Abstract
The biomass waste obtained at the end-of-pipe of the extraction industry can be used as fuel for energy production, aiming at cost reduction/waste disposal issues. However, few systematic investigations into the calorific value of these residues are reported in the literature. In this work, the thermochemical properties of solid residues from different biomasses (residues from citrus peels, leaves, flowers, stems, and poultry feathers used for extraction) as potential biomass fuels have been investigated. The heat of combustion (ΔcH) of the solid residues from citrus (orange, tangerine, lemon, grapefruit, and pomelo), aromatic herbs (rosemary, lavender, thyme, Artemisia vulgaris L. and Ruta chalepensis L.), and poultry feathers biomasses was measured by direct calorimetry. The results were compared with the higher heating values (HHV) calculated using the elemental (CHNOS) and thermogravimetric (TGA) analyses data and with the enthalpy of combustion calculated using the biomass composition predicted by FTIR spectroscopy in tandem with chemometrics. The calculated values match with the corresponding experimental values of ΔcH. The heat of combustion highlights the energetic features of solid residues for their potential uses as alternative biomass for energy production. This information is essential to evaluate the employment of solid residues as fossil fuel substitutes.
1. Introduction
The extraction of added-value products from different waste biomasses is a strategy largely exploited in many industries to maximize profits and minimize the costs of waste disposal [1]. As a matter of example, wastes of the agri-food industry, such as the peels of different fruits (e.g., tangerine, citrus, orange, grapes), are used to extract pectin, flavonoids or carotenoids useful in the food, packaging, cosmetics and pharmaceutical industries [2,3,4] poultry feathers (wastes of the poultry industry) are used as keratin source to form keratin-based biomaterials or composites [5,6,7,8]; aromatic herbs, i.e., rosemary, lavender, thyme, Artemisia vulgaris L. and Ruta chalepensis L. are commonly employed for the extraction of essential oils [9,10,11,12]. The extraction processes leave an end-of-pipe solid residue, which can, in turn, be used for fuel energy production by pyrolysis, gasification, or hydrothermal carbonization processes [13]. The fuel can be stocked or used in an upstream phase of the industrial process, in view of the optimization of a circular process, convenient in terms of costs and environmental preservation. Despite the great economic interests related to the topic, few works on the calorific values of these end-of-pipe waste biomasses have been published.
In this work, we performed a systematic evaluation of the calorimetric properties of the solid residues obtained after extraction performed on orange, tangerine, lemon, grapefruit and pomelo peels, rosemary, lavender, thyme, Artemisia vulgaris L. and Ruta chalepensis L. leaves and poultry feathers, in order to obtain extensive information on their possible usage for energy production (Figure 1). We compared experimental values of the biomass combustion enthalpy measured with an isoperibolic calorimeter [14] (already used in various literature works to calculate the calorific power of various kinds of wastes, e.g., plastics [15] or alternative solid fuels [16]) with the values calculated using two different models. In one case, we calculated the heat of combustion as the higher heating value (HHV) by employing one of the most widely used empirical equations based on the composition of samples obtained by elemental analysis and thermogravimetry under oxygen flux. In the second case, we determined the fraction of cellulose, hemicellulose, and lignin contained in each sample (considered the only comburent parts in the citrus peels and aromatic herb wastes) by FTIR spectroscopy in tandem with chemometrics. The total combustion heat was then calculated as the weighted sum of contributions given by each component, taken from the literature. The HHV of citrus peel [17,18,19,20], poultry feathers [21,22], and many other municipal wastes [23,24] was already reported in the literature, while the second method is new and has never been used to evaluate the calorific power of waste biomasses.
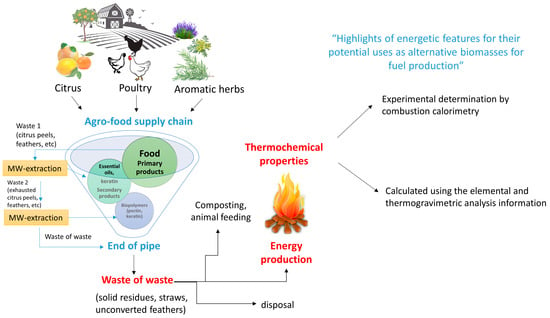
Figure 1.
Scheme of the production process from biomasses to the end-of-pipe residues, highlighting the use of the latter for energy production.
We observed that direct calorimetry and the two indirect methods provided comparable results for each biomass, highlighting the robustness of the models, and confirming the high potential of the end-of-pipe residues for energy production.
2. Materials and Methods
2.1. Materials
The waste biomasses were derived from different extraction processes, grounded, and dried at 60 °C overnight. The citrus peel wastes (orange, tangerine, grapefruit, pomelo, and lemon) represent the final waste residue after the extraction of essential oils and pectin, as reported in Ref. [25]. The aromatic herb wastes (rosemary, lavender, thyme, Artemisia vulgaris L., Ruta chalepensis L.) are the solid waste residues after the extraction of essential oils from aromatic herbs [26]. The poultry feather residue is the fraction of non-soluble keratin recovered after keratin extraction performed in acetic acid (70% w/w), as reported in Ref [5]. Poultry feathers were provided by Consortium SGS (Santa Croce sull’ Arno, Italy), a company that processes animal by-products. Benzoic acid (RPE-ACS reagent) was purchased from Carlo Erba Reagents (Milan, Italy), while hexadecane (ReagentPlus®, 99%) was purchased from Merck-Sigma Aldrich (Milan, Italy).
2.2. Elemental Analysis
The elemental composition (C, N, H, S, and O) of all dried waste biomass samples was obtained by the Elementar Vario MICRO cube instrument (Elementar, Langenselbold, Germany).
2.3. Thermogravimetric Analyses
The ash content of all waste biomasses was determined by thermogravimetric analysis (TGA). TGA experiments were carried out with a TA Instruments Thermobalance model Q5000IR (TA, New Castle, DE, USA, 2010). Measurements were performed at a rate of 10 °C/min, from 30 °C to 900 °C under oxygen flow (25 mL/min). The amount of sample in each TGA measurement varied between 2 and 5 mg. Error was calculated on 3 replicas performed on each biomass sample.
2.4. Experimental Determination by Isoperibolic Calorimeter
Combustion of the samples was carried out using a home-made bomb calorimeter, as previously reported in Ref. [27]. Briefly, a standard stainless-steel bomb (volume = 375 mL) was placed in a calorimetric vessel with 2200 mL of water, equipped with a stirring system and a temperature probe (thermistor). The bath was lodged in an isoperibolic calorimeter. Calorimetric curves were recorded by a computer and corrected to account for heat exchange between the calorimetric system (bomb + calorimeter vessel + water) and the thermostatic bath. The calorimeter was previously calibrated by the benzoic acid-certified standard. One gram of sample was pressed to form a pellet and placed in a crucible with about 0.5 g of hexadecane. An iron wire was then immersed in the hexadecane and connected to electrical terminals. Then, about 0.5 mL of water was put into the bottom of the bomb to saturate the interior with water vapor so that the observed heat of combustion matched the higher heating value. Finally, the bomb was closed and pressurized with oxygen (99.9%, 15 bar) after flushing for about 30 s, placed into the calorimeter, and the sample was ignited. For each experiment, the corresponding ΔU value was obtained from the thermal effect, corrected for heat losses and the stirring effect. The blank contribution due to the combustion of the ignition wire and hexadecane was subtracted. The experiments were carried out at 25 ± 0.1 °C. The enthalpy of combustion can be obtained by Equation (1):
where Δng is the variation of the number of moles of gaseous species due to the combustion. However, since Δng = 0 for both cellulose and hemicellulose, only the contribution due to lignin combustion should be considered. Considering that the molar fraction of lignin in the biomasses here considered is between 0.15 and 0.26, the correction term to calculate ΔcH from ΔcU in the citrus peel and aromatic herb wastes is between −0.003 and −0.005 kJ g−1, which is within the experimental error. For the feathers, mainly composed of keratin, looking at the elemental composition, we estimated a Δng= −0.15 per gram of sample, corresponding to a correction term for going from ΔcU to ΔcH of −0.04 kJ g−1, also in this case within the experimental error. The correction to return ΔcH to the standard value (i.e., to 1 bar pressure) is also negligible. The measurements were repeated 3 times on each biomass sample, and the values of ΔcH are reported as means values with relative standard deviation (RSD%) < 2%. In our case, the samples represent the residual end-of-pipe of a procedure performed on a laboratory scale, so they are homogeneous. The biomasses from industrial treatment are less homogeneous, and this is one of the main issues in reproducibility of HHVs by direct calorimetry [28].
ΔcH (p, T) = ΔcU (p, T) + ΔngRT
2.5. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR) and Chemometric Ftir Spectroscopy Analysis (PLS) of Lignocellulosic Solid Residues
Infrared spectroscopy analysis was performed by using a Perkin-Elmer Frontiers FTIR Spectrophotometer (Waltham, MA, USA), equipped with a universal attenuated total reflectance (ATR) accessory with a diamond crystal and a triglycine sulfate TGS detector. Measurements on lignocellulosic waste biomass samples (citrus peels and aromatic herbs samples) were carried out after background acquisition. For each sample, 32 scans were recorded, averaged, and Fourier-transformed to produce a spectrum with a nominal resolution of 4 cm−1. The determination of lignocellulose composition was performed by using Partial Least Squares (PLS) chemometrics. The PLS model was reconstructed using the FTIR spectra of the lignocellulosic samples in the region of 1900–800 cm−1 (i.e., the fingerprint of wood components). As X matrix, it was used the first derivative of the normalized FTIR spectra, and as Y matrix, the chemical composition of lignin, cellulose, and hemicellulose (determined by Van Soest analysis) [29]. The model was developed using JMP software and seven principal components that account for 98% of the variance in the X matrix and 98.8% of the variance in the Y matrix. The composition prediction of hemicellulose, cellulose, and lignin was then performed by loading their first derivative of the normalized FTIR spectra into the X matrix of the model and by applying the cross-validation technique.
2.6. Calculation of Combustion Enthalpy Knowing the Content of Lignin/Cellulose/Hemicellulose
The standard enthalpy of combustion (ΔcH°) of the solid residues was calculated using the standard enthalpy of formation (ΔfH°) reported for hemicellulose, cellulose, and lignin components [30]. The ΔfH° and monomer units for the three components were:
- hemicellulose: monomer xylose units, MW = 132.12 g mol−1, ΔfH° = −759.2 kJ mol−1;
- cellulose: monomer glucose unit, MW = 161.14 g mol−1, ΔfH° = −1019.0 kJ mol−1;
- lignin: monomer unit, MW = 258.27 g mol−1, ΔfH° = −759.39 kJ mol−1 [30].
The ΔcH°(i) values were calculated by Equation (2):
with i = hemicellulose, cellulose, lignin; −393.51 kJ mol−1 and −285.83 kJ mol−1 are the standard formation enthalpies of carbon dioxide and liquid water, respectively and and are the stoichiometric coefficients of CO2 and H2O in each combustion reaction:
The combustion enthalpy values calculated by Equation (2) for hemicellulose, cellulose and lignin are ΔcH°hemi = −2351.67 kJ mol−1, ΔcH°cell = −2771.21 kJ mol−1 and ΔcH◦lig = −7144.07 kJ mol−1. From the percentage composition in hemicellulose, cellulose, and lignin of the solid residue, calculated by chemometric FTIR spectroscopy, as previously described, and assuming these components as the only burning agents in the sample, the combustion enthalpy of the biomass was calculated following Equation (3), with i = hemicellulose, cellulose, lignin.
The values of ΔcH are reported as means values with relative standard deviation (RSD%) < 5%.
3. Results and Discussion
3.1. Experimental Combustion Enthalpy
The data of experimental combustion enthalpy obtained with an isoperibolic calorimeter are reported in Table 1.

Table 1.
Experimental combustion enthalpy of the studied samples obtained with an isoperibolic calorimeter.
The experimental values obtained range from −15.1 to −19.2 kJ/g. According to data in Table 1, waste biomasses can be classified into three different categories:
- Waste biomasses from citrus residues, which have the lowest combustion enthalpies values (ranging from −15.1 to −17.2 kJ/g);
- Waste biomasses from aromatic herbs with middle combustion enthalpies values (ranging from 15.5 to −18.0 kJ/g);
- Waste biomasses from poultry feathers, which have the highest combustion enthalpies values (ca 20 kJ/g).
All the combustion enthalpies recorded for the citrus waste biomasses are similar except for grapefruit wastes, which present a slightly higher value (−17.2 kJ/g). The measured values are in reasonable agreement with the ones already reported in the literature (orange peels waste: −18.3 kJ/g [17], grapefruit peels waste: −18.3 kJ/g [17], and tangerine peels waste: −16.8 kJ/g [31]) indicating that the pectin and essential oils extraction process to which our samples were subjected did not significantly alter the energy content stored in the biomass. The small differences observed can easily be accounted for by differences in composition/humidity/ash content of the starting material due to the heterogeneous nature of the samples. In the case of the aromatic herbs, the solid residues from rosemary have the highest ΔcH value (−18.0 kJ/g), followed by the solid residues from Ruta chalepensis L. (−17.7 kJ/g), Artemisia vulgaris L. (−16.4 kJ/g), thyme (−16.1 kJ/g) and the lowest ΔcH value for lavender (−15.5 kJ/g). The slight differences in the ΔcH value can be ascribed to the differences in the composition/moisture content of the different biomasses. ΔcH values of aromatic herb waste biomasses are scarcely reported in the literature, with only one paper reporting the ΔcH value for lavender waste stalks after the distillation process (−19.6 kJ/g) [32]. Feather wastes after keratin extraction in acetic acid (70%) present a higher ΔcH value (−19.2 kJ/g), which is anyway lower with respect to the one reported in the literature for “uncleaned” poultry feathers (burned without degreasing) (−26.1 kJ/g) [33], probably because the latter may account for the contribution of lipids and fatty acid compounds presented in the raw substrate which are not present in our degreased sample. Another important point that needs to be taken into account when evaluating the possibility of recovering energy from feathers is that, despite the promising values in terms of ΔcH, feather combustion leads to the emission of gaseous NOx and SO2 and other substances highly dangerous for the environment and human health [34]; thus, it needs to be performed in specific plants which take care also of their disposal.
Overall, all the biomasses show a good energy recovery. It is important to remember that measured heats of combustion represent HHVs and that the lower heat values (LHV) can be roughly estimated, reducing the corresponding HHVs by about 6–10% according to the nature of considered biomass. Even taking into account this reduction, all the biomasses are highly exploitable for energy recovery if compared to the combustion of urban solid residues (LHV of ca 8 kJ/g [35]). Laws usually provide threshold LHV values for the exploitation of biomasses in industrial plants. For instance, Italian law suggests a minimum LHV of 15 kJ/g, which is close to those estimated for biomasses reported in the present work.
3.2. Combustion Enthalpy Calculated by FTIR/Chemometric Method
After the removal by extraction of essential oils, polyphenols and pectin, citrus peels, and aromatic herb wastes are mainly composed of lignocellulosic matter. Figure 2 shows the FTIR spectra of citrus peel waste (Figure 2a) and aromatic herb waste (Figure 2b). The lignocellulosic nature of the substrates was confirmed by characteristic absorption bands as already described elsewhere [29]. In particular, we can distinguish the band at around 1020 cm−1 (C-O stretching of C-O-C group of the anhydroglucose ring of cellulose) and the band at 1237–1239 cm−1 (C-O-C stretching in phenol-ether bonds of lignocellulosic materials). These absorption bands are commonly associated with the overlapping of characteristic bands of cellulose, hemicellulose, and lignin in lignocellulosic materials [29]. The composition of lignocellulose, expressed as a percentage of hemicellulose, cellulose, and lignin, was estimated using the PLS model through the first derivative of the normalized FTIR spectra (fingerprint region 1900–800 cm−1) as reported elsewhere [29]. Figure 2c,d show the lignocellulose composition for citrus peel and aromatic herb wastes, respectively. Citrus peel waste from even different substrates has a very similar composition to each other, with cellulose as the main component (ranging from 40 to 44%) followed by hemicellulose (23–24%) and lignin (18–19%). In the case of aromatic herbal wastes, cellulose was also the main component, but we observed significant differences among different substrates in terms of cellulose and lignin content, while the content of hemicellulose was similar for all the samples.
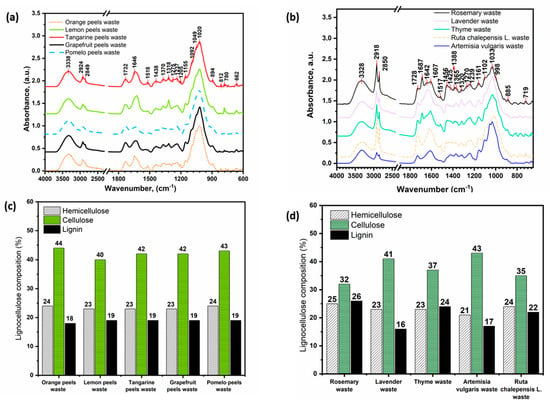
Figure 2.
ATR-FTIR spectra (a,b) and the PLS predicted chemical composition of cellulose, hemicellulose, and lignin (c,d) of citrus-based waste and aromatic herbs waste, respectively.
The percentage values of lignocellulosic components were then used to calculate the ΔcH using Equation (3) (experimental Section 2.6). The results are reported in the following Section 3.4.
3.3. Fuel Capacity Calculated by HHV
The higher heating value (HHV) is a parameter commonly used to assess the thermochemical characteristics of fuels, including biomasses. An estimation of this value can be obtained using a mathematical relationship that takes into account the fuel composition [36,37,38]. In the specific case of biomasses, data on the composition can be obtained by proximate analysis (wt% of moisture, volatile matter, fixed carbon, and ash) or ultimate analysis (wt% of carbon, hydrogen, nitrogen, oxygen, and sulfur). Here, we used a model proposed by S. A. Channiwala and P. P. Parikh (Equation (4)), which needs the data obtained from both analyses [39].
with C, H, S, O, and N, the percentages of carbon, hydrogen, sulfur, oxygen and nitrogen in the sample (obtained by ultimate analysis), and A, the percentage of ash in the sample (obtained by TGA experiments).
The elemental composition and the ash content for each sample are reported in Table 2. Different biomasses have a similar composition in terms of carbon (between 40–50%) and hydrogen percentage (about 6–7%). On the contrary, a remarkable difference is observed in nitrogen and sulfur content between feathers and vegetable wastes due to the proteinaceous nature of the former (N: about 15% against ≈ 1%; S: 2.7% in feathers, below the quantification limit in vegetable waste). A significant difference is also observed in the ash content of aromatic herb wastes, whose values range from 3% up to 7% for Ruta chalepensis L. waste, significantly higher than those of all other biomasses analyzed here. The high ash content in aromatic herbs may be due to the natural bioaccumulation of minerals/inorganic matter during their cultivation or interaction with their growth soils. The HHV values calculated from this information and Equation (4) are reported in the following paragraph.

Table 2.
Elemental composition by ultimate analysis and the ash content determined by TGA analysis of waste biomasses (citrus peels, aromatic herbs and poultry feathers).
3.4. Comparison of the Data Obtained by Different Methodologies
The combustion enthalpies (ΔcH) of the waste biomasses derived from agro-food activities determined experimentally by direct calorimetric measurements and calculated with the two approaches described above are summarized in Table 3.

Table 3.
Experimental combustion enthalpy of different waste biomasses (citrus peels, aromatic herbs and poultry feathers) compared with the values calculated by the different approaches herein described.
The data obtained by direct calorimetry (calorimetric bomb), FTIR chemometrics, and HHV estimation based on elemental and proximate analysis are in good agreement with each other. HHV calculated with the model proposed by Channiwala et al. gave values significantly different with respect to ΔcH experimental values (paired-samples t-test, α = 0.001), probably due to several variables not included in the theoretical equation. The other two data sets were not statistically different (paired-samples t-test, α = 0.001), confirming that the burning part of the citrus peel and aromatic herb wastes is effectively composed mostly of the lignocellulosic fraction. Nonetheless, it is important to remember that the FTIR method can only be used for wastes in which this fraction is the major component, e.g., it is not suitable for poultry feathers.
Both the calculation-based methodologies revealed suitable for the estimation of the samples’ energetic properties, giving ΔcH values in good agreement with those obtained by direct calorimetry, which should always be considered as the golden standard. Overall the obtained values were in reasonable agreement with those reported in the literature for lignocellulose woods (ranging from −18.4 kJ/mol for wheat straw to −20.7 kJ/mol for pine) [40] and solid residues for spices (ΔcH = −17.1 kJ/mol for clove buds) [27].
4. Conclusions
In this paper, we reported the combustion heat (ΔcH) of different kinds of waste biomasses, highlighting their potential for energy production. Experimental values obtained by indirect methods based on both FTIR/chemometrics and proximate analysis matched those determined by direct calorimetry; thus, these methodologies can constitute a reliable and affordable alternative whenever the direct measurement is not possible (for instance, because of instrumentation unavailability. FTIR in tandem with chemometrics seems to be the easiest, cheaper, and fast approach even though this method can be used only on lignocellulosic biomasses and not on other kinds of samples, e.g., for the feathers, which are mostly made of keratin.
Author Contributions
Conceptualization, E.P., J.G.-R., L.B. and C.D.; methodology, E.P., J.G.-R. and E.B.; investigation, E.P., J.G.-R., E.B. and C.P.; resources, C.P., C.D., M.R.T., L.B. and C.F.; data curation, E.P. and J.G.-R.; writing—original draft preparation, E.P., J.G.-R. and C.P.; writing—review and editing, E.B., C.F., L.B., M.R.T. and C.D.; supervision, E.B., C.F., L.B., M.R.T. and C.D.; funding acquisition, C.F. and M.R.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Project INSOLE-Innovazioni agronomiche e tecnologiche per la coltivazione sostenibile di piante officinali e la produzione di oli essenziali di qualità” nell’ambito del PSR Sicilia 2014–2020. Sottomisura 16.1. Sostegno per la costituzione e la gestione di gruppi operativi del PEI in materia di produttività e sostenibilità dell’agricoltura.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors would like to thank Massimo Guelfi and Rita Carosi for their valuable technical support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Taghian Dinani, S.; van der Goot, A.J. Challenges and solutions of extracting value-added ingredients from fruit and vegetable by-products: A review. Crit. Rev. Food Sci. Nutr. 2022, 1–23. [Google Scholar] [CrossRef]
- Russo, C.; Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Barreca, D.; Rapisarda, A.; Cirmi, S.; Navarra, M. The Second Life of Citrus Fruit Waste: A Valuable Source of Bioactive Compounds. Molecules 2021, 26, 5991. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, E.H.; Mazzei, R.; Bazzarelli, F.; Piacentini, E.; Giannakopoulos, V.; Roberts, M.R.; Giorno, L. Agri-Food Industry Waste as Resource of Chemicals: The Role of Membrane Technology in Their Sustainable Recycling. Sustainability 2022, 14, 1483. [Google Scholar] [CrossRef]
- Muller, B.; Laibach, L. Extraction of valuable components from waste biomass. In Waste to Food; Smetana, S., Pleissner, D., Zeidler, V.Z., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2022; Chapter 5; ISBN 978-90-8686-377-8. [Google Scholar]
- Pulidori, E.; Micalizzi, S.; Bramanti, E.; Bernazzani, L.; Duce, C.; De Maria, C.; Montemurro, F.; Pelosi, C.; De Acutis, A.; Vozzi, G.; et al. One-Pot Process: Microwave-Assisted Keratin Extraction and Direct Electrospinning to Obtain Keratin-Based Bioplastic. Int. J. Mol. Sci. 2021, 22, 9597. [Google Scholar] [CrossRef]
- Pulidori, E.; Micalizzi, S.; Bramanti, E.; Bernazzani, L.; De Maria, C.; Pelosi, C.; Tinè, M.R.; Vozzi, G.; Duce, C. Valorization of not soluble byproducts deriving from green keratin extraction from poultry feathers as filler for biocomposites. J. Therm. Anal. Calorim. 2022, 147, 5377–5390. [Google Scholar] [CrossRef]
- Sinkiewicz, I.; Śliwińska, A.; Staroszczyk, H.; Kołodziejska, I. Alternative Methods of Preparation of Soluble Keratin from Chicken Feathers. Waste Biomass Valorization 2017, 8, 1043–1048. [Google Scholar] [CrossRef]
- Rouse, J.G.; Van Dyke, M.E. A Review of Keratin-Based Biomaterials for Biomedical Applications. Materials 2010, 3, 999–1014. [Google Scholar] [CrossRef]
- Lucchesi, M.E.; Chemat, F.; Smadja, J. Solvent-free microwave extraction of essential oil from aromatic herbs: Comparison with conventional hydro-distillation. J. Chromatogr. A 2004, 1043, 323–327. [Google Scholar] [CrossRef]
- Kant, R.; Kumar, A. Review on essential oil extraction from aromatic and medicinal plants: Techniques, performance and economic analysis. Sustain. Chem. Pharm. 2022, 30, 100829. [Google Scholar] [CrossRef]
- Giacometti, J.; Bursać Kovačević, D.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G.; Stulić, V.; Barba, F.J.; Chemat, F.; Barbosa-Cánovas, G.; et al. Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef]
- Duce, C.; Vecchio Ciprioti, S.; Spepi, A.; Bernazzani, L.; Tinè, M.R. Vaporization kinetic study of lavender and sage essential oils. J. Therm. Anal. Calorim. 2017, 130, 595–604. [Google Scholar] [CrossRef]
- Mastellone, M.L. Waste Management and clean Energy Production from Municipal Solid Waste; Nova Science Publishers, Inc.: New York, NY, USA, 2015. [Google Scholar]
- Basu, P. Analytical Techniques. In Biomass Gasification, Pyrolysis and Torrefaction; Elsevier: Amsterdam, The Netherlands, 2018; pp. 479–495. [Google Scholar]
- Knurr, B.J.; Hauri, J.F. An Alternative to Recycling: Measurement of Combustion Enthalpies of Plastics via Bomb Calorimetry. J. Chem. Educ. 2020, 97, 1465–1469. [Google Scholar] [CrossRef]
- Bouabid, G.; Nahya, D.; Azzi, M. Determination of heating value of industrial waste for the formulation of alternative fuels. MATEC Web Conf. 2013, 5, 04031. [Google Scholar] [CrossRef]
- Tamelová, B.; Malaťák, J.; Velebil, J.; Gendek, A.; Aniszewska, M. Energy Utilization of Torrefied Residue from Wine Production. Materials 2021, 14, 1610. [Google Scholar] [CrossRef]
- Picone, A.; Volpe, M.; Giustra, M.G.; Bella, G.D.; Messineo, A. Hydrothermal carbonization of lemon peel waste: Preliminary results on the effects of temperature during process water recirculation. Appl. Syst. Innov. 2021, 4, 19. [Google Scholar] [CrossRef]
- Indulekha, J.; Gokul Siddarth, M.S.; Kalaichelvi, P.; Arunagiri, A. Characterization of Citrus Peels for Bioethanol Production. In Materials, Energy and Environment Engineering; Springer: Singapore, 2017; pp. 3–12. [Google Scholar]
- Selvarajoo, A.; Wong, Y.L.; Khoo, K.S.; Chen, W.-H.; Show, P.L. Biochar production via pyrolysis of citrus peel fruit waste as a potential usage as solid biofuel. Chemosphere 2022, 294, 133671. [Google Scholar] [CrossRef] [PubMed]
- Stan, C. Energy Recovery from Industrial Feather Waste by Gasification. J. Clean Energy Technol. 2018, 6, 401–404. [Google Scholar] [CrossRef]
- Stan, C.; Badea, A. Thermo-physico-chemical analyses and calorific value of poultry processing industry waste. UPB Sci. Bull. Ser. C 2013, 75, 277–284. [Google Scholar]
- Meraz, L.; Oropeza, M.; Dominguez, A. Prediction of the Combustion Enthalpy of Municipal Solid Waste. Chem. Educ. 2002, 7, 66–70. [Google Scholar] [CrossRef]
- Meraz, L.; Domínguez, A.; Kornhauser, I.; Rojas, F. A thermochemical concept-based equation to estimate waste combustion enthalpy from elemental composition. Fuel 2003, 82, 1499–1507. [Google Scholar] [CrossRef]
- González-Rivera, J.; Spepi, A.; Ferrari, C.; Duce, C.; Longo, I.; Falconieri, D.; Piras, A.; Tinè, M.R. Novel configurations for a citrus waste based biorefinery: From solventless to simultaneous ultrasound and microwave assisted extraction. Green Chem. 2016, 18, 6482–6492. [Google Scholar] [CrossRef]
- González-Rivera, J.; Duce, C.; Falconieri, D.; Ferrari, C.; Ghezzi, L.; Piras, A.; Tine, M.R. Coaxial microwave assisted hydrodistillation of essential oils from five different herbs (lavender, rosemary, sage, fennel seeds and clove buds): Chemical composition and thermal analysis. Innov. Food Sci. Emerg. Technol. 2016, 33, 308–318. [Google Scholar] [CrossRef]
- Gonzalez-Rivera, J.; Duce, C.; Campanella, B.; Bernazzani, L.; Ferrari, C.; Tanzini, E.; Onor, M.; Longo, I.; Ruiz, J.C.; Tinè, M.R.; et al. In situ microwave assisted extraction of clove buds to isolate essential oil, polyphenols, and lignocellulosic compounds. Ind. Crops Prod. 2021, 161, 113203. [Google Scholar] [CrossRef]
- Shehab, M.; Stratulat, C.; Ozcan, K.; Boztepe, A.; Isleyen, A.; Zondervan, E.; Moshammer, K. A Comprehensive Analysis of the Risks Associated with the Determination of Biofuels’ Calorific Value by Bomb Calorimetry. Energies 2022, 15, 2771. [Google Scholar] [CrossRef]
- Chen, H.; Ferrari, C.; Angiuli, M.; Yao, J.; Raspi, C.; Bramanti, E. Qualitative and quantitative analysis of wood samples by Fourier transform infrared spectroscopy and multivariate analysis. Carbohydr. Polym. 2010, 82, 772–778. [Google Scholar] [CrossRef]
- Gorensek, M.B.; Shukre, R.; Chen, C.-C. Development of a Thermophysical Properties Model for Flowsheet Simulation of Biomass Pyrolysis Processes. ACS Sustain. Chem. Eng. 2019, 7, 9017–9027. [Google Scholar] [CrossRef]
- Yankovsky, S.A.; Tolokolnikov, A.A.; Cherednik, I.V.; Kuznetsov, G.V. Reasons for tangerine peel utilization in the composition of mixed fuels based on bituminous coal. J. Phys. Conf. Ser. 2019, 1359, 012136. [Google Scholar] [CrossRef]
- Chakyrova, D.; Doseva, N. Analysis of the energy recovery possibilities of energy from lavender straws after a steam distillation process. In IOP Conferene Series: Material Science and Engeneering, Proceedings of the International Scientific Conference of Communications, Information, Electronic and Energy Systems (CIEES 2020), Borovets, Bulgaria, 26–29 November 2020; IOP Publishing Ltd.: Bristol, UK, 2021; p. 012023. [Google Scholar]
- Marculescu, C.; Stan, C. Poultry processing industry waste to energy conversion. Energy Procedia 2011, 6, 550–557. [Google Scholar] [CrossRef][Green Version]
- Stingone, J.A.; Wing, S. Poultry Litter Incineration as a Source of Energy: Reviewing the Potential for Impacts on Environmental Health and Justice. NEW Solut. A J. Environ. Occup. Health Policy 2011, 21, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Del Tedesco, S. Incineration of Municipal Solid Waste with Energy Recovery. Master’s Thesis, University of Padova, Padova, Italy, 2009. [Google Scholar]
- Dashti, A.; Noushabadi, A.S.; Raji, M.; Razmi, A.; Ceylan, S.; Mohammadi, A.H. Estimation of biomass higher heating value (HHV) based on the proximate analysis: Smart modeling and correlation. Fuel 2019, 257, 115931. [Google Scholar] [CrossRef]
- Setyawati, W.; Damanhuri, E.; Lestari, P.; Dewi, K. Correlation Equation to Predict HHV of Tropical Peat Based on its Ultimate Analyses. Procedia Eng. 2015, 125, 298–303. [Google Scholar] [CrossRef][Green Version]
- Galhano dos Santos, R.; Bordado, J.C.; Mateus, M.M. Estimation of HHV of lignocellulosic biomass towards hierarchical cluster analysis by Euclidean’s distance method. Fuel 2018, 221, 72–77. [Google Scholar] [CrossRef]
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Ioelovich, M. Thermodynamics of Biomass-Based Solid Fuels. Acad. J. Polym. Sci. 2018, 2, 555577. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).