine Cycle Systems. In Proceedings of the European Conference on Economics and Management of Energy in Industry, Vilamoura, Portugal, 14–17 April 2009. [Google Scholar]
- Waste Heat Recovery Technologies Largely Used in the European Container Glass Industry to Optimize Energy Consumption and reduce CO2 Emissions. Available online: https://feve.org/case_study/waste-heat-recovery-technologies-largely-used-in-the-european-container-glass-industry-to-optimize-energy-consumption-and-reduce-co2-emissions/ (accessed on 7 June 2023).
- Hibscher, C.; Davies, P.; Davies, M.; Davis, D. A Designer’s Insight Into All-Electric Melting. In Proceedings of the 65th Conference on Glass Problems: Ceramic Engineering and Science Proceedings, Columbus, OH, USA, 19–20 October 2004; pp. 131–143. [Google Scholar]
- Krijgsman, R.; Marsidi, M. Decarbonisation Options for the Dutch Glass Wool Industry; PBL Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2019. [Google Scholar]
- Wesseling, J.H.; Lechtenböhmer, S.; Åhman, M.; Nilsson, L.J.; Worrell, E.; Coenen, L. The Transition of Energy Intensive Processing Industries towards Deep Decarbonization: Characteristics and Implications for Future Research. Renew. Sustain. Energy Rev. 2017, 79, 1303–1313. [Google Scholar] [CrossRef]
- Glass Industry Boss: Replacing Old Windows Can Bring Huge Energy Savings—EURACTIV.Com. Available online: https://www.euractiv.com/section/energy/interview/glass-industry-boss-replacing-old-windows-can-bring-huge-energy-savings/ (accessed on 8 June 2023).
- The Furnace for the Future. Available online: https://feve.org/about-glass/furnace-for-the-future/ (accessed on 8 January 2022).
- Somerhausen, B.; Di Marino, E.; Hunturk, T.; Ceola, S. Benefits for Fiber Glass Producers to Use Calcium Oxide in Their Raw Material. In Proceedings of the ICG Conference 2017, Istanbul, Turkey, 22–25 October 2017. [Google Scholar]
- SORG; Schnurpfeil, D. Some Thoughts about Hydrogen Combustion in Glass Melting. In Proceedings of the Hydrogen in Glass Industry, Hydrogen in Glass Manufacturing, Online, 17 June 2021. [Google Scholar]
- CGEP. Challenges and Solutions for U.S. Industrial Decarbonization. Available online: https://www.energypolicy.columbia.edu/publications/challenges-and-solutions-us-industrial-decarbonization/ (accessed on 8 June 2023).
- Adendorff, M.; Robert (Bob), L. Bell and Hisashi (Sho) Kobayashi Hydrogen Fired Oxyfuel Burners for Glass Melters. In Proceedings of the ICG 2022 Conference, Berlin, Germany, 3–8 July 2022. [Google Scholar]
- redazione. Snam, RINA e Bormioli lanciano il progetto ‘Divina’: Idrogeno per decarbonizzare l’industria del vetro. HydroNews, 21 July 2021. [Google Scholar]
- BV GLAS; ESSEN HyGlass. Hydrogen Utilization as a Decarbonization Measure for Glass Industry. In Proceedings of the Glass International: Hydrogen in Glass Manufacturing, Online, 16 June 2021. [Google Scholar]
- HyGlass—Process Heat for Industrial Applications. H2-Int. 2021. Available online: https://h2-international.com/2021/12/15/hyglass-process-heat-for-industrial-applications/ (accessed on 1 June 2023).
- Fiehl, M.; Leicher, J.; Giese, A.; Görner, K.; Fleischmann, B.; Spielmann, S. Biogas as a Co-Firing Fuel in Thermal Processing Industries: Implementation in a Glass Melting Furnace. Energy Procedia 2017, 120, 302–308. [Google Scholar] [CrossRef]
- Torrijos, M. State of Development of Biogas Production in Europe. Procedia Environ. Sci. 2016, 35, 881–889. [Google Scholar] [CrossRef]
- Impianti|Zignago Power. Available online: http://www.zignagopower.com/impianti (accessed on 4 June 2023).
- Deng, W.; Backhouse, D.J.; Kabir, F.; Janani, R.; Bigharaz, M.; Bingham, P.A.; Marshall, M.; Ireson, R. An Ancient Technology Could Help Deliver Decarbonisation. Glass Int. 2019, 42, 47–49. [Google Scholar]
- Encirc-Admin World’s Most Sustainable Glass Bottles Created in Ground-Breaking Biofuel Trial. Available online: https://www.encirc360.com/2021/02/05/worlds-most-sustainable-glass-bottles-created-in-ground-breaking-biofuel-trial/ (accessed on 7 June 2023).
- Saint-Gobain Glass:—One Week of Carbon-Free Glass Production. Available online: https://www.gw-news.eu/glass/saint-gobain-glass-one-week-carbon-free-glass-production (accessed on 7 June 2023).
- Beerkens, R. Analysis of Elementary Process Steps in Industrial Glass Melting Tanks—Some Ideas on Innovations in Industrial Glass Melting. Ceramics-Silikaty 2008, 52, 206–217. [Google Scholar]
- BEIS. Glass Sector Joint Industry—Government Industrial Decarbonisation and Energy Efficiency Roadmap Action Plan; BEIS: London, UK, 2017. [Google Scholar]
- Available online: https://www.lifeoptimelt.com/ (accessed on 1 June 2023).
- Gonzalez, A.; Solorzano, E.; Laux, S.; Iyoha, U.; Wu, K.t.; Kobayashi, H. Operating Experience with OptimeltTM Regenerative Thermo-Chemical Heat Recovery for Oxy-Fuel Fired Glass Furnaces. In Proceedings of the 76th Conference on Glass Problems, Columbus, OH, USA, 2–5 November 2015; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 17–25, ISBN 978-1-119-28247-1. [Google Scholar]
- HotOxyGlass Life+|HotOxyGlass Life+. Available online: https://www.oxyfuel-heatrecovery.com/ (accessed on 16 June 2023).
- De Diego, J. Hydrogen in Glass Manufacturing—Nippon Gases. Online 16 June 2021. Available online: https://www.glass-international.com/hydrogen-in-glass-manufacturing/view-the-presentations (accessed on 16 June 2023).
- Tapia, J.F.D.; Lee, J.-Y.; Ooi, R.E.H.; Foo, D.C.Y.; Tan, R.R. A Review of Optimization and Decision-Making Models for the Planning of CO2 Capture, Utilization and Storage (CCUS) Systems. Sustain. Prod. Consum. 2018, 13, 1–15. [Google Scholar] [CrossRef]
- Chan, Y.; Petithuguenin, L.; Fleiter, T.; Herbst, A.; Arens, M.; Stevenson, P. Part 1: Technology Analysis; ICF: Reston, VA, USA, 2019. [Google Scholar]
- Sutherland, B.R. Sustainably Heating Heavy Industry. Joule 2020, 4, 14–16. [Google Scholar] [CrossRef]
- Dorn, C.; Behrend, R.; Uhlig, V.; Trimis, D.; Krause, H. A Technology Comparison Concerning Scale Dependencies of Industrial Furnaces. A Case Study of Glass Production. Energy Procedia 2017, 120, 388–394. [Google Scholar] [CrossRef]
- Mandal, A.K.; Sen, R. An Overview on Microwave Processing of Material: A Special Emphasis on Glass Melting. Mater. Manuf. Process. 2017, 32, 1–20. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Kharisov, B.I.; Valdés, J.J.R. Review: The Use of Microwave Irradiation in the Processing of Glasses and Their Composites. Ind. Eng. Chem. Res. 2010, 49, 1457–1466. [Google Scholar] [CrossRef]
- Acevedo, L.; Usón, S.; Uche, J. Local Exergy Cost Analysis of Cullet Glass Heating by Microwaves. Appl. Therm. Eng. 2019, 152, 778–795. [Google Scholar] [CrossRef]
- Mimoso, R.; Albuquerque, D.; Pereira, J.; Pereira, J. Simulation and Control of Continuous Glass Melting by Microwave Heating in a Single-Mode Cavity with Energy Efficiency Optimization. Int. J. Therm. Sci. 2017, 111, 175–187. [Google Scholar] [CrossRef]
- Majdinasab, A.R.; Manna, P.K.; Wroczynskyj, Y.; van Lierop, J.; Cicek, N.; Tranmer, G.K.; Yuan, Q. Cost-Effective Zeolite Synthesis from Waste Glass Cullet Using Energy Efficient Microwave Radiation. Mater. Chem. Phys. 2019, 221, 272–287. [Google Scholar] [CrossRef]
- Yao, Y.; Watanabe, T.; Yano, T.; Iseda, T.; Sakamoto, O.; Iwamoto, M.; Inoue, S. An Innovative Energy-Saving in-Flight Melting Technology and Its Application to Glass Production. Sci. Technol. Adv. Mater. 2008, 9, 025013. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Yatsuda, K.; Yao, Y.; Yano, T.; Matuura, T. Innovative In-Flight Glass-Melting Technology Using Thermal Plasmas. Pure Appl. Chem. 2010, 82, 1337–1351. [Google Scholar] [CrossRef]
- Springer, C.; Hasanbeigi, A. Emerging Energy Efficiency and Carbon Dioxide Emissions-Reduction Technologies for the Glass Industry; Energy Analysis and Environmental Impacts Division, Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2017. [Google Scholar]
- Submerged Combustion Melter. Available online: https://www.combustion-consulting.com/en/SBM-Furnace/ (accessed on 10 June 2023).

| SiO2 | Na2O | CaO | MgO | Al2O3 | K2O | |
|---|---|---|---|---|---|---|
| Weight percentage | 70.0–73.0% | 12.0–14.0% | 8.5–12.0% | 0.0–5.0% | 1.0–3.0% | 0.0–1.5% |
| Silicon (Si) | Calcium (Ca) | Sodium (Na) | Magnesium (Mg) | Aluminum (Al) | Others | |
|---|---|---|---|---|---|---|
| Constituents | 32–35% | 3.5–10.1% | 7.4–11.9% | 0.0–3.7% | 0.0–1.6% | <5.0% |
| Years | ||||||||
|---|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | |
| Hollow glass production (ton) | 3,626,376 | 3,936,885 | 4,061,931 | 4,177,711 | 4,287,283 | 4,485,190 | 4,429,110 | 4,702,984 |
| Flat glass production (ton) | 793,211 | 838,019 | 887,125 | 870,440 | 1,054,763 | 1,034,244 | 965,859 | 1,190,251 |
| Phase of the Production Process | Type of Pollutant Emitted |
|---|---|
| Raw material handling and weighing | Dust |
| Combustion | CO CO2 NOx SOx |
| Decomposition Carbonates | CO2 |
| Decomposition Sulfates | SOx |
| Evaporation Batch and melt | Dust, HCl, HF, Metals |
| Condensation of fumes | Dust (sodium sulfate) |
| Hot End treatment | Sn, HCl, SOx |
| Cold End treatment | Organic compounds |
| Hydrogen Denomination | Technology | Source | Carbon Footprint |
|---|---|---|---|
| Green | Electrolyzer | Sun, wind, water, geothermic, tide | Minimal |
| Purple/Pink | Nuclear | ||
| Yellow | Stored energy from mixed origin | Medium | |
| Blue | Fossil Fuel + Carbon Capture | Fossil fuel (i.e., natural gas/coal) | Low |
| Turquoise | Pyrolysis | Fossil fuel (i.e., natural gas) | Medium |
| Grey | Fossil Fuel | ||
| Brown | Gasification | Brown Coal | High |
| Black | Black Coal |
| Solution/Technology | Maturity | Times of Research/Implementation |
|---|---|---|
| Increased combustion efficiency and reduced residence time of the glass in the furnace | High | Immediate |
| Waste heat recovery from fumes | High | Short term |
| Electric boosting | High | Immediate (for working at maximum available power)/short term (for increasing the maximum boosting power installed) |
| Process electrification | Low | Medium term |
| Pre-calcined raw materials | High/Medium (depending on sector) | Short term |
| Hydrogen Combustion | Low (needs development of the H2 production and transport grid, as well as substantial development of renewable electricity generation capacity) | Short for low percentage blending with natural gas (on-site or in the grid), Medium for 100% hydrogen/air or 100% H2/O2 combustion |
| Biogas Combustion | Medium (subject to availability issues) | Short term |
| Oxygen Combustion | High | Immediate |
| Carbon Capture Storage and Carbon Capture Utilization on site | Medium (needs development of the CO2 transport grid and of sites for geological storage) | Medium Term |
| Other solutions | High for plant efficiency improvement solutions outside the melting tank;|Low for others | Immediate|medium term |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atzori, D.; Tiozzo, S.; Vellini, M.; Gambini, M.; Mazzoni, S. Industrial Technologies for CO2 Reduction Applicable to Glass Furnaces. Thermo 2023, 3, 682-710. https://doi.org/10.3390/thermo3040039
Atzori D, Tiozzo S, Vellini M, Gambini M, Mazzoni S. Industrial Technologies for CO2 Reduction Applicable to Glass Furnaces. Thermo. 2023; 3(4):682-710. https://doi.org/10.3390/thermo3040039
Chicago/Turabian StyleAtzori, Dario, Simone Tiozzo, Michela Vellini, Marco Gambini, and Stefano Mazzoni. 2023. "Industrial Technologies for CO2 Reduction Applicable to Glass Furnaces" Thermo 3, no. 4: 682-710. https://doi.org/10.3390/thermo3040039
APA StyleAtzori, D., Tiozzo, S., Vellini, M., Gambini, M., & Mazzoni, S. (2023). Industrial Technologies for CO2 Reduction Applicable to Glass Furnaces. Thermo, 3(4), 682-710. https://doi.org/10.3390/thermo3040039







